Abstract
The beneficial effects of omega-3 fatty acids are believed to be due in part to selective alteration of arachidonate metabolism that involves cyclooxygenase (COX) enzymes. Here we investigated the effect of eicosapentaenoic acid (EPA) on the proliferation of human non-small cell lung cancer A549 (COX-2 over-expressing) and H1299 (COX-2 null) as well as their xenograft models. While EPA inhibited 50% of proliferation of A549 cells at 6.05 μM, almost 80 μM of EPA was needed to reach similar levels of inhibition of H1299 cells. The formation of PGE3 in A549 cells was almost 3-fold higher than that of H1299 cells when these cells were treated with EPA (25 μM). Intriguingly, when COX-2 expression was reduced by siRNA or shRNA in A549 cells, the antiproliferative activity of EPA was reduced substantially compared to that of control siRNA or shRNA transfected A549 cells. In line with this, dietary menhaden oil significantly inhibited the growth of A549 tumors by reducing tumor weight by 58.8 ± 7.4 %. In contrast, similar diet did not suppress the development of H1299 xenograft. Interestingly, the ratio of PGE3 to PGE2 in A549 was about 0.16 versus only 0.06 in H1299 xenograft tissues. Furthermore, PGE2 up-regulated expression of pAkt, whereas PGE3 downregulated expression of pAkt in A549 cells. Taken together, the results of our study suggest that the ability of EPA to generate PGE3 through COX-2 enzyme might be critical for EPA-mediated tumor growth inhibition which is at least partly due to down-regulation of Akt phosphorylation by PGE3.
Keywords: Antiproliferative activity, NSCLC cells, fish oil EPA, COX-2, Prostaglandins
Introduction
Lung cancer is the single greatest cause of death from malignancy in both men and women in the United States and is greater than for the next 3 leading cancers (prostate, colon, and breast) combined (1). The overall 5-year survival rate for patients with lung cancer is less than 15% and, this has remained largely unchanged for the last 3 decades. Thus, finding an effective prevention and treatment strategy for this cancer is desperately needed. Although smoking cessation is a key factor in halting the lung cancer epidemic, most lung cancers develop in ex-smokers (2). Inflammation has been postulated to play a key role in lung carcinogenesis. Regular use of aspirin and other nonsteroidal anti-inflammatory agents (NSAIDs) is associated with a reduced risk of developing lung cancer in animal models and among smokers (2-5). Among several proinflammatory mediators, cyclooxygenase-2 (COX-2), the inducible form of COX and its arachidonic acid (AA) metabolite, PGE2, have been suggested as novel targets for lung cancer therapy and chemoprevention (6) because COX-2 and PGE2 have been implicated in apoptosis resistance (7), angiogenesis (8,9), decreased host immunity (10), and enhanced invasion and metastasis (11). A phase II clinical trial suggested that treatment with chemotherapy plus celecoxib led to superior survival in patients with high intratumoral COX-2 expression than did chemotherapy alone. This suggests that some non-small cell lung cancers (NSCLCs) (35%) are associated with relative COX-2 enzymatic activity and this may be a pivotal target for this subpopulation of lung cancer patients (12). However, due to the cardio-toxicity believed to be associated with use of selective COX-2 inhibitors in the clinical setting, targeting COX-2 pathways by altering COX-2’s AA metabolism with little or no cardiotoxicity may provide a promising chemopreventive approach for lung cancer, particularly in a patient population with moderate to high expression of COX-2.
While a large body of evidence indicates that n-6 polyunsaturated fatty acids promote the growth of tumor cells, n-3 fatty acids (e.g. EPA and DHA) have actually been shown to inhibit breast, colon, prostate, and lung tumor cell proliferation (Reviews in [13,14]). EPA and DHA have also been shown to induce apoptosis in a number of cell lines, including human lung A549 and A427 cells (15-17). Fish oil inhibited the growth of NSCLC cell lines (H1838 and H1792) by suppressing integrin-linked kinases (18). The anti-inflammatory activity of fish oil was demonstrated by the significantly reduced expression of pro-inflammatory genes or related products, such as LTB4, PI3Kα, IL-1β, IL-10, and IL-23 in the peripheral blood mononuclear cells (PBMC) of a healthy population given fish oil-derived EPA (775 mg/day) for 5 weeks (19). Number of epidemiological studies suggest fish consumption is significantly inversely associated with lung cancer risk and mortality (20-23). Most recently, a study suggested that a combination of n-3 fatty acids (2 g of fish oil, twice daily) and a COX-2 inhibitor (celecoxib, 200 mg) could ameliorate the symptoms and signs associated with Systemic Immune-Metabolic Syndrome in advanced lung cancer (24).
Although the mechanisms of antitumor activity of fish oil are not clear, a number of studies have suggested that the anticancer activity of both EPA and DHA are associated eicosanoid metabolism through their ability to inhibit PGE2 production (25). In contrast to DHA, EPA can function as a substrate of COX for the synthesis of a unique series of PG3-related compounds (26). We reported that human lung cancer cells and pure COX enzymes have the capacity to synthesize PGE2 and PGE3 (Fig S1) when incubated with either AA or EPA, respectively. We have also shown that in human NSCLC A549 cells, formation of PGE3 results mainly from COX-2, but not COX-1, suggesting that PGH3 is an efficient substrate for PGE synthase (27). In comparison to our understanding of the biosynthesis of the n-3 series of PGs, knowledge of the biologic functions of these eicosanoids is limited. Studies from our laboratory indicate that PGE3 inhibits proliferation of A549 cells, whereas PGE2 slightly stimulates the growth of these cells (27). Addition of PGE2 eliminates the inhibitory effect of fish oil, while the addition of PGE3 reduced the invasiveness of hepatoma cells pretreated with safflower oil (28). Significant elevation of PGE3 has been detected in rat colonic mucosa, which may contribute to fish oil-induced apoptosis in azoxymethane alone or in irradiated azoxymethane treated rats (29). Moreover, PGE3 suppresses the induction of angiopoietin-2 and results in inhibition of angiogenesis in HUVEC cells while it also modulates COX-2–mediated invasion and angiogenesis in brain-metastatic melanoma (30,31).
Whether fish oil derived PGE3 contributes to the chemopreventive effect of fish oil on lung cancer has not been comprehensively investigated, we therefore studied the relative effect of fish oil derived EPA on proliferation of COX-2 expressing human lung cancer A549 cells and COX-2 null H1299 cells and their respective xenograft models. A correlation between formation of PGE3 and inhibition of PGE2 in xenograft tumor tissues and the tumor suppressive effect of fish oil in A549 xenograft models was observed. Furthermore, our data suggests that the anti-proliferative effect of EPA or PGE3 might be mediated, at least in part, through down-regulation of phosphorylation of the Akt pathway
Materials and Methods
Materials and reagents
EPA (> 99%) , DHA (> 98%), butylated hydroxytoluene (BHT), EDTA and MTT were purchased from Sigma Chemical Co. (St. Louis, MO). PGE2, PGE2-d4, PGE3 and anti-COX-2 antibody were obtained from Cayman Chemical (Ann Arbor, MI). Calcein acetoxymethyl (CAM) ester was purchased from Molecular Probes (Eugene, OR). Anti–β-actin antibody was also purchased from Sigma.
