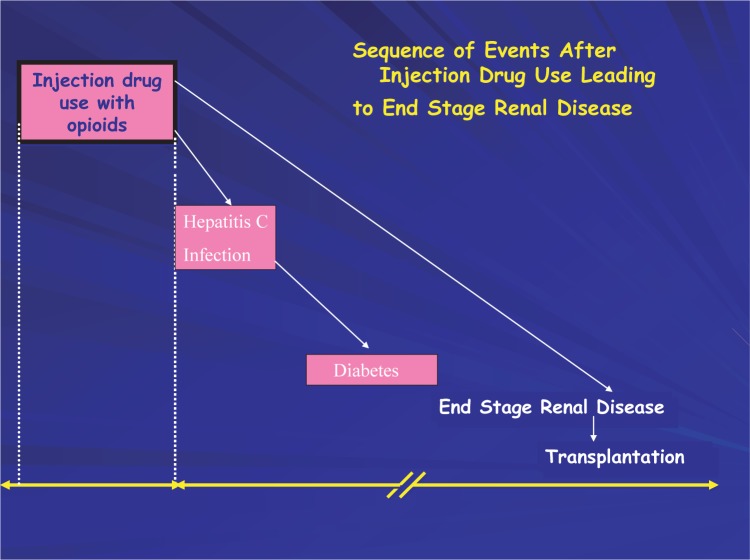
Abstract
Many new and existing cases of viral hepatitis infections are related to injection drug use. Transmission of these infections can result directly from the use of injection equipment that is contaminated with blood containing the hepatitis B or C virus or through sexual contact with an infected individual. In the latter case, drug use can indirectly contribute to hepatitis transmission through the dis-inhibited at-risk behavior, that is, unprotected sex with an infected partner. Individuals who inject drugs are at-risk for infection from different hepatitis viruses, hepatitis A, B, or C. Those with chronic hepatitis B virus infection also face additional risk should they become co-infected with hepatitis D virus. Protection from the transmission of hepatitis viruses A and B is best achieved by vaccination. For those with a history of or who currently inject drugs, the medical management of viral hepatitis infection comprising screening, testing, counseling and providing care and treatment is evolving. Components of the medical management of hepatitis infection, for persons considering, initiating, or receiving pharmacologic therapy for opioid addiction include: testing for hepatitis B and C infections; education and counseling regarding at-risk behavior and hepatitis transmission, acute and chronic hepatitis infection, liver disease and its care and treatment; vaccination against hepatitis A and B infection; and integrative primary care as part of the comprehensive treatment approach for recovery from opioid abuse and dependence. In addition, participation in a peer support group as part of integrated medical care enhances treatment outcomes. Liver disease is highly prevalent in patient populations seeking recovery from opioid addiction or who are currently receiving pharmacotherapy for opioid addiction. Pharmacotherapy for opioid addiction is not a contraindication to evaluation, care, or treatment of liver disease due to hepatitis virus infection. Successful pharmacotherapy for opioid addiction stabilizes patients and improves patient compliance to care and treatment regimens as well as promotes good patient outcomes. Implementation and integration of effective hepatitis prevention programs, care programs, and treatment regimens in concert with the pharmacological therapy of opioid addiction can reduce the public health burdens of hepatitis and injection drug use.
Keywords: hepatitis, methadone, substance abuse treatment, medication assisted treatment
Viral Hepatitis Infections, Liver Disease and Injection Drug Use
Chronic liver disease is a major health problem with chronic liver disease and cirrhosis the thirteenth most frequent cause of death in the United States (CDC, 2007). Chronic liver disease can result from viral hepatitis infection. Hepatitis viruses can be transmitted from one individual to another through shared drug use practices, especially those involving injection of drugs. The disease burden and estimated incident infections of hepatitis A virus (HAV), hepatitis B virus (HBV) and hepatitis C virus (HCV) are summarized in Table 1 (CDC, 2006).
Table 1.
Disease burden from viral hepatitis in the US in 2006.
| Virus | Infections
|
Comment | |
|---|---|---|---|
| New | Chronic | ||
| A (HAV) | 32,000* | none | No chronic disease; one-third of persons in the United States have evidence of past HAV infection |
| B (HBV) | 46,000* acute | 1.25 million acute | About 5 percent of persons in the United States have evidence of past or current HBV infection |
| C (HCV) | 19,000* | 3.2 million | About 1.6 percent of Americans have evidence of past or current HCV infection |
| D (HDV) | Unreliable | Only concurrent with HBV infection | |
Estimated new infections in 2003. Data are influenced by annual incidence fluctuations and variable estimates of unreported and/or undiagnosed cases.
Hepatitis A virus infection
HAV infection in injection drug users can have significant medical consequences. HAV infection in patients already chronically infected with HBV or HCV can result in fulminant liver failure. HAV also will increase the health risks faced by individuals with HIV infection, and a recent national study of human immunodeficiency virus (HIV)-infected individuals in primary care revealed that only 12.5 percent of injection drug users were vaccinated against HAV infection (Tedaldi et al. 2004).
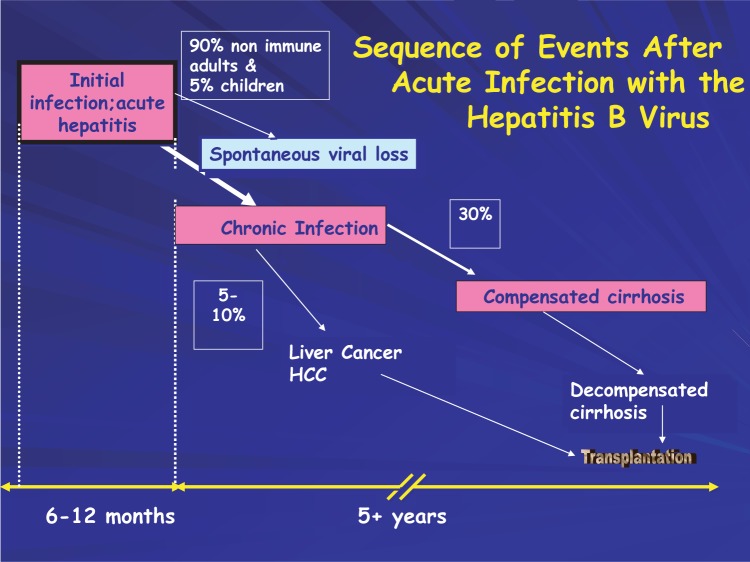
Hepatitis A is a virus transmitted through the fecal-oral route, by consuming contaminated food or water that may have been fecally contaminated, or transmitted directly from person-to-person through poor hygiene and intimate contact. Hepatitis A has an incubation period ranging from 15 days to 50 days, and, in uncomplicated cases, the infection is completely resolved by 6 months after infection (Fig. 1). There is no specific treatment for HAV infection, and most people recover without medical intervention, although supportive measures such as intravenous fluids are occasionally needed.
Figure 1.
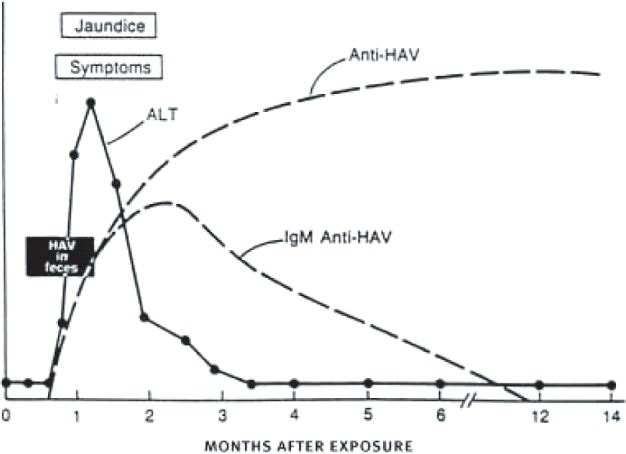
The time course of an uncomplicated HAV infection (from Larson et al. 2005).
As many as a third of all persons in the United States are estimated to have been infected at some time, usually during childhood. The estimated annual incidence of HAV infection has dropped substantially since introduction of an HAV vaccine in 1995, but computer models suggest an estimated annual incidence of 270,000 cases in the United States, more than 10 times the number actually reported (Armstrong and Bell, 2002).
Among injection drug users, HAV can be transmitted intravenously (though rarely) through shared equipment or HAV-contaminated water, but is far more commonly transmitted by the usual fecal-oral route through unhygienic practices during drug preparation and sharing. The fatality rate for HAV infection is generally low (less than 1 percent, or about 100 persons per year), although injection drug users with preexisting chronic liver disease (such as alcoholic liver disease or chronic HCV infection) are at increased risk of liver failure and death. Nearly 20 percent of reported HAV cases have occurred among injection drug users (CDC, 1999), and approximately 6 percent of reported HAV cases occurred among injection drug users during 2002 (CDC, 2004).
In a recent study of young injectors in Juneau, Alaska, 33 percent of those tested were seropositive for antibodies to HAV (Wells et al. 2006). Correlates of infection included having less than a high school education, exposure to HBV, and frequent opioid injection in the last 30 days. In a similar study from Canada, 58 percent of a large cohort of individuals entering opioid detoxification were HAV seropositive (Reimer et al. 2006). In another study of individuals entering a treatment program for opioid dependence, 41.2 percent were seropositive for HAV (Gerlich et al. 2006). Other epidemiologic studies in Europe have shown multiple outbreaks among men having sex with men of infection with a specific genotype of HAV (IA), indicating the exchange of HAV to endemic levels among groups with identifiable behaviors (Stene-Johansen et al. 2007).
HAV infection can be prevented by vaccination, and the Centers for Disease Control and Prevention (CDC) recommends that all injection drug users not previously vaccinated be immunized with the hepatitis A vaccine to protect from severe liver disease. Vaccination is not harmful for persons who have been infected with HAV, and thus pre-vaccinating testing to determine need for vaccine is not recommended unless people are in a stable environment (e.g. in-patient long term drug treatment) where it can be assured they will be around when the test result is received.
Hepatitis B virus infection
HBV infection typically is a self-limited illness, with infected adults recovering fully in approximately 6 months (Fig. 2). However, persons with chronic liver disease from other causes (e.g. chronic HCV infection) may be more likely to develop liver failure from acute HBV infection. Multiple hepatitis infections, or coinfections are common among injection drug users, particularly in the context of observed health disparities among African American and Hispanic drug injectors (Estrada, 2005; Fisher et al. 2006). Injection drug users who may already have underlying liver disease and become infected with HBV are at high risk for serious liver disease. In a series of case studies of injection drug users with acute HBV infection, nearly all those with underlying chronic HCV infection died from fulminant liver failure (Garfein et al. 2004). Thus, prevention of HBV infection among injection drug users is critically important, and the CDC recommends vaccination for all injection drug users who have not been previously vaccinated or known to have been exposed to HBV. Vaccination is not harmful for persons who have been infected with HBV, thus, pre-vaccination testing should not be a barrier to receiving vaccine.
Figure 2.
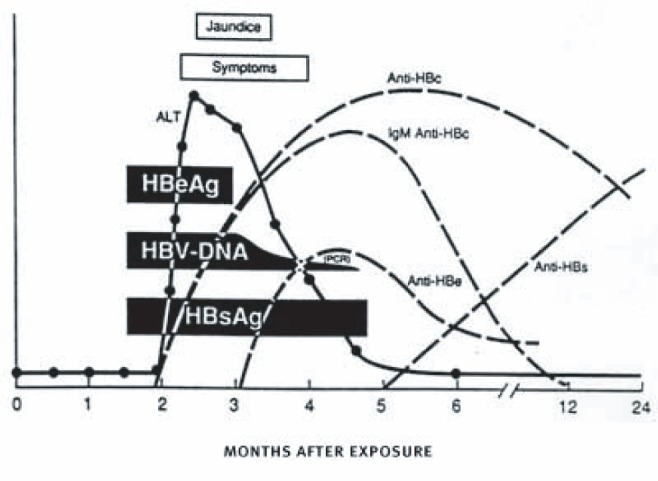
Time course of acute HBV infection showing the expression of viral markers of infection and the immune response to infection among persons who recover from acute infection (from Larson et al. 2005).
Counseling and educating injection drug users about HBV infection and vaccination is also important, as studies have shown that a majority of injection drug users questioned were not able to accurately self-report their vaccination status (de la Fuente et al. 2007; Kuo et al. 2004a). Studies have shown that an HBV vaccination program targeting injection drug users is both feasible and effective (Altice et al. 2005; Burt et al. 2007; Kuo et al. 2004; Quaglio et al. 2002).
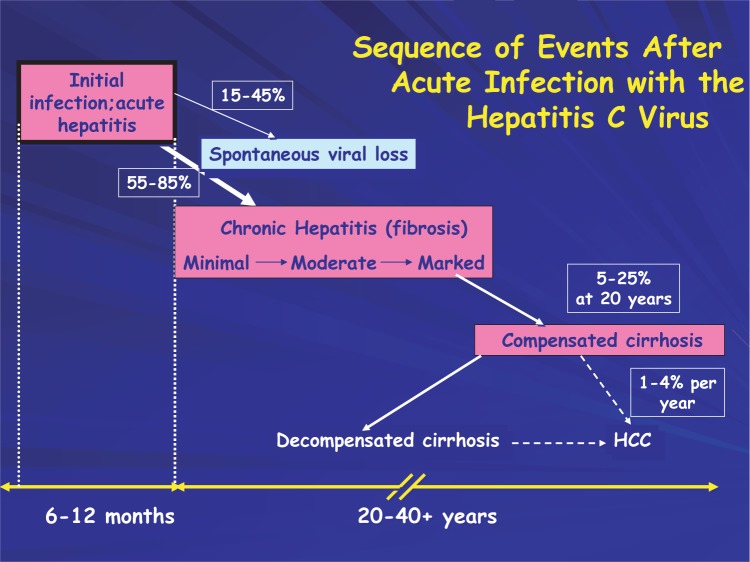
Roughly 5 percent of persons in the United States have been exposed to HBV, and an estimated 51,000 new cases of acute HBV infection occurred in 2005. Because of the success of infant and childhood HBV vaccination programs in the United States, the vast majority of acute HBV infections occur in adults. Most cases result in complete recovery and immunity from future infection. HBV infection may become chronic in only about 5 percent of persons infected as adults (Fig. 3). Chronic HBV infection affects the liver and may lead over time to cirrhosis (yearly incidence of 1.3 percent to 5.9 percent) that may result in liver failure or cancer. The 5-year survival rate of patients with HBV-related cirrhosis ranges from 52 percent to 82 percent. Co-infection with hepatitis D virus (HDV) or HIV or chronic alcohol consumption are the main factors that modify and exacerbate the course of liver disease in chronic infection (Sheng et al. 2007).
Figure 3.
Diagram of the natural history of infection with HBV.
As seen in Table 1, more than one million persons in the United States are chronically infected with HBV. Roughly one in five persons with chronic HBV infection will die prematurely from the consequences of chronic liver disease. Approximately 4,000 persons die each year of HBV-related liver cirrhosis, and 1,500 individuals die of heptocellular carcinoma related to HBV infection each year (CDC, 2002; Fattovitich et al. 2004).
Although sexual contact with an individual chronically infected with HBV is the most common route of transmission, sharing injection drug use equipment contaminated with HBV can also lead to infection. Injection drug users accounted for approximately 12 percent of all cases in 2002, with 40 percent becoming infected with HBV after 1 year of injection drug use and more than 80 percent becoming infected after 10 years (CDC, 2002). Sexual transmission, accounting for half of all HBV infections (41 percent heterosexual, 9 percent men having sex with men; CDC, 2002), also may be a significant route of infection among addicted persons as a result of unsafe behavior, such as exchanging sex for drugs. Other risk factors associated with HBV infection include the presence of HCV co-infection and a history of imprisonment (Backmund et al. 2006).
Treatment of Hepatitis B virus infection
The current goals of treatment of chronic HBV infection are to achieve a sustained suppression of HBV replication and a remission of liver disease (Lok and McMahon, 2007). Reducing the progression of liver disease is important so that liver cancer, cirrhosis, and hepatic failure does not develop and reversing decompensated cirrhosis is important so that the patient is no longer a candidate for liver transplantation (AASLD, 2003; Fung and Lok, 2005; Kanwal et al. 2005). Factors that influence a response to treatment include patient age, severity of liver disease, likelihood of a treatment response, and comorbid complications. Interferon may be used as an initial therapy for a predefined time period, although six drugs can be used for the treatment of chronic HBV: interferon-alpha(2b), pegylated interferon-alpha(2a), lamivudine, adefovir, entecavir, and telbivudine (Ruiz-Sancho et al. 2007). Combination therapy, using two or more approved drugs for chronic HBV infection, is being investigated and may enhance the patient's response to treatment. Additional treatment complications may occur in Asian chronic HBV-infected patients who acquire the virus early in life (Yuen, 2007). Thus, it is important to introduce hepatitis education and prevention programs into a substance abuse treatment setting (Hagedorn et al. 2007; Strauss et al. 2007). For example, the Healthy Liver Program in Minnesota provides screening, education, and vaccination against hepatitis infection, particularly HBV.
Hepatitis B virus and Hepatitis D virus co-infection
New HBV infection may be accompanied by co-infection with HDV, which can replicate only with the aid of a “helper” function of HBV; persons who have chronic HBV infection can subsequently be infected with HDV (called “super-infection”) (Fig. 4). HDV co-infection should be suspected in any patient with severe acute HBV infection. The prevalence of HDV in the United States is relatively low, although injection drug users may be at high risk. HDV infection complicates the liver disease associated with HBV infection and increases the risk of liver cancer two- to sixfold compared to HBV infection alone (Fattovitch et al. 2004). In a recent outbreak of HBV infection in injection drug users with a high prevalence of HDV infection, risk factors for co-infection were having more than one sex partner, injecting more than four times a day, and sharing injection equipment with more than two persons. The important public health issue represented by HBV/HDV co-infection can be seen in this outbreak, as all co-infected individuals died of fulminant liver failure (Bialek et al. 2005).
Figure 4.
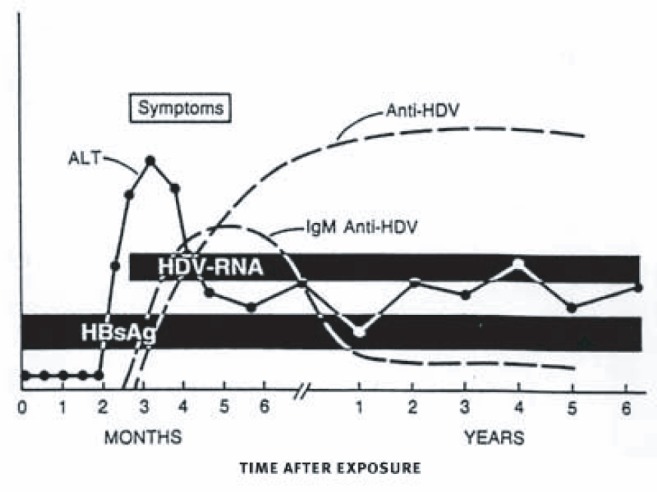
Time course of acute (months) and chronic (years) HDV superinfection (HBV/HDV co-infection) (from Larson et al. 2005).
Interferon therapy in persons co-infected with HBV and HDV is less effective because of the complications associated with this type of infection. While treatment decreases liver enzyme levels and suppresses HBV replication, the virus is not eradicated; typically, HBV infection reasserts itself if treatment is stopped. Initial prevention of HBV infection through vaccination will prevent subsequent infection with HDV.
Hepatitis C virus infection
HCV infection is the most common chronic blood-borne infection in the United States. According the National Health and Nutrition Examination Survey (NHANES), more than four million Americans have been exposed to HCV and therefore exhibit antibodies to the virus. This is approximately four times the number infected with HIV. A diagram of the natural history of chronic HCV is presented in Figure 5. Infection with HCV typically leads to chronic viremia—the existence of viruses in the bloodstream. A recent review of studies of HCV infection indicated that spontaneous clearance of virus occurs in approximately one in four individuals with at least six-months of medical followup after acute HCV infection (Micallef et al. 2006). Spontaneous clearance of HCV infection, or self-cure, is an area of intense research investigation. Recent studies have shown that a competent immune response, comprising neutralizing antibodies and cellular immune responses (CD4 T cells), in acute HCV infection (the first six months after initial viral exposure) is necessary for self-cure (Kaplan et al. 2006; Pestka et al. 2007; Ruys et al. 2008).
Figure 5.
Diagram of the natural history of infection with HCV.
NHANES estimates that an estimated 3.1 million persons in the United States have active chronic HCV infection (Table 1). The peak prevalence at the time of NHANES III (conducted from, 1988 to1994) was in persons 30 to 49 years of age (Alter et al. 1999), and more recent NHANES (1999–2002) shows, as expected, the highest prevalence of chronic infection is now in persons 40–59 years of age. The health care costs of illness and death associated with HCV infection in the United States is estimated to be $5.46 billion annually (Leigh et al. 2001).
Alone or in combination with alcohol consumption, HCV infections account for about 60 percent of all newly diagnosed cases of chronic liver disease and are the leading reason for liver transplantation as well as a major cause of liver cancer in U.S. residents (Chitturi and George, 2000; NIDA, 2000). Overall, HCV is responsible for up to 70 percent of chronic hepatitis cases, 30 percent to 40 percent of cases of cirrhosis and end stage liver disease, and 60 percent of liver cancer cases (CDC, 1998).
Injection drug use is the major high-risk activity associated with HCV infection. Through the implementation of HIV prevention interventions during the late 1980s and early 1990s, and subsequently through HCV education, the spread of HCV infection within the injection drug use risk group has slowed. Prospective studies have shown that 55 percent to 85 percent of exposed persons will develop a chronic infection with the virus, and up to 50 percent of patients, including injection drug users, may clear the virus (spontaneous self-cure) during acute infection (Jauncey et al. 2004). Estimates of self-cures of HCV infection in drug users vary greatly due to a number of parameters, including the difficulty of identifying acute HCV and accessing care for drug users (Amin et al. 2007). In a study of viral clearance in drug-abusing veterans, increasing age at the time of HCV infection, alcohol consumption, and HIV co-infection were associated with decreased likelihood of spontaneous HCV infection clearance (Piasecki et al. 2004). Another study showed the feasibility of using a prison setting or entry into detoxification as an environment in which to successfully identify acute HCV infection and treat those that do not self-cure (McGovern et al. 2006).
Persons who do not clear HCV infection may develop progressive liver disease and HCV-induced cirrhosis, which occurs in up to 20 percent of persons after roughly 20 years of chronic infection (Fig. 5). Approximately one-quarter of persons progressing to cirrhosis may develop end-stage liver disease and become candidates for liver transplantation. Patients who develop decompensated cirrhosis have a high likelihood of dying from complications of liver disease. Currently, an estimated 8,000 to 10,000 persons in the United States die from liver disease as a result of HCV infection each year, and the CDC has predicted that HCV-related mortality could triple over the next two decades (CDC, 1998).
Preventing the development of additional comorbidities by vaccinating against HAV and HBV infections as well as referring for substance abuse treatment are fundamental to the medical management of chronic HCV. For injection drug users, this can best be achieved by integrating prevention, care, and treatment for both substance abuse and HCV infection (Edlin et al. 2005).
National reporting data indicate that injection drug use accounts for the majority of reported acute HCV infections, greatly exceeding all other transmission factors (CDC, 2003a). Sexual exposure is the next highest risk factor accounting for up to 30 percent of cases of HCV infection, and transmission by this route is associated with multiple sexual partners and other sexually transmitted diseases. A recent study of the HCV incidence in a population of men having sex with men showed high incidence of HCV infection associated with HIV infection and ulcerative sexually transmitted diseases and rough sexual techniques (van de Laar et al. 2007). These data suggest a men having sex with men HCV transmission network. Almost all blood transfusion-related cases occurred prior to initiation of blood product screening in 1992. Other transmission routes include health care-related cases (e.g. accidental needle-stick or unclean medical procedure equipment), hemodialysis, non-sterile tattooing, and mother-to-child transmission during birth. In a small but significant number of cases, the etiology cannot be identified (Alter et al. 1999; CDC, 1998).
Treatment of Hepatitis C virus infection
The medical management of HCV has been addressed by consensus statements or clinical practice guideline development groups in the United States (AASLD, 2004; NIH, 2002), Canada (Sherman et al. 1997), France (Galmiche, 1998), and Europe (EASL, 1999). According to current HCV treatment guidelines, all patients with chronic HCV infection are potential candidates for antiviral therapy. Drug users, individuals with a history of drug use, or individuals in drug addiction treatment should not be excluded from needed HCV treatment as a result of drug use issues (AASLD, 2004; Scott, 2005). The latest update of treatment guidelines from NIH does not specify the need for a drug-abstinence period but indicates that patients should participate in drug treatment as an important adjunct to HCV therapy. Generally, patients with biopsy-proven liver disease who are at increased risk for progression to cirrhosis and end-stage liver disease are considered to be treatment candidates. As shown in Table 2, there are factors that influence the outcome of treatment for hepatitis C infection. In addition, those with factors associated with increased risk of rapidly progressive liver disease, such as HIV/HCV or HBV/HCV co-infection, are also candidates for treatment. A large retrospective Veterans Administration study has shown that individuals who are diagnosed with a substance use disorder (SUD) complete and respond to interferon-based HCV treatment regimens at similar rates to veterans without SUD's (Huckans et al. 2007). Because individuals, including injection drug users, with acute HCV infection may be highly responsive to interferon therapy, consideration should be given to early treatment during acute HCV infection (Calleri et al. 2007; Corey et al. 2006). A short course (12 weeks) of pegylated interferon-alfa treatment has been shown to be effective for injection drug users diagnosed with acute HCV infection (Calleri et al. 2007; DeRosa et al. 2007). The criteria for acute HCV infection in theses studies were one of the following: a) HCV antibody sero-conversion in the past 6 months; b) first at-risk exposure to HCV in the past 6 months; or c) elevated liver enzyme levels in the year prior to infection (normal liver enzyme level prior to infection. Treatment response times of as early as four weeks after the initiation of treatment appear to correlate with successful treatment outcomes (Bryan, 2007). Individuals with cirrhosis can be offered pharmacotherapy for HCV. However, those with signs of hepatic decompensation (such as ascites, persistent jaundice, wasting, variceal hemorrhage, or hepatic encephalopathy) are at high risk for treatment-related complications and death and should be referred for clinical trials or liver transplantation.
Table 2.
Factors influencing HCV treatment outcomes.
| Host factors | Viral factors |
|---|---|
| • High degree of fibrosis | • Genotype 1 |
| • Age >40 or 50 at time of infection | • Viral load > 2 million copies/ml |
| • Male sex | |
| • Weight >75 kg; 165 lbs | • Large number of quasispecies (a measure of the HCV genome heterogeneity) |
| • African-American | |
| • Long duration of infection | |
| • HIV co-infection |
New pharmacologic agents and combination treatments introduced during the past decade have made treatment of chronic HCV infection increasingly effective. Sustained virologic response (SVR) is the benchmark of treatment success; it is defined as an undetectable viral load 6 months after the end of treatment. Approximately 55 percent of uncomplicated patients treated with current antiviral regimens can expect a sustained virological response. Follow-up studies of these patients show that nearly all have remained free of the virus (Kjaergard et al. 2001; Lang, 2007). Responses as high as 90 percent have been achieved in select populations. However, the development of similarly effective treatment options for patient groups at high risk for treatment-related complications and progressive liver failure remains an ongoing challenge (Davis and Rodrigue, 2001; Manns and Wedemeyer, 2001).
To date, the standard therapy for chronic HCV infection is the combination of pegylated (long acting) interferon and ribavirin; this combination has improved overall sustained virological response to greater than 50 percent (Davis and Rodrigue, 2001). Maximium sustained virological responses may occur in treatment with pegylated interferon and weight-based ribavirin (Torriani et al. 2004). Induction regimens, lengthier treatment regimens, consensus interferon, albumin interferon, and gamma interferon have all shown efficacy in preliminary trials. The use of mycophenolate mofetil and amantadine as adjunctive agents is also under study.
Reports at the American Association for the Study of Liver Disease, 2007 meeting indicated the greatest potential for new treatment breakthrough lies in orally available small molecules that target the HCV protease or polymerase (Sigal and Jacobson, 2007). Phase II clinical trials of HCV protease inhibitors and HCV polymerase inhibitors are revealing rapid declines in HCV levels (Afdhal et al. 2004; Reesink et al. 2006; Chu et al. 2004). However, due to the generation of resistant viruses, the antiviral therapies are provided in combination with current interferon-based treatment regimens (Lang, 2007a; Sarrazin et al. 2007). Other potential therapies are in development, such as synthetic oligodeoxynucleotides, and it will be several years before the efficacy of these newer products can be determined through clinical trials and become standard treatment regimens (McHutchison et al. 2007).
Hepatitis C treatment: Complementary and alternative medicine
Approximately one-third of patients with chronic liver disease have been reported to use complementary and alternative medicines, and many use them without consulting their physicians (Seeff et al. 2001). NIH's National Center for Complementary and Alternative Medicine (NCCAM) is careful to note that “no complementary medicine or alternative medicine therapies have been scientifically proven to cure or even ease symptoms of hepatitis C” (NCCAM, 2000, p. 2).
Silymarin (milk thistle) is the complementary medication most frequently used, but St. John's wort, ginkgo biloba, ginseng, garlic extract, echinacea, and “Liverite” (a liver hydrolysate containing amino acids, vitamin B12, choline, inositol, lecithin, phosphatidylethanolamine, and phosphatidylinositol) are also commonly taken in an attempt to minimize the liver damage caused by HCV infection (Modi et al. 2007; NCCAM, 2000;). Milk thistle extracts have been shown to be have anti-inflammatory and anti-viral properties in addition to being well tolerated with minimal adverse effects (Polyak et al. 2007; Tamayo and Diamond, 2007). However, the interactions of these agents with interferon-based treatment regimens and adjunctive medications (e.g. methadone, antidepressants, etc.) are not known, but such “drug-drug interactions” may be significant, and certain alternative medications such as kava-kava have been associated with the development of fulminant liver failure.
