Abstract
Neuregulin-1 (NRG-1) and its signaling receptors, erythroblastic leukemia viral oncogene homologs (ErbB) 2, 3, and 4, have been implicated in both cardiomyocyte development and disease, as well as in homeostatic cardiac function. NRG-1/ErbB signaling is involved in a multitude of cardiac processes ranging from myocardial and cardiac conduction system development to angiogenic support of cardiomyocytes, to cardioprotective effects upon injury. Numerous studies of NRG-1 employ a variety of platforms, including in vitro assays, animal models, and human clinical trials, with equally varying and, sometimes, contradictory outcomes. NRG-1 has the potential to be used as a therapeutic tool in stem cell therapies, tissue engineering applications, and clinical diagnostics and treatment. This review presents a concise summary of the growing body of literature to highlight the temporally persistent significance of NRG-1/ErbB signaling throughout development, homeostasis, and disease in the heart, specifically in cardiomyocytes.
Keywords: neuregulin-1, ErbB receptors, cardiomyocyte, therapeutic, cardiac regeneration, stem cells
Introduction
Neuregulin-1 (NRG-1), a member of the neuregulin growth factor family, has been implicated in a number of cellular processes via paracrine and juxtacrine signaling through receptor tyrosine kinases termed erythroblastic leukemia viral oncogene homologs (ErbBs), of the family of epidermal growth factor receptors.1 The discovery of neuregulins was made in the context of cancer and neural research, unrelated to the cardiovascular system.2–6 However, in the late 1990s, several groups performing studies to disrupt NRG/ErbB signaling discovered its crucial role in cardiac development.7–9 NRG remained for the most part in the realm of neurological research, but in recent years, it resurfaced in the cardiovascular field due to the adverse side effects of chemotherapeutics that target this signaling pathway.10,11
Numerous studies of NRG-1/ErbB signaling in the heart have been carried out since the discovery of its critical roles in both cardiac development and disease. Results have indicated its ubiquitous importance in the heart from the womb to the tomb (Fig. 1); however, the specifics of developmental, homeostatic, and cardioprotective mechanisms are broadly scattered and sometimes contradictory. This review aims to weave together many threads of information that have been gained about NRG/ErbB signaling during different spatial and temporal contexts into a cohesive and coherent time line. This is done to emphasize the potential of NRG-1 as a therapeutic agent throughout this time course: from a biostimulant in stem cell therapy, to a possible biomarker or drug during cardiac disease. Because this review will follow NRG-1 through development, homeostasis, and disease with a focus on cardiomyocyte biology, readers are directed to other reviews for additional details and a broader perspective of NRG/ErbB signaling.12–14
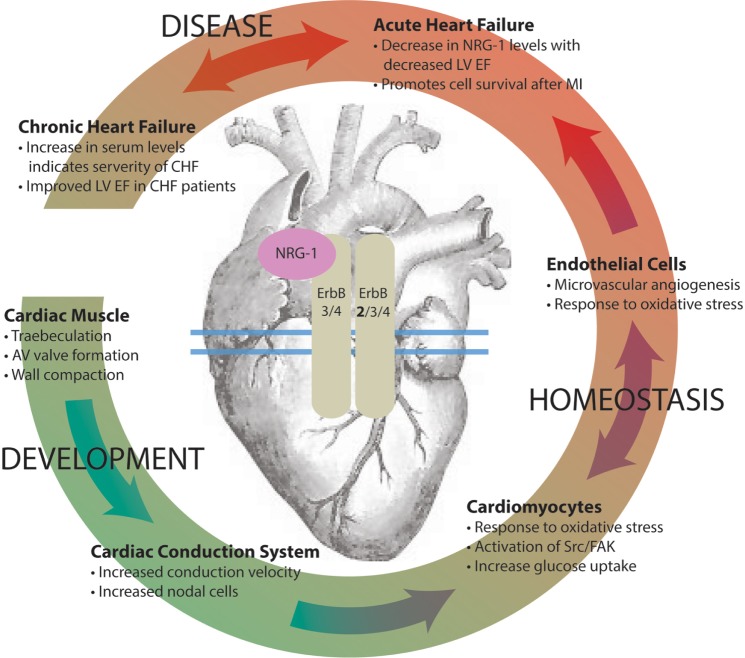
Figure 1.
NRG-1/ErbB signaling has diverse, context-dependent physiological effects in the heart during development, homeostasis, and disease.