Cell lines
Human non-small cell lung cancer H1299 and A549 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained in a humidified atmosphere containing 5% CO2 at 37°C. H1299 cells were routinely cultured in MEM medium (Invitrogen Corp, Grand Island, NY) supplemented with 10% heat inactivated fetal bovine serum ([FBS] Hyclone Laboratories Inc., Logan, UT), 50 IU/ml penicillin and 50 μg/ml streptomycin, and 2 mM L-glutamine from GIBCO (Invitrogen). A549 cells were routinely grown in DMEM-F12 medium (Invitrogen) supplemented with 5% heat inactivated FBS, (Hyclone Laboratories Inc., Logan, UT), 50 IU/ml penicillin and 50 μg/ml streptomycin, and 2 mM L-glutamine from GIBCO (Invitrogen). All cell lines were authenticated via microscopic morphology check and DNA characterization.
Cytotoxicity determination
Cells were grown at a density of 1 × 104 cells/well in their relevant media as indicated above. After a 24 hr incubation period, cells were washed with serum free medium prior to treatment with various concentrations of EPA and DHA (1 to 100 µM). After an additional 72 hr, inhibition of cellular proliferation was assessed by MTT assay (32). Absorbance was read at a wavelength of 570 nm and a reference wavelength of 650 nm using a V-Max Micro-plate Reader by Molecular Devices, Inc. (Sunnyvale, CA).
Western blot analysis
Cells were washed with cold PBS and scraped free in the presence of lysis buffer (20 mM MOPS, 2 mM EGTA, 5 mM EDTA, 30 mM NaF, 40 mM β-glycerophosphate, 20 mM sodium pyruvate, 0.5% Triton X-100, and 1 mM sodium orthovanadate with protease inhibitor cocktail). Cell lysates were then sonicated on ice for 3 min, incubated for an additional 10 min at 4°C prior to centrifugation at 14,000 × g (10 min at 4°C). Protein levels were quantified via the BioRad Dc protein assay (BioRad, Inc., Hercules, CA). Equal levels of protein (50 μg) were applied to BioRad precast gels or NuPAGE Novex precast bis-tris mini-gels (Invitrogen, Carlsbad, CA) and then transferred onto polyvinylidene diflouride membranes, according to standard methods. Following a 1- to 2-hr incubation period in 5% nonfat dry milk blocking buffer prepared in Tris-buffered saline with 0.1% Tween 20, membranes were probed with primary antibodies to COX-1 (Abcam Inc, Cambridge, MA), COX-2 (Cayman Chemical), Akt and pAkt (Cell Signaling Technologies, Inc., Danvers, MA) diluted 1:2,000 in blocking buffer. Protein bands were visualized via chemluminesence using the ECL+ detection kit and hyper-film (Amersham Biosciences, Piscataway, NJ). Equal loading of samples was illustrated by Western blotting for the presence of β-actin. Protein bands were quantified using Alpha DigiDoc 1000 software (Alpha Innotech Corp., San Leandro, CA).
Development of COX-2 transient and stable knockdowns in A549 cells
A549 cells were plated in 6 and 48 well plates and allowed to attach overnight. Transient transfection of non-specific siRNA (control siRNA) and COX-2 siRNA molecules was carried out using siPORT™ Amine Transfection Agent (Ambion, Austin, TX) and COX-2 silencing RNA (0.2-0.4 µM) (Santa Cruz Biotechnology, Inc. Santa Cruz, CA) following the manufacturer’s instructions. Twenty-four hr after transfection, cells were treated with 50 to 200 µM EPA for 24 hr. Protein was collected from the 6 well plates 72 hr after transfection for Western blot analysis. Assessment of cell viability affected by different treatments was carried out by Calcien AM staining (33).
For the development of stable COX-2 knockdown A549 cells, cells were set up in 6 well plates with complete media and incubated overnight. Media containing 5 µg/ mL polybrene (Santa Cruz Biotechnology) was added to the cells. COX-2 shRNA Lentiviral Particles (Santa Cruz Biotechnology) was added to the culture at a concentration of 1.0 × 104 infectious units of virus (10 µL of stock) and incubated for 24 hours. The culture media was replaced with 1 ml of complete medium (without Polybrene) and incubated overnight. Cells were then split 1:3 and then further incubated 24-48 hours in complete medium. COX-2 silenced cells were selected by using 8 µg/mL puromycin dihydrochloride (Santa Cruz Biotechnology). Fresh puromycin-containing medium was added every 3-4 days until resistant colonies were identified. Individual colonies were isolated and maintained in a medium containing puromycin (8 µg/mL). The expression of COX-2 in the stably transfected A549 cells was determined by western blot analysis. The anti-proliferative effect of EPA in the COX-2 stably knockdown A549 cells was evaluated with BrDU assays (Calbiochem).
Eicosanoid analyses
Levels of eicosanoids in the lung cancer cells and xenograft tissues were determined according to the method of Kempen et al. (34) and Yang et al. (35,36). In brief, A549 or H1299 (5 × 106) were harvested by trypsinization and washed with PBS and then resuspended in 0.5 ml of PBS containing 1 mM CaCl2. For exogeneous eicosanoid analysis, samples were incubated with 2.5 μL of calcium ionophore A23187 (1 mM) for 2 min, followed by addition of an aliquot of 2.5 μL EPA (10 to 50 μM). Samples were then incubated for an additional 10 min. The reaction was terminated by addition of aliquots of 40 μL of 1 N citric acid and 5 μL of 10% BHT. An aliquot (10 μL) of the PGE2-d4 (100 ng/mL) was added as an internal standard to the reaction mixtures. Eicosanoids were extracted using 2 mL of hexane-ethyl acetate (1:1; v/v) and the extraction was repeated three times. The upper organic phases were pooled and evaporated to dryness under a stream of nitrogen at room temperature. All extraction procedures were performed under conditions of minimal light. Samples were then reconstituted in 100 μL of methanol/10 mM ammonium acetate buffer (70:30, v/v) (pH 8.5) prior to analysis by liquid chromatography/tandem mass spectrometry (LC/MS/MS).
For eicosanoid analysis of xenografts, frozen tissue (25–50 mg) was ground to a fine powder using a liquid nitrogen–cooled mortar (Fisher Scientific). Samples were then transferred to sealed microcentrifuge tubes, and ice-cold PBS buffer, containing 0.1% BHT and 1 mM EDTA, three times the volume of the samples was added. The mixture was then homogenized by using an ultrasonic processor (Misonix, Farmingdale, NY) at 0°C for 3 min. A 100-μL aliquot of the homogenate was transferred to a glass tube (13 × 100 mm) and subjected to extraction of eicosanoids using the method of Yang et al. (36).