Hepatitis and HIV co-infection
Viral hepatitis and HIV infections are intersecting epidemics among injection drug users and possess many shared public health and treatment concerns (Bonacini, 2002; Peters, 2005). One survey of 295 patients entering an Opioid Treatment Program (OTP) found a prevalence of markers for HCV, HBV, and HIV of 80, 65, and 32 percent, respectively. Among the HIV-positive patients, 88 percent also were positive for HCV or HBV exposure (Chamot et al. 1992). Thus, viral hepatitis and HIV co-infection may be common among patients seeking or receiving treatment for opioid dependence.
HBV-HIV co-infection
Among patients infected with HIV, rates of chronic HBV infection range from 7 to 10 percent, with 80 percent of patients showing evidence of past or current HBV infection (Osborn et al. 2007). In injection drug use cohorts rates approaching 70 percent have been reported (Shire and Sherman, 2005). A study of HBV and HIV transmission has shown that HBV is sexually transmitted nearly nine times more efficiently than HIV (Kingsley et al. 1990). Therefore, sexual transmission of HBV and the intravenous inoculation of HBV through injection drug use need to be considered as potential transmission routes.
HIV infection modifies the natural history of HBV infection. Individuals with HIV infection are less likely to spontaneously clear or resolve HBV infection and therefore more likely to become chronic carriers of HBV. The ability to spontaneously clear HBV infection is dependent on generating an immune response to infection. For individuals infected with HIV, immune competence is a function of their CD4 count. Thus, managing HIV infection and maintaining elevated CD counts can be keys to managing the early stages of HIV/HBV co-infection. However, HIV induced immunodeficiency can reduce the immune mediated liver disease induced by HBV infection, but promote HBV replication. Reconstituting an immune response in HIV/HBV chronically co-infected patients through the use of antiretroviral therapy may result in enhanced liver damage and an initial flare up in liver enzymes. Studies of HBV/HIV infected patients show higher rates of liver-related mortality as well as increased progression of HIV infection (Konopnicki et al. 2005; Shen et al. 2004; Thio et al. 2002).
Treatment of HBV-HIV co-infection
Advances in antiretroviral therapy have prompted a renewed interest in the medical management of HBV/HIV co-infection (Alberti et al. 2005; Nunez and Soriano, 2005; Peters, 2005; Shire and Sherman, 2005; Soriano et al. 2005). For co-infected patients, control of HIV infection is the priority. With the control of HIV, patients who are candidates for HBV therapy have the same treatment goals as individuals infected with HBV alone. Although there are currently no FDA approved drugs for the treatment of HBV/HIV co-infection, pharmacotherapy options include interferon-a (pegylated), lamivudine, adefovir, tenofovir, emtricitabine, and entecavir. The multiple antiviral options available allow for combination regimens and salvage therapy once drug resistant virus develops.
Table 3 provides representative treatment options targeting specific aspects of co-infection for patients with HBV/HIV co-infection based on either the U.S. Public Health Service Treatment Guidelines (Benson et al. 2004), the Spanish Consensus Conference recommendations (Soriano et al. 2004), the European Consensus Conference Guidelines (Alberti et al. 2005) or the recommendations of an International Panel of Experts (Soriano et al. 2005). The various treatment options, available guidelines, and treatment challenges have resulted in a variety of treatment practices for the management of HIV/HBV coinfection (Gaglio et al. 2006).
Table 3.
Treatment targets and possible options for patients with HBV/HIV co-infection.
| Co-infection target | Treatment option |
|---|---|
| Hepatitis B virus (HBeAg+) | interferon-a (pegylated); entecavir; or Adefovir |
| Hepatitis B virus (HBeAg−) | Interferon-a (pegylated); Entecavir; Adefovir |
| Hepatitis B virus and HIV | antiretroviral (HIV) regimen, including lamivudine or emtricitabine with tenofovir or adefovir |
| To control HIV | antiretroviral (HIV) regimen + adefovir or entecavir antiretroviral (HIV) regimen + one HBV antiviral drug to avoid immune reconstitution induced liver disease |
HCV-HIV co-infection
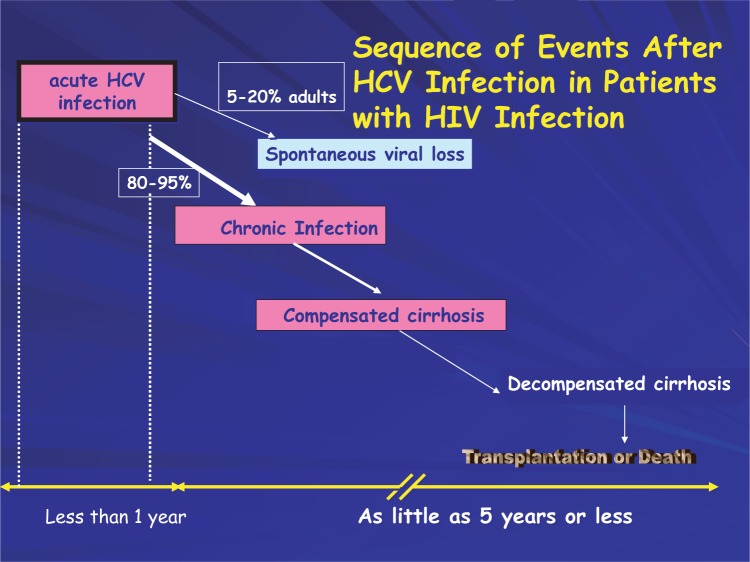
Eighty percent or more of injection drug users infected with HIV also test positive for exposure to HCV. The mode of transmission is through sharing of injection equipment resulting in the intravenous inoculation of virus. The majority (80 percent to 85 percent) of those exposed to HCV will become chronically infected (Fig. 6).
Figure 6.
Diagram of the natural history of HCV infection in patients with HIV infection.
Most research studies indicate that HCV-positive persons co-infected with HIV tend to have more rapid declines in health, even when they receive antiretroviral therapy for HIV infection (Alvarez and Latorre, 2004; Greub et al. 2000; Sulkowski et al. 2007). HIV co-infection has also been shown to shorten the survival time of patients with HCV-related decompensated cirrhosis (Pineda et al. 2005). An investigation has shown that in a population of patients, HCV co-infection did not alter some health parameters: the risk of dying, developing acquired immune deficiency syndrome, or responding immunologically to antiretroviral therapy (Sulkowski et al. 2002). Prior to implementation of antiretroviral therapy, life expectancies were shorter and progressive liver disease was less evident in co-infected injection drug users. In the antiretroviral therapy era, life spans of patients with HIV infection are increasing, and end-stage liver disease is emerging as a major cause of morbidity and mortality in this population.
Treatment of HCV-HIV co-infection
There is growing experience with treating HCV infection in HIV co-infected persons (Dore and Thomas, 2005; Mauss and Rockstroh, 2005; Mehta et al. 2006; Soriano et al. 2007). The medical management of patients infected with HIV and HCV remains a significant medical problem (Mehta et al. 2006). Medical management and treatment recommendations for HCV infection in HIV-infected individuals are available from the Hepatitis C Resource Centers (Department of Veterans Affairs, 2005), the Health Resources and Services Administration, HIV/AIDS Bureau (Swan, 2006), and a HCV-HIV international panel (Soriano et al. 2007). HCV-related liver disease in patients with HCV/HIV co-infection is a significant medical management issue. Thus, treatment guidelines for the management of HCV recommend that patients with HIV/HCV undergo medical evaluation for HCV-related liver disease. Liver biopsy remains the gold standard for the evaluation of liver disease (Sterling, 2005), but efforts are underway to develop noninvasive surrogate markers to accurately stage mild versus advanced liver disease in patients with HIV/HCV co-infection (Kelleher et al. 2005).
The level of liver disease is a consideration for HCV treatment (Aranzabal et al. 2005). Treatment of patients with HIV/HCV co-infection is further complicated by the relatively high prevalence of other medical and psychiatric comorbidities as well as the influence of each infection on the other. Compared with infection only with HCV, HCV/HIV co-infection results in a shorter interval for the appearance of clinically relevant liver disease, accelerated progression of liver disease, and increased mortality as a result of HCV-induced liver disease (Fig. 6). The treatment of HIV with antiretroviral regimens may result in an increase of HCV viral load and liver toxicity. Individuals, who develop a hypersensitivity to nevirapine during the course of treatment for HIV, have a sevenfold increase in their risk of death (Phillips et al. 2007). Other HIV treatment issues involve coin-fected patients receiving pharmacotherapies as part of their substance abuse treatment plan. They include substantial drug-drug interaction between methadone and antiretroviral medications as well as pharmacokinetic interactions between buprenorphine and efavirenz (McCance-Katz, 2005). Elevated HCV viral loads do not predict good outcomes for HCV treatment and are associated with post treatment relapse of coinfection (Nunez et al. 2007). Effective interferon-based treatment of HCV/HIV-infected individuals correlates with an early virologic response at 12 weeks of treatment (Montserrat et al. 2007); thus, allowing an early stoppage of treatment for those patients not responding to treatment.
A reduced treatment response, compared to HCV monoinfection, to HCV treatment in HCV/HIV populations is clearly evident from the data of three large-scale treatment trials (Carrat et al. 2004; Chung et al. 2004; Torriani et al. 2004). These three clinical trials reported similar sustained viro-logic response despite having diverse clinical trials designs. The APRICOT international clinical trial provided the best SVR to HCV treatment of HCV/HIV co-infected patients at 40 percent (Carrat et al. 2004). This study shows the importance of maximizing ribavirin concentration in combination with interferon treatment for patients with minimal liver disease to produce a SVR.
The NIH-supported AIDS Clinical Trials Group (ACTG5071) study reported a 27-percent SVR for co-infected patients with a history of drug use and minimal liver disease (Chung et al. 2004). This study reported a sustained virologic response of 15 percent for the patients infected with HCV genotype 1, the major HCV genotype observed in injection drug users.
The RIBAVIC study, a European study with a majority of injection drug users, reported a 27-percent SVR in patients who completed treatment, with a SVR for genotype 1 of less than 10 percent (Torriani et al. 2004). In this study, nearly half the patients were unable to complete the treatment regimen, a fact that underscores the difficulty facing health care providers in providing HCV treatment for co-infected individuals and the need for support services for these patients to maximize successful outcomes. Premature treatment discontinuation continues to be a prominent aspect of treatment clinical trials for HIV/HCV coinfected patients (Soriano et al. 2007a). Thus, HCV/HIV co-infected individuals who are in most need of effective treatment regimens find treatment difficult to complete and are least likely to respond to interferon-based treatment regimens.
Efficacy of treatment of acute HCV infection in patients with HIV infection has been shown in a pilot study (Dominquez et al. 2006). In this study, a 71-percent sustained virologic response was obtained in patients who were treated with peginterferon alfa-2a who had detectable HCV RNA 12 weeks after diagnosis. Thus, in this study, early treatment of acute HCV infection was highly successful.
An important measure in the response to treatment in HCV/HIV co-infected patients may be immune competence (Graham et al. 2005). However, the hallmark of HIV infection is the gradual loss of CD4+ cells as the infection progresses from acute to chronic. Progression of liver disease in the immunocompromised host is accelerated, but the immunopathogenic events that take place during this progression are poorly understood. The presence of CD4+ cells may be required for HCV clearance and self-limited disease (Post et al. 2004). A weak or limited CD4+ response to HCV antigens has been shown to be associated with a rapid progression of liver disease related to HCV infection, both in transplantation and nontransplantation settings. In patients with HCV/HIV co-infection, CD4+ T cell proliferative immune responses to HCV antigens are lower than in HCV monoinfected patients. Thus, in HCV/HIV co-infection, there may be a loss of recognition of HCV antigens and/or the loss of CD4+ helper function to induce CD8+ cytolytic cells which neutralize cells infected with HCV (Einav and Koziel, 2002). Immune enhancement strategies may be important in HCV/HIV co-infection to reduce the depletion of CD4+ cells thereby promoting both host defense mechanisms and enhanced responses to therapeutic regimens.
Treatment of Viral Hepatitis Infection in the Context of Pharmacologic Therapy Provided in Opioid Treatment Programs
There are many types of substance abuse treatment programs that provide a variety of services for HIV/AIDS, hepatitis infection, and other sexually transmitted diseases (Brown et al. 2006). Opioid treatment programs (commonly referred to as OTPs or methadone programs) help individuals dependent on opioids abstain from illicit drug use through the dispensing of opiate agonist pharmacotherapies and other wrap around services. These programs range in the number of services provided and some programs provide a comprehensive therapeutic program that incorporates primary medical care, psychosocial counseling, vocational rehabilitation, HIV testing and counseling, viral hepatitis education and testing, and other vital medical and social services. Comprehensive “one stop shopping” integrated health service programs for injection drug users have been shown to promote good clinical treatment outcomes (Grebely et al. 2007; Sylvestre and Zweben, 2007).
Substance use disorders are complex chronic brain diseases with vast social costs that include crime, poverty, and devastating impacts on individuals and families (Brown, 2004) and “one stop shopping” integrated health service program can impact the ability of injection drug users to address social issues. Injection drug use—common among men and women who abuse or are dependent on heroin, cocaine, methamphetamine, and prescription opioids—often involves shared needles, non-sterile conditions, and other high-risk behaviors that can result complex health problems and require substantial care and services for the medical management of the consequences of substance abuse or dependence (Table 4).
Table 4.
Comorbidities associated with substance abuse and dependence.
| • Medical: HCV infection, HBV infection, tuberculosis and other pulmonary disease, immune deficiency, human immunodeficiency virus infection, sexually transmitted diseases, sexual disorders, dental and periodontal disease, nutrient deficiency, cardiovascular disease, sleep disorders, chronic pain syndromes |
| • Psychiatric: Axis I spectrum disorders such as depression, anxiety, post-traumatic stress disorder, personality disorder, bipolar disorder, attention deficit hyperactivity disorder, schizophrenia, cognitive dysfunction; Axis II personality and developmental disorders |
| • Social: poverty, homelessness, family dysfunction, corrections/prison, violence, sexual abuse, drug-using peer groups, easy drug access, lack of occupation and skills |
| • Other Addictions and Abuse: alcohol, nicotine, stimulants, cocaine, hallucinogens, marijuana, prescription drug, internet, gambling |
Drug or alcohol abuse/dependence can cause direct damage to the liver, and substance-induced liver disease may be compounded by infection with viruses which may result in liver disease that requires medical care and is difficult to treat. The Centers for Disease Control and Prevention (CDC) current hepatitis C fact sheet indicated that most cases of HCV infection are due to injection drug use while the National Prevention Plan indicated that 60%–80% of persons who have injected drugs for at least 5 years are infected with HCV (CDC Viral Hepatitis C Fact Sheet; CDC National Prevention Plan).
Many patients dependent on opiates also abuse other drugs and may meet criteria for several substance use disorders. For example, patients dependent on heroin also have high rates of comorbid alcohol and/or cocaine abuse (Conway et al. 2003; Costenbader et al. 2007; Watson et al. 2007). For these individuals, the combination of medication, brief/behavioral interventions, and network therapy/peer support groups—which utilize family members and/or friends to support compliance with drug treatment (Galanter et al. 2004)—may be most useful to reduce risk-taking behavior and enhance their quality of life. Alcohol consumption exacerbates co-occurring liver disease, and patients with viral hepatitis infection should not consume alcohol (Kulig and Beresford, 2005). Alcohol is associated with elevated viral loads among patients infected with HCV, and the combination of elevated HCV loads and alcohol use is associated with a poorer therapeutic response to treatment (Finucane et al. 2007). Alcohol consumption reduces the survival rate among patients with hepatocellular carcinoma, a liver cancer that can result from chronic HBV or HCV infection (Wong et al. 2005).
Care and treatment of hepatitis infection and opioid abuse and dependence
Prevention of and medical care for liver disease should be provided to patients in drug treatment and recovery programs in a comprehensive fashion to promote positive medical outcomes (Birkhead et al. 2007; van Beek, 2007). Enrollment in primary care and patient education about liver disease and prevention of infectious diseases are important in the medical management of viral liver infections (Edlin et al. 2005). Counseling/education of young injection drug users about prevention of infectious disease is particularly important. A recent study evaluating a behavioral intervention for young injection drug users comprising peer HIV and HCV education skills has shown that interventions that provide information, enhance risk-reduction skills and motivate behavior change can reduce injection risk behaviors (Garfein et al. 2007). Young injection drug users are seldom vaccinated to prevent viral hepatitis infection (Elefsiniotis et al. 2006; Kuo et al. 2004), even though vaccination may be the best course of action (Baral et al. 2007). Counseling individuals who already have hepatitis infection about a healthy lifestyle promotes treatment readiness among patients with progressive liver disease (Zweben, 2001). Treatment readiness interventions are important for injection drug users in their access to care and treatment. Injection drug users do not receive treatment for hepatitis C infection in great numbers (Grebely et al. 2008). Developing treatment readiness through patient education and counseling with the use of peer educators and support groups can reduce the number of patients refusing treatment for hepatitis infection (Moirand et al. 2007; Schackman et al. 2007; Sylvestre and Zweben, 2007). Providing integrated primary care and pharmacologic treatment for opioid dependence can facilitate both recovery from opioid dependence and medical treatment of co-occurring conditions, such as viral hepatitis infections (Fiellin et al. 2003; Litwin et al. 2007).
Current clinical practice guidelines recommend care and treatment for patients infected with viral hepatitis who might benefit from treatment and virus eradication. However, barriers seriously limit this care and treatment for injection drug users (Edlin et al. 2005; Grebely et al. 2008; Nguyen et al. 2007; NIH, 2002; Schaefer et al. 2004). One significant barrier is the need for more data on program structure and elements supporting effective HCV treatment for injection drug users. A review of the research clinical trials literature published between 1987 and 2004 and focusing on the treatment of chronic HCV infection describes only 10 clinical trials involving patients with drug abuse (Robaeys and Buntinx, 2005). None of the published clinical trials randomized patients and only one used pegylated interferon, the medication currently considered the standard of care for HCV treatment. Recently, more prospective, controlled clinical trials of standard-of-care treatments for HCV infection in patients who are injection drug users have been performed. In 2007, seven treatment trials were published that included injection drug users (Belfiori et al. 2007; Calleria et al. 2007; DeRosa, et al. 2007; Grebely et al. 2007; Huckans et al. 2007; Krook et al. 2007; Schaefer et al. 2007) while other studies investigated factors related to the successful treatment of injection drug users including drug-drug interactions with methadone (Berk et al. 2007; Gupta et al. 2007), immune responses of injection drug users (Sergi et al. 2007), and medication adherence (Sylvestre and Clements, 2007). More studies are needed to enhance the development of effective treatment programs and clinical guidelines.
There are numerous reasons for the lack of large clinical trials resulting in clinical guidelines for the medical management of co-occurring hepatitis infection and drug abuse/dependence. These include the generalized stigma and prejudice associated with substance-dependent persons, their disenfranchisement from the medical community, their complex medical management issues, health-care providers' lack of current treatment knowledge about patients who are injection drug users, the design of clinical trials to exclude injection drug user participation, as well as a lack of infrastructure to deliver effective care and treatment to injection drug users (Dore and Thomas, 2005; Edlin et al. 2001; Rauch et al. 2005). Thus, substance abusers are rarely able to meet the strict eligibility criteria established for entry into many studies using interferon therapy. Consequently, treatment for chronic liver disease may be delayed or withheld for current or former substance-dependent patients, as well as for those in recovery who are receiving treatment for drug abuse. Individuals with untreated hepatitis infection are at risk of progressing to end-stage liver disease or decompensated cirrhosis, leaving liver transplantation as the only life-saving alternative. More liver transplants are performed for HCV-related infection (30–46 percent of transplants) than for alcohol-related disorders (23–25 percent) (Botero, 2004; Mandayam et al. 2004).
Patient/provider relationship and care and treatment for hepatitis infection
Care and treatment of hepatitis infection and other comorbidities associated with injection drug use is complex, and numerous barriers prevent high-quality care and positive medical outcomes. Patients vary over a wide range of engagement in care and treatment, as well as various stages of readiness to seek care. Some patients do not know whether or not they have hepatitis infection or other comorbidities. Others, with known hepatitis infection, may not have been referred for medical evaluation with care and treatment or did not follow though on the referral. Others are actively involved in treatment for hepatitis infection and other comorbidities.
Injection drug users and those who are at risk for viral hepatitis are more likely than the general population to suffer psychiatric disorders such as major depression, anxiety disorder, and bipolar disorder, and some patients use drugs or pharmaceuticals in an attempt to self-medicate an underlying psychological illness. Such untreated co-occurring disorders may increase risk-taking behaviors, and this scenario is further complicated by negative experiences of injection drug users with the health care system (Davis and Rodrigue, 2001; Golub et al. 2004; Stein et al. 2003).
A trusting relationship with a member of the healthcare team who can help patients anticipate, plan for, and endure the difficulties that arise in the medical management of drug abuse/dependence and its associated comorbidities is fundamental for drug users who seek care. This engaging relationship can be facilitated by peer support groups (Sylvestre and Zweben, 2007). A patient-provider relationship that will support a dialogue in which both parties are able to communicate openly about their expectations and frustrations is critical. However, the health care system may not support such a dialogue (Shine, 1996). Drug users often believe that the health care they receive is judgmental and condescending, unresponsive to their needs, and often delivered without respect. As a result, drug users may display individual barriers to accepting therapy and fail to follow through with medical advice, or take prescribed medication, or keep appointments (Mehta et al. 2005).
The extensive experience gained from treating injection drug users for medical conditions, especially HIV infection, has led to the development of effective principles for engaging drug users in health care relationships. Successful programs have a respectful approach to drug users, understand the medical and behavioral aspects and consequences of drug abuse and dependence, refrain from moral judgments and utilize a multidisciplinary team approach (Batki and Sorensen, 1999; O'Connor et al. 1994; Robertson, 1998; van Beek, 2007). These strategies embody a client-centered approach in which a care provider works with a client to identify changes that the client is motivated to make to enhance health and well-being (Brands et al. 2003). Even if global behavior change (such as ceasing all drug use) is not possible, other measures can reduce the medical consequences of high-risk behavior (Des Jarlais et al. 1993). In this setting, health care providers can work with the patient to develop a care and treatment regimen that is able to fit the lifestyle of the patient (e.g. once-daily therapy) rather than attempting to restructure the patient's lifestyle.
Misunderstandings about the nature of substance use disorders as chronic, potentially recurring diseases influence the nature of the relationship between the patient and the provider. Relapse during care often is perceived as failure in drug treatment rather than a characteristic of the disease. In fact, relapse may actually be relatively benign, with brief “lapses” of sobriety or abstinence sometimes called “slips” (Finney et al. 1999; Graham et al. 2002). In the context of ongoing substance abuse treatment, neither lapses nor relapses represent permanent barriers to recovery.
Strong patient-provider relationships are essential, because treatment regimens for chronic HBV and HCV infections are difficult and stressful for patients. Drug users should be presented with a comprehensive health program that incorporates high-quality hepatitis prevention and care, and substance abuse treatment. Hepatitis prevention and care should include outreach to drug users through peer educators/support groups, screening and counseling for at-risk behavior, HCV infection testing and genotyping, HBV infection testing, HIV infection testing, prevention counseling and hepatitis education, vaccination against HAV and HBV infections (if eligible), and evaluation for comorbidities (Edlin et al. 2005). This evaluation should include determining the need for substance abuse services, psychiatric care, and social support. It also should include an effort to engage the patient in primary care, as well as a liver evaluation and an assessment for treatment of chronic HBV infection and/or HCV infection. Treatment trials of chronic HCV infection have shown that programs that employ a multidisciplinary team and address co-occurring psychological disorders result in excellent treatment outcomes compared with programs that do not address treatment barriers (Bargiacchi et al. 2005; Broers et al. 2005; Sylvestre and Zweben, 2007).
It is possible—and important—to prevent progression to injection drug use by encouraging individuals who abuse noninjection drugs to enter treatment. Specific risk factors appear to lead individuals to make the transition to injection drug use. For example, individuals who engage in inhalant drug use in early teenage years tend to have a higher likelihood of progressing to injection drug use. In addition, certain factors appear to influence whether those who inhale drugs progress to injection drug use: Those who inhale drugs and who also have an intact family structure appear less likely to progress to injection drug use; homeless individuals and those whose sex partners inject drugs are more likely to progress to injection drug use (Lankenau and Clatts, 2004; Maxwell et al. 2004; Storr et al. 2005).
The importance of preventing individuals from progressing to injection drug use can be vividly seen in data comparing the HCV infection incidence among injection and noninjection drug users (Fuller et al. 2004). This longitudinal surveillance study in New York City showed an annual incidence rate of HCV infection in young noninjectors of 0.4/100 person years, compared with 35.9/100 person years for injection drug users. Delaying or preventing the transition to injection drug use may have a significant health benefit by reducing the risk of comorbid conditions associated with injection drug use and drug abuse/dependence.
A comprehensive substance abuse treatment plan and pharmacologic therapy
Substance abuse is a complex physiologic, social, and behavioral disorder that often coexists with psychiatric illness as well as comorbid medical conditions. For this reason, screening substance users for comorbid psychiatric illness should be considered an integral part of any medical intervention and comprehensive substance abuse treatment program (Sylvestre et al. 2004). It may be difficult to determine which comorbidity—substance abuse, mental illness, or infectious disease—should be addressed first. However, medical treatment of substance-related disorders often is necessary to create sufficient stability to begin treatment of other conditions. Stability is further increased when mental health services and substance abuse treatment are combined, enhancing the medical outcomes of treatment for comorbidities. Substance Abuse Treatment for Persons with Co-Occurring Disorders, Treatment Improvement Protocol (TIP) 42, (SAMHSA, 2005a) provides up-to-date information about co-occurring substance use and mental disorders as well as recommended best practices in the treatment of these disorders.
Understanding that substance abuse is a complex multifactor disorder, it is appropriate to develop, through case management, a comprehensive substance abuse treatment plan that comprises behavioral, social rehabilitative components, and biological (pharmacological) treatments (Table 5).
Table 5.
Pharmacotherapy and behavioral therapy comprising a comprehensive substance abuse treatment plan.
| Pharmacotherapy |
| • Opioid Dependence Methadone—Federally regulated through OTP; opioid receptor agonist for pharmacological therapy Buprenorphine—office-based opioid treatment or OTPs; Federally regulated, partial opioid receptor agonist for pharmacological therapy Naltrexone—office-based and substance abuse treatment programs; used when opioid abstinence is possible without significant relapse risk; opioid receptor antagonist for relapse prevention |
| • Alcohol Dependence Naltrexone—an “anti-craving” agent, opioid receptor antagonist; reduced reward effect with daily use; new forms are long acting Acamprosate—an “anti-craving” agent that normalizes glutamatergic neurotransmission; slow acting, attenuates relapse Disulfiram—a “vicarious” aversive medication supporting complete abstinence to alcohol that blocks complete oxidation of alcohol with accumulation of acetaldehyde and resultant unpleasant “allergic” physical symptoms when alcohol is absorbed (e.g. flushing, headache, and vomiting) |
| • Nicotine Dependence Nicotine replacement therapy—many over-the-counter regimens, such as patches, gum, and inhalers, are used to replace the daily physical requirement for nicotine and may be used for nicotine withdrawal or maintenance Buproprion—an antidepressant also found to be an “anti-craving” agent that reduces the psychological craving for tobacco |
| Behavioral therapy |
| • Brief interventions for 1 to 3 visits (low intensity); for early drug use and substance abuse; available in many different outpatient settings |
| • Motivational enhancement interviewing and therapy |
| • 12-step facilitation |
| • Stage-of-change model interventions |
| • Long-term, multimodal, and multidimensional comprehensive therapies and interventions to restructure belief and cognitive systems; enhance coping strategies; and change friendships, environment, and behavior |
| • Individual interpersonal one-on-one therapy, such as cognitive behavioral therapy and insight-oriented psychotherapy |
| • Group therapy—such as family or faith-based, Therapeutic Communities |
| • “12-step” programs and “clean and sober” recovery living environments in which peer groups interested in sobriety mutually help one another stay sober |
Pharmacological treatments have been developed and approved for specific drug addictions. Currently, addiction treatment medications are available for nicotine, alcohol, and opiates. Medications are now being developed for dependence and abuse of stimulants, like cocaine and methamphetamine. Several marketed medications—disulfiram, baclofen, modafinil, naltrexone, ondansetron, tiagabine, and topiramate—have shown efficacy to reduce cocaine use in initial clinical trials (Vocci and Ling, 2005). To date, no medications tested in clinical trials have shown efficacy to reduce methamphetamine use.
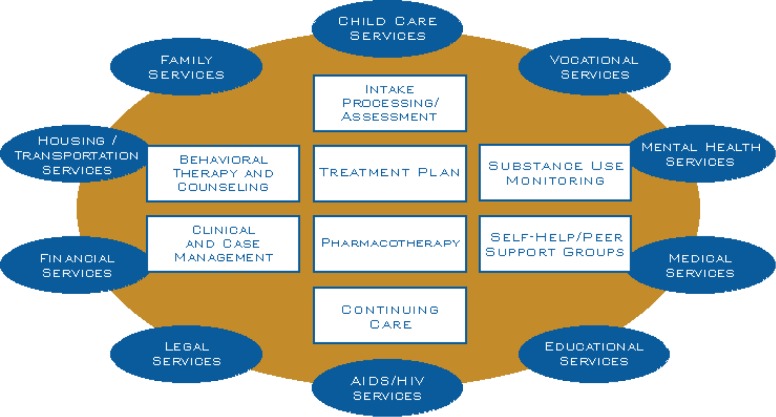
Medications are a proven component of comprehensive substance abuse treatment plans that reduce drug use and provide an opportunity for improvement in health and social functioning for individuals with opioid dependence (Fig. 7; Gowing et al. 2004; Johnson and McCaugh, 2000; NIDA, 2000a). Two recent TIPs from SAMHSA, TIP 40 and TIP 43, provide the best practices guidelines for the use of either methadone or buprenorphine as part of a comprehensive treatment plan for opioid abuse/dependence (SAMHSA, 2004; SAMHSA, 2005).