Notes: Upon binding of NRG-1 to its ErbB3/4 receptor, dimerization with another ErbB2 (preferred), ErbB3, or ErbB4 receptor initiates a cascade of downstream signals in the heart. Signaling plays an important role in the following: development of the ventricular wall, arteriovenous (AV) valves, and the cardiac conduction system; homeostatic function of the heart via cardiomyocyte adaptability to stress and microvascular support; and the adaptation to disease such as myocardial infarction (MI) in both acute and chronic heart failure (CHF), as reflected by changes in left ventricular ejection fraction (LVEF).
NRG-1 Structure and Signaling
NRG-1 is a member of the neuregulin family of signaling proteins that act on NRG-responsive cells by binding to the extracellular domain of ErbB receptor tyrosine kinases; it was discovered in 1992 during the search for ligands for ErbB2 in mammary tumor cells.1,3 Fifteen isoforms of NRG have been subsequently identified,15 indicative of the host of biological targets of the protein in vivo. Importantly, these isoforms are distinguished by their N-terminal sequence (NRG type I, II, or III), EGF-like domain (α or β, resulting from alternative splicing at the C-terminal, which determines receptor affinity), and synthesis sites (transmembranous or nonmembranous).1 Type I NRG-1 is transmembranous and requires proteolytic cleavage by three transmembrane proteases (meltrin,16 memapsin,17 and tumor necrosis factor-α-converting enzyme18) to be active and secreted, with the EGF-like domain available for ErbB binding.19 This type of neuregulin is endogenous in the heart at high levels and will be the focus of this review. Type II NRG-1 expresses an N-terminal secretory signal peptide and is thus an active ligand upon secretion; it is not present at significant levels in the heart, and type II-specific research is mostly focused on its role in psychological disorders.20,21 Type III NRG-1 is, for the most part, expressed in neuronal cells and will not be discussed further. Henceforth, in this review, NRG-1 will refer to only type I NRG-1.
NRG-1 acts by means of paracrine signaling through ErbB2, 3, and 4.22 NRG-1 binds to the extracellular ligand-binding domain of ErbB3 and ErbB4 (but not ErbB2), which induces a conformational change in ErbB3 and 4 and subsequent dimerization with either ErbB2 or activated ErbB3 or ErbB4. The many splice variants of NRG-1 constitute a source of possible redundancy in ErbB signaling, with the NRG-1α isoform displaying 100-fold weaker binding affinity than NRG-1β.23 Genetic variants in NRG-1 and ErbB4 have been associated with sudden cardiac death and congenital left ventricular (LV) outflow tract defects, respectively.24,25 Upon NRG-1 binding and dimerization, ErbB2, 3, and 4 receptors’ intracellular kinase domains phosphorylate their dimerization partner’s C-terminus. This initiates a host of downstream signaling pathways, leading to, among other phenomena, migration, growth, adhesion, and differentiation in a context-dependent manner. Investigation of these downstream effects has led not only to important discoveries in the cardiovascular realm but also to insights into cancer pathology.26 Moving forward, the spatial context examined will be within the heart, and the temporal context will span development to heart failure.
NRG-1/ErbB Signaling in Development
Cardiac muscle development
The first associations made between NRG-1/ErbB signaling and the heart implicated it in cardiac muscle development in vivo. When ErbB47 or ErbB28 was deleted in mice, a significant deficit in ventricular trabeculation and endocardial cushion formation (from which the atrioventricular valves develop) was observed, and mice died during midembryogenesis. Similar results were seen upon deletion of NRG-1 in mice: these mice also died during embryogenesis.9 A less severe phenotype was observed in zebrafish, where erbb2−/− cardiomyocytes exhibited irregular myofibril organization, deviating from the spatiotemporal organization of wild-type cardiomyocytes.27 Significant trabeculation did not occur in erbb2−/− zebrafish, and by 4.5 days post fertilization, myofibrils were more dense at the base and less dense at the apex of the heart versus wild-type controls, accompanied by suppressed whole heart function as measured by fractional shortening (%FS). Although mutant zebrafish survived through early stages of development, defects resulting from ErbB2 removal caused early death. Further into cardiac development, NRG-1, synergistically with insulin-like growth factor 1 (IGF-1), was shown to be necessary for compact zone expansion in mouse embryos, in addition to previously described effects.28 In vitro, treatment of neonatal rat ventricular myocytes (NRVMs) with NRG-1 resulted in increased protein production (indicated by >2-fold increase in S6 kinase peptide phosphorylation), F-actin organization, and subsequent hypertrophy (both assessed qualitatively through phalloidin staining).29 Rapamycin inhibited these effects, indicating that phosphatidylinositol 3-kinase (PI3K)/p70S6K activation is necessary for NRG-1-induced protein synthesis and myofibrillogenesis. Taken together, this evidence demonstrates that NRG-1/ErbB signaling is crucial for proper heart formation, a process that must be at least partially recapitulated in stem cell therapies aimed to regenerate the heart.