Reverse-phase HPLC electrospray ionization mass spectrometry (MS) was used to determine eicosanoid (PGE2 and PGE3) levels in cells or tissues using previously published methods reported by our laboratory (34,37). A Micromass Quattro Ultima tandem mass spectrometer (Waters, Milford, MA) was equipped with an Agilent 1100-HP binary pump high-pressure liquid chromatography (LC) inlet for use in these studies. Eicosanoids were separated using a Luna 3-μ phenyl-hexyl (2 × 150 mm) analytical LC column (Phenomenex, Torrance, CA). The mobile phase consisted of 10 mM ammonium acetate (pH 8.5) and methanol; the flow rate was 250 μL/minute, the column temperature was 50°C, and the sample injection volume was 25 μL. Samples were kept at 4°C in an autosampler prior to injection into the column. The mass spectrometer was operated in the electrospray negative-ion mode with a cone voltage of 100 V. Fragmentation of all compounds was performed using argon as the collision gas at a cell pressure of 2.1 × 10–3 torr. The collision energy was 19 V. All eicosanoids were detected using negative ionization and multiple-reaction monitoring of the transition ions for eicosanoid products and their internal standards (36). The concentration of these eicosanoids was normalized by the amount of protein or number of cells.
Animal Study
All animal experiments were approved by The University of Texas MD Anderson Cancer Center Animal Care and Use Committee. The human lung xenograft A549 and H1299 tumor models were developed using a modified version of the method described by Raben et al. (38). Five- to 6-week-old male BALB/c athymic (Nu/Nu) mice were purchased from Charles River Laboratories (Wilmington, MA) and acclimated to the institutional animal care facility for 1 week. They were then randomized to three different groups based on diets: regular AIN-76 mouse chow, soybean oil and menhaden oil diets (Research Diet Inc., New Brunswick, NJ). The composition of the diets is listed in Table 1. Diets were vacuum sealed in small quantities prior to their arrival to the animal facility to prevent auto-oxidataion. Upon arrival, diets were kept at −20°C. Food was replaced every other day to prevent consumption of oxidized lipids. After 10 days, the mice were then subjected to subcutaneous injections of human non-small cell lung cancer A549 (1 × 107) or H1299 (5 × 106) cells suspended in 100 μl of cell culture medium. Body weight were measured every three days. Four weeks after inoculation, the mice were sacrificed, tumor was collect and weighed. Tumor tissues were rapidly collected from mice and flash-frozen in liquid nitrogen for further analysis.
Table 1.
Experimental Diet Compositiona
| Component | AIN -76 | Soybean oil | Menhaden Oil |
|---|---|---|---|
| Casein | 200 | 200 | 200 |
| DL-Methionine | 3 | 3 | 3 |
| Corn Starch | 150 | 50 | 50 |
| Maltodexrin | 0 | 100 | 100 |
| Sucrose | 500 | 309 | 309 |
| Corn oil | 50 | 0 | 0 |
| Soybean oil | 0 | 135 | 45 |
| Menhaden oilb | 0 | 0 | 90 |
All diets (g/kg) contain 35 g of mineral mix, 10 g of vitamin mix, 50 g of cellulose, and 2 g choline Bitartrate
Menhaden oil contains 32% of n-3 fatty acids with an EPA and DHA ratio of 3: 2.
Diet
Both soybean and menhaden oil diets were made by Research Diet Incorporation (NJ). They are composed of 15% fat by weight, which equates to 30% of energy per day for humans. The diets were modified based on AIN-76 dietary content. The soybean oil diet contained 15 g of soybean oil replacing 5 g of corn oil within every 100 g of regular AIN-76 diet. In comparison, the menhaden oil diets contained 10 g of menhaden oil/100 g diet and 5.5 g of soybean oil per 100 g of diet to deliver the levels of the essential fatty acid linolenic acid that is necessary to protect against essential fatty acid deficiency. The amount of sucrose was proportionally reduced. Additionally, 2 mg of tertiary butylhydroquinone was added per 100 g of diet as an antioxidant. Fresh diets were prepared as necessary and kept at −20º C for long-term storage (months) or 4º C for short-term storage (weeks) to prevent lipid oxidation.
Immunohistochemical analysis
Immunohistochemical examination of cleaved caspase 3 was performed in the frozen section of the tissues. Sections were treated with treated with primary antibody against cleaved caspase-3 (1:250 diluted; Santa Cruz Biotechnology). Images were collected randomly at ×40 using a EVOS microscope Westover Scientific’s Advanced Microscopy Group). The quantitation of cleaved caspase-3 positive cells were performed by counting the cells in 8 selected field.
Statistical analysis
Student’s t-test was used to determine the statistical differences between various experimental groups; a value of P ≤ 0.05 was considered to be significant.
Results
The antiproliferative effect of EPA in NSCLC cells is correlated with the level of COX-2 expression
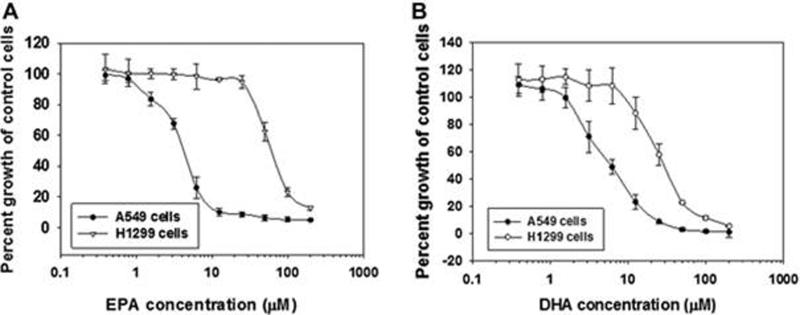
In order to test whether EPA and DHA differentially inhibit the proliferation of NSCLC, we selected two human non-small cell lung cancer cell lines: A549 and H1299 cells. As shown in Fig. 1A, EPA inhibited the proliferation of A549 cells with an IC50 value of 6.05 μM. In contrast, the inhibitory effect of EPA on H1299 cells was relatively weaker as evidenced by a 13-fold higher IC50 value (80 μM). In comparison, the inhibitory effect of DHA on the growth of A549 cells (IC50, 9.7 μM) was only 5-fold greater than that of H1299 cells (IC50, 50 μM) (Fig. 1B).
Figure 1.
The anti-proliferative effect of EPA and DHA in human non-small cell lung cancer A549 (COX-2 constitutively expressed) and H1299 (COX-2 lacking) cells. A. Exposure of A549 and H1299 cells to EPA for 72 hours produced an 8-fold stronger inhibition of cell proliferation to A549 cells than that in H1299 cells. B. Both A549 and H1299 cells were treated with DHA for 72 hrs. The anti-proliferative effect of DHA in A549 cells was only 5-fold stronger than that in H1299 cells.
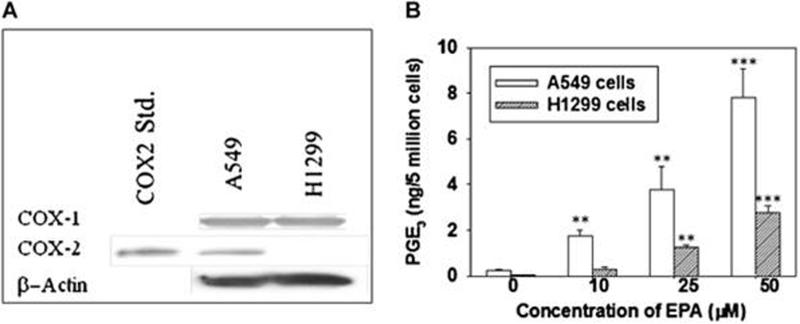
Because the anti-inflammatory activity of fish oils such as EPA and DHA have been reported to be due, at least in part, to inhibition of formation of PGE2, the relative expression of COX-2 was examined in these cell lines. Western blotting results (Fig.2A) demonstrated that the level of COX-1 protein expression was similar in the two cell lines. In contrast, A549 cells expressed a higher amount of COX-2 than H1299 cells, suggesting the differential anti-proliferative effect of EPA and DHA in NSCLC cells might be associated with the level of expression of COX-2 and consequently PGE2. Similar phenomena were also observed in EPA treated premalignant (1198) and tumorigenic (1170) lung cells, which showed much less sensitivity to EPA treatment than did A549 cells due to lower expression of COX-2 in these cells (data not shown).