Figure 7.
Diagram of the elements of a comprehensive substance abuse treatment plan (from NIDA 2000a).
Treatment services for drug abuse/dependence that follow recommended best medical practices are more likely to manage the care and treatment of hepatitis successfully and to prevent progressive liver disease. The medications used in the management of opiate dependence are metabolized through the liver, and therapeutic blood levels can be affected by liver disease. Two pharmacologic therapies, methadone and buprenorphine, illustrate the interaction between appropriate pharmacotherapy and the possible impact on liver disease.
Methadone
Methadone is the mainstay of pharmacotherapy treatment for opioid dependence and helps dependent individuals abstain from illicit drug use and achieve recovery. Methadone is a synthetic mu-opioid receptor agonist with pharmacological properties qualitatively similar to morphine. Administered daily as an oral dose, methadone should be present in the blood at levels sufficient to eliminate symptoms of opioid dependence during a 24-hour period, without episodes of opioid overmedication or withdrawal (Payte and Zweben, 1998). The blood level and elimination of methadone may be influenced by factors such as poor absorption, variable metabolism, other medications, diet, physical condition, patient age or pregnancy, and vitamins or herbal products such as St. John's wort. Therefore, considerable flexibility in dosing is required to stabilize patients and an adequate physiologic methadone level is critical for therapeutic success (Eap et al. 2002).
Methadone is safe when used as indicated (SAMHSA, 2005). Research studies have not demonstrated liver toxicity in patients with underlying liver disease. Serious adverse reactions or cumulative organ damage has not been reported when daily methadone is used in appropriate dosages. Mortality rates of patients in methadone treatment from all causes are typically one-third those of untreated opioid addicts (SAMHSA, 2004a). However, fatal overdoses with methadone, as well as deaths of clients in methadone treatment, have been reported (Clarck et al. 1995; Maxwell et al. 2005; Shah et al. 2005; Fugelstad et al. 2007). Data from Stockholm, Sweden show that patients receiving methadone treatment had a lower mortality rate and that leaving methadone treatment resulted in a 20 times increase risk in death due to drug overdose (Fugelstad et al. 2007). A study of patient deaths in methadone treatment in Texas (Maxwell et al. 2005) revealed 20 percent of deaths due to liver disease, 18 percent of deaths due to cardiovascular disease, and 14 percent due to drug overdose or trauma. In New Mexico, (Shah et al. 2005), 50.3 percent of deaths of patients in treatment between 1998 and 2002 were from methadone in combination with illicit drugs, 23.8 percent were from methadone in combination with prescription drugs (possible pain management patients), and 3.5 percent due to methadone in combination with alcohol. These data show the importance of other addictive drugs in combination with methadone in unintentional methadone-related deaths. In treatment, methadone-associated deaths can occur during the induction phase when a patient's level of tolerance to opioids is not correctly assessed or when a patient continues to use other central nervous system depressant drugs in combination with methadone.
Buprenorphine
Buprenorphine is a partial muopioid receptor agonist (Ling and Smith, 2002; see Table 5). At higher doses, buprenorphine reaches a plateau in its similarity to opioid properties. This limitation on agonist effects results in an improved safety profile compared with a full agonist such as methadone. Specifically, buprenorphine has a favorable “ceiling effect” on respiratory depression (Walsh at al. 1994). In addition to improved safety, flexible dosing (e.g. thrice weekly) is feasible since buprenorphine has a high binding affinity for the opiate receptor and dissociates slowly.
A report from France has noted an elevation in measures of abnormal liver function after the use of intravenous buprenorphine. This report was limited by the small sample size, retrospective analysis, and short time in which buprenorphine was given (Petry et al. 2002). However, the use of buprenorphine in individuals with known liver disease is of concern and many clinicians have avoided buprenorphine in this patient population. Since, 2001, no additional reports of liver toxicity with buprenorphine have been reported, despite increasing use of buprenorphine in treating opioid dependence. In 2002, the Food and Drug Administration (FDA) approved two sublingual buprenorphine products for use in the United States as a treatment for opioid dependence, and large-scale use of buprenorphine continues in Europe.
Buprenorphine may be a component of the substance abuse treatment plan for individuals infected with HCV (Alford et al. 2007; Belfiori et al. 2007; Bruce and Altice, 2007; Krook et al. 2007). Among patients treated through a mobile outreach intervention in New Haven, Connecticut, 36 individuals infected with HCV and HIV have been treated with buprenorphine. Liver function measures show no adverse effect from buprenorphine treatment in this co-infected population (Kresina et al. 2005). In a recent study of co-infected homeless opioid-dependent individuals, buprenorphine treatment was effectively implemented with comparable outcomes to housed patients treated with buprenorphine (Alford et al. 2007). Although monitoring is required when any medication is added to a patient's medication regimen, the presence of HIV/HCV co-infection or use of antiretroviral therapy does not rule out use of buprenorphine.
Pharmacotherapy for Alcohol Dependence
Patients with co-occurring injection drug use, alcoholism, and liver disease may need treatment aimed at ending alcohol use. Medications for alcohol dependence include acamprosate, naltrexone (vivitrex), or disulfiram (Fiellin et al. 2004). Acamprosate and naltrexone have different mechanisms of action and modify different behavioral aspects of dependence. Acamprosate, a long-acting compound, prolongs periods of abstinence by normalizing glutamate neurotransmission that is disrupted during chronic alcohol consumption and withdrawal. Naltrexone, a fast-acting opioid receptor antagonist with a long half-life, can reduce heavy drinking by decreasing alcohol's rewarding effects. Safety and effectiveness of treatment using both drugs for alcohol addiction have been shown in double blind studies (Littleton and Zieglgansberger, 2003). A long-acting formulation of naltrexone, which would allow treatment of alcohol dependence with a monthly injection, is now FDA approved (Garbutt et al. 2005; Johnson, 2006). Disulfiram is designed to help motivate patients to remain abstinent from alcohol through “vicarious aversive therapy”—the patient who has taken disulfiram and then ingests alcohol experiences a series of unpleasant allergic-like symptoms (e.g. flushing, headache, and vomiting). The drug works by blocking the oxidation of alcohol at the acetaldehyde stage in its metabolism. Incorporating Alcohol Pharmacotherapies into Medical Practice, Treatment Improvement Protocol (TIP) 48, (SAMHSA, 2007) provides up-to-date information about the use of medications currently approved for treating alcohol use disorders.
Integration of addiction treatment with hepatitis prevention, screening and treatment
Individual OTPs provide a range of services and some programs provide a comprehensive blend of therapies—primary medical care, psychosocial counseling, vocational rehabilitation, HIV testing and counseling, HCV education and testing, and other vital medical and social services—needed to effectively treat substance abuse, dependence and its associated comorbidities. Substance abuse treatment programs that offer a broader array and greater frequency of services report longer time in treatment and improved treatment outcomes. Programs that respond to the severity of drug abuse during the first stages of drug treatment show positive treatment outcomes related to longer retention in treatment and patient satisfaction with treatment services (Hser et al. 2004). Entry of injection drug users into substance abuse treatment is facilitated by program outreach and case management as well as the patient characteristics of not being homeless, having less problems with alcohol consumption and advancing though the stages of behavior change (Corsi et al. 2007). Maximum retention time in methadone treatment is associated with comprehensive treatment and provision of frequent health services, as well as appropriate methadone dosing (Booth et al. 2004).
Comprehensive services for hepatitis infection include hepatitis prevention, care, and treatment. Elements of hepatitis prevention and care for drug users include screening for at-risk behavior; HAV, HBV, HCV, and HIV testing; prevention counseling and education; vaccination against HAV and HBV infections; and evaluation for comorbidities, including the need for substance abuse services, psychiatric care, social support, liver disease evaluation, and interferon-based HCV treatment.
Injection drug use can lead to HCV infection. A recent incident infection study showed that women, new injection initiates and injection drug users recruited through outreach are at increased risk for HCV infection (Maher et al. 2006). Prevalence estimates of HCV infection derived from surveys of patients in methadone treatment programs range from 72 percent to more than 90 percent (CDC, 1998; Inglesby, 1999; McCarthy and Flynn, 2001; NIDA, 2000; Stein et al. 2001), compared with 1.8 percent in the overall U.S. population (Alter et al. 1999; CDC, 1998). In one study of 306 OTP patients, 82 percent had not received prior HCV testing and 87 percent were infected with HCV (Stein et al. 2001). The CDC recommends routine HCV testing for individuals who have ever injected illegal drugs as part of a national strategy to identify HCV-infected individuals and to enter into care to prevent the consequences of their infection (CDC, 1998). In addition, testing for hepatitis infection and peer-driven counseling can change injection drug users' risky behaviors that increase the risk of transmitting HCV (Aitken et al. 2002; Garfein et al. 2007; Latka et al. 2007; Tucker et al. 2004).
HCV treatment studies demonstrate that roughly one in five current alcohol and/or drug abusers do not comply with HCV treatment monitoring or are lost to followup. Thus, a consequence of continued drug use may be an increased viral load and reduced response to treatment (Davis and Rodrigue, 2001; Sylvestre, 2002). However, patients with co-occurring HCV infection and substance use can complete interferon-based treatment with careful monitoring and aggressive intervention. HCV treatment providers who integrate early interventions for drug use and other comorbidities into their HCV treatment plan improve the likelihood of good outcomes. HCV patients can successfully be treated with interferon-based therapy even if they have histories (or current incidence) of significant substance use disorders (Dore and Thomas, 2005; Grebely et al. 2007; Hopwood and Treloar, 2007; Sylvestre et al. 2005).
In the past, patients receiving methadone have not been included in clinical studies of HCV treatments because methadone treatment has been considered a confounding factor in determining treatment efficacy, the OTP population has been viewed as atypical HCV patients, and researchers have feared that some former and current injection drug users would not adhere to treatment. Recently, however, a growing number of studies (Berk et al. 2007; Gupta et al. 2007; Mauss et al. 2004; Robaeys et al. 2006; Schaefer et al. 2003; Sergio et al. 2007; Sylvestre et al. 2005; Van Thiel et al. 2003; Verrando et al. 2005) have found that interferon-based treatment regimens are safe and effective for patients receiving methadone treatment, that dosing of interferon or ribavirin is not altered by methadone, and that patients who discontinue HCV therapy while receiving methadone do so early in the course of HCV treatment.
Patients receiving methadone treatment should not be withdrawn from methadone prior to HCV treatment, as continued methadone maintenance can be helpful in enhancing quality of life through stabilization during HCV treatment. However, additional research is needed to better understand the natural history of HCV infection in patients receiving pharmacotherapy for substance use. Recent studies have added to the growing body of evidence indicating that interferon-based therapy is effective in substance abuse treatment settings for patients receiving methadone or buprenorphine as part of their treatment for opioid dependence (Belfiori et al. 2007; Krook et al. 2007). Therefore, AASLD Clinical Practice Guidelines recommend that HCV treatment not be withheld from individuals seeking or receiving substance abuse treatment. (Strader et al. 2004).
Early screening, testing, and treatment for HCV infection
The best methods for detecting HCV infection are to screen populations for a history of at-risk behaviors and to test individuals who have an identified risk behavior or factor for HCV exposure (AASLD, 2004). Injection drug use is the chief mode of HCV transmission in the United States; therefore, anyone with a history of injecting drugs should be tested for HCV infection (CDC, 1998). Regardless of substance abuse status, individuals with HCV infection should receive counseling, education, medical evaluation, care, and needed treatment (Alter et al. 2004). Early treatment studies have shown high sustained virologic response in patients treated within 3 months of testing positive for HCV infection or 8 weeks post-exposure, reporting a sustained virologic response of greater than 90 percent (Calleri et al. 2007; Corey et al. 2006; Jaeckel et al. 2001; Normura et al. 2004). Treating acute HCV infection expeditiously is likely to prevent complications, such as cirrhosis, and to be cost-effective (Santantonio, 2004).
Unfortunately, it is difficult to identify recent or acute HCV infection in opioid-addicted persons first entering substance abuse treatment programs because other health problems or barriers may be present (Chitturri and George, 2000; Leavitt, 2001; Sylvestre, 2002; Watson et al. 2007). Exposure to HCV is determined by the presence of serum antibody to HCV through use of an enzyme immunoassay. HCV infection is determined by identifying HCV virus in samples of blood serum using molecular tests such as polymerase chain reaction and/or transcription-mediated amplification (NIH, 2002). In a study of 493 patients exposed to HCV who were in opioid treatment, 77 percent were found to have HCV infection as determined by polymerase chain reaction analysis. The only statistically significant clinical feature distinguishing those with HCV infection from others was abnormal levels of the liver enzyme alanine aminotransferase (Sylvestre et al. 2005). Fewer than half (30–40 percent) of patients display symptoms prior to testing positive for HCV exposure; the initial marker for HCV exposure may not be present in symptomatic patients. For some patients, acute HCV infection occurs without any signs and symptoms, which may not be apparent until cirrhosis develops. Once end-stage liver disease develops, prospects for survival are limited (Wong et al. 2004). There are important benefits to starting treatment during early or acute stages of HCV infection, but an accurate evaluation using appropriate laboratory screening techniques is needed, as laboratory methods to identify early HCV infection are not readily available.
Hepatitis education
Drug treatment programs can provide a variety of services including education of patients about hepatitis infection, but patients may not be aware or utilize such a service (Strauss et al. 2007). OTPs are more likely than are drug-free treatment programs to provide hepatitis education materials to patients and to educate most or all staff about hepatitis infection (Astone et al. 2003; Strauss et al. 2003). Education materials provided in OTPs are more comprehensive and encompass topics such as viral transmission, testing, treatment options, and HIV co-infection (Strauss et al. 2004). SAMHSA supports a hepatitis education program for OTPs, and the American Association for the Treatment of Opioid Dependence (AATOD) has developed a curriculum for hepatitis education and participated in its dissemination through The Hepatitis Education Training for Opioid Treatment Providers Program (www.AATOD.org/hepatitis. html).
Other hepatitis curricula, developed by Federal and State agencies, are available (see Table 6) and emphasize differing aspects of HCV infection. For example, the New York State Department of Health curriculum includes a 2-day workshop that emphasizes integrating HCV into substance abuse treatment settings; the Veterans Administration curriculum is vast and provides great detail regarding HCV and alcohol consumption; and the Health Resources and Services Administration has developed a curriculum as technical assistance for HIV care providers that treat patients with co-occurring HCV infection.
Table 6.
Salient Hepatitis C curricula developed by federal and state agencies.
| Agency | Program and website |
|---|---|
| Substance Abuse and Mental Health Services Administration (SAMHSA) | HIV and Hepatitis Matrix www.samhsa.gov/Matrix/matrix_HIV.aspx American Association for the Treatment of Opioid Dependence web site www.AATOD.org/hepatitis.html |
| Centers for Disease Control and Prevention (CDC) | The Division of Viral Hepatitis—Hepatitis C Toolkit, brochures, posters, Counseling and Training resources www.cdc.gov/ncidod/diseases/hepatitis/c/#materials The National Commission on Correctional Health Care Curriculum www.cdc.gov/ncidod/diseases/hepatitis/resources/training/ncchc_man.htm |
| Department of Veterans Affairs (VA) | VA National Hepatitis C Program Educational resources. Patients corner, VA information www.hepatitis.va.gov |
| Health Resources and Services Administration (HRSA) | Integrating HIV and HCV services www.hab.hrsa.gov/catie/list.asp?ref=256 |
| New York City Department of Health and Mental Hygiene | Understanding Hepatitis C www.nyc.gov/health |
| Massachusetts Department of Health | Massachusetts Hepatitis C Program www.mass.gov/dph/cdc/masshepc/default.htm |
| New York State Department of Health | National Virus Hepatitis Training |
| Texas Department of State Health Services | Texas Hepatitis C Initiative Counselor Training Manual www.tdh.state.tx.us/ideas/hepatitis Also required continuing nursing education through the CDC, Texas Nurses Association or National Center of Continuing Education www.tdh.state.tx.us/ideas/hepatitis/heaptitis_c/professional/cne |
Peer-driven counseling and education using a brief behavioral intervention and peer-driven counseling coupled to testing for hepatitis infection can change risky behavior associated with the HCV transmission among injection drug users (Aitken et al. 2002; Garfein et al. 2007; Tucker et al. 2004). These education and counseling efforts are important components of a comprehensive substance abuse treatment plan for injection drug users because, when implemented, they increase the patient's knowledge of HCV and promote treatment readiness (Evans et al. 2005; Sylvestre and Zweben, 2007; Walley et al. 2005). However, not every substance abuse treatment program serving injection drug users provides these needed services (Brown et al. 2006; Vassilev et al. 2004).
Clinical research: Hepatitis C treatment studies and pharmacotherapy
Available data indicate that HCV treatment outcomes of patients receiving methadone treatment or buprenorphine, as part of their treatment for opioid dependence, can be equivalent to those reported in studies of patient populations that exclude patients receiving methadone treatment. Schafer (2001) found that 50 percent of patients receiving methadone experienced a viral response to interferon-ribavirin treatment after 6 months, compared to 39 percent of patients in a control population. Schafer concluded that patient compliance and retention in therapy were critical factors and that an interdisciplinary OTP setting with adequate patient support facilitated safe and successful treatment.
Blechman et al. (1999) compared interferon therapy for chronic HCV infection in 26 patients receiving methadone treatment with a control group of 22 patients not receiving methadone. Disease severity, response to interferon, side effects, and treatment compliance were equivalent in both groups. The authors concluded that stable patients receiving methadone treatment should not be excluded from HCV treatment trials and are candidates for antiviral therapy as noted in current Clinical Practice Guidelines (AASLD, 2004).
Hagan and colleagues (1999) examined interferon therapy administered to 19 HCV-infected patients in an OTP. Of the 14 (74 percent) who completed the study, 79 percent had a treatment response at 3 months. Only two patients were discontinued because of medication nonadherence; two left the OTP; and one discontinued interferon. This study showed that delivering interferon-based therapy in the OTP clinic setting is a feasible option.
A study of HCV treatment in 76 recovering heroin users maintained on methadone found that neither current drug use nor short duration of abstinence before treatment led to significant reductions in outcomes for patients receiving standard regimens of interferon plus ribavirin (Fig. 8). The authors concluded that injection drug users can be safely and effectively treated for HCV despite multiple barriers to treatment when they are treated in a setting that can address their special needs (Sylvestre et al. 2005).
Figure 8.
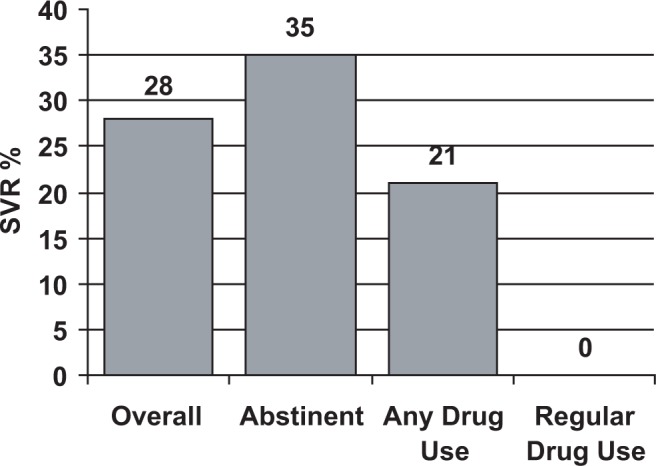
Sustained virologic response (SVR) to interferon-ribavirin treatment in 66 HCV-positive patients receiving methadone treatment (Sylvestre et al. 2005).
Another study investigated whether patients still injecting opioids and infected with HCV could be detoxified from opioids and successfully treated with interferon-ribavirin combination therapy. Fifty injection drug users, including a majority variously dependent on alcohol, cocaine, and/or benzodiazepines, underwent a 28-day opioid withdrawal program and received treatment for HCV infection (Backmund et al. 2001). Overall, there was a sustained virologic response in 36 percent of subjects who exhibited excellent medication compliance and clinic attendance. However, 80 percent of patients who had completed 28-day detoxification had one or more injection-drug relapses, including 10 of the 18 patients with a sustained virologic response. Thirty percent of the patients who relapsed had entered treatment at an OTP, and 53 percent of these patients receiving methadone treatment had a sustained virologic response. This response was higher than the rate in the overall group (53 percent versus 17 percent) and the rate in patients who remained abstinent without a drug relapse (53 percent versus 40 percent) who did not enter an OTP (Fig. 9). While substantial, these differences did not reach statistical significance, possibly because of the small numbers of subjects involved and the low statistical power.
Figure 9.
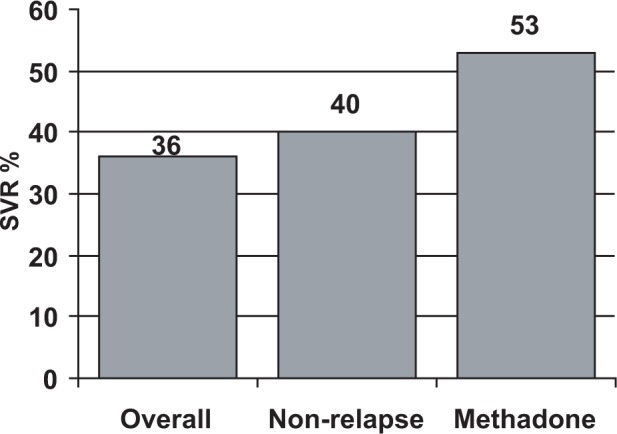
Sustained virologic response (SVR) among HCV-positive injection drug users (data from Backmund et al. 2001).
Although this study indicates that injection drug users may be treated successfully for HCV infection in the face of continuing drug abuse, the researchers emphasize that this group of subjects was younger and had a relatively shorter duration of HCV infection than in other comparative studies. The study reported high relapse rates with no HCV re-infections. This may be due, in part, to subjects using the sterile syringes and needles provided for home injection of interferon. Other reports suggest that re-infection may not be as rapid as previously thought. In a study of 27 former injection drug users not in an OTP who had been treated successfully for HCV infection, one-third returned to injecting drugs and only one was found to be re-infected at 64-months followup. In another study that followed injection drug users for a mean of 33.8 months, 15 of 18 participants remained HCV RNA-negative (Dalgard et al. 2002; Backmund et al. 2004). Further studies are needed to clearly assess the risk of HCV re-infection in patients who relapse to injection drug use.
Stein and colleagues (2001) observed that the typical stability of persons in an OTP makes them good candidates for the rigors of interferon-based therapy. Of 306 HCV-positive patients in an OTP that they surveyed, 53 percent were eager to participate in interferon therapy even though they understood that interferon-based treatment requires injections, is only partially efficacious, may produce adverse reactions, and may require a liver biopsy.
Robaeys and colleagues (2006) showed in a retrospective cohort study of 406 patients with chronic HCV that noncompliance in injection drug users was not different from noninjection drug users. Moreover, the injection drug users group had a higher sustained virologic response (46.6 percent) than the noninjection drug users group (34.6 percent).
Two recent studies of patients receiving either methadone or buprenorphine in combination with psychosocial support showed effective treatment of HCV infection with peg interferon alfa 2a or 2b plus ribavirin (Belfiori et al. 2007; Krook et al. 2007). In the Norwegian study, treatment compliance was 100 percent, all patients responded to HCV treatment and 94 percent of patients obtained a sustained virological response (Krook et al. 2007). In the Italian study, three-fourths of patients were drug dependent for longer than 5 years prior to HCV treatment, 17 percent dropped out of treatment relapsing to drug use while 39 percent obtained a sustained virological response.
Thus, a growing body of evidence indicates that patients' receiving either methadone or buprenorphine, as part of their treatment for opioid dependence, can comply with interferon-based HCV treatment regiments with treatment outcomes producing a sustained virological response. In addition, patients receiving treatment at OTPs may exhibit good HCV treatment adherence and retention, with limited side effects or adverse events. AALSD Clinical Treatment Guidelines recommend that HCV treatment not be withheld from patients participating in substance abuse treatment programs. A period of abstinence from alcohol and illicit drugs may be beneficial for maximizing treatment responses but is not necessary or required to initiate interferon-based treatment of chronic HCV infection. Two critical factors in pharmacotherapy treatment for drug dependence and concurrent HCV treatment are (1) maintaining adequate methadone/buprenorphine dosages to avert potential drug-relapse both prior to, during, and following treatment for HCV infection and (2) conducting ongoing supportive psychosocial and psychotherapies for patients through the course of treatment.
Viral hepatitis and the health care provider: Occupational and health care facility-related exposure to viral hepatitis
It is possible that HCV can be transmitted to patients being treated in a health care setting. In substance abuse treatment programs, both the health care provider and patient must be aware of this risk and of possible courses of action if viral transmission should occur.
The route of transmission for approximately 10 percent to 15 percent (Fig. 7) of all hepatitis infections remains undetermined. Nosocomial infections (i.e. infections that are a result of treatment in a hospital or hospital-like setting) and patient-to-patient transmission during treatment are important issues for substance abuse treatment programs. HBV and HCV have been documented to be transmitted from an infected health care worker or patient through percutaneous or mucosal exposure to blood and other body fluids (CDC, 2003). The opportunity for occupational or nosocomial exposure to hepatitis in a substance abuse treatment program increases as more wraparound services, such as infectious disease testing, immunizations, pain management, and treatment clinical trials, are performed at the treatment site as part of the comprehensive substance abuse treatment plan (Comstock et al. 2004; Krause et al. 2003; Larghi et al. 2002).
This transmission poses personal, legal, and professional risks to patients, health care workers and treatment programs. Providing education—particularly to health care staff on the Occupational and Safety and Health Administration's Blood Borne Pathogens Standard (www.osha.gov/SLTC/bloodbornepathogens/index.html) and use of the latest safety devices—is central to effective efforts to prevention viral transmission. Strict adherence to universal safety precautions is equally important, and dedicated space, equipment, and staff for wraparound services also can significantly reduce hepatitis transmission (Saxena et al. 2003). HAV and HBV vaccination of health care workers and all non-immune individuals in the substance abuse treatment setting also is an important prevention measure and has been shown to be cost-effective (Jacobs et al. 2004).
Protocols for post-exposure treatment and follow-up have been developed (CDC, 2001; Delwaide, 2003; Sounder et al. 2005; West, 2001). For exposure to HBV, the exposed individuals should undergo serological testing. Active (immunization) and passive (anti-HBV antibody) vaccines are effective against HBV infection, if the individual is not vaccine immune, and should be provided within 24 hours of exposure. Antiviral treatment is not recommended for persons diagnosed with acute HBV infection if they have healthy immune systems. Immune globulin and interferon-based antiviral treatment are not recommended for exposure to HCV; instead, the CDC recommends determining the HCV status of the source and the exposed person and follow-up HCV testing to determine if infection has occurred (CDC, 2001).
HCV is detectable as early as 1–2 weeks after exposure, while hepatitis C antibodies can be detected in blood approximately 8 weeks after exposure. Elevations of liver enzymes may be detected 6–12 weeks after exposure (Kim and Saab, 2005). Studies have shown that individuals with symptomatic acute HCV infection have a high likelihood of self clearance of virus or self-cure. The only controlled clinical trial of initiation of interferon-based treatment in acute HCV infection (8 weeks post-exposure) showed a sustained virologic response of 100 percent (Normura et al. 2004). Thus, addressing HCV exposure in acute infection predicts a good outcome either through self-cure or short-term interferon-based treatment.
Medical Complications and Psychiatric Comorbidities of Hepatitis Infection, Treatment, and Dependence
Side effects of treatment for hepatitis C infection
Treatment side effects associated with HCV pharmacotherapies have been found to impair quality of life to the extent that approximately 15 percent to 20 percent of patients discontinue treatment. However, as many as 15 percent of patients experience no side effects (Ryder and Beckingham, 2001). Interferon therapy may be associated with side effects such as fatigue, muscle aches, nausea, vomiting, headaches, low-grade fever, and low platelet and neutrophil counts. Although such adverse reactions usually are mild to moderate and can be managed, they may be sufficiently troublesome to influence patient noncompliance or withdrawal from treatment (Table 7; Strader et al. 2004).
Table 7.
Common side effects of interferon-alpha and ribavirin.
| Flu-like Symptoms- fever, headache, myalgia, fatigue, asthenia, rigors, dizziness, influenza-like symptoms |
| Gastrointestinal- diarrhea, anorexia, nausea, vomiting, abdominal pain |
| Neuropsychiatric- depression, impaired concentration, irritability, insomnia |
| Skin/Appendages- alopecia, pruritus, rash |
| Hematologic- decreased hemoglobin, decreased white blood cell count, decreased platelet count |
Natural and recombinant interferons have short biological half-lives and therefore require daily or thrice-weekly injections. Fluctuations in serum concentrations may undermine both efficacy and tolerance. Pegylated interferon has a longer half-life, a characteristic that improves tolerance and permits less frequent injection. Compared with treatment with interferon alone, interferon plus ribavirin combinations typically result in reduced side effects, with the result that fewer patients discontinue treatment (McHutchison et al. 1998). A recombinant form of interferon, albumin-interferon alfa-2b, has a longer half-life and initial studies show some efficacy in patients who previously failed their interferon therapy (Balan et al. 2006). In these patients, there were no treatment discontinuations due to adverse events and the most common (30–50 percent of patients) side effects of albumin-interferon treatment were headache, fatigue, injection site erythema, and arthralgias.