Cardiac conduction system development
In vivo experiments have demonstrated the importance of NRG-1 in the development of the cardiac conduction system (CCS). Injection of mouse embryos with NRG-1 induced ectopic CCS development in a dose-dependent manner (with no increased response above 2.5 pM) and increased both the number of pacemaker cells as well as the conduction velocity.30 Because trabeculation, endocardial cushion formation, compact zone expansion, and CCS development occur sequentially during development, these studies, when examined as a whole, demonstrate the temporal importance of NRG-1/ErbB signaling and the multitude of downstream effectors of ErbB activation. When the nuances of these sequential effects are fully elucidated, NRG-1 has the potential to become a tool with multiple specific uses in cardiac stem cell therapy. Administered at the appropriate time, it could direct cardiomyocyte subpopulations into desired phenotypes during cardiac development.
Cardiomyocyte differentiation from pluripotent stem cells
Some progress has been made in untangling NRG-1/ErbB signaling effects in the stem cell environment. Importantly, NRG-1 also exhibits developmental effects in pluripotent stem cell (PSC)-derived cardiomyocytes in vitro; however, treatment results vary and can be contradictory. By examining the context and time dependence of NRG-1/ErbB signaling, seemingly disparate effects of NRG-1 administration in vitro instead become a road map of NRG-1’s diverse effects on cardiomyocyte development. Initial reports suggested that NRG-1 globally increased cardiomyocyte differentiation from mouse embryonic stem cells (mESCs).31 More specifically, NRG-1 was shown to increase cardiomyogenesis, in particular “working-type” atrial and ventricular cardiomyocytes, via ErbB signaling in mESCs when administered on days 5 through 9 of differentiation.32 When treated with NRG-1 on days 1 through 3 of differentiation, mESCs were reported to preferentially differentiate into “nodal-type” pacemaker cardiomyocytes.33 During development in vivo, the CCS develops after heart trabeculation and compaction, which contradicts the aforementioned preferential differentiation of nodal-type cardiomyocytes (found almost exclusively in the CCS) with early NRG-1 treatment. Because differentiation methods vary among studies and the uniformity and time course of differentiation vary depending on the differentiation method used, it is difficult to make comparisons of the timing of NRG-1 treatments, and comprehensive time-course studies are necessary. The in vitro platform will clearly not perfectly mimic in vivo processes, and the mechanisms of this preferential differentiation may lead to further understanding of NRG-1’s uses in stem cell therapy (Table 1).
Table 1.
Studies conducted on NRG-1/ErbB signaling during cardiac development.
| MODEL | ANIMAL | TREATMENT | RESULTS |
|---|---|---|---|
| In Vivo | Mouse | ErbB 4 deleted7 | Death in utero, decreased trabeculation and AV cushion formation |
| ErbB 2 deleted8 | Death in utero, decreased trabeculation and AV cushion formation | ||
| NRG-1 deleted9 | Death in utero, decreased trabeculation and AV cushion formation | ||
| Zebrafish | NRG-1 deleted27 | Myofibrilar disorganization | |
| Mouse Embryo | NRG-1 & IGF-1 injected E11.5-E12.528 | GFs necessary for compact zone expansion and AV cushion formation | |
| NRG-1 9.5–11.5 dpc30 | NRG-1 necessary for full CCS development | ||
| In Vitro | NRVMs | NRG-1, 24 hours29 | Increased F-actin organization and hypertrophy |
| mESCs | NRG-1 d1-d3 of differentiation33 | Preferrential differentiation to nodal-type cardiomyocytes | |
| NRG-1 d1-d3 of differentiation35 | Increased cardiac induction via ErbB3/ErbB2 signaling | ||
| NRG-1 d3-d7 of differentiation31 | Global increase in cardiogenesis | ||
| NRG-1 d5-d7 of differentiation34 | Increased cardiogenesis via ErbB4 signaling, increased contractile components | ||
| NRG-1 d5-d9 of differentiation32 | ErbB signaling increases working-type cardiogenesis | ||
| hESCs | NRG-1 Agonist d5-d12 of differentiation37 | Increased proportion of working type cardiomyocytes |
Notes: Studies include in vivo and in vitro platforms in mice, zebrafish, and mouse embryos, as well as rat, mouse, and human cell lines. Different experimental results are observed depending on platform, timing, and duration of treatment.