EPA inhibits formation of PGE2 and increases production of PGE3 in A549 cells to a greater extent than in H1299 cells
We have previously demonstrated that EPA can serve as a substrate for COX-2 and give rise to PGE3 in A549 cells (29). Here we tested the ability of EPA to form PGE3 in H1299 cells in comparison to that in A549 cells. Human NSCLC H1299 cells (lacking expression of COX-2) and A549 cells were treated with different concentrations of EPA (10, 25 and 50 µM) and the relative formation of PGE2 and PGE3 in these cell lines was determined. The level of PGE3 formation in A549 cells was 2.02 ± 0.24 ng/5 million cells when these cells were treated with 10 µM EPA. Interestingly, H1299 cells treated with a similar concentration of EPA generated only 0.32 ± 0.04 ng/5 million cells, which was only 16% of that generated by A549 cells. The level of PGE3 in A549 and H1299 cells treated with 50 µM EPA was 7.98 ± 1.75 ng/5 million cells and 2.5 ± 0.3 ng/5 million cells, respectively. The formation of PGE3 by EPA in both A549 and H1299 cells was concentration dependent (Fig. 2B).
Figure 2.
The expression of COX-1 and COX-2 in A549 and H1299 cells and the relatively formation of PGE2 and PGE3 is shown for both cell line. A. A549 and H1299 cells were collected, lysed, and protein levels of COX-1 and COX-2 determined by Western blotting with relevant antibodies. B. Cells (5 × 106) were treated with EPA (25 to 100 μM) for 20 min at 37°C followed by extraction with hexane and ethyl acetate (1:1) as described in Material and Methods. The formation of PGE3 by EPA in A549 cells was markedly higher than that in H1299 cells. * P < 0.05, ** P < 0.01, and *** P < 0.005 versus control vehicle treated. Data are presented as the means ± SDs of three independent experiments.
Reduced COX-2 expression in A549 cells decreased the anti-proliferative effect of EPA
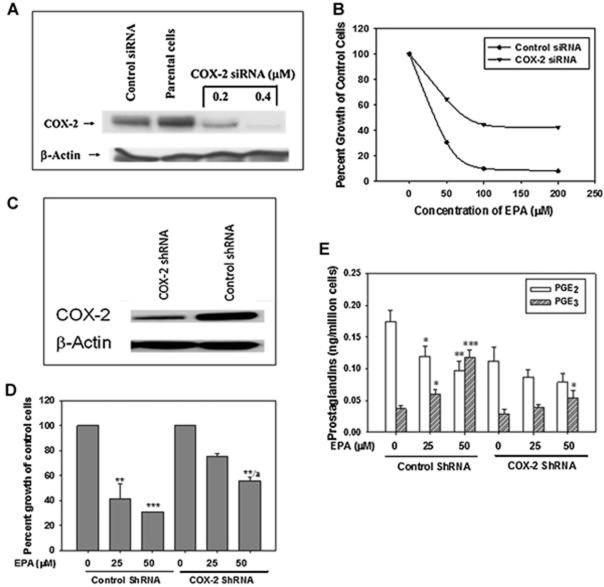
Previously, we reported that exposure of lung cancer cells to PGE2 (1 μM) slightly increased cell proliferation, whereas PGE3 (1 μM) significantly inhibited cell proliferation by 40% (29). In order to further confirm that the presence of the COX-2 enzyme is a critical factor in explaining the EPA-elicited anti-proliferative effect, A549 cells were transfected with COX-2 siRNA. The anti-proliferative activity of EPA in both control siRNA and COX-2 siRNA transfected A549 cells was then determined. As shown in Fig 3, when the expression of COX-2 protein was reduced by COX-2 siRNA by more than 90% (Fig. 3A), the inhibitory effect of EPA (50 µM) with regard to the growth of A549 cells was reduced by 40% compared to that of control siRNA transfected A549 cells, suggesting that the anti-proliferative effect of EPA was markedly reduced as a result of decreased expression of COX-2 in these particular cells (Fig. 3B). A similar phenomenon was observed when stable COX-2 knockdown A549 cells were treated with EPA. As shown Fig. 3C, the stable COX-2 knockdown A549 cells expressed markedly reduced COX-2 protein content compared to that of parental A549 cells (reduced over 90%). When the control-shRNA and shRNA COX-2 stably transfected A549 cells were treated with EPA for 24 hrs, the suppression of growth of stable COX-2 knockdown A549 cells by EPA (50 µM) was significantly reduced to 34% compared to that of control-shRNA treated group (inhibition was about 65%) (P < 0.05) (Fig. 3D).
Figure 3.
Decreasing the expression of COX-2 protein and formation of PGE3 in A549 cells affects cell sensitivity to EPA. A, Cells were transfected for 24 hr with either control siRNA or COX-2 siRNA (0.2-0.4 μM). Cells transfected with COX-2 siRNA resulted in approximately an 85% reduction of COX-2 protein compared to control siRNA transfected cells. The reduction of the COX-2 expression was concentration dependent. B. Cells exhibiting knockdown of COX-2 protein were less sensitive to EPA implying that the COX-2 target is necessary to retain sensitivity of A549 cells to EPA treatment. C. COX-2 expression was noticeably reduced in stably COX-2 knockdown A549 cells; D. COX-2 stably knockdown A549 cells were much less sensitive to EPA treatment than that in control-ShRNA transfected A549 cells. * P < 0.05, ** P < 0.01, and *** P < 0.005 versus control vehicle treated. a p< 0.05 versus control shRNA treated with 50 μM EPA. Data are presented as the means ± SDs of three independent experiments. E. The effect of EPA in the formation of PGE2 and PGE3 in control shRNA and COX-2 shRNA transfected A549 cells. While EPA was able to inhibit PGE2 formation and concomitantly increased production of PGE3 in the control shRNA transfected A549 cell, the effect of EPA on reduction of PGE2 and formation of PGE3 in COX-2 shRNA transfected A549 cells were relatively weaker comparing to that of control shRNA transfected cells. * P < 0.05, ** P < 0.01, and *** P < 0.005 versus control vehicle treated. Data are presented as the means ± SDs of three independent experiments.
Intriguingly, while EPA (50 μM) inhibited the formation of PGE2 in control ShRNA and COX-2 shRNA transfected A549 cells by 46.1% and 34.1%, respectively, EPA gave rise to much lower levels of PGE3 (0.068 ng/million cells) in COX-2 shRNA transfected A549 cells than that in control shRNA transfected A549 cells (0.126 ng/million cells) (p < 0.05). These effects were also concentration dependent (Fig. 3E).