Psychiatric illness comorbidity
Manufacturers' package inserts for interferon warn of the potential for neuropsychiatric side effects, the most common being depression and the possibility of psychiatric relapse after beginning therapy. Other neuropsychiatric side effects of treatment include mood alterations, irritability, anxiety, and acute manic episodes (Dell'Osso et al. 2007). Dose-dependent and reversible neuropsy-chiatric effects have been reported to occur in 30 percent to 40 percent of patients during interferon treatment. Treatment with ribavirin may also contribute to interferon-induced depression (Asnis and De la Garza, 2006). The neuropsychiatric comorbidities of interferon alfa-2a or -2b treatment may be severe, limiting treatment in 10 percent to 20 percent of cases and are more than twice as likely in persons with histories of psychiatric disorders than in those without (Davis and Rodrigue, 2001; Ho et al. 2001; Neri et al. 2006). Psychiatric diagnoses, rather than substance-abuse disorders, are a prominent reason for treatment ineligibility unless the patient has been treated and stabilized (Loftis et al. 2006; Muir et al. 1999; Sylvestre et al. 2004). Psychiatric support, including the use of antidepressants, such as selective serotonin reuptake inhibitors or anxiolytics, is frequently required during interferon-based therapy.
Preexisting psychiatric comorbidity has been observed in up to half of all persons entering OTPs (Brooner et al. 1997; SAMHSA, 2005a; Kamal et al. 2007). The management of these illnesses is an important component of the OTPs therapeutic milieu that can promote abstinence and help reduce adverse reactions and risks of drug relapse associated with interferon-based treatments (Ho et al. 2001; SAMHSA, 2005; Kamal et al. 2007). Furthermore, prospective clinical studies demonstrate that a concurrent diagnosis of mental disorder does not preclude effective interferon-based treatment, provided the patient is receiving appropriate psychiatric care and psychotropic drug therapy (Huckans et al. 2006; Pariante et al. 1999; Sylvestre et al. 2004). A study of patients treated with peginterferon alfa-2b and ribavirin while in methadone treatment showed no serious psychiatric events due to interferon-based treatment (Mauss et al. 2004).
An earlier small, prospective, controlled study in Europe examined psychiatric complications during interferon-based combination therapy (Schafer, 2001). Depression increased from baseline in only 16 percent of patients receiving methadone treatment and was found to be mild or moderate. However, during interferon-based treatment, many OTP patients requested increased methadone doses.
The Hepatitis C Resource Centers of the Veterans Administration have produced a monograph—Management of Psychiatric and Substance Use Disorders in Patients with Hepatitis C: A Reference for Hepatitis C Care Providers—that provides an algorithm for screening for psychiatric and substance use disorders and addressing suicidal ideation, depression, and post-traumatic stress disorder in the context of substance use and abuse and treatment for HCV infection. The full text can be found at www.hepatitis.va.gov.
Medication adherence
As with all illnesses treated with prescribed medications, adherence to the HCV treatment regimen is fundamental to a positive treatment outcome. Medication adherence is an important factor in the observation of a sustained virologic response (McHutchison et al. 2001). Multiple recent studies have shown that individuals receiving methadone or buprenorphine treatment, in a supportive medical environment, are adherent to interferon treatment resulting in a high percentage of sustained virologic responses (Krook et al. 2007; Schaefer et al. 2007; Samba et al. 2007; Sylvestre and Clements, 2007). Sustained virologic responses are observed in numerous patients with psychiatric illness with the initiation of psychiatric medications.
In a study of treatment outcomes in a HCV clinical practice, a significantly higher sustained virologic response (53 percent versus 20 percent) was observed in patients who received greater than 80 percent of the recommended dose of interferon-based therapy (Shehab et al. 2004). In an analysis of HCV treatment studies published through 2004 in which patients were treated for HCV infection in combination with substance abuse treatment, sustained virologic response and adherence data in HCV-infected methadone treated patients were comparable to control groups (Schaefer et al. 2004). However, patients with former or current drug abuse were more likely to discontinue treatment early compared to control groups. Patients in methadone treatment have also been shown to discontinue HCV treatment early in the course of treatment (Mauss et al. 2004). Patients who are likely to discontinue treatment early need supportive interventions, including medication adherence interventions (Bacon, 2004). These may include the management of drug-drug interactions and treatment of side effects. Other interventions, such as taking into account lifestyle factors and daily activities or using directly observed treatment protocols, can enhance medication adherence in drug users (Conway et al. 2004; Kresina et al. 2004; Wagner and Ryan, 2004).
Medication adherence interventions are particularly important in the early phase of interferon-based combination therapies. A stronger predictor of a sustained virologic response to combination therapy is the early virologic response—a reduction in detectable HCV RNA during the first 12 weeks of treatment (Fried, 2004). Two studies among patients infected with both HIV and HCV have shown early virologic response to have a value in predicting whether patients will obtain a sustained virologic response through HCV treatment (Ballesteros et al. 2004; Soriano et al. 2004a). Thus, supportive interventions initiated during the first 12 weeks of HCV treatment, such as medication adherence interventions, are particularly important in the medical management of patients with chronic HCV.
Interventions that help patients adhere to treatment are important and necessary in treating of comorbidities associated with substance abuse. Nonadherence with antiviral treatment is common in persons actively abusing substances—particularly alcohol, cocaine, or both—who do not receive adherence interventions (Samet et al. 2004; Weiss, 2004). However, research shows that drug use is not a predictor of noncompliance with treatment regimens and that past drug dependence does not preclude favorable adherence to antiviral therapies (Lucas et al. 2001; Murphy et al. 2004). A prospective longitudinal study of 74 HCV-infected patients receiving interferon treatment, with and without ribavirin, found that adherence was not influenced by sociodemographic factors or source of hepatitis infection. In particular, a history of injection drug abuse was not linked significantly with compliance difficulties (Kraus et al. 2001).
Experience with populations receiving effective substance abuse treatment indicates that stabilized patients tend to be exceptionally compliant, even with unusually burdensome treatment requirements such as reporting to a clinic multiple times each week to receive methadone or to attend therapy groups. Patients in substance abuse treatment programs tend to be resilient, perhaps as a result of the rigors of former addictive lifestyles. With adequate preparation and motivation, they readily endure difficult therapeutic regimens. Furthermore, these patients' frequent contacts with the health care system through OTP's that provide comprehensive services or other treatment venues also promote ongoing compliance monitoring and long-term followup (Backmund et al. 2001; Borg et al. 1999).
Drug-drug interactions: Addiction pharmacotherapy and HCV treatment
Concerns about therapeutic drug interactions with methadone have been cited as medical justification to exclude patients in substance abuse treatment from receiving treatment for hepatitis and from pharmaceutical trials. Drug interactions, when anticipated and monitored, can be managed by adjustments to medication dosage. Monitoring methadone levels is a critical step in achieving and continuing abstinence in OTPs. Any medication that could alter methadone pharmacokinetics might contribute to drug relapse and modify treatment outcomes.
Methadone and currently used interferon-based treatments do not interact significantly with each other, although the full extent of interactions has not been investigated rigorously. A recent study of 20 adult patients receiving long term methadone treatment showed that peginterferon alfa-2b treatment is associated with minor increases in exposure to methadone that are unlikely to be clinically meaningful (Gupta et al. 2007). A study evaluating interaction between methadone and peginterferon-2a in 24 patients infected only with HCV receiving methadone showed that methadone concentrations were elevated 10 percent to 15 percent but were not clinically significant and that no dose reductions occurred (Sulkowski et al. 2005). There is some evidence that interferon-alfa may mildly inhibit CYP450 enzymes involved in methadone metabolism (Rebetron, 1998). A recent prospective, nonrandomized crossover study of HCV/HIV co-infected patients receiving methadone showed that the pharmacokinetic and the pharmacodynamic properties of methadone were not changed by interferon-alfa-2b treatment (Berk et al. 2007).
Most patients in OTPs who receive methadone also receive prescribed medications for various co-occurring disorders and treatment side effects. Psychotropic agents also often are prescribed to counter the side effects of interferon. Potential drug interactions should be considered carefully to help avoid unintended increases or decreases in methadone concentrations. The number of compounds known to interact with the CYP450 system is large and comedications that influence enzyme activity may lead to decreased methadone concentrations and produce opioid withdrawal symptoms. Conversely, enzyme inhibitors may cause abnormally high methadone levels precipitating toxic adverse reactions (Wolff et al. 2000). For a list of common CYP450 enzyme substrates, inhibitors, and inducers see http://medicine.iupui.edu/flockhart.
Diseases with hepatic involvement could disrupt hepatic metabolic function, down-regulate CYP450 enzymes, and result in slower rates of drug clearance. Viral infections also stimulate cytokine production, which has been associated with suppressed CYP enzyme activity. Thus, conditions suppressing CYP function could produce higher than expected methadone serum levels in HCV-infected patients receiving methadone treatment. However, studies of patient dosing of methadone at OTPs show that patients with HCV infection receive higher methadone doses (Maxwell et al. 2002). However, in one examination of 228 patients receiving methadone treatment, of whom 149 (65 percent) were HCV-infected, no significant differences in dose were found between those who had or did not have HCV (Litwin and Gourevitch, 2001).
Further research is needed to define the interaction of HCV and subsequent liver damage with methadone dose variability. Few studies specifically have measured the effects of hepatitis virus on methadone serum levels. Meanwhile, appropriate monitoring and individualization of methadone dose appear to be essential in patients with HCV infection who are receiving methadone treatment.
Hepatototoxicity, liver disease, and liver function tests
There are numerous laboratory tests, referred to as liver function tests, which are useful in providing information on liver function/dysfunction. Liver function tests serve as noninvasive markers of liver function and can be used as screening tools to assess liver dysfunction in patients who have unsuspected liver disorders, such as acute viral hepatitis, cirrhosis, or partial bile obstruction. Liver function tests, when performed over time, can also detect a change in liver function or characterize a patterned liver dysfunction. For example, liver function tests can distinguish between liver disorders such as viral hepatitis and cholestatic syndromes. Liver function tests alone cannot be diagnostic for a specific liver disease (Knight, 2005). However, liver function tests allow the health care provider to characterize and follow the course of the liver disease. Although some patients with serious liver disease can have normal test values, liver function tests can be a component of data that may allow the health care provider to predict an outcome early in course of the disease (Peng et al. 2005).
The liver performs hundreds of biochemical/biological functions and, thus, one liver function test cannot accurately assess the total functional capacity of the liver. There are many tests for liver function that can be grouped as follows: tests for the liver's capacity to transport organic anions and metabolize drugs; tests that detect injury or death of hepatocytes (hepatic necrosis); tests of the liver's biosynthetic capacity; and tests that detect chronic inflammation in the liver, altered immunoregulation, or viral hepatitis markers (Kaplan, 1993). A listing of salient liver function tests and their clinical use is presented in Table 8.
Table 8.
Common liver function tests and their clinical value.
| Liver function test | Normal value* | Clinical value |
|---|---|---|
| Alanine aminotransferase (ALT) | 10–31 U/L | ALT lower than AST in alcoholism |
| Albumin | 3.5–5.2 g/dL | Assess severity/chronicity measures liver protein synthesis |
| Alkaline phosphatase | 25–112 U/L | Diagnose cholestasis and hepatic infiltrations |
| Aspartate aminotransferase (AST) | 10–44 U/L | Early diagnosis and monitoring of hepatic necrosis |
| Bilirubin (Total) | 0.1–1.0 mg/dL | Assess severity of cholestatic liver disease |
| Gamma glutamyl transpeptidase | 16–74 U/L | Diagnose alcohol abuse Marker of cholestasis |
| Prothrombin time | 11.3–16.5 sec | Assess severity of liver disease; measure of liver protein synthesis |
Normal values vary with the laboratory reporting them (adapted form Larson et al. 2005).
Liver function tests are used in multiple ways in the medical management of addiction and the pharmacologic treatment of opioid dependence. Methadone and buprenorphine are metabolized in the liver and tests that measure liver drug metabolism are important in maintaining therapeutic medication levels that do not fall low enough to promote relapse to heroin abuse (Ferrari et al. 2004). Therapeutic drug monitoring for methadone is being investigated for establishing adequate dosing and for monitoring drug diversion (Mercolini et al. 2007). It is routine for determining individual drug pharmacokinetics for patients taking antiretroviral medication for HIV infection (Cone and Preston, 2002; Fraaji et al. 2004).
For injection drug users, determining the presence of viral hepatitis and other underlying liver disease is important in the medical management of opioid dependence. Tests that identify antibodies to hepatitis viruses indicate past exposure of the patient to infection or vaccination. Tests that determine the presence of virus, viral replication, or viral genes and their gene products indicate a current infection with hepatitis virus. Although the liver contains thousand of enzymes, indicators of liver cell injury and acute hepatocellular diseases are the serum levels of aminotransferase enzymes found in hepatocytes. A routine chemistry liver function panel consists of determinations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), alkaline phosphatase, lactate dehydrogenase, gamma glutamyl transpeptidase, bilirubin (total and conjugated), and albumin levels in a blood sample.
The ALT and AST (and their ratio) are the most frequently measured indicators of liver disease. The measures of serum ALT and AST are an indication of enzyme leakage from tissues rich in the enzymes into the blood. In the case of the liver, the enzymes leak through the damaged cellular (hepatocyte) plasma membrane and, thus, measure cellular damage or death in the liver. Tissues rich in AST are the liver, heart, skeletal muscle, pancreas, and lungs. ALT is primarily present in the liver and kidney; thus, elevated levels of enzymes are not necessarily specific for liver injury or necrosis. However, both AST and ALT are typically elevated in all liver disorders that include acute and chronic hepatitis, cirrhosis, alcoholic liver disease, and liver cancer. Elevations up to eight times the upper limit of normal serum concentrations (Table 8) are nonspecific—they can be found in any of the liver disorders or found in specific patient populations (Celona et al. 2004). The highest elevations occur with liver insults and associated hepatocellular injury, such as drug and alcohol hepatotoxicity or viral hepatitis. Seventy to 90 percent of patients can present with determinations over eight times normal values in liver enzymes. However, as shown in Figure 4, the elevated values may not occur constantly over time in acute or chronic liver disease or even during HIV/HCV coinfection (Maida et al. 2007). Thus, single determinations of liver enzymes may not reflect the level of liver disease. Elevated liver enzymes are considered consistently elevated with a pattern of at least three consecutive monthly elevated readings.
There is a poor correlation between the extent of liver cell necrosis and the elevation of AST and ALT, with the absolute elevation of AST and ALT not a prognostic indicator of liver disease outcome. However, decreases in AST and ALT levels may indicate a liver disease recovery process. In most liver disease, AST and ALT are equally elevated with ALT usually slightly higher than AST. The exception to this observation is alcoholic liver disease in which an AST/ALT ratio of 2 or greater is suggestive of alcoholic liver disease. This is the result of an alcohol-induced deficiency of ALT. Thus, alcohol as a hepatotoxin enhances AST levels but may also reduce ALT levels through cellular metabolic deficiencies (Kaplan, 1993).
As there is a poor correlation between the extent of liver cell necrosis and the elevation of liver enzymes, other laboratory tests have been studied to identify clinical chemistry markers as noninvasive markers of progressive liver disease, especially hepatitis fibrosis (Lichtinghagen and Bahr, 2004; Poynard et al. 2005). These clinical chemistry markers are combined to form multiparameter scores or combined with measures of products of the hepatic extracellular matrix, such as hyaluronic acid; laminin; matrix metalloproteases, or their inhibitors; or collagen degradation products. These studies seek to find biological scores for routine clinical use that are easily obtainable, specific for liver disease, and accurately reflect the stage of liver fibrosis. At a minimum, scores need to accurately differentiate minimal liver disease from advanced liver cirrhosis (Kelleher et al. 2005). With the ability to accurately stage liver disease, biomarkers may replace the current use of liver biopsy in the assessment of liver inflammation and fibrosis due to chronic liver disease (Afdahl, 2004). In addition, serum markers, at that point in the future, may also be used to determine responses to therapy for hepatitis as well as to evaluate disease progression over time (Colletta et al. 2005).
Liver biopsy and methadone treatment
Liver biopsy remains the “gold standard” or only scientifically proven assessment of liver inflammation and fibrosis resulting from chronic liver disease, including HBV or HCV infection (Gebo et al. 2002a). Thus, liver biopsy is used by medical care providers to determine the grade and stage of liver disease. There are both risks and benefits of a liver biopsy. As with any invasive procedure, the benefits gained from an accurate assessment of liver disease must outweigh the small but definitive risks associated with the biopsy. Risks include bleeding, pain, and puncture of organs, sampling error, patient anxiety, costs, and a low risk of adverse events including death (Sterling, 2005). Contraindications for liver biopsy are an uncooperative patient, impaired coagulation, thrombocytopenia, ascites, biliary obstruction, and vascular tumors. Methadone treatment is not a contraindication for liver biopsy, and studies of HCV and substance abuse have routinely utilized liver biopsy to assess the level of liver disease of patients in methadone treatment (Cournot et al. 2004; Jowett et al. 2001; Van Thiel et al. 2003).
Current HCV treatment recommendations approved by AASLD (AASLD, 2004) are that therapy should be individualized for persons with liver biopsy evidence of no or minimal-to-mild fibrosis, while treatment is indicated for persons with more than portal fibrosis. Because 14 percent to 24 percent of individuals have normal amino-transferases values on liver enzyme panels but show portal fibrosis on liver biopsy, the current HCV treatment recommendations are the following: regardless of the level of aminotransferases, a liver biopsy should be performed when the results will influence whether treatment is recommended, but a biopsy is not mandatory in order to initiate therapy. The value (risk versus benefit) of a liver biopsy has been questioned, (the requirement for a biopsy in Australia was removed in, 2006) particularly for HCV treatment decisions for individuals infected with HCV genotypes 2 or 3, as there is a high likelihood of a sustained virologic response using current standard-of-care treatment regimens (Andriulli et al. 2004; AASLD 2004).
Continued alcohol use
Alcohol use and alcoholism is a serious medical problem among individuals with opioid dependence (Costenbader et al. 2007; Kosten and O'Connor, 2003; Watson et al. 2007). Heavy alcohol use has been shown in a substantial number of persons first entering OTPs (Chatham et al. 1995; NIAAA 1988; Teplin et al. 2007) as well as individuals in long term methadone treatment (Watson et al. 2007). The Treatment Episode Data Set (TEDS), which summarizes data on admissions to substance abuse treatment programs, reported in 2000 that 23.3 percent of patients entering an OTP indicated the use of alcohol along with heroin (SAMHSA, 2002a). Continued alcohol use can result in decreased medication adherence, drug-drug interactions that modify treatment pharmacokinetics, progressive liver disease, and continued at-risk behavior for infectious disease comorbidity. Excessive alcohol use can reduce the quality of life for those recovering from opioid dependence and lessen their satisfaction with methadone treatment (Senbanjo et al. 2007). Consensus recommendations for medication-assisted treatment are for OTP staff to be trained to recognize the pharmacologic and psychosocial effects of both opioid and nonopioid substances of abuse, including alcohol (SAMHSA, 2005). Thus, patients seeking, entering, or receiving substance abuse treatment for opioid dependence should be carefully screened for alcohol dependence using a validated instrument, such as the CAGE, AUDIT, or MAST questionnaire, with alcohol abuse treatment options available either directly or through referral (Senbanjo et al. 2007; Teplin et al. 2007).
Treatment for alcohol dependence that maximizes good treatment outcomes consists of pharmacotherapy, counseling interventions, and participation in social mutual-help group (Boothby and Doering, 2005; SAMHSA 2005; SAMHSA, 2007). A recent study has shown that individuals receiving methadone treatment substantially reduced heavy alcohol drinking levels when also receiving a brief intervention for alcohol (Watson et al. 2007). Another recent study has shown that methadone can modify the blood alcohol level of individuals who consume alcohol after taking methadone (Clark et al. 2006). In addition, high dose buprenorphine treatment of individuals, who are dependent on both heroin and alcohol, show reductions in use of alcohol as well as heroin (Ciccocioppo et al. 2007). The medical management of withdrawal from alcohol and opiates is complex, and there are substantial differences in severe complications (Kosten and O'Connor, 2003). Medications used in detoxification from one class of addictive drugs may mask symptoms of another class of drugs. Depending on the emergence of serious complications, detoxification of a poly-substance-dependent individual may require inpatient treatment. For individuals who casually use alcohol and who are opioid-dependent, once the patient receives methadone treatment alcohol use has been shown to decrease with time (Caputo et al. 2002).
Patients participating in OTPs have frequently been excluded from fully participating in Alcoholic Anonymous meetings and denied admission to and treatment in traditional addiction and chemical dependency programs (Kipnis et al. 2001). Pilot programs that educate the medical and counseling staff at addiction treatment centers on integrating methadone treatment into the traditional addiction treatment framework have met with success. Thus, patients receiving methadone can complete traditional chemical dependency treatment programs through integration of services at an addiction treatment center (Kipnis et al. 2001).
Alcohol consumption is also a medical issue for patients who are opioid-dependent and have a viral hepatitis infection. Research studies to date have not been able to determine a safe level of alcohol consumption for individuals infected with viral hepatitis. Alcohol consumption of greater than 30 grams/day in men (3–4 drinks, with an average drink comprising 13 grams of alcohol) and 20 grams/day in women increases the risk of liver disease progression and reduces responses to interferon therapy; more than 80 grams/day may seriously compromise HCV treatment (NIH, 2002; Schiff, 1997). For individuals with HBV infection, alcohol consumption has been shown to increase the risk of the development of hepatocellular carcinoma (liver cancer). For those successfully cured of HCV infection, moderate alcohol consumption has been shown to increase the risk of developing liver cancer (Tokita et al. 2005). Alcohol-induced enhancement of viral replication or increased susceptibility of liver cells to viral injury has been suggested as the means through which liver disease may progress (CDC, 1998). The Consensus Conference Statement of the European Association for the Study of the Liver (EASL, 1999) notes that heavy alcohol intake increases HCV viremia and decreases medication adherence with injection drug users at-risk of HCV re-infection.
The most recent NIH and AASLD guidelines acknowledge that HCV treatment for infected injection drug users is feasible and can be effective. However, the guidelines recommend that interferon-based therapy be performed in the context of efforts to address drug/alcohol use, abuse or dependence including participation in OTPs (AASLD, 2004; NIH, 2002). However, continued drug or alcohol use is not a medically valid, or ethically valid (Scott 2005), reason for withholding HCV treatment to individuals in immediate need (AASLD, 2004). As with all medical interventions, HCV treatment decisions need to be made based on an assessment of risks and benefits to the patient. The risk, in this case, is that ongoing alcohol use/abuse enhances liver toxicity resulting in decompensated liver disease or end-stage liver disease. The benefit is amelioration of HCV infection and potential reversal of progressive liver disease.
HCV treatment studies have reported that approximately one fifth of current alcohol/drug abusers do not comply with HCV treatment monitoring or are lost to followup, and ongoing drug use may increase viral load and reduce virologic response to treatment (Davis and Rodrigue, 2001; Sylvestre, 2002). However, patients with co-occurring HCV infection and substance use may complete interferon treatment with careful monitoring and aggressive intervention. Treatment providers must integrate early interventions for drug use and other comorbidities into their HCV treatment algorithm. Using this treatment paradigm, patients with current and past histories of significant substance use disorders are able to successfully complete a course of interferon-based therapy and that sustained virologic response rates are similar to those without such difficulties.
Liver transplantation
Liver transplantation is standard treatment for individuals with end-stage liver disease. A substance abuse-related diagnosis, either HCV or alcoholic liver disease, is the leading cause (46 percent of cases) and next leading cause (25 percent of cases) for liver transplantation, respectively. The current use of addictive drugs is an absolute contraindication for acceptance into liver transplantation programs (Keeffe, 2000). An abstinence period of at least 6 months is required and patients fully recovered from drug use can be considered for liver transplantation.
A 2001 survey of liver transplantation programs found that 32 percent of programs required patients to discontinue methadone treatment (Koch and Banys, 2001). Approximately 6 percent of all individuals with HCV infection are prescribed methadone. However, 85 percent of all methadone patients are HCV-infected. Studies do not support withholding the provision of liver transplantation from patients receiving methadone, although patients who undergo liver transplantation while receiving methadone do have substantial medical complications. One study described the outcomes of five patients receiving methadone who had been abstinent from illicit drugs and alcohol for at least 6 months. Three patients had end-stage liver disease resulting from HCV infection, one from HBV infection and one from alcoholic liver disease. No patient returned to illicit drug use post-transplantation, and mean patient survival time, at publication, was 1,250 days (over 3 years). The study concluded that acceptable patient survival rates can be achieved in patients receiving methadone as long as patients receive counseling, psychotherapy, and services post-transplantation to enhance retention in care (Kanchana et al. 2002). The largest reported series of patients provided outcomes for 35 patients receiving methadone. The study reported a higher than normal rate of rejection, but patient graft and survival times were comparable to national averages. Eleven percent of patients relapsed to isolated episodes of heroin use post-transplant (Liu et al. 2003).
In a more recent report, 10 patients receiving methadone were compared with 19 nonmethadone, nonopioid-dependent patients, post-transplantation. Patients receiving methadone required significantly more intraoperative anesthesia and postoperative analgesia as well as methadone dose increases (preoperatively compared to postoperatively). Survival time was not different between groups. However, post-transplantation, 20 percent of patients used alcohol or illicit drugs. The authors conclude that liver transplantation patients receiving methadone pose a greater challenge to their medical management but should not be withheld from liver transplantation waiting lists (Weinrieb et al. 2004). Other case reports of patients receiving methadone treatment and a liver transplant show 5 years post-transplant that the patients are stable and continuing on methadone treatment (Hancock et al. 2007). Information on the organ transplantation process and waiting lists for transplantation can be found at the United Network for Organ Sharing Web site www.unos.org.
Hepatitis-HIV co-infected patients and liver transplantation
Co-infection of hepatitis and HIV viruses greatly accelerates the progression of liver disease associated with viral hepatitis. As an emerging problem, persons with co-infection face insurmountable obstacles to treatment or transplantation for their liver disease (Nadler, 2001; Stock and Roland, 2007). Life expectancy in HIV-infected individuals has been extended due to advances in antiret-roviral therapy, and HIV infection now can be considered a chronic illness, rather than an absolute exclusion to organ transplantation. Liver transplantation is being evaluated as a therapeutic option for patients with controlled HIV infection and end-stage liver disease resulting from HCV infection, HBV infection, or drug-induced hepatotoxicity (acute liver failure). Liver transplants have been successfully performed in at 11 centers worldwide between 1990 and 2001. The one caveat has been the potential for drug interactions between anti-retroviral agents and immunosuppressive drugs and the need for dose adjustments.
Clinical trials at 10 centers in the United States are underway to assess the impact of HIV infection (and co-infection with HCV or HBV in some patients) on liver transplant outcomes. While relapse of HCV infection is common after transplantation, initial reports indicate that transplantation is effective with an 85-percent, 1-year survival rate, which is similar to the non-HIV HCV-infected patient survival rate (Castells et al. 2006; Neff et al. 2005).
The accumulated experience in Europe and the United States indicates that the 3-year survival in HIV-positive liver transplantation recipients is similar to that of HIV-negative recipients. Guidelines for the selection of patients with HIV-infection for liver transplantation have been generated in the United Kingdom and Spain (O'Grady et al. 2005; Miro et al. 2005). Methadone treatment is not an exclusion criterion.
Diabetes, kidney disease, opioids, and kidney transplantation
Injection drug use, HCV infection, and type 2 diabetes mellitus each independently, and in concert, can result in chronic kidney (renal) disease and the need for transplantation. Data gathered through NHANES III show that the prevalence of type 2 diabetes in adults aged 20-59 with HCV infection was 3.4 percent (Behrendt and Ruiz, 2005). HCV infection and type 2 diabetes was associated with a family history of diabetes, as well as advanced liver fibrosis, but not the classical phenotype for diabetes (overweight individuals with coronary heart disease). HCV infection has been associated with insulin resistance among older adults and those at risk of HIV infection (Howard et al. 2007). Opium addiction has been shown to enhance the metabolic abnormalities associated in patients with type 2 diabetes (Karam et al. 2004). Numerous studies have described the clinical and pathological features of renal disease associated with injection drug use of heroin, cocaine, morphine, amphetamine, and other narcotics (reviewed in Dettmeyer et al. 2005). Drug addiction neuropathy, including renal failure associated with oxycodone addiction, constitutes an important cause of end-stage renal disease that can be augmented by a genetic predisposition to diabetes as well as HCV infection (Hill et al. 2002). Thus, for the injection drug user seeking treatment, there is a spectrum of diseases and infections that can exacerbate addiction-associated renal disease leading to end-stage renal disease (Fig. 10).
Figure 10.
Enhancement of heroin-associated nephropathy by HCV infection and diabetes.
Methadone has been shown to be safe and effective in patients with renal disease who are undergoing dialysis (Dean, 2004). Renal transplantation of HIV-positive patients has been performed (Kumar et al. 2005; Roland, 2004). Post-transplant survival has improved in these individuals with the use of antiretroviral therapy to control HIV infection. Current experience in renal transplantation in HIV-infected patients in the United States indicates that the 3-year survival rate is similar to that of HIV-negative transplant recipients, with virological and immunological control of the infection by anti-retroviral treatment. There has been no increase in the number of opportunistic infections or tumors due to immunosuppression post-transplantation. The criteria for selecting HIV-positive transplantation candidates include no opportunistic infections, CD4 cell counts greater than 200 per μl. and control of HIV infection with an undetectable viral load. In Spain, where most of these patients are former drug abusers, a 2-year period of abstinence from cocaine and heroine abuse is also required, although patients are permitted to participate in methadone treatment (Trullas et al. 2005). Problems post-transplantation include interactions between anti-retroviral drugs and immunosuppressive drugs, management of HCV coinfection, and progressive liver disease, as well as acute graft rejection.