To reconcile the sometimes contradictory results, differential receptor activation has been examined in mESCs treated with NRG-1. ErbB4 receptors were implicated in increased cardiomyocyte differentiation (>20% increase in beating embryoid bodies [EBs] relative to controls) in mESCs treated days 5 through 7 of differentiation via the hanging drop method. NRG-1 treatment also increased EB mRNA expression levels of cardiomyocyte contractile components cardiac troponin T (cTNT) and myosin light chain 2a (MLC2a), as well as protein expression levels of cTNT, Nkx2.5 (an important cardiac lineage transcription factor), and connexin 40 (a major component of gap junctions in the CCS).34 However, the ErbB3/ErbB2 receptors were shown to be necessary for cardiac induction of mESCs when treated with NRG-1 during days 1 through 3 of differentiation.35 Interestingly, in rat cardiac myocytes, ErbB2 and ErbB4 expression has been shown to persist into adulthood, whereas ErbB3 expression ceases.36 These studies help reconcile the different effects of and the cardiomyocyte subtypes produced by NRG-1 treatment during cardiomyocyte differentiation and help to illustrate the importance of the time dependence of NRG-1’s effects, particularly with respect to the receptor binding to ErbB3 or ErbB4. The differential downstream effects of specific ErbB receptor binding by NRG-1 need further investigation.
The use of mESCs to study NRG-1 in the context of differentiation and development has led to important insights into timing and means of signaling, and yet it is important to utilize human cell platforms in order to orient the capabilities of NRG-1 toward tissue engineering and therapeutic applications in the clinical setting. When human ESC (hESC)-derived cardiomyocytes were treated with NRG-1 on day 10 of differentiation, an increase in the proportion of working-type cardiomyocytes was reported by patch clamp measurements of action potential, and when NRG-1/ErbB signaling was inhibited in these hESC-derived cardiomyocytes, an increase in nodal-type cardiomyocytes was observed.37 Interestingly, the time dependence of these results does not always coincide with the time line of cardiac development in vivo, as mentioned above, demonstrating the need for a more robust study of temporal NRG-1/ErbB signaling in human cardiomyocytes.
NRG-1/ErbB Signaling in Homeostasis
Cardiomyocyte metabolic activity
Although the levels of NRG-1 decrease in the heart after development, NRG-1 is still present and plays an important role in many homeostatic processes. NRG-1/ErbB signaling has been implicated in mitochondrial activity in several contexts. The cardioprotective actions of NRG-1/ErbB signaling have been studied in part because of the cardiotoxic side effects of chemotherapeutic agents.38 Anthracyclines are some of the most effective anticancer drugs that have been developed, but cardiac complications began to be reported within 1–3 years of their introduction.39,40 Anthracyline-induced cardiotoxicity, which at the molecular level increases oxidative stress in the heart, manifests clinically with a variety of symptoms ranging from arrhythmias and sudden cardiac death to LV dysfunction and congestive heart failure.38 ErbB4 expression and its heterodimerization with ErbB2 were found to be downregulated in neonatal rat cardiomyocytes treated with the anthracycline doxorubicin.41 In rat cardiomyocytes, NRG-1/ErbB signaling was able to attenuate myocardial damage resulting from anthracycline-associated oxidative stress.39,42
The mechanisms of the cardioprotective effects of NRG-1/ErbB signaling have been further investigated and found in adult rat cardiomyocytes to be at least in part due to increased voltage-gated ion channel expression, mitochondrial turnover, and fatty acid transporter mRNA suppression.43 Similar results have been observed in rats in vivo, wherein administration of NRG-1 has rescued mitochondrial function, reduced oxidative stress, and inhibited cytochrome c release in coronary ligation-induced heart failure.44 Conversely, when a Cre–loxP system was implemented in mice to delete ErbB2 in cardiomyocytes, attenuating NRG-1/ErbB signaling, the number and disorganization of mitochondria increased.45 A second mechanism of NRG-1’s cardioprotective effect is the downstream activation of Src/focal adhesion kinase (FAK). NRG-1β-specific Src and FAK phosphorylation in cardiomyocytes resulted in increased sarcomere organization (via p130CAS and CRB adaptor proteins), myosin light chain activation (via Erk1/2), cell survival, and cell–cell junction formation at intercalated disks.46,47