PGE3-induced cell growth inhibition may be associated with inhibition of PI3K/Akt pathways
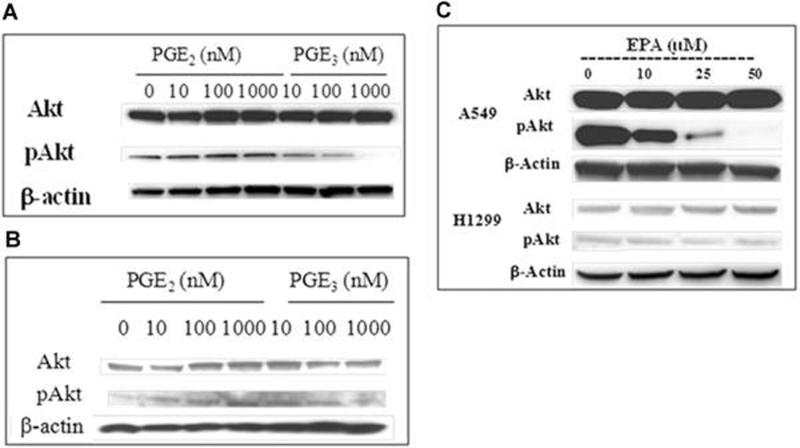
Since studies have suggested that one of the mechanisms of action for PGE2-mediated cell proliferation involves increased activity of PI3K and consequent elevation of Akt phosphorylation as well as increased MAPK activation in human colon cancer cells (43), we examined the effect of EPA, PGE2 and PGE3 on Akt and pAkt protein expression in both A549 and H1299 cells. Fig. 4A and B show that PGE2 (10−7 to 10−8 M) markedly increased phosphorylation of Akt in both A549 and H1299 cells in a concentration-dependent manner, whereas PGE3 failed to enhance Akt phosphorylation in either cell line. Rather, a marked reduction of pAkt expression was observed in A549 cells exposed to PGE3, suggesting that in contrast to PGE2, this fish oil-derived eicosanoid has the ability to result in inhibition of phosphorylation of Akt. The effect of PGE2 and PGE3 on PI3k activity was similar in both A549 and H1299 cells regardless of the differential status of COX-2 enzyme content. Interestingly, when A549 cells were treated with EPA (10 – 50 μM) for 24 hr, Akt phosphorylation was significantly reduced in a concentration-dependent manner, whereas no such change was observed in H1299 cells when they were treated with similar concentrations of EPA (Fig. 4B). These data suggest again that the lack of antiproliferative activity of EPA in H1299 cells may be associated with the relative lack of formation of PGE3 due to a lower level of COX-2 expression.
Figure 4.
The effect of PGE2, PGE3, and EPA on expression of Akt and pAkt in H1299 and A549 cells. Cells (2 × 106) were plated and allowed to attach overnight. They were then serum starved for 24 hrs followed by treatment with PGE2, PGE3 (10 to 1000 nM) or EPA (10 to 50 uM) for an additional 24 hrs. Cells were then harvested, proteins extracted and subjected to measurement of Akt and pAkt proteins by immunoblotting. A. A549 cells treated with PGE2 and PGE3; B; H1299 cells cells treated with PGE2 and PGE3; C A549 and H1299 treated with EPA. The data are representative of two sets of experiments with similar results.
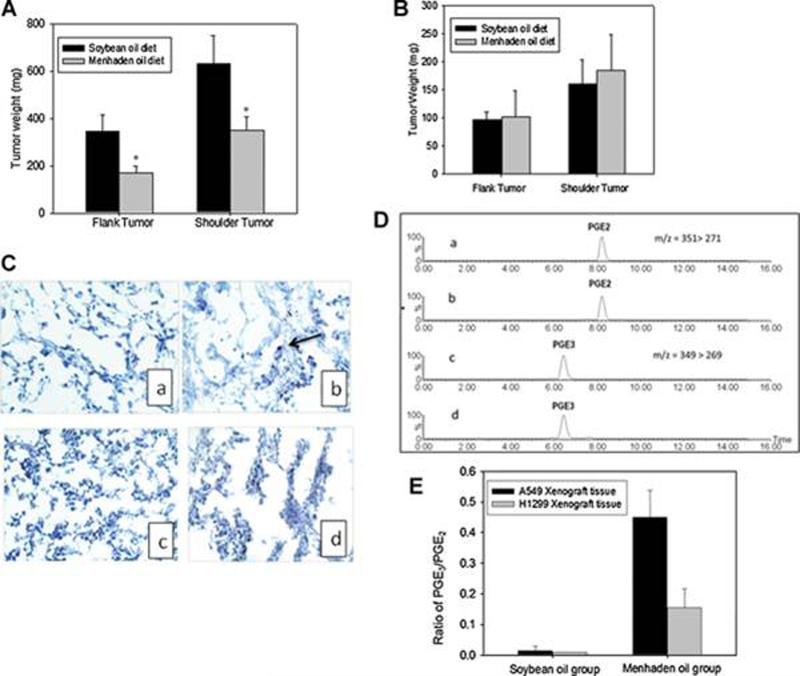
Menhaden oil inhibited growth of A549, but not H1299, xenografts
In order to test the hypothesis that COX-2 expression and formation of PGE2 as opposed to PGE3 is an important determinant of anticancer activity of fish oil, we tested the antitumor activity of menhaden oil, a commonly consumed fish oil, in both A549 and H1299 mouse xenograft models. Mice were fed a control soybean oil or a menhaden oil (EPA: DHA, 3:2) diet (containing 3% n-3 fatty acids, w/w) 10 days before they were subcutaneously inoculated with either A549 (10 × 106) or H1299 (5 × 106) cells. Mice continued receiving the diets for 4 weeks after tumor inoculation. As shown in Fig. 5, menhaden oil diets significantly inhibited the growth of A549 tumors demonstrated by a reduction in tumor weight of 58.8 ± 9.0 % and 48.8 ± 7.4 % (flank or shoulder tumor), respectively (Fig. 5A). In contrast, the tumor weights of mice on the menhaden oil diet in the H1299 xenograft model were similar to that of mice on soybean oil diet (Fig. 5B). Because we have previously reported that EPA could induce apoptotic cell death in A549 cells (27), we then examined whether tumor suppressive effect of menhaden oil was mediated through apoptosis by measuring the cleaved caspase 3 in the tumor tissues of A549 and H1299 xenograft. Fig 5C showed that of cleaved caspase 3 positive cells in A549 xenograft treated with menhaden oil was approximately 6.0 ± 3.4 per field comparing to that treated with soybean oil (not detected), whereas no noticeable cleaved caspase 3 positive cells were detected in either soybean or menhaden treated H1299 xenograft tissues.These in vivo data further support the hypothesis that fish oil exerts a stronger anti-proliferative activity when COX-2 is over-expressed than when COX-2 is absent.
Figure 5.
Antitumor efficacy of the menhaden oil in human non-small cell lung cancer A549 and H1299 xenograt models. A. Menhaden oil significantly inhibited the tumor growth implanted in both flank and shoulder positions in comparison to that of soybean oil treated A549 group (n = 10) ; B. the tumor weight of both flank and shoulder in H1299 bearing mice fed on the menhaden oil diet was similar to that of the control group (n=10). C. IHC staining of cleaved caspase 3 in A549 (a & b) and H1299 (c&d) xengraft tissues. a. A549 soybean; b A549 menhaden oil; c. H1299 soybean; d. H1299 menhaden oil. D. Representive of ion chromatogram of PGE2 and PGE3 in A549 xenograft tumor tissues. E. Endogenous levels of PGE2 and PGE3 in both A549 and H1299 xenograft tissues measured by LC/MS/MS method as described in Material and Methods. Menhaden oil markedly enhanced the ratio of PGE3 over PGE2 in A549 xenograft tissues while only a moderately enhancement of this ratio was observed in H1299 xenograft tissues (n=5). * P < 0.05 versus soybean diet treated group. Data are presented as mean ± SD.