Dependence and immune modulation
Both basic research studies and clinical observational studies indicate that opiates and opiate abuse have a broad influence on immune networks and their function (Donahoe and Vlahov, 1998; Sharp, 2003). Opioid abuse may alter immune function (Alonzo and Bayer, 2002; Ryan et al. 2004). Abnormalities have been observed in immune responses of heroin injection drug users, including diseased lymph glands, elevated white cell counts, increased antibodies, and false-positive tests for syphilis, rheumatoid arthritis, and other illness. T cells, mediators of cell-mediated immunity, have been shown to express cell surface opioid receptors (Sharp, 2003). These receptors, when bound by opioids, would reduce the immune response induced by T cells, thereby suppressing overall immune responses.
Clinical investigations demonstrate that immune response abnormalities can be moderated or eliminated by methadone treatment (Donahoe, 1993; Novick et al. 1991; SAMHSA, 1993; Sergio et al. 2007). Methadone treatment has been reported to normalize immune function and stress responses in former injection drug users (Zajicova et al. 2004). Research also suggests however, that inadequate methadone-maintenance doses or withdrawal from methadone may create extraordinary stress, potentially altering immune system function (McLachlan et al. 1993).
Dependence and cancer
One out of every two men and one of three women in the United States develop cancer at some point in their lifetime (DHHS, 2005). Cancer arises from a loss of the normal regulatory control of how and when cells grow, divide, and proliferate. The loss of the regulatory control of cellular and division is a multistep process called carcinogenesis and has a strong genetic component (Hall et al. 2001). However, research studies show that environmental factors, such as lifestyle behaviors, can contribute to the process of carcinogenesis and, for instance, trigger the malignant transformation of a precancerous lesion to form cancer. A well-recognized behavior that is closely associated with an increased risk of cancers in various organs of the body is tobacco use resulting from nicotine addiction (Nishikawa et al. 2004). Chronic alcoholic beverage consumption is also a significant risk factor for cancer of the digestive tract (oral-pharynx, larynx, esophagus, liver, and colon) as well as breast (Poschl et al. 2004). Alcohol consumption in association with cigarette smoking, which may occur in a significant number of individuals receiving substance abuse treatment, may have a significant impact on carcinogenesis (Poschl and Sietz, 2004). The additive or synergistic effect of two or more agents leading to cancer is termed co-carcinogenesis. Co-carcinogenesis has been proposed for virus-chemical interactions to cause cancer. Nicotine and/or alcohol may be important co-carcinogens in patients with substance use disorders, particularly if these patients are infected with HBV or HCV.
The Eleventh Edition of the Report on Carcinogens (DHHS, 2005; available at http://ntp.niehs.nih.gov) has for the first time listed HCV and HBV as known human carcinogens. Both HBV and HCV infections are listed as a cause of liver cancer. Thus, substance abuse treatment programs that address lifestyle behaviors such as smoking and alcohol consumption, as well as infections related to IDU such as HBV and HCV, are preventing the occurrence of additional comorbidities in their patient population. Primary prevention programs for cancer are focusing on lifestyle changes, which include diet and exercise, to promote better health for their patients (Festi et al. 2004; Martinez, 2005).
Resources for Care and Treatment
Medical education, access to care and treatment, and multidisciplinary service teams
Hepatologists, gastroenterologists, infectious disease specialists, primary care providers, general and family practitioners, psychiatrists, and addiction treatment specialists would benefit from continuing medical education related to the care and treatment of chronic HCV infection in injection drug users. Further understanding of substance dependency and the stages of addiction recovery, particularly with respect to methadone and buprenorphine treatment, are needed. Substance abuse treatment programs need to address hepatitis infection, liver disease, and provide community resources to support patient care and treatment. Information and education resources are readily available on the Internet. A listing of salient resources is provided in Table 9.
Table 9.
Salient internet resources: methadone, Hepatitis, co-infection.
| •Addiction Treatment Forum www.ATForum.com •American Association for the Study of Liver Diseases (AASLD) www.aasld.org •American Association for the Treatment of Opioid Dependence (AATOD) www.AATOD.org/ •American College of Gastroenterology (ACG) www.acg.gi.org • American Gastroenterological Association www.gastro.org/ • American Liver Foundation (ALF) www.liverfoundation.org • American Society of Addiction Medicine (ASAM) www.asam.org • Centers for Dis. Control and Prevention (CDC) www.cdc.gov/ncidod/diseases/hepatitis • Center for Substance Abuse Treatment (CSAT), SAMHSA ww.samhsa.gov/centers/csat2002/csat_frame.html • Cleveland Clinic Foundation. Hepatitis C Management www.clevelandclinicmeded.com/hcv • Department of Veterans Affairs. Hepatitis C Resource Centers. www.hepatitis.va.gov • Division of Pharmacologic Therapies, CSAT, SAMHSA www.samhsa.gov/centers/csat/content/dpt/index.html • GastroHep.com www.gastrohep.com • Hepatitis B Foundation www.hepb.org • Hepatitis c Advocacy www.hepcadovcacy.org • Hepatitis C Connection www.hepc-connection.org • Hepatitis C in Me (Maine) www.hepatitiscnme.org • Hepatitis Foundation International (HFI) www.hepfi.org • HIV and Hepatitis. www.hivandhepatitis.com |
•Infectious Disease Society of America. www.idsociety.org/content/navigationMenu/Practice_Guidelines/Guidelines_by_topic/hepatitiscLibrary, Alcohol and Drug Abuse Institute, University of Washington http://lib.ada.washington.edu • Matrix Institute on Addictions www.matrixinstitute.org • Medscape HIV/AIDS from WebMD www.medscape.com • National Alliance of Methadone Advocates www.methadone.org/ • National Center for Complementary and Alternative Medicine (NCCAM), NIH nccam.nih.gov • National Digestive Diseases Information Clearing-house (NDDIC), NIDDK, NIH www.niddk.nih.gov • Natl. Foundation for Infectious Diseases (NFID) www.nfid.org/ • National Institute of Diabetes and Digestive and Kidney Disease (NIDDK), NIH:www.niddk.nih.gov/health/digest/pubs/hep/ • National Institute on Alcohol Abuse and Alcoholism, NIH www.niaaa.nih.gov/ • National Institute on Drug Addiction, NIH www.nida.nih.gov/ • National Institutes of Health (NIH) www.nih.gov/ • New York Department of Health AIDS Institute Substance Use Guidelines http://hivguidelines.org/public_html/center/clincial-guidelines/sub-gl/substance.html • Organization to Achieve Solutions in Substance Abuse (O.A.S.I.S.) www.oasisclinic.org/ • Projects in Knowledge. Initiatives in Gastroenterology www.projectsinknowledge.com/recent/indexG.html • Texas Department of State Health Services-Public Information kit-Hepatitis C Initiative www.tdh.state.tx.us/ideas/hepatitis/hepatitis_c/overview/public_info_kit/ • University of California, San Francisco Center for Information. Coinfection with hepatitis and HIV http://hivinsite.ucsf.edu/ |
All sites were accessed and active as of September 2007.
The development of multidisciplinary service provider teams and regular interaction among addiction-treatment providers, liver-treatment specialists, infectious disease specialists, primary care providers, case managers and patient advocates as well as peer outreach workers has been recommended to better coordinate hepatitis testing, patient care, and access to care. For injection drug users receiving care in an urban primary care clinic, only 55 percent were tested for hepatitis infection (Trooskin et al. 2007). Follow-up medical care of individuals testing positive for exposure to hepatitis infection is essential. For HCV-positive injection drug users receiving a referral for medical care, a recent study has shown that only 24 percent returned for that care (Reynolds et al. 2006). Of that small group, less than half further sought specialty care for liver disease. Factors that predicted further seeking care included participating in residential drug treatment. Referral to a hepatology clinic for HCV patients receiving methadone treatment was more successful, with 63 percent of patients attending followup appointments (Hallinan et al. 2007). Public health-sponsored hepatitis testing programs report similar referral success, with 70 percent of those tested contacted after HCV testing, 55 percent of those contacted receiving a medical evaluation, and 12 percent receiving HCV care and treatment (Mark et al. 2007).
To foster improved testing, access to care, and treatment for hepatitis, a cooperative and interactive approach is necessary. Components of this approach include interventions to promote testing and reduce barriers to care and interventions to promote adherence to medication regimens, treatment for coexisting physical and psychiatric conditions, and implementation of strategies to prevent relapse during and following treatment (Davis and Rodrigue, 2001; NIH, 2002). Integrating delivery of substance abuse treatment with prevention, care and treatment of liver disease has been demonstrated as an important strategy for improving attendance at medical visits and optimizing treatment outcomes (Litwin et al. 2005; Muir et al. 1999; Sylvestre and Zweben, 2007; Willenbring et al. 2004).
Models of co-location of prevention, care, and treatment services
The co-location of medical/health services or so-called “one stop shopping” for health care results in an increased utilization of services, enhanced medical outcomes and patient statisfaction, as well as enhanced knowledge, communication, and collaborations among co-locating groups (Jackson et al. 2007). For services related to substance abuse treatment and the prevention, care and treatment of hepatitis infection, co-location of services can occur either in the substance abuse treatment setting or in the primary care/medical setting. Regardless of the site, integration of services has been shown to enhance the use of services for those who are medically disadvantaged as well as provide a venue for addressing gender issues in the context of substance abuse treatment (Ayalon et al. 2007; Najavits et al. 2007; Passey et al. 2007).
Co-location of services in a substance abuse treatment setting
Co-location of HCV and HIV prevention, care, and treatment services, as well as other wrap around services, in substance abuse treatment settings fosters access to care for patients with multiple comorbidities, many of whom likely would not be accessing needed care (Dore and Thomas, 2005; Gunn et al. 2005; Kresina et al. 2005). Co-location of services has also been shown to lead to high rates of adherence with liver biopsy and initiation of antiviral therapy (Litwin et al. 2005). Many substance abuse treatment programs are developing model programs of integrated service delivery to significantly improve patient outcomes for this difficult-to-treat patient population. To facilitate this process the American Liver Foundation has developed HIT'M, a manual for training staff to integrate hepatitis prevention into HIV/AIDS, STD, and drug treatment programs.
In the United States, methadone treatment is federally regulated though the Code of Federal Regulations which require OTP's to dispense methadone dosages in a highly rigorous and structured fashion (CFR, 2001). However, these regulations do not preclude the integration of medical and social services to provide a comprehensive therapeutic milieu, comprising primary medical care, psychosocial and infectious disease counseling, vocational rehabilitation, ongoing performance monitoring, street outreach, prevention services and patient education. Prevention, care, and treatment programs are being implemented that integrate general and HCV and/or HIV-related medical, prevention, or mental health services into methadone treatment programs (Gourevitch et al. 2007; Litwin et al. 2005; McLaughlin, 2007; Strauss et al. 2005; Sylvestre et al. 2004). Pilot model programs range from interventions, such as smoking cessation or case management (Sorensen et al. 2006; Stein et al. 2006) to the development of methadone health care provider teams that include a physician (internist or family practitioner), a mid-level provider (PA or NP), psychiatrist, a social worker/case manager, nurse, substance abuse treatment counselors with peer support groups. The co-location of medical care with methadone treatment has been shown to reduce emergency department and inpatient care, increase ambulatory patient care with no net increase in expenditures (Gourevitch et al. 2007). The co-location of buprenorphine treatment with methadone treatment and a chemical dependency program that in combination provide substance abuse counseling, social services, medical and psychiatric treatment has shown to maintain patient retention and abstinence (Whitley et al. 2007).
Co-location of services in a primary care or HIV care and treatment setting
In the United States, the regulation of methadone treatment prohibits the implementation of methadone treatment in a primary care setting. However, a model program received a regulatory exemption and provided methadone treatment through a public hospital for a period of one year (Merrill et al. 2005). An evaluation of the program revealed high patient statisfaction and retention in the program, enhanced medical service utilization by the patients and a change in medical provider stigma toward methadone. In countries where methadone is available in a primary care setting, two recent studies (Grebely et al. 2007a; van Beek, 2007) have show that successful treatment of HCV or HIV infection using directly observed therapy in a primary care setting that treats injection drug users with methadone.
Pharmacotherapy for injection drug users in a primary care setting is available in the United States in the form of treatment with buprenorphine. ‘Real world' clinical practice of using buprenorphine to treat opioid dependent patients in a primary care practice are reported as good, with only 32 percent of patients dropping out of treatment from 2003–2005 (Magura et al. 2007). Mainly through the funding of the Health Services Resources Administration (HRSA), numerous pilot projects have been initiated to develop the best clinical practices that integrate buprenorphine treatment regimens into HIV primary care programs (Altice et al. 2006; Kresina et al. 2005a; Lum and Tulsky, 2006; Mitty et al., 2007; Sullivan et al. 2006; Taylor, 2005; Turner et al. 2005). Integrating care for HIV infection and treatment for opioid dependence optimizes outcomes for patients with both disorders. In addition, buprenorphine treatment in primary care is associated with a reduction in drug-related HIV risk (Sullivan et al. 2007). Other model programs have shown that buprenorphine treatment is effective in homeless opioid dependent patients despite their social instability, greater comorbidities, and severe chronic drug use (Alford et al. 2006). However, barriers to adopting buprenorphine treatment in HIV primary are many and include policy and finance issues as well as infectious disease physician access to substance abuse treatment experts (Cunninghan et al. 2007; Schackman et al. 2006; Turner et al. 2005).
Outreach, referral networks, and community programs
Individuals who abuse heroin, cocaine and alcohol frequently utilize emergency departments for needed health care (O'toole et al. 2007). Developing programs that address the underlying needs of individuals who use emergency departments for medical care and linkage to substance abuse treatment can reduce hospital use (Friedman et al. 2006). Drug treatment linkage strategies vary but both vouchers and case management have been shown to be effective in enhancing access to drug treatment services, including methadone treatment programs (Alexander et al. 2007; Barnett et al. 2006; Sorensen et al. 2005; Zaller et al. 2006). A referral intervention for women that promoted quality of services also enhanced access to outpatient drug treatment (Passey et al. 2007). Referral programs from sexually transmitted disease clinics that offer an initial screen for drug use has also been shown to be successful (Yu et al. 2007). Linking methadone treatment programs to prison release programs can address the high risk of overdose and disease transmission in recently released inmates for (McMurran, 2007; Rich et al. 2005).
Community programs can also enhance the use of medical services for the prevention, care and treatment of substance abuse and hepatitis infection. Programs can utilize peer outreach workers/educators to enhance knowledge of hepatitis infection and access to prevention and care (Sylvestre ands Zweben, 2007). Community interventions that address risk reduction motivation and behavioral skills in the context of pharmacotherapy for opioid dependence have been shown to be successful (Copenhaver and Lee, 2006). Community-based partnerships comprising community teams can recruit participants for family-based drug treatment and prevention interventions (Spoth et al. 2007).
Other community based programs can involve state and county health departments for the provision of resources to support HCV services. For example, in Oregon, the Multnomah County Health Department Viral Hepatitis C Integration Program is dedicated to building capacity within the county health department and in the community through integrating services and creating community services and resource linkages. Five primary areas are targeted HIV prevention and outreach to build street outreach and education for hepatitis; sexually transmitted disease clinics to implement HCV testing, post-test counseling, and hepatitis A and B vaccinations; social work for individuals who test positive for HCV, short-term case management, and social service support; development of a referral system among primary care and family services addiction specialists; health education for the development and dissemination to the community of hepatitis curricula; and community planning processes to establish community planning groups for development and implementation of a HCV strategic plan. In this program, consumers of hepatitis services are part of the community planning process and interface with institutional decision makers and the county health department policymakers. Academic health centers can provide novel programs to address the medical management of chronic hepatitis infection through a partnership of nurse practitioners, primary care physicians, physician assistants, pharmacists, psychiatrists, and substance abuse treatment providers (Arora et al. 2007).
Community planning and health services referral networks are fundamental to providing needed medical and social services not available at OTPs (Fletcher et al. 2003). To help achieve a viable referral network, substance abuse treatment programs need to identify health care providers in the community willing and trained to provide medical and social services to their patients (Novick, 2000; Sweeney et al. 2004). Greater improvement in post-treatment outcomes has been shown in programs that tailor frequency and type of service to unique client needs (Rowan-Szal et al. 2000). Substance abuse treatment programs should be advocates for their patients needing and seeking care and treatment, thereby providing a support network throughout the course of therapy.
Summary
Individuals receiving pharmacologic therapies for opioid abuse/dependence often have co-occurring disorders that can complicate substance abuse treatment regimens. Addressing co-occurring disorders and co-infections in a comprehensive fashion through screening, testing, and medical management can facilitate successful patient outcomes of substance abuse treatment.
Outreach workers and peer advocates working with health care providers should encourage HCV-infected injection drug users to access treatment for opioid abuse/dependence whether or not they are receiving treatment for HCV infection. Treatment for chronic HCV infection has been successful when patients are provided daily methadone and methadone treatment has been shown to reduce risky behaviors that can spread HCV infection. Limited data are available on the treatment of viral hepatitis infections for patients who inject drugs and who are not in drug treatment programs with reported clinical outcomes less successful.
The decision of whether to treat a patient for hepatitis infection should be made considering the anticipated risks and benefits for the individual. Treatment for hepatitis infections should not be denied to any patient needing it, and efforts encompassing proven supportive interventions may be required for these individuals to become ready for treatment. Individuals stabilized through treatment using methadone or buprenorphine and otherwise well engaged in an opioid treatment program are candidates for treatment for chronic HCV infection. Physicians are not required to cease treatment for opioid dependence in order to begin treatment for HCV infection. Substance abuse treatment programs that provide comprehensive services for drug users in a supportive/accepting environment promote compliance with therapeutic treatment regimens. These programs can facilitate medical follow-up and foster social, psychological, and vocational rehabilitation as well as a viable way to prepare injection drug users for pharmacotherapies for chronic liver disease and help promote medication compliance and favorable treatment outcomes. Patients receiving medication for opioid dependence through office-based treatment services may require treatment for hepatitis infections during their substance abuse treatment. Therefore supportive environments that promote hepatitis prevention, care, and treatment programs need to be available and integrated into all venues providing pharmacotherapy for opioid dependence.
Acknowledgments
The authors acknowledge the helpful comments and review by Dr. Joanna Buffington, Center for Disease Control and Prevention and the initial developmental research performed by Stewart B. Leavitt MA PhD, under contract 01M00859901D from the Substance Abuse and Mental Health Services Administration.
Footnotes
Conflict of Interest: Diana L. Sylvestre, MD, has received fees for non-CME programs from Roche and Schering. Others -none.
References
- AASLD, (American Association for the Study of Liver Disease) Chronic Hepatitis B AASLD Practice Guidelines. 2003. Available at: https://www.aasld.org/eweb/docs/chronichep_B.pdf.
- AASLD, (American Association for the Study of Liver Disease) Diagnosis Management Treatment of Hepatitis C, AASLD Practice Guideline. Hepatology. 2004;39:1147–71. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- Afdhal NH. Biopsy or biomarkers: is there a gold standard for diagnosis of liver fibrosis? Clin Chem. 2004;50:1299–300. doi: 10.1373/clinchem.2004.035899. [DOI] [PubMed] [Google Scholar]
- Afdhal NH, Godofsky E, Dienstag J, et al. Final Phase I/II trial results for NM283, a new polymerase inhibitor for hepatitis C: Antiviral efficacy and tolerance in patients with HCV-1 infection, including previous interferon failures; Presentation at the American Association for the Study of Liver Disease 55th Annual Meeting and Postgraduate Course; October 29, 2004; Boston, Massachusetts. 2004. Abstract LB-03. [Google Scholar]
- Aitken CK, Kerger M, Crofts N. Peer-delivered hepatitis C testing and counseling: a means of improving the health of injection drug users. Drug Alcohol Rev. 2002;21:33–7. doi: 10.1080/09595230220119327. [DOI] [PubMed] [Google Scholar]
- Alberti A, Clumeck N, Collins, et al. Short statement of the First European Consensus Conference on the Treatment of chronic Hepatitis B. and C in HIV co-infected patients. J Hepatol. 2005;42:615–24. doi: 10.1016/j.jhep.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Alexander JA, Pollack H, Nahra T, et al. Case management and client access to health and social services in outpatient substance abuse treatment. J Behavior Health Serv Res. 2007;34:221–36. doi: 10.1007/s11414-007-9072-4. [DOI] [PubMed] [Google Scholar]
- Alford DP, LaBelle CT, Richardson JM, et al. Treating homeless opioid patients with buprenorphine in an office—based setting. J Gen Intern Med. 2006;22:171–6. doi: 10.1007/s11606-006-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo NC, Bayer BM. Opioids, immunology and host defenses of intravenous drug abusers. Infect Dis Clin North Am. 2002;16:553–69. doi: 10.1016/s0891-5520(02)00018-1. [DOI] [PubMed] [Google Scholar]
- Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C infection in the United States, 1988 through 1994. New Engl J Med. 1999;341:556–62. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- Alter MJ, Seeff LB, Bacon B, et al. Testing for hepatitis C virus infection should be routine for persons at increased risk for infection. Ann Int Med. 2004;141:715–9. doi: 10.7326/0003-4819-141-9-200411020-00013. [DOI] [PubMed] [Google Scholar]
- Altice FL, Bruce RD, Walton MR, et al. Adherence to hepatitis B. virus vaccination at syringe exchange sites. J Urban Health. 2005;82:151–61. doi: 10.1093/jurban/jti016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Sullivan LE, Smith-Rohrberg 1, et al. The potential role of buprenorphine in the treatment of opioid dependence in HIV-infected individuals and in HIV infection prevention. Clin Infect Dis. 2006;43:S178–83. doi: 10.1086/508181. [DOI] [PubMed] [Google Scholar]
- Alvarez DF, Latorre JS. Hepatitis C virus and HIV coinfection: Clinical management and new strategies. AIDS Read. 2004;14(10 Suppl):S16–S21. [PubMed] [Google Scholar]
- Amin J, Law MG, Micallef J, et al. Potential biases in estimates of hepatitis C RNA clearance in newly acquired hepatitis C infection among a cohort of injection drug users. Epidemiol Infect. 2007;135:144–50. doi: 10.1017/S0950268806006388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriulli A, Perisco M, Lacobellis A, et al. Treatment of patients with HCV infection with or without liver biopsy. J Viral Hepatol. 2004;11:536–42. doi: 10.1111/j.1365-2893.2004.00519.x. [DOI] [PubMed] [Google Scholar]
- Aranzabal L, Casado JL, Moya J, et al. Influence of liver fibrosis on highly active antiretroviral therapy-associated hepatotoxicity in patients with HIV and hepatitis C virus confection. Clin Infect Dis. 2005;40:588–93. doi: 10.1086/427216. [DOI] [PubMed] [Google Scholar]
- Armstrong GL, Bell BP. Hepatitis A virus infections in the United States: model-based estimates and implications for childhood immunization. Pediatrics. 2002;109:839–45. doi: 10.1542/peds.109.5.839. [DOI] [PubMed] [Google Scholar]
- Arora S, Geppert CMA, Kalishman S, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82:154–60. doi: 10.1097/ACM.0b013e31802d8f68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASAM (American Society of Addiction Medicine) Public Policy Statement on the Rights and Responsibilities of Physicians in the Use of Opioids for the Treatment of Pain. ASAM News. 1997;12(4) [Google Scholar]
- Asnis GM, de la Garza R. Interferon-induced depression in chronic hepatitis C: A review of its prevalence, risk factors, biology and treatment approaches. J Clin Gastroenterol. 2006;40:322–35. doi: 10.1097/01.mcg.0000210099.36500.fe. [DOI] [PubMed] [Google Scholar]
- Astone J, Strauss SM, Vassilev ZP, et al. Provision of hepatitis C education in a nationwide sample of drug treatment programs. J Drug Educ. 2003;33:107–17. doi: 10.2190/YEGL-GX4W-HGRA-EDC7. [DOI] [PubMed] [Google Scholar]
- Ayalon L, Arean PA, Linkins K, et al. Integration of mental health services into primary care overcomes ethnic disparities in access to mental health services between black and white elderly. Am J Geriatr Psychiatry. 2007;15:906–12. doi: 10.1097/JGP.0b013e318135113e. [DOI] [PubMed] [Google Scholar]
- Backmund M, Meyer K, Edlin BR. Infrequent reinfection after successful treatment for hepatitis C virus infection in injection drug users. Clin Inf Dis. 2004;39:150–153.4. doi: 10.1086/425361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backmund M, Meyer K, Henkel C, et al. Risk factors and predictors of human immunodeficiency virus infection among injection drug users. Eur Addict Res. 2005;11:138–44. doi: 10.1159/000085549. [DOI] [PubMed] [Google Scholar]
- Backmund M, Meyer K, Schuetz C, et al. Factors associated with exposure to hepatitis B virus in injection drug users. Drug Alcohol Depend. 2006;84:154–9. doi: 10.1016/j.drugalcdep.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Backmund M, Meyer K, Von Zielonka M, et al. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001;34(1):188–93. doi: 10.1053/jhep.2001.25882. [DOI] [PubMed] [Google Scholar]
- Bacon BR. Managing hepatitis. C Am J Manag Care. 2004;10(2Suppl):S30–S40. [PubMed] [Google Scholar]
- Balan V, Nelson DR, Sulkowski MS, et al. A phase I/II study evaluating escalating doses of recombinant human albumin-interferon-alpha fusion protein in chronic hepatitis C patients who have failed previous interferon-alpha-based therapy. Antivir Ther. 2006;11:35–45. [PubMed] [Google Scholar]
- Ballesteros AL, Franco S, Fuster D, et al. Early HCV dynamics on Peg-interferon and ribavirin in HIV/HCV co-infection: Indications for the investigation of new treatment approaches. AIDS. 2004;18:59–66. doi: 10.1097/00002030-200401020-00007. [DOI] [PubMed] [Google Scholar]
- Baral S, Sherman SG, Millson P, Beyrer C. Vaccine immunogenicty in injection drug users: a systematic review. Lancet Infect Dis. 2007;7:667–74. doi: 10.1016/S1473-3099(07)70237-2. [DOI] [PubMed] [Google Scholar]
- Bargiacchi O, Audagnotto S, Garazzino S, et al. Treatment of acute C hepatitis in intravenous drug users. J Hepatol. 2005;43:184–7. doi: 10.1016/j.jhep.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Barnett PG, Masson CL, Sorensen JL, et al. Linking opioid-dependent hospital patients to drug treatment: Health care use and costs 6 months after randomization. Addiction. 2006;101:1797–804. doi: 10.1111/j.1360-0443.2006.01636.x. [DOI] [PubMed] [Google Scholar]
- Basu S, Smith-Rohrberg D, Bruce D, et al. Models for integrating buprenorphine therapy into the primary HIV care setting. Clin Infect Dis. 2006;42:716–21. doi: 10.1086/500200. [DOI] [PubMed] [Google Scholar]
- Batki SL, Sorensen JL. Care of injection drug users with HIV. In: Cohen PT, Sande MS, Volberding PA, editors. The AIDS knowledge base: A textbook on HIV disease from the University of California, San Francisco and San Francisco General Hospital. 3rd ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 1999. Available at: http://hivinsite.ucsf.edu/InSite. [Google Scholar]
- Beers MK, Berkow R, editors. Chapter 42: Hepatitis. 17th ed. Whitehouse Station, NJ: Merck Research Laboratories; 1999. The Merck Manual of Diagnosis and Therapy; pp. 377–86. [Google Scholar]
- Behrendt CE, Ruiz RB. Hyperglycemia among persons with hepatitis C: .Not the classic diabetic phenotype. Diabetes Res Clin Pract. 2006;71:68–74. doi: 10.1016/j.diabres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Belfiori B, Chiodera A, Cileigi P, et al. Treatment for hepatitis C virus in injection drug users on opioid replacement therapy: A prospective multicentre study. Eur J Gastroenterol Hepatol. 2007;19:731–2. doi: 10.1097/01.meg.0000280175.79379.04. [DOI] [PubMed] [Google Scholar]
- Benson CA, Kaplan JE, Masur H, et al. Treating opportunistic infections among HIV-infected adults and adolescents. Morbid Mortal Wkly Rep MMWR. 2004;53:54–8. [PubMed] [Google Scholar]
- Berk SI, Litwin AH, Arnsten JH, et al. Effects of pegylated interferon alfa-2b on the pharmacokinetic and pharmacodynamic properties of methadone: A prospective, nonrandomized, crossover study in patients coinfected with hepatitis C and HIV receiving methadone maintenance treatment. Clin Ther. 2007;29:131–8. doi: 10.1016/j.clinthera.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Bhamb B, Brown D, Hariharan J, et al. Survey of select practice behaviors by primary care physicians on the use of opioids for chronic pain. Curr Med Res Opin. 2006;22:1859–65. doi: 10.1185/030079906X132398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialek SR, Bower WA, Mottram, et al. Risk factors for hepatitis B. in an outbreak of hepatitis B. and D among injection drug users. J Urban Health. 2005;82:468–78. doi: 10.1093/jurban/jti094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhead GS, Klein SJ, Candelas AR, et al. Integrating multiple programme and policy approaches to hepatitis C prevention and care for injection drug users: A comprehensive approach. Int J Drug Policy. 2007;18:417–25. doi: 10.1016/j.drugpo.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Blechman MB, Charney JA, Friedman P, et al. Interferon-alfa (IFN.-() therapy in patients with chronic hepatitis C (CHC) on methadone. Hepatology. 1999;30:482A. Abstract 1287. [Google Scholar]
- Bonacini M. Management issues in patients coinfected with hepatitis C virus and HIV. AIDS Read. 2002;12:19–26. [PubMed] [Google Scholar]
- Booth RE, Corsi KF, Mikulich-Gilbertson SK. Factors associated with methadone maintenance treatment retention among street-recruited injection drug users. Drug Alcohol Depend. 2004;74:177–85. doi: 10.1016/j.drugalcdep.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Boothby LA, Doering PL. Acamprosate for the treatment of alcohol dependence. Clin Ther. 2005;27:695–714. doi: 10.1016/j.clinthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Borg L, Khuri E, Wells A, et al. Methadone-maintained former heroin addicts, including those who are anti-HIV-1 seropositive, comply with and respond to hepatitis B. vaccination. Addiction. 1999;94:489–93. doi: 10.1046/j.1360-0443.1999.9444894.x. [DOI] [PubMed] [Google Scholar]
- Botero RC. Should patients with chronic hepatitis C infection be transplanted? Transplant Proc. 2004;36:1449–54. doi: 10.1016/j.transproceed.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Brands B, Blake J, Marsh D. Impact of methadone program philosophy changes on early treatment outcomes. J Addict Dis. 2003;22:19–38. doi: 10.1300/J069v22n03_03. [DOI] [PubMed] [Google Scholar]
- Broers B, Helbling B, Francois A, et al. Barriers to interferon-ά therapy are higher in intravenous drug users than in other patients with acute hepatitis C. J Hepatol. 2005;42:323–28. doi: 10.1016/j.jhep.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- Brown LR, Kritz SA, Goldsmith RJ, et al. Characteristics of substance abuse treatment programs providing services for HIV/AIDS, hepatitis C virus infection, and sexually transmitted infections: The National Drug Abuse Treatment Clinical Trials Network. J Sub Abuse Treat. 2006;30:315–21. doi: 10.1016/j.jsat.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. Heroin dependence. Wisconsin Med J. 2004;103:20–6. [PubMed] [Google Scholar]
- Bruce RD, Altice FL. Case series on the safe use of buprenorphine/naloxone in individuals with acute hepatitis C infection and abnormal hepatitis liver transaminases. Am J Drug Alcohol Abuse. 2007;33:869–74. doi: 10.1080/00952990701653875. [DOI] [PubMed] [Google Scholar]
- Bryan J. Where next for the treatment of hepatitis C? Future Virol. 2007;2:331–4. [Google Scholar]
- Burt RD, Hagan H, Garfein RS, et al. Trends in hepatitis B. virus, hepatitis C virus, and human immunodeficiency virus prevalence, risk behaviors and preventive measures among Seattle injection drug users aged 18–30 years, 1994–2000. J Urban Health. 2007;84:436–54. doi: 10.1007/s11524-007-9178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleri G, Cariti G, Gaiottino F, et al. A short course of pegylated interferon—alfa in acute HCV hepatitis. J Viral Hepat. 2007;14:116–21. doi: 10.1111/j.1365-2893.2006.00802.x. [DOI] [PubMed] [Google Scholar]
- Caputo F, Addolorato G, Domenicali M. Short-term methadone administration reduces alcohol consumption in non-alcoholic heroin addicts. Alcohol Alcoholism. 2002;37:164–8. doi: 10.1093/alcalc/37.2.164. [DOI] [PubMed] [Google Scholar]
- Carrat F, Bani-Sadr F, Pol S, et al. Pegylated interferon alfa-2b versus standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: A randomized controlled trial. JAMA. 2004;292:2909–13. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- Castells L, Esteban JI, Bilbao I, et al. Early antiviral treatment of hepatitis C virus recurrence after liver transplantation in HIV-infected patients. Antivir Ther. 2006;11:1061–70. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR. 1998;47(RR-19):1–33. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Prevention of hepatitis A through active or passive immunization: Recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR. 1999;48(RR-12):1–12. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Updated US Public Health Services Guidelines for the Management of Occupational Exposure to HBV, HCV, and HIV and Recommendations for Postexposure Prophylaxis. MMWR. 2001;50(RR-11):1–43. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Epidemiology and Prevention of Vaccine Preventable Diseases (“Pink Book”) 7th Ed. Waldorf, MD: Public Health Foundation; 2002. pp. 169–76. (Hepatitis B). Available at: http://www.cdc.gov. [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Transmission of hepatitis B. and C viruses in outpatient settings-New York, Oklahoma, and Nebraska, 2000–2002. MMWR. 2003;52:901–6. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Hepatitis C slide set. 2003a. available at: www.cdc.gov/ncidod/diseases/hepatitis/slideset/hep_c/hcv_epi_for_distrib_000925.ppt#298,10.