Studies of NRG/ErbB signaling in both cardiac and non-cardiac cells have shed light on its role in metabolic activity. Glucose uptake was stimulated by NRG-1 treatment in neonatal rat ventricular myocytes.48 The same phenomenon was observed in L6E9 rat skeletal muscle cells, wherein chronic NRG-1 treatment enhanced oxidative metabolism and mitochondrial activity by stimulating the expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and peroxisome proliferator-activated receptor delta (PPARδ).49 In ErbB4-expressing PC12 cells, NRG-1 treatment was found to regulate elevation of reactive oxygen species by H2O2-induced oxidative stress via the PI3K-PKB/Akt pathway.50 ErbB2 signaling was found to regulate mitochondrial activity in a temporal manner via the mitochondrial inner membrane protein UCP2. Upon constitutive overexpression of ErbB2 in MCF7 cells, UCP2 levels were increased, which contributes to mitochondrial uncoupling and has been shown to increase cardiomyocyte viability during oxidative stress.51 Taken together, evidence from studies covering a wide scope of contexts clearly implicates NRG/ErbB signaling as a crucial player in regulating metabolic activity through multiple pathways.
Maintaining cardiac homeostasis
NRG-1/ErbB signaling is critical for cardiomyocyte response to physiological stress. During exercise-induced physiological hypertrophy in rats, NRG-1 was upregulated at both the mRNA and protein expression levels in ventricular cardiomyocytes in conjunction with hypertrophy (assessed by heart weight and cardiomyocyte diameter) and proliferation (by an increase in mononucleated bromodeoxyuridine [BrdU]-positive cardiomyocytes, indicating a true increase in new, rather than multinucleated, cells). In the same study, isolated c-kit-positive embryonic cardiac stem cells showed >3-fold incorporation of BrdU relative to control.52 These results are supported by a similar study in which NRG-1 treatment increased hypertrophy in both neonatal and adult rat ventricular myocytes (ARVMs) and increased proliferation in only neonatal ventricular cardiomyocytes, with emphasis here on the temporal context dependence of signaling.36 As cardiomyocyte proliferation peaks during development in utero and declines drastically to negligible levels in adulthood, the ability to stimulate cardiomyocyte proliferation in NRVMs over ARVMs may be a result of developmental biology of the cells themselves.53 During pregnancy-induced physiological hypertrophy in mice and rats, phosphorylation levels of NRG-1 and ErbB2 and ErbB4 increased in the mother, and partial inhibition of NRG/ErbB signaling reduced the LV FS and enhanced pregnancy-induced LV dilatation.54 In vivo, and to some extent in vitro, models clearly demonstrate that NRG/ErbB signaling responds to physiological changes during homeostasis and affects cardiomyocyte physiology.
NRG-1 has also been implicated in regulating the cardiac response to β-adrenergic activity. When myocytes isolated from heterozygous NRG-1-knockout mice underwent β-adrenergic stimulation to increase contractility, presence of a muscarinic agonist was unable to decrease contractility, indicating that NRG-1 plays an important role in modulating β-adrenergic stimulation via inhibitory parasympathetic activity.55 In isolated rabbit papillary muscle, NRG-1 treatment decreased sensitivity to the β-adrenergic agonist isoproterenol, presenting a negative inotropic effect (evidenced by a decreased peak twitch active tension response to isoproterenol of papillary muscles simultaneously treated with NRG-1). This response was attenuated by a nitric oxide synthase inhibitor, suggesting that nitric oxide, to some extent, mediates the negative inotropic effect of NRG-1.56 When stimulated with the β-adrenergic agonist norepinephrine, ARVMs were protected from apoptosis by NRG-1 treatment only when simultaneously undergoing electrical stimulation.57 NRG-1/ErbB signaling responds to both physiological and pathological stresses via multiple, nonredundant pathways and permeates heart function throughout adulthood.