Fish oil diets alter the tumor-derived PGE2 and PGE3 formation in A549 and H1299 xenograft tumor models
To further confirm that the formation of PGE3 was associated with COX-2 expression, we determined the formation of both PGE2 and PGE3 in A549 and H1299 xenograft tissues. As illustrated in Fig. 5D, both PGE2 and PGE3 can be detected in A549 xenograft tumor tissues. Interestingly, the endogenous levels of PGE2 in A549 tumor tissues were significantly reduced from 4.8 ± 0.7 ng/mg protein in the vehicle control group to 2.3 ± 0.7 ng/mg protein in the menhaden diet group (p < 0.05). In contrast, the formation of PGE2 in the vehicle control and menhaden oil treated groups in H1299 xenograft tissues was 0.17 ± 0.038 and 0.11 ± 0.02 ng/mg protein, respectively. Furthermore, menhaden oil increased the formation of PGE3 from an undetectable level in the vehicle control group to 0.89 ± 0.12 ng/mg protein in menhaden oil group in the A549 xenograft model, whereas only a small amount of PGE3 was detected in H1299 xenograft tissues. The ratio of PGE3 to PGE2 in menhaden oil fed mice with A549 xenografts was almost 3-fold higher than that generated in H1299 xenografts (Fig. 5E). It appears that the ratio of PGE3 to PGE2 is correlated with the antitumor efficacy of the fish oil in both A549 and H1299 cells. These data suggest again that the level of PGE3 synthesis may be directly linked to the expression of COX-2 proteins.
Discussion
There is extensive documentation on the beneficial health effects of fish oil, especially with respect to cancer prevention and treatment. These effects are mainly contributed by the fish oil-derived omega-3 fatty acids, EPA and DHA. The anti-proliferative activity of fish oil may actually be due to its actions on multiple targets of cell signaling pathways (13). Among these mechanisms, the most important and well appreciated is its effect on eicosanoid metabolism (13). We and other investigators have demonstrated that EPA has the ability to give rise to PGE3 and that PGE3 antagonizes the effect of PGE2 on cell proliferation in both NSCLC A549 and colon cancer HT29 cells (27, 42). In this study, we confirmed that the differential anti-proliferative effect of EPA and fish oil against NSCLC cells A549 and H1299 was associated with expression of COX-2 and consequent formation of PGE3 in those cells and their relevant xenograft models. The important link of COX-2 to EPA elicited anti-proliferative activity in these cells was further evidenced by the inhibitory effect of EPA being markedly reduced in COX-2 knockdown A549 cells compared to that of control siRNA or shRNA transfected cells. The correlation of COX-2 status and antitumor efficacy of fish oil as well as the alteration of PGE3 over PGE2 was also demonstrated in this study. More intriguingly, while we have shown that PGE2 enhanced activity of Akt/PkB kinase denoted by increased Akt phosphorylation, it is to the best of our knowledge the first time to demonstrate that PGE3 inhibited Akt/PKB activity by reducing the phosphorylation of Akt in both A549 and H1299 cells. Thus, the effects of PGE3 on inhibition of the Akt/PKB pathway may play an important role in EPA elicited anti-proliferative activity.
A numbers of studies have documented that fish oil-derived fatty acids have anti-inflammatory or anti-proliferative activity through reduction of COX-2 expression as well as the suppression of formation of the pro-inflammatory lipid mediator PGE2 (14). The impact of fish oil on reducing development of cancer and its association with COX-2 status has been further enlightened by a recent study on the link between omega-3 fatty acids and prostate cancer risk (41). Fradet V., et al recently reported the increasing intake of omega-3 fatty acids was strongly associated with a decreased risk of aggressive prostate cancer. The OR (Odd ratios, 95% confidence interval) for prostate cancer comparing the highest with the lowest quartile of n-3 intake, was 0.37 (0.25-0.54). This inverse association was even stronger among men carrying the COX-2 variant SNP (s4648310 [+8897 A/G], flanking the 3¶ region of COX-2) (41). However, exactly how the relative expression of COX-2 affects the anti-proliferative activity of fish oil derived omega-3 fatty acids, particularly EPA, in cancer is far from clear. We have reported previously that EPA administration to A549 cells that constitutively express COX-2 led to the synthesis of PGE3. Furthermore, the relative formation of PGE3 and its effect on cell proliferation was significantly reduced when the cells were co-treated with a COX-2 inhibitor (27). A similar observation has been made in human pancreatic cancer BxPC3 cells (42). Additionally, we have also shown that administration of EPA to rats significantly reduced formation of PGE2 while concomitantly increasing formation of PGE3 in the colon mucosa [29]. More recently, Hawcroft G, et al, also demonstrated higher levels of PGE3 in EPA treated COX-2 positive human colorectal cancer HCA-7 cells (40). Taken together, these studies collectively support the concept that EPA, in contrast to DHA, has the ability to generate PGE3 through COX-2 pathways in various human cancer cells. In order to further support the notion of COX-2 status as a critical target for EPA-elicited anti-proliferative activity, it is critical to delineate how COX-2 status affects the sensitivity of cancer cells to EPA treatment and how it links to the balance of PGE2 and PGE3 in those cells, something that was lacking from previous studies. We demonstrated that COX-2 expressing NSCLC A549 cells or its xenograft models were more sensitive to EPA or menhaden treatment, respectively than H1299 cells, a COX-2 null NSCLC cell line and its xenograft model. The stronger anticancer activity of EPA or fish oil in A549 cells or xenografts than that in H1299 cells or its xenografts is associated with a higher ratio of PGE3 over PGE2 in A549 than in H1299 cells. Furthermore, when COX-2 expression was knocked down by COX-2 siRNA or shRNA, the anti-proliferative effect of EPA against the COX-2 knockdown cells was reduced by 40% in comparison to that of control siRNA or shRNA transfected A549 cells suggesting that the anti-proliferative effect of EPA in NSCLCs was at least partially mediated through COX-2. Thus, taken together, the results of our in vitro and in vivo studies strongly suggest that the antiproliferative effect of EPA is mediated at least partially by modulation of COX-2 metabolism in NSCLCs.