- CDC (Centers for Disease Control and Prevention) Hepatitis Surveillance Report. Atlanta GA: United States Department of Health and Human Services, Centers for Disease Control and Prevention; 2005. www.cdc.gov/ncidod/diseases/hepatitis/resource/PDFs/disease_burden2005.pdf. [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Deaths: final Data for 2004. National Vital Statistics Reports. 2007 2007 Aug 21;55(19) Available at ; www.cdc.gov/nchs/data/nvsr/nvsr55/nvsr55_19.pdf. [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Viral Hepatitis C Fact Sheet. available at : www.cdc.gov/ncidod/diseases/hepatitis/c/fact.htm.
- CDC (Centers for Disease Control and Prevention) National Hepatitis C Prevention Strategy. Available at www.cdc.gov/ncidod/diseases/hepatitis/c/plan/HCV_infection.htm.
- Celona AF, Yu MC, Prakash M, et al. Hepatitis C in a Los Angeles public hepatitis clinic: Demographic and biochemical differences associated with race-ethnicity. Clin Gastroenterol Hepatol. 2004;2:459–62. doi: 10.1016/s1542-3565(04)00160-0. [DOI] [PubMed] [Google Scholar]
- CFR.-Code of Federal Regulations. United States of America Opioid drugs in maintenance and detoxification treatment of opiate addiction; Final Rule 42: Part 8. Federal Register. 2001;66:4076–102. [PubMed] [Google Scholar]
- Chamot E, de Saussure Ph, Deglon JJ, et al. Incidence of hepatitis C, hepatitis B. and HIV infections among drug users in a methadone-maintenance programme. AIDS. 1992;6:430–31. [PubMed] [Google Scholar]
- Chatham LR, Rowan-Szal GA, Joe GW, et al. Heavy drinking in a population of methadone-maintained clients. J Stud Alcohol. 1995;56:417–22. doi: 10.15288/jsa.1995.56.417. [DOI] [PubMed] [Google Scholar]
- Chitturi S, George J. Predictors of liver-related complications in patients with chronic hepatitis C. Ann Med. 2000;32:588–91. doi: 10.3109/07853890009002028. [DOI] [PubMed] [Google Scholar]
- Chu HM, McNair L. Results of a phase I single-dose escalation study of the hepatitis C protease inhibitor VX-950 in health volunteers; Presentation at the American Association for the Study of Liver Disease 55th Annual Meeting and Postgraduate Course; October 29; Boston, MA. 2004. Abstract LB-02. [Google Scholar]
- Chung R, Anderson J, Volberding P, et al. A PEG interferon-α 2a plus ribavirin versus interferon-α 2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–9. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Rimondi R, et al. Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biol Psychiatry. 2007;61:4–12. doi: 10.1016/j.biopsych.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarck JC, Milroy CM, Forrest ARW. Deaths from methadone use. J Clin For Med. 1995;2:143–4. doi: 10.1016/1353-1131(95)90082-9. [DOI] [PubMed] [Google Scholar]
- Clark NC, Dietze P, Lenne MG, et al. Effect of opioid substitution therapy on alcohol metabolism. J Subst Abuse Treat. 2006;30:191–6. doi: 10.1016/j.jsat.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Colletta C, Smirne C, Fabris C, et al. Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology. 2005;42:838–45. doi: 10.1002/hep.20814. [DOI] [PubMed] [Google Scholar]
- Comstock RD, Mallonee S, Fox JL. A large nosocomial outbreak of hepatitis C and hepatitis B. among patients receiving pain medications. Infect Control Hosp Epidemiol. 2004;25:576–83. doi: 10.1086/502442. [DOI] [PubMed] [Google Scholar]
- Cone EJ, Preston KL. Toxological aspects of heroin substation treatment. Ther Drug Monit. 2002;24:210–21. doi: 10.1097/00007691-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Conway B, Prasad J, Reynold R, et al. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clin Infect Dis. 2004;38(Suppl 5):S402–S408. doi: 10.1086/421404. [DOI] [PubMed] [Google Scholar]
- Conway KP, Kane RJ, Ball SA, et al. Personality, substance of choice, and polysubstance involvement among substance dependent patients. Drug Alcohol Depend. 2003;71:65–75. doi: 10.1016/s0376-8716(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Copenhaver MM, Lee I_C. Optimizing a community-friendly HIV risk reduction intervention for injection drug users in treatment: A structural equation modeling approach. J Urban Health. 2006;83:1132–42. doi: 10.1007/s11524-006-9090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey KE, Ross AS, Wurcel A, et al. Outcomes and treatment of acute hepatitis C virus infection in a United States population. Clin Gastroenterol Hepatol. 2006;4:1278–82. doi: 10.1016/j.cgh.2006.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi KF, Kwiatowski CF, Booth RE. Treatment entry and predictors among opiate-using injection drug users. Am J Drug Alcohol Abuse. 2007;33:121–7. doi: 10.1080/00952990601091093. [DOI] [PubMed] [Google Scholar]
- Costenbader EC, Zule WA, Coomes CM. The impact of illicit drug use and harmful drinking on quality of life among injection drug users at high risk for hepatitis C infection. Drug Alcohol Dep. 2007;89:251–58. doi: 10.1016/j.drugalcdep.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournot M, Gilbert A, Castel F, et al. Management of hepatitis C in active drug users: Experience of an addiction care hepatology unit. Gastroenterol Clin Biol. 2004;28:1101–5. doi: 10.1016/s0399-8320(04)95008-7. [DOI] [PubMed] [Google Scholar]
- Cunningham CO, Kunins HV, Roose RJ, et al. Barriers to obtaining waivers to prescribe buprenorphine for opioid addiction treatment among HIV physicians. J Gen Intern Med. 2007;22:1325–29. doi: 10.1007/s11606-007-0264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgard O, Bjoro K, Hellum K, et al. Treatment of chronic hepatitis C in injection drug users: 5 years' follow-up. Eur Addict Res. 2002;8:45–49. doi: 10.1159/000049487. [DOI] [PubMed] [Google Scholar]
- Davis GL, Rodrigue JR. Treatment of chronic hepatitis C in active drug users. N Engl J Med. 2001;345:215–7. doi: 10.1056/NEJM200107193450312. [DOI] [PubMed] [Google Scholar]
- de la Fuente L, Toro C, Brugal MT, et al. Poor validity of self-report of HBV vaccination among young heroin users in Spain supports the policy “don't ask, draw a blood sample, vaccinate and try to schedule another visit”. J Clin Virol. 2007;38:87–90. doi: 10.1016/j.jcv.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28:497–504. doi: 10.1016/j.jpainsymman.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Dell'Osso L, Pini S, Maggi L, et al. Subthreshold mania as predictor of depression during interferon treatment in HCV+ patients without current or lifetime psychiatric disorders. J Psychosom Res. 2007;62:349–55. doi: 10.1016/j.jpsychores.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Delwaide J. Postexposure management of hepatitis A, B, or C: treatment, postexposure prophylaxis and recommendations. Acta Gastroenterol Belg. 2003;66:250–4. [PubMed] [Google Scholar]
- Department of Health and Human Services. National Institues of Health. National Institue of Environmental Health Sciences . Report on Carcinogens. 11th Edition. National Toxicology Program; 2005. Available at http://ntp.niehs.nih.gov/index.cfm?objectid=72016262-BDB.7-CEBA-FA60E922B.18C2540. [Google Scholar]
- Department of Veterans Affairs Hepatitis C Resource Centers Management and Treatment of Hepatitis C Virus Infection in HIV-Infected Adults: Recommendations from the Veterans Affairs Hepatitis C Resource Center Program and National Hepatitis C Program Office. 2005. Available at www.hepatitis.va.gov. [DOI] [PubMed]
- DeRosa FC, Bargiacchi O, Audagnotto S, et al. Twelve-week treatment of acute hepatitis C virus with pegylated interferon-ά-2b in injection drug users. Clin Infect Dis. 2007;45:583–8. doi: 10.1086/520660. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Friedman SR, Ward TP. Harm reduction: A public health response to the AIDS epidemic among injecting drug users. Ann Rev Public Health. 1993;14:413–50. doi: 10.1146/annurev.pu.14.050193.002213. [DOI] [PubMed] [Google Scholar]
- Dettmeyer RB, Preuss J, Wollerson H. Heroin-associated nephropathy. Expt Opin Drug Saf. 2005;4:19–28. doi: 10.1517/14740338.4.1.19. [DOI] [PubMed] [Google Scholar]
- Di Bisceglie AM, McHutchison J, Rice C. New therapeutic strategies for hepatitis C. Hepatology. 2002;35:224–31. doi: 10.1053/jhep.2002.30531. [DOI] [PubMed] [Google Scholar]
- Di Martini A, Weinrieb R. Liver transplantation for methadone-maintained opiate dependents: making the case for cautious optimism. Am J Transplant. 2003;3:1183–4. doi: 10.1046/j.1600-6143.2003.00217.x. [DOI] [PubMed] [Google Scholar]
- Dominguez S, Ghosn J, Valantin MA, et al. Efficacy of early treatment of acute hepatitis C infection with pegylated interferon and ribavirin in HIV-infected patients. AIDS. 2006;20:1157–61. doi: 10.1097/01.aids.0000226956.02719.fd. [DOI] [PubMed] [Google Scholar]
- Donahoe RM. Neuroimmunomodulation by opiates: relationship to HIV infection and AIDS. Adv Neuroimmunol. 1993;3:31–46. [PubMed] [Google Scholar]
- Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infection to AIDS. J Neuroimmunol. 1998;83:77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- Dore GJ, Thomas DL. Management and treatment of injection drug users with hepatitis C virus (HCV) infection and HCV/human immunodeficiency virus coinfection. Sem Liver Dis. 2005;25:18–32. doi: 10.1055/s-2005-864779. [DOI] [PubMed] [Google Scholar]
- Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: Implications for the treatment of opioid dependence. Clin Pharmacokin. 2002;41:1153–93. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- EASL (European Association for the Study of the Liver) International Consensus Conference on Hepatitis C: Paris, 26–27 February 1999 Consensus statement. J Hepatol. 1999;31(suppl 1):3–8. [PubMed] [Google Scholar]
- Edlin BR, Kresina TF, Raymond DB, et al. Care for hepatitis C disease and barriers to care among injection drug users. Clin Infect Dis. 2005;40:S276–85. doi: 10.1086/427441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin BR, Seal KH, Lorvick J, et al. Is it justifiable to withhold treatment for hepatitis C from illicit-drug users? N Engl J Med. 2001;345:211–4. doi: 10.1056/NEJM200107193450311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einav S, Koziel MJ. Immunopathogenesis of hepatitis C virus in the immunosuppressed host. Transpl Infect Dis. 2002;4:85–92. doi: 10.1034/j.1399-3062.2002.t01-2-02001.x. [DOI] [PubMed] [Google Scholar]
- Elefsiniotis IS, Pantazis KD, Ketikoglou ID, et al. Changing serological status and low vaccination-induced protection rates against hepatitis B. characterize chronic hepatitis C virus-infected injection drug users in Greece: need for immunization policy. Eur J Gastroent Hepatol. 2006;18:1227–31. doi: 10.1097/01.meg.0000236888.51838.36. [DOI] [PubMed] [Google Scholar]
- Estrada AL. Health disparities among African-American and Hispanic drug injectors-HIV, AIDS, hepatitis B. virus and hepatitis C virus: review. AIDS. 2005;19(suppl 3):S47–52. doi: 10.1097/01.aids.0000192070.95819.7c. [DOI] [PubMed] [Google Scholar]
- Evans M, Hokanson PS, Augsburger J, et al. Increasing knowledge and HIV and hepatitis C during substance abuse treatment. Addict Dis Treat. 2005;4:71–6. [Google Scholar]
- Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology. 2004;127(Suppl 2):S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Coccia CP, Bertolini A, et al. Methadone-metabolism, pharmacokinetics and interactions. Pharmcol Res. 2004;50:551–9. doi: 10.1016/j.phrs.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Festi D, Colecchia A, Sacco, et al. Hepatitis steatosis in obese patients: Clinical aspects and prognostic significance. Obes Rev. 2004;5:27–42. doi: 10.1111/j.1467-789x.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- Fiellin DA. Substance use disorders in HIV-infected patients: impact and new treatment strategies. Top HIV Med. 2004;12:77–82. [PubMed] [Google Scholar]
- Fiellin DA, Rosenheck RA, Kosten TR. Coordination of medical care and opioid dependence treatment in primary care: A case report. Subst Abuse. 2003;24:43–6. doi: 10.1080/08897070309511532. [DOI] [PubMed] [Google Scholar]
- Finney JW, Moos RH, Timko C. The course of treated and untreated substance use disorders: Remission and resolution, relapse and mortality. In: McCrady BS, Epstein EE, editors. Addictions: A Comprehensive Guidebook. New York: Oxford University Press; 1999. pp. 30–49. [Google Scholar]
- Finucane MM, Samet JH, Horton NJ. Translational methods in biostatistics: linear mixed effect regression models of alcohol consumption and HIV disease progression over time. J Subst Abuse Treat epub. 2007 doi: 10.1186/1742-5573-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DG, Reynolds GL, Jaffe A, et al. Hepatitis and human immunodeficiency virus co-infection among injection drug users in Los Angeles County, California. J Addict Dis. 2006;25:25–32. doi: 10.1300/J069v25n02_04. [DOI] [PubMed] [Google Scholar]
- Fletcher BW, Broome KM, Delany PJ, et al. Patient and program factors in obtaining supportive services in DATOS. J Subst Abuse Treat. 2003;25:165–75. doi: 10.1016/s0740-5472(03)00126-0. [DOI] [PubMed] [Google Scholar]
- Fraaji PL, Rakhmanina N, Burger DM, et al. Therapeutic drug monitoring in children with HIV/AIDS. Ther Drug Monitor. 2004;26:122–6. doi: 10.1097/00007691-200404000-00006. [DOI] [PubMed] [Google Scholar]
- Fried MW. Viral factors affecting the outcome of therapy for chronic hepatitis C. Rev Gastroenterol Disord. 2004;4:S8–S13. [PubMed] [Google Scholar]
- Freidmann PD, Hendrickson JC, Gertein DR, et al. Do mechanisms that link addition treatment patients to primary care influence subsequent utilization of emergency and hospital care? Med Care. 2006;44:8–15. doi: 10.1097/01.mlr.0000188913.50489.77. [DOI] [PubMed] [Google Scholar]
- Fugelstad A, Stenbacka M, Leifman A, et al. Methadone maintenance treatment: the balance between life-saving treatment and fatal poisonings. Addiction. 2007;102:406–12. doi: 10.1111/j.1360-0443.2006.01714.x. [DOI] [PubMed] [Google Scholar]
- Fuller CM, Ompad DC, Galea S, et al. Hepatitis C incidence—A comparison between injection and noninjection drug users in New York City. J Urban Health. 2004;81:20–24. doi: 10.1093/jurban/jth084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SK, Lok ASF. Hepatitis B virus Genotypes: Do they play a role in the outcome of HBV infection? Hepatology. 2004;40:790–792. doi: 10.1002/hep.1840400407. [DOI] [PubMed] [Google Scholar]
- Gaglio PJ, Sterling R, Daniels E, et al. Hepatitis B. virus and HIV coinfection: Results of a survey on treatment practices and recommendations for therapy. Clin Infect Dis. 2006;45:618–23. doi: 10.1086/520751. [DOI] [PubMed] [Google Scholar]
- Galanter M, Dermatis H, Glickman I, et al. Network therapy: Decreased secondary opioid use during buprenorphine maintenance. J Subst Abuse Treat. 2004;26:313–8. doi: 10.1016/j.jsat.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Galmiche JP. French consensus conference on hepatitis C: Screening and treatment. Gut. 1998;42:892–8. doi: 10.1136/gut.42.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O'Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: A randomized controlled trial. JAMA. 2005;293:1617–25. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Garfein RS, Bower WA, Loney CM, et al. Factors associated with fulminant liver failure during an outbreak among injection drug users with acute hepatitis B. Hepatology. 2004;40:865–73. doi: 10.1002/hep.20383. [DOI] [PubMed] [Google Scholar]
- Garfein RS, Golub ET, Greenberg AE, et al. A peer-education intervention to reduce injection risk behaviors fro HIV and hepatitis C virus infection in young injection drug users. AIDS. 2007;21:1923–32. doi: 10.1097/QAD.0b013e32823f9066. [DOI] [PubMed] [Google Scholar]
- Gebo KA, Herlong HF, Torbenson MS, et al. Role of liver biopsy in the management of chronic hepatitis C: A systematic review. Hepatology. 2002a;26:S161–S172. doi: 10.1053/jhep.2002.36989. [DOI] [PubMed] [Google Scholar]
- Gebo K, Jenckes M, Chander G, et al. AHRQ. Rockville, MD: Agency for Healthcare Research and Quality; 2002. Jul, 2002. Management of chronic hepatitis C. Evidence Report/Technology Assessment No. 60 (Prepared by the Johns Hopkins University Evidence-based Practice Center under Contract No. 290--97-0006) (Publication No. 02-E030). Available at http://www.ahcpr.gov/clinic/epcix.htm. [Google Scholar]
- Gerlich M, Gschwend P, Uchtenhagen A, et al. Prevalence of hepatitis and HIV infections and vaccination rates in patients entering the heroin-assisted treatment in Switzerland between 1994–2002. Eur J Epidemiol. 2006;21:545–49. doi: 10.1007/s10654-006-9023-z. [DOI] [PubMed] [Google Scholar]
- Golub ET, Latka M, Hagan H, et al. Screening for depressive symptoms among HCV-infected injection drug users: Examination of the utility of the CES-D and the Beck Depression Inventory. J Urban Health. 2004;81:278–90. doi: 10.1093/jurban/jth114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutevitch MN, Chatterji P, Deb N, et al. On-site medical care in methadone maintenance: Associations with health care use and expenditures. J Subst Abuse Treat. 2007;32:143–51. doi: 10.1016/j.jsat.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell M, Bornemann R, et al. Substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2004:CD004145. doi: 10.1002/14651858.CD004145.pub2. [DOI] [PubMed] [Google Scholar]
- Graham AW, Schultz TK, Mayo-Smith M, et al. Principles of Addiction Medicine. 3rd ed. Chevy Chase, MD: American Society of Addiction Medicine; 2002. [Google Scholar]
- Graham CS, Wells A, Liu T, et al. Antigen-specific immune responses and liver histology in HIV and hepatitis C coinfection. AIDS. 2005;19:767–73. doi: 10.1097/01.aids.0000168970.80551.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebely J, Genoway K, Khara, et al. Treatment uptake and outcomes among current and former injection drug users receiving directly observed therapy within a multidisciplinary group model for the treatment of hepatitis C virus infection. Int J Drug Policy. 2007;18:437–43. doi: 10.1016/j.drugpo.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Grebely J, Raffa JD, Meagher C, et al. Directly observed therapy for the treatment of hepatitis C virus infection in current and former injection drug users. J Gastroenterol Hepatol. 2007a;22:1519–25. doi: 10.1111/j.1440-1746.2007.05032.x. [DOI] [PubMed] [Google Scholar]
- Grebely J, Genoway KA, Raffa, et al. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend. 2008;93:141–7. doi: 10.1016/j.drugalcdep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: The Swiss HIV Cohort Study. Lancet. 2000;356(9244):1800–5. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- Gunn RA, Lee MA, Callahan DB, et al. Integrating hepatitis, STD, and HIV services into a drug rehabilitation program. Am J Prev Med. 2005;29:27–33. doi: 10.1016/j.amepre.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Sellers E, Somoza E, et al. The effect of multiple doses of peginterferon alfa-2b on the steady-state pharmacokinetics of methadone in patients with chronic hepatitis C undergoing methadone maintenance therapy. J Clin Pharmacol. 2007;47:604–12. doi: 10.1177/0091270007299760. [DOI] [PubMed] [Google Scholar]
- Hagan H, Haynes H, Willson RA, et al. Feasibility of alpha-interferon administration to opiate addicts in a methadone treatment setting. Hepatology. 1999;30(4 Part 2):624A. [Google Scholar]
- Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis preventions services into a substance use disorder clinic. J Subst Abuse Treatment. 2007;32:391–8. doi: 10.1016/j.jsat.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Hall SJ, Kresina TF, Trauger R, et al. In: Gene therapy in the treatment of cancer IN An Introduction to Molecular Medicine and Gene Therapy. Kresina TF, editor. John Wiley and Sons, Inc; Publisher, New York, New York: 2001. pp. 235–62. [Google Scholar]
- Hallinan R, Byrne A, Agho K, et al. Referral for chronic hepatitis C treatment from a drug dependency treatment setting. Drug Alcohol Depend. 2007;88:49–53. doi: 10.1016/j.drugalcdep.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Hancock MM, Prosser CC, Ransibrahmanakul K, et al. Liver transplant and hepatitis C in methadone maintenance therapy; A case report. Subst Abuse Treat Prev Policy. 2007;2:5. doi: 10.1186/1747-597X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill P, Dwyer K, Kay T, Murphy B. Severe chronic renal failure in association with oxycodone addiction: A new form of fibrillary glomerulopathy. Human Pathol. 2002;33:783–7. doi: 10.1053/hupa.2002.126185. [DOI] [PubMed] [Google Scholar]
- Ho SB, Nguyen H, Tetrick LL, et al. Influence of psychiatric diagnoses on interferon-alfa treatment for chronic hepatitis C in a veteran population. Am J Gastroenterol. 2001;96:157–64. doi: 10.1111/j.1572-0241.2001.03468.x. [DOI] [PubMed] [Google Scholar]
- Hopwood M, Treloar C. The drugs that dare not speak their name: Injecting and other illicit drug use during treatment for hepatitis C infection. Int J Drug Policy. 2007;18:374–80. doi: 10.1016/j.drugpo.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Howard AA, Klein RS, Schoenbaum EE. Association of hepatitis C infection and antiretroviral use with diabetes mellitus in drug users. Clin infect Dis. 2003;36:1318–23. doi: 10.1086/374838. [DOI] [PubMed] [Google Scholar]
- Howard AA, Lo Y, Floris-Morre M, et al. Hepatitis C virus infection is associated with insulin resistance among older adults with or at risk of HIV infection. AIDS. 2007;21:633–41. doi: 10.1097/QAD.0b013e3280464db7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang D, et al. Relationship between drug treatment services, retention, and outcomes. Psychiat Serv. 2004;55:767–774. doi: 10.1176/appi.ps.55.7.767. [DOI] [PubMed] [Google Scholar]
- Huckans MS, Blackwell AD, Harms TA, et al. Management of hepatitis C disease among VA patients with schizophrenia and substance use disorder. Psychiat Serv. 2006;57:403–6. doi: 10.1176/appi.ps.57.3.403. [DOI] [PubMed] [Google Scholar]
- Huckans MS, Loftis JM, Blackwell AD, et al. Interferon alpha therapy for hepatitis C: Treatment completion and response rates among patients with substance use disorders. Subst Abuse Treat Prev Policy. 2007;2:4–12. doi: 10.1186/1747-597X-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglesby TV, Rai R, Astemborski J. A prospective, community-based evaluation of liver enzymes in individuals with hepatitis C after drug use. Hepatology. 1999;29:590–6. doi: 10.1002/hep.510290219. [DOI] [PubMed] [Google Scholar]
- Intron [interferon alfa-2b package insert] Kenilworth, NJ: Schering Corp; 1998. [Google Scholar]
- Jackson CL, Nicholson C, Tweeddale M, et al. Creating an integrated vision by collocating health organizations: Hearding cats or a meeting of minds? Aust Health Rev. 2007;31:256–66. doi: 10.1071/ah070256. [DOI] [PubMed] [Google Scholar]
- Jacobs RJ, Gibson GA, Meyerhoff AS. Cost-effectiveness of hepatitis A-B. vaccine versus hepatitis B vaccine for healthcare and public safety workers in the western United States. Infect Control Hosp Epidemiol. 2004;25:563–9. doi: 10.1086/502440. [DOI] [PubMed] [Google Scholar]
- Jaeckel E, Cornberg M, Wedemeyer H, et al. Treatment of acute hepatitis with interferon alfa-2b. N Engl J Med. 2001;345:1452–7. doi: 10.1056/NEJMoa011232. [DOI] [PubMed] [Google Scholar]
- Janssen HLA, van Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAG-positive chronic hepatitis B: A randomized trial. Lancet. 2005;365:123–9. doi: 10.1016/S0140-6736(05)17701-0. [DOI] [PubMed] [Google Scholar]
- Jauncey M, Micallef JM, Gilmour S, et al. Clearance of hepatitis C virus after newly acquired infection in injection drug users. J Infect Dis. 2004;190:1270–4. doi: 10.1086/423943. [DOI] [PubMed] [Google Scholar]
- Johnson RE, McCaugh JC. Buprenorphine and naloxone for heroin dependence. Curr Psychiatry Rep. 2000;2:519–26. doi: 10.1007/s11920-000-0012-8. [DOI] [PubMed] [Google Scholar]
- Jowett Sl, Agarwal K, Smith BC, et al. Managing chronic hepatitis C acquired through intravenous drug use. QJM. 2001;94:153–8. doi: 10.1093/qjmed/94.3.153. [DOI] [PubMed] [Google Scholar]
- Kamal F, Flavin S, Cambell F, et al. factors affecting the outcome of methadone maintenance treatment in opiate dependence. Ir Med J. 2007;100:393–7. [PubMed] [Google Scholar]
- Kanchana TP, Kaul V, Manzarbeitia C, et al. Liver transplantation for patients on methadone maintenance. Liver Transplant. 2002;8:778–82. doi: 10.1053/jlts.2002.33976. [DOI] [PubMed] [Google Scholar]
- Kanwal F, Gralnek IM, Martin P, et al. Treatment alternatives for chronic hepatitis B. infection: a cost effectiveness analysis. Ann Intern Med. 2005;142:821–31. doi: 10.7326/0003-4819-142-10-200505170-00007. [DOI] [PubMed] [Google Scholar]
- Kaplan DE, Sugimoto K, Newton K, et al. Discordant role of CD4 T-cell responses relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:801–5. doi: 10.1053/j.gastro.2006.11.044. [DOI] [PubMed] [Google Scholar]
- Kaplan MM. Laboratory Tests. In Diseases of the Liver, seventh edition. edited by Schiff L and Schiff ER. JB Lippincott Publishers, Philadelphia PA. 1993:108–44. [Google Scholar]
- Karam GA, Reisi M, Kaseb AA, et al. Effects of opium addiction on some serum factors in addicts with non-insulin dependent diabetes mellitus. Addict Biol. 2004;9:53–8. doi: 10.1080/13556210410001674095. [DOI] [PubMed] [Google Scholar]
- Keeffe E. Liver transplantation at the millennium. Past, present, and future. Clin Liver Dis. 2000;4:241–55. doi: 10.1016/s1089-3261(05)70106-9. [DOI] [PubMed] [Google Scholar]
- Kelleher TB, Mehta SH, Bhaskar R, et al. Prediction of hepatic fibrosis in HIV/HCV coinfected patients using serum fibrosis markers: the SHASTA index. J Hepatol. 2005;43:78–84. doi: 10.1016/j.jhep.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Kim AI, Saab S. Treatment of hepatitis C. Am J Med. 2005;118:808–15. doi: 10.1016/j.amjmed.2005.01.073. [DOI] [PubMed] [Google Scholar]
- Kingsley LA, Rinaldo CR, Jr, Lyter DW, et al. Sexual transmission efficacy of hepatitis B. virus and human immunodeficiency virus among homosexual men. JAMA. 1990;264:230–4. [PubMed] [Google Scholar]
- Kipnis SS, Herron A, Perez J, et al. Integrating the methadone patient in traditional addiction impatient rehabilitation programs-Problems and solutions. Mt Sinai J Med. 2001;68:28–32. [PubMed] [Google Scholar]
- Kjaergard LL, Krogsgaard K, Gluud C. Interferon alfa with or without ribavirin for chronic hepatitis C: Systemic review of randomized trials. Brit Med J. 2001;323:1151–5. doi: 10.1136/bmj.323.7322.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JA. Liver function tests: Their role in the diagnosis of hepatobiliary disease. J Infus Nurs. 2005;28:108–17. doi: 10.1097/00129804-200503000-00004. [DOI] [PubMed] [Google Scholar]
- Koch M, Banys P. Liver transplantation and opioid dependence. JAMA. 2001;285:1056–8. doi: 10.1001/jama.285.8.1056. [DOI] [PubMed] [Google Scholar]
- Konopnicki D, Mocroft A, DeWit S, et al. Hepatitis B. and HIV: Prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:593–601. doi: 10.1097/01.aids.0000163936.99401.fe. [DOI] [PubMed] [Google Scholar]
- Kornblat KM, Berk PD. Hyperbilirubinemia in the setting of antiviral therapy. Clin Gastroenterol Hepatol. 2005;3:303–10. doi: 10.1016/s1542-3565(05)00083-2. [DOI] [PubMed] [Google Scholar]
- Kosten TR, O'Connor PG. Management of drug and alcohol withdrawal. N Engl J Med. 2003;348:1786–95. doi: 10.1056/NEJMra020617. [DOI] [PubMed] [Google Scholar]
- Kraus MR, Schafer A, Csef H, et al. Compliance with therapy in patients with chronic hepatitis C: Associations with psychiatric symptoms, interpersonal problems, and mode of acquisition. Dig Dis Sci. 2001;46:2060–5. doi: 10.1023/a:1011973823032. [DOI] [PubMed] [Google Scholar]
- Krause G, Trepka MJ, Whisenhunt RS, et al. Nosocomial transmission of hepatitis C virus associated with the use of multidose saline vials. Infect Control Hosp Epidemiol. 2003;24:122–7. doi: 10.1086/502176. [DOI] [PubMed] [Google Scholar]
- Kresina TF, Bruce D, Cargill V, et al. Integrating HCV Care and HIV Primary Care for the Injection Drug User Coinfected with HIV and HCV. Clin Inf Dis. 2005;41(Suppl 1):S–83–8. doi: 10.1086/429502. [DOI] [PubMed] [Google Scholar]
- Kresina TF, Eldred L, Bruce RD, et al. Integration of pharma-cotherapy for opioid addiction into HIV primary care for HIV/hepatitis C virus co-infected patients. AIDS. 2005a;19(Suppl 3):S221–6. doi: 10.1097/01.aids.0000192093.46506.e5. [DOI] [PubMed] [Google Scholar]
- Kresina TF, Normand J, Khalsa J, et al. Addressing the need for treatment paradigms for drug-abusing patients with multiple morbidities. Clin Infect Dis. 2004;38(Suppl 5):S398–401. doi: 10.1086/421403. [DOI] [PubMed] [Google Scholar]
- Krook AL, Stokka D, Heger B, et al. Hepatitis C treatment of opioid dependants receiving maintenance treatment: Results of a Norwegian pilot study. Eur Addict Res. 2007;13:216–21. doi: 10.1159/000104884. [DOI] [PubMed] [Google Scholar]
- Kullig CC, Bereford TP. Hepatitis C in alcohol dependence: Drinking versus disulfiram. J Addict Dis. 2005:77–89. doi: 10.1300/J069v24n02_07. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Sierka DR, Damask AM, et al. Safety and success of kidney transplantation and concomitant immunosuppression in HIV-positive patients. Kidney Int. 2005;67:1622–9. doi: 10.1111/j.1523-1755.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- Kuo I, Mudrick DW, Strathdee SA, et al. Poor validity of self-reported hepatitis B. virus infection and vaccination status among young drug users. Clin Infect Dis. 2004a;38:587–590. doi: 10.1086/381440. [DOI] [PubMed] [Google Scholar]
- Kuo I, Sherman SG, Thomas DL, et al. Hepatitis B. virus infection and vaccination among young injection and non-injection drug users: Missed opportunities to prevent infection. Drug Alcohol Depend. 2004;73:69–78. doi: 10.1016/j.drugalcdep.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Lang L. HCV findings presented at EASL: long-term follow-up and the criteria of a cure. Gastroenterology. 2007;132:2282–3. doi: 10.1053/j.gastro.2007.04.062. [DOI] [PubMed] [Google Scholar]
- Lang L. Combination therapy with telaprevir and pegylated interferon suppresses both wild-type and resistant hepatitis C virus. Gastroenterology. 2007a;132:5–6. doi: 10.1053/j.gastro.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Lankenau SE, Clatts MC. Drug injection practices among high-risk youth: The first shot of ketamine. J Urban Health. 2004;81:232–48. doi: 10.1093/jurban/jth110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larghi A, Zuin M, Crosignani A, et al. Outcome of an outbreak of acute hepatitis C among healthy volunteers participating in pharmacokinetics studies. Hepatology. 2002;36:993–1000. doi: 10.1053/jhep.2002.36129. [DOI] [PubMed] [Google Scholar]
- Larson A, Murakami C, Willson R. The evaluation of abnormal liver function tests and jaundice. University of Washington Gastrointestinal Practice Guidelines. 2005. Chapter 9 available at www.uwgi.org/guidelines/ch_09/Ch09txt.htm.