Heterotypic interactions with endothelial cells
Cardiac vascular endothelium is a critical component of cardiac maintenance; it physically and biochemically communicates with cardiomyocytes, in addition to being susceptible to the effects of NRG/ErbB signaling. Endothelial cells are themselves the major producers of NRG-1, and interrupting this signaling in mouse endothelium has led to capillary loss, increased expression of hypoxia-inducible transcription factor, and decreased cardiac function.58,59 Isolated cardiac microvascular endothelial cells have been reported to release NRG-1 in a dose-dependent response to oxidative stress by H2O2 treatment, and this activity was shown to subsequently decrease H2O2-induced apoptosis in ARVMs.60 The protective effects of cardiac microvascular endothelium (CMVE) against cardiomyocyte apoptosis were diminished when ErbB2 signaling was blocked in a similar study, indicating, once again, a receptor dependence of the protective action of NRG-1 signaling.61 Endothelial cells can themselves use autocrine NRG/ErbB signaling to induce angiogenesis in a metalloproteinase-dependent manner.62 In vitro and in vivo endothelial cell platforms have also indicated that hypoxia-induced endothelial NRG-1 expression increases angiogenesis via paracrine upregulation of vascular endothelial growth factor (VEGF).63 The importance of NRG-1 in CMVE provides yet another avenue for exploration, which could reveal therapeutic applications of NRG-1 limited not just to cardiomyocytes but to their support system as well.
NRG-1/ErbB Signaling in Heart Disease
Acute heart failure
NRG-1/ErbB signaling is ubiquitous throughout cardiac development and homeostasis, and its role in heart disease (both acute and chronic heart failure [CHF]) has been the focus of a major portion of NRG-1-related research. An indication of the role of NRG-1 in acute heart failure is the decrease in ErbB2 and NRG-1 expression associated with the need for inotropic support and decreased ejection fraction (EF), respectively, in clinical data.64 In acute heart failure models, NRG-1 can often exert a cardioprotective effect by means of pathways discussed above. NRG-1 and phosphorylated ErbB4 levels have been reported to increase directly after ischemic/reperfusion injury in rats, and preconditioning with NRG-1 intravenously, 20 minutes before injury, reduces the resulting infarct in a PI3K/Akt-dependent manner.65 Endothelial cell-derived NRG-1 has been implicated in this cardioprotective mechanism; when NRG-1 was selectively deleted in ECs in an ischemic mouse model, systolic function was significantly impaired.66 In a rat myocardial infarct (MI) model, intravenous NRG-1 administration 4 weeks after injury reduced mitochondrial dysfunction and myocyte apoptosis, thus reducing LV remodeling after injury.44 When human NRG-1 was introduced in a similar rat MI model using lentiviral gene transduction at the infarcted border zone, also 4 weeks after injury, an increase in microvasculature was observed in the infarcted myocardium, accompanied by an upregulation of VEGF.67 When NRG-1 was administered 4 days post-MI for sustained periods using microparticle delivery (shown to stably release growth factors for 4 weeks in vitro) in rats, overall cardiac function was improved, as indicated by increased LVEF and LV mass, as well as the increase in end-systolic and end-diastolic diameters (LVESD and LVEDD, respectively) and volumes.68 More specifically, cardiomyocyte proliferation increased, as demonstrated by an increased number of Ki67-positive cells in the infarcted and peri-infarcted zones, and apoptosis and fibrotic remodeling decreased. Clearly, the beneficial effects of NRG-1 treatment and the detrimental effects of its removal in acute heart failure models provide solid evidence of its importance in the heart’s response to injury.
Several mechanisms have been proposed to further elucidate the beneficial effects of NRG-1 during acute heart failure. Some studies have suggested that the positive effects of NRG-1 treatment after MI may be due to increased cardiomyocyte proliferation68,69; however, definitive evidence of this phenomenon is yet to be presented. Another study sought to explain NRG-1’s mechanism of action using a rat MI model and found that NRG-1 treatment significantly upregulated cardiac MLC kinases and phosphorylation of ventricular MLC2, which accompanied an increase in cardiac function (characterized by increased EF, %FS, LVEDD, and LVESD).70 NRG-1 may also exert beneficial effects by downregulating genes involved in fibrosis (eg, matricellular proteins, collagens, and basal lamina components).71 All these studies report an increase in cardiac function, and it is clear that NRG-1 acts through a multitude of pathways to protect and help restore heart function during the remodeling process that follows ischemic injury.