The importance of prostaglandins in carcinogenesis and chronic inflammation is supported by population studies, clinical trials as well as animal experiments. Among the prostaglandins of interest, PGE2 has been consistently documented to act as a tumor promoting mediator in various types of cancers including colon, lung, breast, head and neck cancers (43). PGE2 induces a proliferative effect through modulation of multiple cell signaling pathways in an autocrine or paracrine fashion. For example, it has been demonstrated that PGE2 promotes colon tumor cell survival through its action on the PI3K-Akt-PPARδ cascade in Apcmin mice as well as colon cancer cells (44). It promotes proliferation of colon and lung cancer cells by acting on Ras-Erk and glycogen synthase kinase-3β - β catenin pathways (45). Additionally, a PGE2-induced anti-apoptotic effect has been linked to upregulation of Bcl-2 expression and induction of nuclear factor - κB (NF-κB) transcriptional activity (38, 46). We have observed that PGE2 increased Akt/PKB activity by upregulating Akt phosphorylation in both A549 and H1299 cells in a concentration dependent manner regardless of the COX-2 status of these two cell lines. Intriguingly, PGE3 did not enhance Akt phosphorylation in either of these two cell lines compared to that of PGE2. In fact, PGE3 actually down regulated Akt/PKB activity as denoted by a reduced phosphorylation of Akt. Our finding demonstrates for the first time that PGE2 might promote proliferation of NSCLC cells through up-regulation of Akt/PKB pathways and that PGE3 inhibits the activity of this particular pathway. Given that the PI3K/Akt pathway is critically important in oncogenes, the results of our study suggest that different types of prostaglandins could exert distinctly different effects on this particular pathway and on proliferation of NSCLC cells. EPA treatment only caused marked down regulation of Akt phosphorylation in A549 cells, but not on H1299 cells. These data suggest again that the relatively stronger inhibition of proliferation of A549 cells by EPA might be mediated through increased formation of PGE3 and subsequent downregulation of phosphorylation of Akt; whereas, the relative poor sensitivity of H1299 cells to EPA treatment is likely due to the reduced formation of PGE3. These findings collectively suggest that EPA-elicited anti-proliferative activity is mediated through PGE3 formation by COX-2 enzymes and downregulation of PI3K pathways in NSCLC cells.
Taken as a whole, these data suggest that fish oil derived n-3 fatty acid EPA exerts a relatively stronger anti-proliferative activity in COX-2 expressing NSCLC A549 cells and in associated xenograft tissue than that of COX-2 deficient H1299 cells and tumors. These differential effects of EPA on the proliferation of A549 and H1299 tumor are likely due to enhanced formation of PGE3 in A549 cells, but not H1299 cells. In light of the fact that COX-2 overexpression was observed in approximately 70% of lung adenocarcinomas (47) and a decreased incidence of lung cancer was observed in patients who regularly take aspirin (48), agents like fish oil EPA with the capability of altering COX-2 metabolism, while at the same time conferring cardiovascular health benefits (14), could provide a natural and effective treatment or prevention for lung cancer. Furthermore, while emerging evidence suggests that most epithelial-derived tumors constitutively express COX-2, most normal tissues have at most a low level of expression of this enzyme (49). This suggests that n-3 fatty acids may preferentially be growth inhibitory to developing cancers and not normal tissues. This differential effect makes fish oil an ideal chemopreventive agent.
Acknowledgment
The study was support in part by 1R21CA102411-01 and 1R01CA144053-01A1.
Abbreviations
- PGE2
Prostaglandin E2
- PGE3
Prostaglandin E3
- EPA
eicosapentaenoic acid
- COX
cyclooxygenase
- AA
arachidonic acid
- DHA
docosahexaenoic acid
- BHT
bytulated hydroxytoluenen
- LC/MS/MS
liquid chromatography and tandem mass spectrometry
- DAPI
4’ 6-dimidino-2-phenylindole
- Calcein AM
acetoxymethyl ester of calcein
- NSAIDs
nonsteroidal anti-inflammatory agents
- NSCLC
Non-small cell lung cancer cells
- MTT
3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide)
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Smith CJ, Perfetti TA, King JA. Perspectives on pulmonary inflammation and lung cancer risk in cigarette smokers. Inhal Toxicol. 2006;18:667–677. doi: 10.1080/08958370600742821. [DOI] [PubMed] [Google Scholar]
- 3.Moysich KB, Menezes RJ, Ronsani A, et al. Regular aspirin use and lung cancer risk. BMC Cancer. 2002;2:31. doi: 10.1186/1471-2407-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JM, Yanagawa J, Peebles KA, Sharma S, Mao JT, Dubinett SM. Inflammation in lung carcinogenesis: new targets for lung cancer chemoprevention and treatment. Crit Rev Oncol Hematol. 2008;66:208–217. doi: 10.1016/j.critrevonc.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris RE, Beebe-Donk J, Doss H, Burr Doss D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review) Oncol Rep. 2005;13:559–583. [PubMed] [Google Scholar]
- 6.Hampton T. New studies target lung cancer prevention, imaging, and treatment. JAMA. 2008;300:267–268. doi: 10.1001/jama.2008.49. [DOI] [PubMed] [Google Scholar]
- 7.Krysan K, Dalwadi H, Sharma S, Pold M, Dubinett S. Cyclooxygenase 2-dependent expression of survivin is critical for apoptosis resistance in non-small cell lung cancer. Cancer Res. 2004;64:6359–6362. doi: 10.1158/0008-5472.CAN-04-1681. [DOI] [PubMed] [Google Scholar]
- 8.Gately S. The contributions of cyclooxygenase-2 to tumor angiogenesis. Cancer Metastasis Rev. 2000;19:19–27. doi: 10.1023/a:1026575610124. [DOI] [PubMed] [Google Scholar]
- 9.Leahy KM, Koki AT, Masferrer JL. Role of cyclooxygenases in angiogenesis. Curr Med Chem. 2000;7:1163–1170. doi: 10.2174/0929867003374336. [DOI] [PubMed] [Google Scholar]
- 10.Baratelli F, Lin Y, Zhu L, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 11.Brown JR, DuBois RN. Cyclooxygenase as a target in lung cancer. Clin Cancer Res. 2004;10(12):4266s–4269s. doi: 10.1158/1078-0432.CCR-040014. Pt 2. [DOI] [PubMed] [Google Scholar]
- 12.Edelman MJ, Watson D, Wang X, et al. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy--Cancer and Leukemia Group B Trial 30203. J Clin Oncol. 2008;26:848–855. doi: 10.1200/JCO.2007.13.8081. [DOI] [PubMed] [Google Scholar]
- 13.Wendel M, Heller AR. Anticancer actions of omega-3 fatty acids--current state and future perspectives. Anticancer Agents Med Chem. 2009;9:457–470. doi: 10.2174/1871520610909040457. [DOI] [PubMed] [Google Scholar]
- 14.Riediger ND, Othman RA, Suh M, Moghadasian MH. A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc. 2009;109:668–679. doi: 10.1016/j.jada.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Arita K, Kobuchi H, Utsumi T, et al. Mechanism of apoptosis in HL-60 cells induced by n-3 and n-6 polyunsaturated fatty acids. Biochem Pharmacol. 2001;62:821–828. doi: 10.1016/s0006-2952(01)00723-7. [DOI] [PubMed] [Google Scholar]
- 16.Hardman WE, Avula CP, Fernandes G, Cameron IL. Three percent dietary fish oil concentrate increased efficacy of doxorubicin against MDA-MB 231 breast cancer xenografts. Clin Cancer Res. 2001;7:2041–2049. [PubMed] [Google Scholar]