- Latka MH, Hagan H, Kapadia F, et al. Effects of a randomized intervention trial to reduce lending of used injection equipment among HCV-infected injection drug users. Am J Pub Health (in press) 2007 doi: 10.2105/AJPH.2007.113415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt SB. Liver disease in MMT: treatment and transplant Part 1: Critical concerns. Addiction Treatment Forum. 10; 2001. Available at: http://www.atforum.com. [Google Scholar]
- Leigh JP, Bowlus CL, Leistikow BN, et al. Costs of hepatitis C. Arch Intern Med. 2001;161:2231–7. doi: 10.1001/archinte.161.18.2231. [DOI] [PubMed] [Google Scholar]
- Levy MJ, Herrera JL, DiPalma JA. Immune. globulin and vaccine therapy to prevent hepatitis A infection. Am J Med. 1998;105:416–23. doi: 10.1016/s0002-9343(98)00296-4. [DOI] [PubMed] [Google Scholar]
- Lichtinghagen R, Bahr MJ. Noninvasive diagnosis of fibrosis in chronic liver disease. Exp Rev Molec Diag. 2004;4:715–26. doi: 10.1586/14737159.4.5.715. [DOI] [PubMed] [Google Scholar]
- Ling W, Smith D. Buprenorphine: blending practice and research. J Subst Abuse Treat. 2002;23:87–92. doi: 10.1016/s0740-5472(02)00257-x. [DOI] [PubMed] [Google Scholar]
- Littleton J, Zieglgansberger W. Pharmacological mechanisms of naltrexone and acamprosate in the prevention of relapse in alcohol dependence. Am J Addict. 2003;12(Suppl 1):S3–11. doi: 10.1111/j.1521-0391.2003.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Litwin AH, Gourevitch M. Does hepatitis C (HCV) virus infection alter methadone dose requirements?; Presented at: 129th Annual Meeting of American Public Health Association; October 23, 2001; Atlanta, GA. 2001. Abstract, #26390. [Google Scholar]
- Litwin AH, Kunins HV, Berg KH, et al. Hepatitis C management by addiction medicine physicians: results from a national survey. J Subst Abuse Treat. 2007;33:99–105. doi: 10.1016/j.jsat.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin AH, Soloway I, Gourevitch M. Integrating hepatitis C services with methadone maintenance. Clin Infect Dis. 2005;40:S339–45. doi: 10.1086/427450. [DOI] [PubMed] [Google Scholar]
- Liu L, Schiano TD, Lau N, et al. Survival and risk of recidivism in methadone-dependent patients undergoing liver transplantation. Am J Transpl. 2003;3:1273–7. doi: 10.1046/j.1600-6143.2003.00199.x. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Matthews AM, Hauser P. Psychiatric and substance use disorders in individuals with hepatitis C: epidemiology and management. Drugs. 2006;66:155–74. doi: 10.2165/00003495-200666020-00003. [DOI] [PubMed] [Google Scholar]
- Lok AS. Prevention of hepatitis B. virus-related hepatocellular carcinoma. Gastroenterology. 2004;127(Suppl 2):S303–S309. doi: 10.1053/j.gastro.2004.09.045. [DOI] [PubMed] [Google Scholar]
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–39. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Cheever LW, Chaisson RE, et al. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. J Acquir Immune Defic Syndrome. 2001;27:251–9. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- Lum PJ, Tulsky JP. The medical management of opioid dependence in HIV primary care settings. Curr HIV/AIDS Rep. 2006;3:195–204. doi: 10.1007/s11904-006-0016-z. [DOI] [PubMed] [Google Scholar]
- Magura S, Lee SJ, Salsitz EA, et al. Outcomes of buprenorphine maintenance in office-based practice. J Addict Dis. 2007;26:13–23. doi: 10.1300/J069v26n02_03. [DOI] [PubMed] [Google Scholar]
- Maher L, Jalaludin B, Chant KG, et al. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction. 2006;101:1499–508. doi: 10.1111/j.1360-0443.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- Maida I, Soriano V, Barreiro P, et al. Liver fibrosis stage and HCV genotype distribution in HIV-HCV coinfected patients with persistently normal transaminases. AIDS Res Hum Retroviruses. 2007;23:801–4. doi: 10.1089/aid.2006.0085. [DOI] [PubMed] [Google Scholar]
- Mandayam S, Jamal MM, Morgan TR. Epidemiology of alcoholic liver disease. Semin Liver Dis. 2004;24:217–32. doi: 10.1055/s-2004-832936. [DOI] [PubMed] [Google Scholar]
- Mark KE, Murray PJ, Callahan DB, et al. Medical care and alcohol use after testing hepatitis C antibody positive at STD clinic and HIV test site screening programs. Public Health Rep. 2007;122:37–43. doi: 10.1177/003335490712200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez ME. Primary prevention of colorectal cancer: Lifestyle, nutrition, exercise. Recent Results Cancer Res. 2005;166:177–211. doi: 10.1007/3-540-26980-0_13. [DOI] [PubMed] [Google Scholar]
- Mauss S, Berger F, Goelz J, et al. A prospective controlled study of interferon-based therapy of chronic hepatitis C in patients on methadone maintenance. Hepatology. 2004;40:120–4. doi: 10.1002/hep.20279. [DOI] [PubMed] [Google Scholar]
- Mauss S, Rockstroh JK. HCV/HIV coinfection-is there a state of the art after APRICOT and RIBAVIC? J Antimicrob Chemother. 2005;56:615–8. doi: 10.1093/jac/dki277. [DOI] [PubMed] [Google Scholar]
- Maxwell JC, Bohman TM, Spence RT. Differences in characteristics of heroin inhalers and heroin injectors at admission to treatment: A preliminary study using a large database of client records. Subst Use Misuse. 2004;39:993–1012. doi: 10.1081/ja-120030896. [DOI] [PubMed] [Google Scholar]
- Maxwell JC, Pullum TW, Tannert K. Deaths of clients in methadone treatment in Texas: 1994–2002. Drug Alcohol Depend. 2005;78:73–81. doi: 10.1016/j.drugalcdep.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Maxwell S, Shinderman MS, Miner A, et al. Correlation between hepatitis C serostatus and methadone dose requirement in 1,163 methadone-maintained patients. Her Add Rel Clin Prob. 2002;4:5–10. [Google Scholar]
- McCance-Katz EF. Treatment of opioid dependence and coinfection with HIV and hepatitis C virus in opioid-dependent patients: The importance of drug interactions between opioids and antiretroviral agents. Clin Infect Dis. 2005;41:S89–95. doi: 10.1086/429503. [DOI] [PubMed] [Google Scholar]
- McCarthy JJ, Flynn N. Hepatitis C in methadone maintenance patients: Prevalence and public policy implications. J Addictive Dis. 2001;20:19–31. doi: 10.1300/J069v20n01_03. [DOI] [PubMed] [Google Scholar]
- McGovern BH, Wurcel A, Kim AY, et al. Acute hepatitis C virus infection in incarcerated injection drug users. Clin Infect Dis. 2006;42:1663–70. doi: 10.1086/504327. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Bacon BR, Gordon SC, et al. Phase 1B, randomized, double blind, dose-escalation trial of CPG10101 in patients with chronic hepatitis C virus. Hepatology Epub. 2007 doi: 10.1002/hep.21773. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Manns M, Harvey J, et al. Adherence to therapy enhances sustained response in chronic hepatitis C patients receiving PEG-interferon alfa-2b plus ribavirin. J Hepatology. 2001;34(Suppl 1):2–3. [Google Scholar]
- McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–92. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- McLachlan C, Crofts N, Wodak A, et al. The effects of methadone on immune function among injecting drug users: A review. Addiction. 1993;88(2):257–63. doi: 10.1111/j.1360-0443.1993.tb00809.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin P. Viral hepatitis vaccination in an opioid treatment program: Harford, connecticut, 2002–2005. Public Health Rep. 2007;122(suppl 2):48–51. doi: 10.1177/00333549071220S209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurran M. What works in substance misues treatments for offenders? Crim Behav Ment Health. 2007;17:225–33. doi: 10.1002/cbm.662. [DOI] [PubMed] [Google Scholar]
- Mehta SH, Lucas GM, Kirel LB, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006;20:2361–9. doi: 10.1097/QAD.0b013e32801086da. [DOI] [PubMed] [Google Scholar]
- Mercolini L, Mandrioli R, Conti M, et al. Simultaneous determination of methadone, buprenorphine and nor buprenorphine in biological fluids for therapeutic drug monitoring purposes. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;847:95–102. doi: 10.1016/j.jchromb.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Merrill JO, Jackson TR, Schulman BA, et al. Methadone medical maintenance in primary care. An implementation evaluation. J Gen Intern Med. 2005;20:344–9. doi: 10.1111/j.1525-1497.2005.04028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: A systematic review of longitudinal studies. J Viral Hepatitis. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- Millson PE, Challacombe L, Villeneuve PJ, et al. Self-perceived health among Canadian opiate users: A comparison to the general population and to other chronic disease populations. Can J Public Health. 2004;95:99–103. doi: 10.1007/BF03405775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miro JM, Montejo M, Rufi G, et al. Liver transplantation in patients with HIV infection: A reality in 2004. Enferm Infec Microbiol Clin. 2004;22:529–38. doi: 10.1016/s0213-005x(04)73155-9. [DOI] [PubMed] [Google Scholar]
- Miro JM, Torre-Cisneros J, Moreno A, et al. Consensus document from GESIDA/GESITRA-SEIMS, SPNS, and ONT on solid organ transplantation for patients with HIV infection in Spain(March 2005) Enferm Infec Microbial Clin. 2005;23:353–62. doi: 10.1157/13076175. [DOI] [PubMed] [Google Scholar]
- Mitty J, Macleod C, Loewenthal H, et al. An integrated program of buprenorphine in the primary care setting for HIV(+) persons in Rhode Island. Med Health RI. 2007;90:145–7. [PubMed] [Google Scholar]
- Mizuno Y, Purcell D, Borkowski TM, et al. The life priorities of HIV-seropositive injection drug users: Findings from a community-based sample. AIDS Behav. 2003;7:395–403. doi: 10.1023/b:aibe.0000004731.94734.77. [DOI] [PubMed] [Google Scholar]
- Modi AA, Wright EC, Seeff LB. Complementary and alternative medicine (CAM) for the treatment of chronic hepatitis B. and C: a review. Antivir Ther. 2007;12:285–95. [PubMed] [Google Scholar]
- Moirand R, Bilodeau M, Brissette S, et al. Determinants of antiviral treatment initiation in a hepatitis C-infected population benefiting from universal health care coverage. Can J Gastroenterol. 2007;21:355–61. doi: 10.1155/2007/576765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar BE, Buka SL, Kessler RC. Child sexual abuse and subsequent psychopathology: results from the National Comorbidity Survey. Am J Public Health. 2001;91:753–60. doi: 10.2105/ajph.91.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montserrat L, Larrouse M, Murillas J, et al. Predictive value of early virolgic response in HIV/Hepatitis C virus coinfected patients treated with an interferon-based regimen plus ribavirin. J Acquir Immun Def Syn. 2007;44:174–78. doi: 10.1097/QAI.0b013e31802b812d. [DOI] [PubMed] [Google Scholar]
- Muir AJ, Provenzale D, Killenberg PG. An evaluation of eligibility for therapy among veterans with hepatitis C. Hepatology. 1999;30(Suppl 1):190A. [Google Scholar]
- Murphy DA, Marelich WD, Hoffman D, et al. Predictors of antiretroviral adherence. AIDS Care. 2004;16:471–84. doi: 10.1080/09540120410001683402. [DOI] [PubMed] [Google Scholar]
- Mutimer D, Feraz-Neto BH, Harrison R, et al. Acute liver graft failure due to emergence of lamivudine resistant hepatitis B. virus: Rapid resolution during treatment with adefovir. Gut. 2001;49:860–3. doi: 10.1136/gut.49.6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JP. HIV and hepatitis C—Another perspective. Infect Med. 2001;18:292–6. [Google Scholar]
- Najavits LM, Rosier M, Nolan AL, et al. A new gender-based model for women's recovery from substance abuse: Results of a pilot outcome study. Am J Drug Alcohol Abuse. 2007;33:5–11. doi: 10.1080/00952990601082597. [DOI] [PubMed] [Google Scholar]
- NCCAM (National Center for Complementary and Alternative Medicine) Hepatitis C: Treatment alternatives. 2000. (Publication Z-04). Available at: http://nccam.nih.gov/fcp/factsheets/
- Neff GW, Bonham A, Tzakis AG, et al. Orthotopic liver transplantation in patients with human immunodeficiency virus and end-stage liver disease. Liver Transpl. 2003;9:239–47. doi: 10.1053/jlts.2003.50054. [DOI] [PubMed] [Google Scholar]
- Neff GW, Sherman KE, Eghtesad B, et al. Review article: Current status of liver transplantation in HIV-infected patients. Aliment Pharmacol Ther. 2004;20:993–1000. doi: 10.1111/j.1365-2036.2004.02232.x. [DOI] [PubMed] [Google Scholar]
- Neff GW, Shire NJ, Rudich SM. Outcomes among patients with end-stage liver disease who are coinfected with HIV and hepatitis C virus. Clin Infect Dis. 2005;41:S50–5. doi: 10.1086/429496. [DOI] [PubMed] [Google Scholar]
- Neri S, Pulvirenti D, Bertino G. Psychiatric symptoms induced by antiviral therapy in chronic hepatitis C: comparison between interferon-alpha 2a and interferon—alpha-2b. Clin Drug Investig. 2006;26:655–62. doi: 10.2165/00044011-200626110-00005. [DOI] [PubMed] [Google Scholar]
- Nguyen OK, Dore GJ, Kaldore JM, et al. Recruitment and follow-up of injection drug users in the setting of early hepatitis C treatment: Insights from the ATAHC study. Int J Drug Policy. 2007;18:447–51. doi: 10.1016/j.drugpo.2007.01.007. [DOI] [PubMed] [Google Scholar]
- NIAAA (National Institute on Alcohol Abuse and Alcoholism) Methadone maintenance and patients in alcoholism treatment. Alcohol Alert. 1988 Aug;:1. [Google Scholar]
- NIDA (National Institute on Drug Abuse) Hepatitis C NIDA Community Drug Alert Bulletin. Washington DC: US Department of Health and Human Services; 2000. (NIH Publication No. 00–4663). [Google Scholar]
- NIDA (National Institute on Drug Abuse) Principles of Drug Addiction Treatment: A Research Based Guide. Washington, DC: US Department of Health and Human Services; 2000a. (NIH Publication No. 00–4180). [Google Scholar]
- NIH (National Institutes of Health) 1997Effective Medical Treatment of Opiate Addiction NIH Consensus Statement Bethesda, MD: National Institutes of Health; November19–191561–38.See also JAMA. 1998; 280: 1936–43 [Google Scholar]
- NIH (National Institutes of Health) Consensus Development Conference Statement: Management of Hepatitis C: Final statement. 2002. Aug 26, 2002. Available at: http://consensus.nih.gov.