- 17.Yam D, Peled A, Shinitzky M. Suppression of tumor growth and metastasis by dietary fish oil combined with vitamins E and C and cisplatin. Cancer Chemother Pharmacol. 2001;47:34–40. doi: 10.1007/s002800000205. [DOI] [PubMed] [Google Scholar]
- 18.Han S, Sun X, Ritzenthaler JD, Roman J. Fish oil inhibits human lung carcinoma cell growth by suppressing integrin-linked kinase. Mol Cancer Res. 2009;7:108–117. doi: 10.1158/1541-7786.MCR-08-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weaver KL, Ivester P, Seeds M, Case LD, Arm JP, Chilton FH. Effect of dietary fatty acids on inflammatory gene expression in healthy humans. J Biol Chem. 2009;284:15400–15407. doi: 10.1074/jbc.M109.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takezaki T, Hirose K, Inoue M, et al. Dietary factors and lung cancer risk in Japanese: with special reference to fish consumption and adenocarcinomas. Br J Cancer. 2001;84:1199–1206. doi: 10.1054/bjoc.2001.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Temme EH, Kesteloot H. Fish consumption is inversely associated with male lung cancer mortality in countries with high levels of cigarette smoking or animal fat consumption. Int J Epidemiol. 2000;29:615–621. doi: 10.1093/ije/29.4.615. [DOI] [PubMed] [Google Scholar]
- 22.Veierod MB, Laake P, Thelle DS. Dietary fat intake and risk of lung cancer: a prospective study of 51,452 Norwegian men and women. Eur J Cancer Prev. 1997;6:540–549. doi: 10.1097/00008469-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Darby S, Whitley E, Doll R, Key T, Silcocks P. Diet, smoking and lung cancer: a case-control study of 1000 cases and 1500 controls in South-West England. Br J Cancer. 2001;84:728–735. doi: 10.1054/bjoc.2000.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerchietti LC, Navigante AH, Castro MA. Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids from fish oil and preferential Cox-2 inhibition on systemic syndromes in patients with advanced lung cancer. Nutr Cancer. 2007;59:14–20. doi: 10.1080/01635580701365068. [DOI] [PubMed] [Google Scholar]
- 25.MacLean CH, Newberry SJ, Mojica WA, et al. Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA. 2006;295:403–415. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- 26.Smith WL. Cyclooxygenases, peroxide tone and the allure of fish oil. Curr Opin Cell Biol. 2005;17:174–182. doi: 10.1016/j.ceb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Yang P, Chan D, Felix E, et al. Formation and antiproliferative effect of prostaglandin E(3) from eicosapentaenoic acid in human lung cancer cells. J Lipid Res. 2004;45:1030–1039. doi: 10.1194/jlr.M300455-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Hagi A, Nakayama M, Miura Y, Yagasaki K. Effects of a fish oil-based emulsion on rat hepatoma cell invasion in culture. Nutrition. 2007;23:871–877. doi: 10.1016/j.nut.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Vanamala J, Glagolenko A, Yang P, et al. Dietary fish oil and pectin enhance colonocyte apoptosis in part through suppression of PPARdelta/PGE2 and elevation of PGE3. Carcinogenesis. 2008;29:790–796. doi: 10.1093/carcin/bgm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denkins Y, Kempf D, Ferniz M, Nileshwar S, Marchetti D. Role of omega-3 polyunsaturated fatty acids on cyclooxygenase-2 metabolism in brain-metastatic melanoma. J Lipid Res. 2005;46:1278–1284. doi: 10.1194/jlr.M400474-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Szymczak M, Murray M, Petrovic N. Modulation of angiogenesis by omega-3 polyunsaturated fatty acids is mediated by cyclooxygenases. Blood. 2008;111:3514–3521. doi: 10.1182/blood-2007-08-109934. [DOI] [PubMed] [Google Scholar]
- 32.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 33.Yang P, Menter DG, Cartwright C, et al. Oleandrin-mediated inhibition of human tumor cell proliferation: importance of Na,K-ATPase alpha subunits as drug targets. Mol Cancer Ther. 2009;8:2319–2328. doi: 10.1158/1535-7163.MCT-08-1085. [DOI] [PubMed] [Google Scholar]
- 34.Kempen EC, Yang P, Felix E, Madden T, Newman RA. Simultaneous quantification of arachidonic acid metabolites in cultured tumor cells using high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Anal Biochem. 2001;297:183–190. doi: 10.1006/abio.2001.5325. [DOI] [PubMed] [Google Scholar]
- 35.Yang P, Felix E, Madden T, Fischer SM, Newman RA. Quantitative high-performance liquid chromatography/electrospray ionization tandem mass spectrometric analysis of 2- and 3-series prostaglandins in cultured tumor cells. Anal Biochem. 2002;308:168–177. doi: 10.1016/s0003-2697(02)00218-x. [DOI] [PubMed] [Google Scholar]
- 36.Yang P, Chan D, Felix E, et al. Determination of endogenous tissue inflammation profiles by LC/MS/MS: COX- and LOX-derived bioactive lipids. Prostaglandins Leukot Essent Fatty Acids. 2006;75:385–395. doi: 10.1016/j.plefa.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Yang P, Collin P, Madden T, et al. Inhibition of proliferation of PC3 cells by the branched-chain fatty acid, 12-methyltetradecanoic acid, is associated with inhibition of 5-lipoxygenase. Prostate. 2003;55:281–291. doi: 10.1002/pros.10243. [DOI] [PubMed] [Google Scholar]
- 38.Raben D, Bianco C, Damiano V, et al. Antitumor activity of ZD6126, a novel vascular-targeting agent, is enhanced when combined with ZD1839, an epidermal growth factor receptor tyrosine kinase inhibitor, and potentiates the effects of radiation in a human non-small cell lung cancer xenograft model. Mol Cancer Ther. 2004;3:977–983. [PubMed] [Google Scholar]
- 39.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 40.Hawcroft G, Loadman PM, Belluzzi A, Hull MA. Effect of Eicosapentaenoic Acid on E-type Prostaglandin Synthesis and EP4 Receptor Signaling in Human Colorectal Cancer Cells. Neoplasia. 12:618–627. doi: 10.1593/neo.10388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fradet V, Cheng I, Casey G, Witte JS. Dietary omega-3 fatty acids, cyclooxygenase-2 genetic variation, and aggressive prostate cancer risk. Clin Cancer Res. 2009;15:2559–2566. doi: 10.1158/1078-0432.CCR-08-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Funahashi H, Satake M, Hasan S, et al. Opposing effects of n-6 and n-3 polyunsaturated fatty acids on pancreatic cancer growth. Pancreas. 2008;36:353–362. doi: 10.1097/MPA.0b013e31815ccc44. [DOI] [PubMed] [Google Scholar]
- 43.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D, Wang H, Shi Q, et al. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell. 2004;6:285–295. doi: 10.1016/j.ccr.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Krysan K, Reckamp KL, Dalwadi H, et al. Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Res. 2005;65:6275–6281. doi: 10.1158/0008-5472.CAN-05-0216. [DOI] [PubMed] [Google Scholar]
- 46.Poligone B, Baldwin AS. Positive and negative regulation of NF-kappaB by COX-2: roles of different prostaglandins. J Biol Chem. 2001;276:38658–38664. doi: 10.1074/jbc.M106599200. [DOI] [PubMed] [Google Scholar]
- 47.Hida T, Yatabe Y, Achiwa H, et al. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- 48.Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5:138–146. doi: 10.1097/00001648-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]