- Nishikawa A, Mori Y, Lee IS, et al. Cigarette smoking, metabolic activation and carcinogenesis. Curr Drug Metab. 2004;5:363–73. doi: 10.2174/1389200043335441. [DOI] [PubMed] [Google Scholar]
- Nomura H, Sou S, Tanimoto H, et al. Short-term interferon –alpha therapy for acute hepatitis C: a randomized controlled trial. Hepatology. 2004;39:1213–9. doi: 10.1002/hep.20196. [DOI] [PubMed] [Google Scholar]
- Novick DM, Ochshorn M, Kreek MJ. In vivo and in vitro studies of opiates and cellular immunity in narcotic addicts. In: Friedman H, Specter S, Klein TW, editors. Drugs of Abuse, Immunity, and Immunodeficiency. New York: Plenum Press; 1991. pp. 159–70. [DOI] [PubMed] [Google Scholar]
- Novick DM. The impact of hepatitis C virus infection on methadone maintenance treatment. Mt Sinai J Med. 2000;67:437–43. [PubMed] [Google Scholar]
- Nunez M, Marino A, Mirralles C, et al. Baseline serum hepatitis C virus (HCV) RNA level and response at week 4 are the best predictors of relapse after treatment with pegylated interferon plus ribavirin in HIV/HCV coinfected patients. J Acquir Immune Defic Syndr. 2007;45:439–44. doi: 10.1097/QAI.0b013e318061b5d9. [DOI] [PubMed] [Google Scholar]
- Nunez M, Soriano V. Management of patients co-infected with hepatitis B. virus and HIV. Lancet Infect Dis. 2005;5:374–82. doi: 10.1016/S1473-3099(05)70141-9. [DOI] [PubMed] [Google Scholar]
- O'Connor PG, Selwyn PA, Schottenfeld RS. Medical care for injection-drug users with human immunodeficiency virus infection. N Engl J Med. 1994;331:450–9. doi: 10.1056/NEJM199408183310707. [DOI] [PubMed] [Google Scholar]
- O'Grady J, Taulor C, Brook G. Guidelines for liver transplantation in patients with HIV infection. HIV Medicine. 2005;6(suppl 2):159–53. doi: 10.1111/j.1468-1293.2005.00303.x. [DOI] [PubMed] [Google Scholar]
- Ompad DC, Ikeda RM, Shah N, et al. Childhood sexual abuse and age at initiation of injection drug use. Am J Public Health. 2005;95:703–9. doi: 10.2105/AJPH.2003.019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONDCP (Office of National Drug Control Policy) Drug Abuse in America [slide presentation] Washington, DC: Office of National Drug Control Policy; 2002. Available at: http://www.whitehousedrugpolicy.gov. [Google Scholar]
- Osborn MK, Guest JL, Rimland D. Hepatitis B. virus and HIV coinfection: relationship of different serological patterns to survival and liver disease. HIV Medicine. 2007;8:271–79. doi: 10.1111/j.1468-1293.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- O'Toole TP, Polloni R, Gray P. Factors identifying high-frequency and low-frequency health service utilization among substance-using adults. J Subst Abuse Treat. 2007;33:51–9. doi: 10.1016/j.jsat.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Orru MG, Baita A, et al. Treatment with interferon-alpha in patients with chronic hepatitis and mood or anxiety disorders. Lancet. 1999;354(9173):131–2. doi: 10.1016/S0140-6736(98)04793-X. [DOI] [PubMed] [Google Scholar]
- Passey M, Sheldrake M, Leitch K, et al. Impact of case management on rural women's quality of life and substance use. Rural Remote Health. 2007;7:710–25. [PubMed] [Google Scholar]
- Payte JT, Zweben JE. Opioid maintenance therapies. In: Graham AW, Schultz TK, editors. Principles of Addiction Medicine. 2nd ed. Chevy Chase, MD: American Society of Addiction Medicine, Inc; 1998. pp. 557–70. [Google Scholar]
- PEG-Intron™ [peginterferon alfa-2b package insert] Kenilworth, NJ: Schering Corp; 2001. [Google Scholar]
- Peng WK, Sheikh Z, Paterson-Brown S, et al. Role of liver function tests in predicting common bile duct stones in acute calculous cholecystitis. Br J Surg. 2005;92:1241–7. doi: 10.1002/bjs.4955. [DOI] [PubMed] [Google Scholar]
- Pestka JM, Zeisel MB, Blaser E, et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad SciUSA. 2007;104:6025–30. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M. Managing hepatitis B. coinfection in HIV-infected patients. Curr HIV/AIDS Report. 2005;2:122–6. doi: 10.1007/s11904-005-0004-8. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Piasecki D. Elevated liver enzymes levels in opioid-dependent patients with hepatitis treated with buprenorphine. Am J Addict. 2000;9:265–9. doi: 10.1080/10550490050148099. [DOI] [PubMed] [Google Scholar]
- Phillips E, Gutierrez S, Jahnke N, et al. Determinants of nevirap-ine hypersensitivity and its effects on the association between hepatitis C status and mortality in antiretroviral drug-naïve HIV-postive patients. AIDS. 2007;21:1561–8. doi: 10.1097/QAD.0b013e3282170a9d. [DOI] [PubMed] [Google Scholar]
- Piasecki BA, Lewis JD, Reddy KR, et al. Influence of alcohol use, race, and viral coinfections on spontaneous HCV clearance in a US veteran population. Hepatology. 2004;40:892–899. doi: 10.1002/hep.20384. [DOI] [PubMed] [Google Scholar]
- Pineda JA, Romero-Gomez M, Diaz-Garcia F, et al. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005;41:779–89. doi: 10.1002/hep.20626. [DOI] [PubMed] [Google Scholar]
- Polyak SJ, Morishima C, Shuhart MC, et al. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized silymarin Gastroenterology. 2007;132:1925–36. doi: 10.1053/j.gastro.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Poschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol. 2004;39:155–165. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- Poschl G, Stickel F, Wang XD, et al. Alcohol and cancer : Genetic and nutritional aspects. Proc Nutr Soc. 2004;63:65–71. doi: 10.1079/PNS2003323. [DOI] [PubMed] [Google Scholar]
- Post JJ, Pan Y, Freeman AJ, et al. Clearance of hepatitis C viremia associated with cellular immunity in the absence of seroconversion in the hepatitis C incidence and transmission in prisons study cohort. J Infect Dis. 2004;189:1846–55. doi: 10.1086/383279. [DOI] [PubMed] [Google Scholar]
- Poynard T, Imbert-Bismut F, Munteanu M, et al. FibroTest-FibroSURE: towards a universal biomarker of liver fibrosis? Exp Rev Molec Diag. 2005;5:15–21. doi: 10.1586/14737159.5.1.15. [DOI] [PubMed] [Google Scholar]
- Puigdollers E, Domingo-Salvany A, Brugal MT, et al. Characteristics of heroin addicts entering methadone maintenance treatment: Quality of life and gender. Subst Use Misuse. 2004;39:1353–1368. doi: 10.1081/ja-120039392. [DOI] [PubMed] [Google Scholar]
- Pybus OG, Cochrane A, Holmes EC, et al. The hepatitis C virus epidemic among injecting drug users. Infect Genetics Evolution. 2005;5:131–139. doi: 10.1016/j.meegid.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Quaglio G, Talamini G, Lugoboni F, et al. Compliance with hepatitis B. vaccination in 1175 heroin users and risk factors associated with lack of vaccine response. Addiction. 2002;97:985–92. doi: 10.1046/j.1360-0443.2002.00147.x. [DOI] [PubMed] [Google Scholar]
- Qureshi NA, al-Ghamdy YS, al-Habeeb TA. Drug addiction: a general review of new concepts and future challenges. East Mediterr Health J. 2000;7:723–33. [PubMed] [Google Scholar]
- Raj V. Treatment of hepatitis B. Clin Cornerstone. 2001;3:24–36. doi: 10.1016/s1098-3597(01)90076-6. [DOI] [PubMed] [Google Scholar]
- Rauch A, Egger M, Reichen J, et al. Chronic hepatitis C in HIV-infected patients: low eligibility and applicability of therapy with pegylated interferon-alpha plus ribavirin. Acquir Immune Defic Syndr. 2005;38:238–40. doi: 10.1097/01.qai.0000148535.97081.72. [DOI] [PubMed] [Google Scholar]
- Rebetron [ribavirin + peginterferon alfa-2b package insert] Kenilworth, NJ: Schering Corp; [Google Scholar]
- Reesink HW, Zeuzem S, Weegink CJ, et al. Rapid decline of viral RNA in hepatitis with VX-950: A phase 1b, placebo-controlled, randomized study. Gastroenterology. 2006;131:997–1002. doi: 10.1053/j.gastro.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Reimer J, Lorenzen J, Baetz B, et al. Multiple viral hepatitis in injection drug users and associated risk factors. J Gastroenterol Hepatol. 2007;22:80–5. doi: 10.1111/j.1440-1746.2006.04358.x. [DOI] [PubMed] [Google Scholar]
- Reynolds GL, Fisher DG, Jaffe A, et al. Follow-up for medical care among drug users with hepatitis C. Eval Health Prof. 2006;29:355–66. doi: 10.1177/0163278706296003. [DOI] [PubMed] [Google Scholar]
- Rich JD, McKenzie M, Shield DC, et al. Linkage with methadone treatment upon release from incarceration: A promising opportunity. J Addict Dis. 2005;24:49–59. doi: 10.1300/J069v24n03_04. [DOI] [PubMed] [Google Scholar]
- Robaeys G, Van Vlierberghe H, Mathei C, et al. Similar compliance and effect of treatment in chronic hepatitis C resulting from intravenous drug use in comparison to other infectious causes. Eur J Gastroentrol Hepatol. 2006;18:159–66. doi: 10.1097/00042737-200602000-00008. [DOI] [PubMed] [Google Scholar]
- Robertson R, editor. Management of drug users in the community: A practical handbook. London: Arnold; 1998. [Google Scholar]
- Roland ME. Solid-organ transplantation in HIV-infected patients in the potent antiretroviral therapy era. Top HIV Med. 2004;12:73–6. [PubMed] [Google Scholar]
- Rowan-Szal GA, Chatham LR, Joe GW, et al. Services provided during methadone treatment A gender comparison. J Subst Abuse Treat. 2000;19:7–14. doi: 10.1016/s0740-5472(99)00091-4. [DOI] [PubMed] [Google Scholar]
- Ruiz-Sancho A, Sheldon J, Soriano V. Telbivudine: a new option for the treatment of chronic hepatitis B. Expert Opin Biol Ther. 2007;7:751–61. doi: 10.1517/14712598.7.5.751. [DOI] [PubMed] [Google Scholar]
- Ruys TA, Naniohy NM, Van den Berg, et al. HCV-specific T cell responses in injection drug users: evidence for previous exposure to HCV and a role for CD4+ T cells focusing on nonstructural proteins in viral clearance. J Viral Hepat epub. 2008 doi: 10.1111/j.1365-2893.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- Ryan LA, Brester M, Bohac D, et al. Up-regulation of soluble tumor necrosis factor receptor two in plasma of HIV-seropositive individuals who use opiates. AIDS Res Hum Retrovirus. 2004;20:41–5. doi: 10.1089/088922204322749486. [DOI] [PubMed] [Google Scholar]
- Ryder SD, Beckingham IJ. Chronic viral hepatitis. Br Med J. 2001;322:219–21. doi: 10.1136/bmj.322.7280.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, et al. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28:572–7. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- SAMHSA (Substance Abuse and Mental Health Services Administration) The National Survey on Drug Use and Health (NSDUH): National Findings. 2002. Available at http://oas.samhsa.gov/nhsda/2k2nsduh/Results/2k2Results.htm#chap2. [PubMed]
- SAMHSA (Substance Abuse and Mental Health Services Administration) Treatment Episode Data Set (TEDS): 1992–2000. Rockville, MD: Office of Applied Studies, SAMHSA; 2002a. (National Admissions to Substance Abuse Treatment Services, DAIS Series S-17. DHHS Publication No. (SMA) 02–3727). [Google Scholar]
- SAMHSA (Substance Abuse and Mental Health Services Administration) Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. A Treatment Improvement Protocol TIP 40. 2004. Available at: http://www.kap.samhsa.gov/products/manuals/index.htm. [PubMed]
- SAMHSA (Substance Abuse and Mental Health Services Administration) Methadone-Associated Mortality: Report of a National Assessment. 2004a. (CSAT Publication No. 28–03). Available at: http://www.dpt.samhsa.gov/reports/index.htm. [Google Scholar]
- SAMHSA (Substance Abuse and Mental Health Services Administration) Treatment Improvement Protocol (TIP) 43. Rockville Maryland: 2005. Medication Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. (DHHS Publication No. 05–4048). http://www.dpt.samhsa.gov/reports/index.htm. [PubMed] [Google Scholar]
- SAMHSA (Substance Abuse and Mental Health Services Administration) Treatment Improvement Protocol (TIP) 42. Rockville Maryland: 2005a. Substance abuse treatment for persons with co-occurring disorders. (DHHS Publication No. 05–3993). http://www.dpt.samhsa.gov/reports/index.htm. [PubMed] [Google Scholar]
- SAMHSA (Substance Abuse and Mental Health Services Administration) Treatment Improvement Protocol (TIP) 48. Rockville Maryland: 2007. Incorporating Alcohol Pharmacotherapies into Medical Practice. (DHHS Publication 07–1993). http://www.dpt.samhsa.gov/reports/index.htm. [Google Scholar]
- Santantonio T. Treatment of acute hepatitis C. Curr Pharm Res. 2004;10:2077–80. doi: 10.2174/1381612043384222. [DOI] [PubMed] [Google Scholar]
- Sarrazin C, Kieffer TL, Bartels D, et al. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology. 2007;132:1767–77. doi: 10.1053/j.gastro.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Saxena AK, Panhotra BR, Sundaram DS, et al. Impact of dedicated space, dialysis equipment, and nursing staff on the transmission of hepatitis C virus in a hemodialysis unit in the Middle East. Am J Infect Control. 2003;31:26–33. doi: 10.1067/mic.2003.55. [DOI] [PubMed] [Google Scholar]
- Schackman BR, Merill JO, McCarty D, et al. Overcoming policy and financing barriers to integrated buprenorphine and HIV primary care. Clin Infect Dis. 2006;43:S247–53. doi: 10.1086/508190. [DOI] [PubMed] [Google Scholar]
- Schackman BR, Teixeira PA, Beeder AB. Offers of hepatitis C care do not lead to treatment. J Urban Health. 2007;84:455–8. doi: 10.1007/s11524-007-9180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Heinz A, Backmund M. Treatment of chronic hepatitis C in patients with drug dependence: Time to change the rules? Addiction. 2004;99:1167–1175. doi: 10.1111/j.1360-0443.2004.00821.x. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Hinzpeter A, Mohmand A, et al. Hepatitis C treatment in “difficult-to-treat” psychiatric patients with pegylated interferon alpha and ribavirin: response and psychiatric side effects. Hepatology. 2007;46:991–8. doi: 10.1002/hep.21791. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Schmidt F, Folwaczny C, et al. Adherence and mental side effects during hepatitis C treatment with interferon alpha and ribavirin in psychiatric risk groups. Hepatology. 2003;37:443–51. doi: 10.1053/jhep.2003.50031. [DOI] [PubMed] [Google Scholar]
- Schafer M. ychiatric patients, methadone patients, and earlier drug users can be treated for HCV when given adequate support services; Presentation at Digestive Disease Week; May 20–23; Atlanta, GA. 2001. [Google Scholar]
- Schiff ER. Hepatitis C and alcohol. Hepatology. 1997;26(Suppl 1):39S–42S. doi: 10.1002/hep.510260707. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Walsh SL, Bigelow GE, et al. Buprenorphine, morphine, and naloxone effects during ascending morphine maintenance in humans. J Pharmacol Exp Ther. 1996;278:836–46. [PubMed] [Google Scholar]
- Scott LD. Treatment of hepatitis C in injection drug users. Am J Gastroentrol. 2005;100:5–7. doi: 10.1111/j.1572-0241.2005.41294.x. [DOI] [PubMed] [Google Scholar]
- Seeff LB, Lindsay KL, Bacon BR, et al. Complementary and alternative medicine in chronic liver disease. Hepatology. 2001;34:595–603. doi: 10.1053/jhep.2001.27445. [DOI] [PubMed] [Google Scholar]
- Semba RD, Ricketts EP, Mehta SF, et al. Adherence and retention of female injection drug users in a phase III clinical trial in inner city Baltimore. Am J Drug Alcohol Abuse. 2007;33:71–80. doi: 10.1080/00952990601082696. [DOI] [PubMed] [Google Scholar]
- Senbanjo R, Wolff K, Marshall J. Excessive alcohol consumption is associated with reduced quality of life among methadone patients. Addiction. 2007;102:257–63. doi: 10.1111/j.1360-0443.2006.01683.x. [DOI] [PubMed] [Google Scholar]
- Sergio N, Salvatore T, Gaetano B, et al. Immune. response in addicts with chronic hepatitis C treated with interferon and ribavirin. J Gastroenterol Hepatol. 2007;22:74–9. doi: 10.1111/j.1440-1746.2006.04423.x. [DOI] [PubMed] [Google Scholar]
- Shad JA, McHutchinson JG. Current and future therapies of hepatitis C. Clin Liver Dis. 2001;5:335–359. doi: 10.1016/s1089-3261(05)70169-0. [DOI] [PubMed] [Google Scholar]
- Shah N, Lathrop SL, Landen MG. Unintentional methadone-related overdose deaths in New Mexico (USA) and implications for surveillance, 1998–2002. Addiction. 2005;100:176–88. doi: 10.1111/j.1360-0443.2004.00956.x. [DOI] [PubMed] [Google Scholar]
- Sharp BM. Opioid receptor expression and intracellular signaling by cells involved in host deference and immunity. Adv Exp Med Biol. 2003;521:98–105. [PubMed] [Google Scholar]
- Shehab TM, Fontana RJ, Oberhelman K, et al. Effectiveness of interferon alpha 2b and ribavirin combination therapy in the treatment of naive chronic hepatitis C patients in clinical practice. Clin Gastroenterol Hepatol. 2004;2:425–31. doi: 10.1016/s1542-3565(04)00018-7. [DOI] [PubMed] [Google Scholar]
- Sheng WH, Chen MY, Hseih SM, et al. Impact of chronic heaptitis B. virus (HBV) infection on outcomes of patients infected with HIV in an area where HBV is hyperendemic. Clin Infect Dis. 2004;38:1471–77. doi: 10.1086/420744. [DOI] [PubMed] [Google Scholar]
- Sheng WH, Hung CC, Kao JH, et al. Impact of hepatitis D virus infection on the long term outcomes of patients with hepatitis B. virus and HIV coinfection in the era of highly active antiretroviral therapy: a matched cohort study. Clin Infect Dis. 2007;44:988–95. doi: 10.1086/511867. [DOI] [PubMed] [Google Scholar]
- Sherman M, the CASL Hepatitis Consensus Group Management of viral hepatitis: Clinical and public health perspectives—a consensus statement. Can J Gastroenterol. 1997;11:407–16. doi: 10.1155/1997/454278. [DOI] [PubMed] [Google Scholar]
- Shine D. Drug abuse. In: Noble J, editor. Textbook of primary care medicine. St. Louis, MO: Mosby; 1996. pp. 1709–32. [Google Scholar]
- Shire NJ, Sherman KE. Management of HBV/HIV coinfected patients. Sem Liver Dis. 2005;25:48–57. doi: 10.1055/s-2005-915646. [DOI] [PubMed] [Google Scholar]
- Sigal S, Jacobson I. Future therapies for hepatitis C: where do we go from here? Nature Clin Pract Gastroenterol Hepatol. 2007;4:60–1. doi: 10.1038/ncpgasthep0736. [DOI] [PubMed] [Google Scholar]
- Sorensen JL, Masson CL, Delucchi K, et al. Ramdonized trial of drug abuse treatment—linkage strategies. J Consult Clin Psychol. 2005;73:1026–35. doi: 10.1037/0022-006X.73.6.1026. [DOI] [PubMed] [Google Scholar]
- Soriano V, Miralles C, Berdun MA, et al. Premature treatment discontinuation in HIV/HCV coinfected patients receiving pegylated interferon plus weight-based ribavirin. Antivir Ther. 2007a;12:469–76. [PubMed] [Google Scholar]
- Soriano V, Miro JM, Garcia-Samaniego J, et al. Consensus conference on chronic viral hepatitis and HIV infection: Updated Spanish recommendations. J Viral Hepatol. 2004;11:2–17. doi: 10.1046/j.1365-2893.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- Soriano V, Nunez M, Camino N, et al. Hepatitis C virus-RNA clearance in HIV-coinfected patients with chronic hepatitis C treated with pegylated interferon plus ribavirin. Antivir Ther. 2004a;9:505–9. [PubMed] [Google Scholar]
- Soriano V, Puoti M, Bonacini M, et al. Care of patients with chronic hepatitis B. and HIV co-infection: recommendations from an HIV-HBV international panel. AIDS. 2005;19:221–40. [PubMed] [Google Scholar]
- Soriano V, Puoti M, Sulkowski M, et al. Care of patients coinfected with HIV and hepatitis C virus : 2007 updated recommendations from the HCV-HIV international panel. AIDS. 2007;21:1073–89. doi: 10.1097/QAD.0b013e3281084e4d. [DOI] [PubMed] [Google Scholar]
- Sounder GJ, Regez RM, Brinkman K, et al. Prophylaxis and follow-up after possible exposure to HIV, hepatitis B virus and hepatitis C virus outside hospital: Evaluation of policy 2000–3. Brit Med J. 2005;330:825–9. doi: 10.1136/bmj.330.7495.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoth R, Clair S, Greenberg M, et al. Toward dissemination of evidence-based family interventions: Maintenance of community-based partnership recruitment results and associated factors. J Fam Psychol. 2007;21:137–46. doi: 10.1037/0893-3200.21.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Maksad J, Clarke J. Hepatitis C disease among injection drug users: Knowledge, perceived risk and willingness to receive treatment. Drug Alcohol Dep. 2001;61:211–5. doi: 10.1016/s0376-8716(00)00144-7. [DOI] [PubMed] [Google Scholar]
- Stein MD, Solomon DA, Herman DS. Depression Severity and Drug Injection HIV Risk Behaviors. Am J Psych. 2003;160:1659–62. doi: 10.1176/appi.ajp.160.9.1659. [DOI] [PubMed] [Google Scholar]
- Stein MD, Weinstock MC, Herman D, et al. A smoking cessation intervention for the methadone-maintained. Addiction. 2006;101:5999–607. doi: 10.1111/j.1360-0443.2006.01406.x. [DOI] [PubMed] [Google Scholar]
- Stene-Johansen K, Tjon G, Schreier E, et al. Molecular epidemiological studies show that hepatitis A virus is endemic among active homosexual men in Europe. J Med Virol. 2007;79:356–65. doi: 10.1002/jmv.20781. [DOI] [PubMed] [Google Scholar]
- Stephenson J. Former addicts face barriers to treatment for HCV. JAMA. 2001;285:1003–5. doi: 10.1001/jama.285.8.1003. [DOI] [PubMed] [Google Scholar]
- Sterling RK. The role of liver biopsy in the evaluation of hepatitis C virus in human immunodeficiency virus coinfection. Clin Inf Dis. 2005;40(Suppl 5):S270–5. doi: 10.1086/427439. [DOI] [PubMed] [Google Scholar]
- Storr CL, Westergaard R, Anthony JC. Early onset inhalant use and risk for opiate initiation by young adulthood. Drug Alcohol Depend. 2005;78:253–61. doi: 10.1016/j.drugalcdep.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Strader DB, Wright T, Thomas DL, et al. Diagnosis, management and treatment of hepatitis C AASLD Practice Guideline. Hepatology. 2004;39:1147–71. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- Straley SD, Terrault NA. Chronic hepatitis B. Curr Treat Options Gastroenterol. 2004;7:477–89. doi: 10.1007/s11938-004-0007-3. [DOI] [PubMed] [Google Scholar]
- Strauss SM, Astone JM, Des Jarlais DC, et al. Integrating hepatitis C services into existing HIV services: the experience of a sample of US drug treatment units. AIDS Patient Care STD. 2005;19:78–88. doi: 10.1089/apc.2005.19.78. [DOI] [PubMed] [Google Scholar]
- Strauss SM, Astone JM, Hagan H, et al. The content and comprehensiveness of hepatitis C education in methadone maintenance and drug free treatment units. J Urban Health. 2004;81:38–47. doi: 10.1093/jurban/jth086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss SM, Astone-Twerall J, Munoz-Plaza CE, et al. Drug treatment program patients hepatitis C virus (HCV) education needs and their use of available HCV education services. BMC Health Serv Res. 2007;7:39–49. doi: 10.1186/1472-6963-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss SM, Astone J, Vassilev ZP, et al. Gaps in the drug-free and methadone maintenance treatment program response to hepatitis C. J Subst Abuse Treat. 2003;24:291–97. doi: 10.1016/s0740-5472(03)00037-0. [DOI] [PubMed] [Google Scholar]
- Sulkowski M, Mehta S, Torbenson M, et al. Rapid fibrosis progression among HIV/hepatitis C virus co-infected adults. AIDS. 2007;21:2209–16. doi: 10.1097/QAD.0b013e3282f10de9. [DOI] [PubMed] [Google Scholar]
- Sulkowski MS, Moore RD, Mehta SH, et al. Hepatitis C and progression of HIV disease. JAMA. 2002;288:199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- Sulkowski MS, Wright T, Rossi S, et al. Peginterferon alfa-2a does not alter the pharmacokinetics of methadone in patients with chronic hepatitis C undergoing methadone maintenance therapy. Clin Pharmacol Ther. 2005;77:214–24. doi: 10.1016/j.clpt.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Moore BA, Chawarski MC, et al. Buprenorphine/naloxone treatment in primary care is associated with decreased human immunodeficiency virus risk behaviors. J Subst Abuse Treat epub. 2007 doi: 10.1016/j.jsat.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan T. Care and treatment for hepatitis C and HIV coinfection. Publication of the US Department of Health and Human Services, Health Resources and Services Administration, HIV/AIDS Bureau; 2006. available at : http://hab.hrsa.gov/tools/coinfection/index.html. [Google Scholar]
- Sweeney LP, Samet JH, Larson MJ, et al. Establishment of a multidisciplinary health evaluation and linkage to primary care (HELP) clinic in a detoxification unit. J Addict Dis. 2004;23:33–45. doi: 10.1300/J069v23n02_03. [DOI] [PubMed] [Google Scholar]
- Sylvestre DL. Treating hepatitis C in methadone maintenance patients: An interval analysis. Drug Alcohol Dep. 2002;67:117–23. doi: 10.1016/s0376-8716(02)00010-8. [DOI] [PubMed] [Google Scholar]
- Sylvestre DL, Clements BJ. Adherence to hepatitis C treatment in recovering heroin users maintained on methadone. Eur J Gastroenterol Hepatol. 2007;19:741–7. doi: 10.1097/MEG.0b013e3281bcb8d8. [DOI] [PubMed] [Google Scholar]
- Sylvestre DL, Litwin AH, Clements BJ, et al. The impact of barriers to HCV treatment in recovering heroin users maintained on methadone. J Sub Abuse Treat. 2005;29:159–65. doi: 10.1016/j.jsat.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Sylvestre DL, Loftis JM, Hauser P, et al. Co-occurring hepatitis C, substance use and psychiatric illness: Treatment issues and developing integrated models of care. J Urban Health. 2004;81:719–34. doi: 10.1093/jurban/jth153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvestre DL, Zweben JE. Integrating HCV services for drug users: A model to improve engagement and outcomes. Int J Drug Policy. 2007;18:406–10. doi: 10.1016/j.drugpo.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Tamayo C, Diamond S. Review of clinical trials evaluating safety and efficacy of milk thistle (silybum marianum [L] Gaetn) Integr Cancer Ther. 2007;6:146–57. doi: 10.1177/1534735407301942. [DOI] [PubMed] [Google Scholar]
- Taylor L. Delivering care to injection drug users coinfected with HIV and hepatitis C virus. Clin Infect Dis. 2005;40:S255–61. doi: 10.1086/427453. [DOI] [PubMed] [Google Scholar]
- Tedaldi EM, Baker RK, Moorman AC, et al. Hepatitis A and B. vaccination practices for ambulatory patients infected with HIV. Clin Infect Dis. 2004;38:1478–84. doi: 10.1086/420740. [DOI] [PubMed] [Google Scholar]
- Tennant F. Hepatitis C, B, D, and A: Contrasting features and liver function abnormalities in heroin addicts. J Addict Dis. 2001;20:9–17. doi: 10.1300/J069v20n01_02. [DOI] [PubMed] [Google Scholar]
- Teplin D, Raz B, Daiter J, et al. Screening for alcohol use patterns among methadone maintenance patients. Am J Drug Alcohol Abuse. 2007;33:179–33. doi: 10.1080/00952990601091184. [DOI] [PubMed] [Google Scholar]
- Thio CL. Hepatitis B. in the human immunodeficiency virus-infected patient: epidemiology, natural history, and treatment. Semin Liver Dis. 2003;23:125–36. doi: 10.1055/s-2003-39951. [DOI] [PubMed] [Google Scholar]
- Thio CL, Seaberg EC, Solasky R, Jr, et al. HIV-1, hepatitis B. virus, and risk of liver related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- Tokita H, Fukui H, Tanaka A, et al. Risk factors for the development of hepatocellular carcinoma among patients with chronic hepatitis C who achieved sustained virolgical response to interferon therapy. J Gastroenterol Hepatol. 2005;20:752–8. doi: 10.1111/j.1440-1746.2005.03800.x. [DOI] [PubMed] [Google Scholar]
- Torriani FJ, Rodriguez-Torres M, Rockstroh J, et al. Peginterferon -α-2a + ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- Trooskin SB, Navarro VJ, Winn RJ, et al. Hepatitis C risk assessment, testing and referral for treatment in urban primary care: Role of race and ethnicity. World J Gastroenterol. 2007;13:1074–78. doi: 10.3748/wjg.v13.i7.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trullas JC, Miro JM, Barril G, et al. Renal transplantation in patients with HIV infection. Enferm Infec Microbiol Clin. 2005;23:363–74. doi: 10.1157/13076177. [DOI] [PubMed] [Google Scholar]
- Tucker T, Fry CL, Lintzeris N, et al. Randomized controlled trial of a brief behavioral intervention for reducing hepatitis C virus risk practices among injection drug users. Addiction. 2004;99:1157–66. doi: 10.1111/j.1360-0443.2004.00809.x. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Laine C, Lin Y-t, et al. Barriers and facilitators to primary care or human immunodeficiency virus clinics providing methadone or buprenorphine for the management of opioid dependence. Arch Intern Med. 2007;165:1769–76. doi: 10.1001/archinte.165.15.1769. [DOI] [PubMed] [Google Scholar]
- van Beek I. Case study: Accessible primary health care-a foundation to improve health outcomes for people who inject drugs. Int J Drug Policy. 2007;18:329–32. doi: 10.1016/j.drugpo.2006.11.005. [DOI] [PubMed] [Google Scholar]
- van de Laar TJW, van der Bij AK, Prins M, et al. Increase in HCV incidence among men who have sex with men in Amsterdam most likely caused by sexual transmission. J Infect Dis. 2007;196:230–8. doi: 10.1086/518796. [DOI] [PubMed] [Google Scholar]
- Van Thiel DH, Anantharaju A, Creech S. Response to treatment of hepatitis C in individuals with a recent history of intravenous drug abuse. Am J Gastroent. 2003;98:2281–8. doi: 10.1111/j.1572-0241.2003.07702.x. [DOI] [PubMed] [Google Scholar]
- Vassilev ZP, Strauss SM, Astone J, et al. Injection drug users and the provision of hepatitis C-related services in a nationwide sample of drug treatment programs. J Behavior Health Serv Res. 2004;31:208–16. doi: 10.1007/BF02287383. [DOI] [PubMed] [Google Scholar]
- Verrando R, Robaeys G, Mathei C. Methadone and buprenorphine maintenance therapies for patients with hepatitis C virus infected after intravenous drug use. Acta Gastroenterol Belg. 2005;68:81–5. [PubMed] [Google Scholar]
- Villano SA, Vlahov D, Nelson KE, et al. Persistence of viremia and the importance of long-term follow-up after acute hepatitis C infection. Hepatology. 1999;29:908–14. doi: 10.1002/hep.510290311. [DOI] [PubMed] [Google Scholar]
- Vocci F, Ling W. Medications development: Successes and challenges. Pharmacol Ther. 2005;108:94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Wagner GJ, Ryan GW. Relationship between routinization of daily behaviors and medication adherence in HIV-positive drug users. AIDS Patient Care STDS. 2004;18:385–93. doi: 10.1089/1087291041518238. [DOI] [PubMed] [Google Scholar]
- Walley AY, White MC, Kushel MB, et al. Knowledge of and interest in hepatitis C treatment at a methadone clinic. J Sub Abuse Treat. 2005;28:181–7. doi: 10.1016/j.jsat.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Walsh KM, Woodall T, Lamy P, et al. Successful treatment with adefovir dipivoxil in a patient with fibrosing cholestatic hepatitis and lamivudine resistant hepatitis B. virus. Gut. 2001;49:436–40. doi: 10.1136/gut.49.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–76. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Watson B, Conigrave KM, Wallace C, et al. Hazardous alcohol consumption and other barriers to antiviral treatment among hepatitis C positive people receiving opioid maintenance treatment. Drug Alcohol Rev. 2007;26:231–9. doi: 10.1080/09595230701247681. [DOI] [PubMed] [Google Scholar]
- Weinrieb RM, Barnett R, Lynch KG, et al. A matched comparison study of medical and psychiatric complications and anesthesia and analgesia requirements in methadone-maintained liver transplant recipients. Liver Transplant. 2004;10:97–106. doi: 10.1002/lt.20003. [DOI] [PubMed] [Google Scholar]
- Weinrieb RM, Van Horn DH, McLellan AT, et al. Interpreting the significance of drinking by alcohol-dependent liver transplant patients: Fostering candor is the key to recovery. Liver Transpl. 2000;6:769–76. doi: 10.1053/jlts.2000.18497. [DOI] [PubMed] [Google Scholar]
- Weiss RD. Adherence to pharmacotherapy in patients with alcohol and opioid dependence. Addiction. 2004;99:1382–92. doi: 10.1111/j.1360-0443.2004.00884.x. [DOI] [PubMed] [Google Scholar]
- Wells R, Fisher D, Fenaughty A, et al. Hepatitis A prevalence among injection drug users. Clin Lab Sci. 2006;19:12–7. [PubMed] [Google Scholar]
- West K. CDC post-exposure guidelines: What providers and management needs to know. Emerg Med Serv. 2001;30:57–63. [PubMed] [Google Scholar]
- Whitley SD, Kunins HV, Arnsten JH, et al. Collocating buprenorphine with methadone maintenance and outpatient chemical dependency services. J Subst Abuse Treat. 2007;33:85–90. doi: 10.1016/j.jsat.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Willenbring ML, Hagedorn HJ, Postier AC, et al. Variations in evidence-based clinical practices in nine United States Veterans Administration opioid agonist therapy clinics. Drug Alcohol Depend. 2004;75:97–106. doi: 10.1016/j.drugalcdep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Wolff K, Rostami-Hodjegan A, Hay AWM, et al. Population-based pharmacokinetic approach for methadone monitoring of opiate addicts: Potential clinical utility. Addiction. 2000;95:1771–83. doi: 10.1046/j.1360-0443.2000.951217717.x. [DOI] [PubMed] [Google Scholar]
- Wong LL, Limm WM, Tsai N, et al. Hepatitis B and alcohol affect the survival of hepatocellular carcinoma patients. World Gastroenterol. 2005;11:3491–7. doi: 10.3748/wjg.v11.i23.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LL, Tsai N, Limm W, et al. Liver transplant for hepatocellular cancer: A treatment for select few. Clin Transplant. 2004;18:205–210. doi: 10.1046/j.1399-0012.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- Yu J, Appel PW, Warren BE, et al. Substance abuse intervention services in public STD clinics: A pilot experience. J Subst Abuse Treat E pub. 2007 doi: 10.1016/j.jsat.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Yuen MF. Revisiting the natural history of chronic hepatitis B: Impact of new concepts on clinical management. J Gastroenterol Hepatol. 2007;22:973–6. doi: 10.1111/j.1440-1746.2007.04938.x. [DOI] [PubMed] [Google Scholar]
- Zaller ND, Thurmond P, Brett J, et al. Linkage to methadone treatment from acute opiate detoxification treatment. J Opioid Manag. 2006;2:341–6. doi: 10.5055/jom.2006.0050. [DOI] [PubMed] [Google Scholar]
- Zajicova A, Wilczek H, Holan V. The alternations of immunological reactivity in heroin addicts and their normalization in patients maintained on methadone. Folia Bio (Praha) 2004;50:24–28. [PubMed] [Google Scholar]
- Zweben JE. Hepatitis C: Education and counseling issues. J Addict Dis. 2001;20:33–42. doi: 10.1300/J069v20n01_04. [DOI] [PubMed] [Google Scholar]