As has been a common thread in the discussion of NRG-1/ErbB signaling thus far, the timing of NRG-1 administration in acute heart failure appears to be important. Temporal dependence of treatment was investigated in a rat MI model when NRG-1 was injected via the tail vein 1 or 8 weeks after injury. A decreased sensitivity to NRG-1 was observed with increasing time post-MI for NRG-1 administration, which may be caused by a temporary increase in ErbB2 expression immediately after injury. In addition to improving heart function, NRG-1 was reported to “normalize” expression of genes related to metabolic function back to the expression levels in uninjured animals.72 In a similar model of rat MI, intravenous NRG-1 treatment, either 1 or 8 weeks post-MI, improved hemodynamic function (increased mean arterial pressure, LV pressure, and rate of contraction and relaxation relative to the same in untreated rats) and showed an additive effect when administered with an angiotensin-converting enzyme inhibitor.73 Both studies suggest that activation of NRG-1/ErbB signaling at various time points after acute MI can attenuate adverse remodeling and improve heart function, implying the temporally broad therapeutic benefits of NRG-1 administration.
Chronic heart failure
NRG-1/ErbB signaling not only is limited to acute injury in the heart but also has been implicated during CHF. Although there are fewer studies in this context, NRG-1 continues to exhibit cardioprotective effects in CHF, as previously described for acute heart failure. In a study examining 899 systolic heart failure patients, serum NRG-1 levels were found to be independently associated with the severity of heart failure.74 Circulating level of NRG-1 may be a biomarker for heart failure as increased levels indicate increased compensatory efforts of the cardiovascular system. Increased level of circulating NRG-1, however, is accompanied with decreased expression of ErbB2 and ErbB4 receptors in failing human myocardium.75 Expression levels can be rescued upon implantation of ventricular assist devices, indicating that ErbB levels are modulated by the degree of overload in CHF.76 This phenomenon has been reported by the same group in an aortic stenosis rat model, where ErbB2 and ErbB4 levels were stable after 6 weeks of stenosis but significantly decreased 22 weeks after stenosis.77 Because CHF is associated with depressed ErbB receptor levels, administration of NRG-1 alone may no longer be sufficient to improve cardiac function in the long term. These studies emphasize the importance of uncovering the mechanisms of NRG-1 action over the course of disease progression in order to harness its benefits most effectively.
Therapeutic administration of NRG-1 has been shown to improve cardiac function in a variety of in vivo models of CHF. NRG-1 improved heart function and survival in a mouse myocarditis model.73 Similar beneficial effects were observed in a canine chronic pacing model, wherein NRG-1 injections for 5 days improved LV end diastolic and systolic pressure, cardiac contractility, and relaxation only in chronically paced animals and not in the control group, indicating that elevated levels of NRG-1 alone are not sufficient to elicit such responses.73 In a study examining diabetic cardiomyopathy in rats, NRG-1 had similar beneficial effects, which were attributed to attenuation of myocardial interstitial fibrosis.78 Although much work is yet to be done to untangle the mechanisms of NRG-1 action in clinical and preclinical models of CHF, existing studies suggest that NRG-1 imparts benefits on heart function across a broad spectrum of disease pathologies.
NRG-1 in Clinical Trials
Because of the cardioprotective role of NRG-1/ErbB signaling and the dire consequences when this signaling is inhibited, recombinant human NRG-1 (rhNRG-1) has been used in two clinical trials with reported results and in several other ongoing clinical trials.79–82 A phase II, randomized, double-blind trial enrolled 44 CHF patients, characterized by an LVEF ≤40%. CHF patients received rhNRG-1 intravenously, 8 hours a day for 10 days and LVEF improved 31.99% in treated patients compared with a 15.05% increase in patients treated with placebo for 90 days, indicating that short-term administration can have long-term effects on the inhibition of adverse remodeling.83 In a single-center, prospective, non-randomized, open-label study, rhNRG-1 was administered intravenously to 15 CHF patients (LVEF: ≤40%) 6 hours a day for 11 days, and both acute and sustained hemodynamic responses were measured by Swan–Ganz catheterization.84 Significant changes in cardiac function were observed within 8 hours of rhNRG-1 treatment, including increased cardiac output, stroke volume, and mean arterial pressure; however, mean arterial pressure was the only hemodynamic parameter to display a significant change at 84 days posttreatment. The promising results of these two trials suggest that therapeutic use of rhNRG-1 will continue to be investigated in the CHF patient population. As scientific research continues to unravel the specific mechanisms of NRG-1/ErbB cardioprotection and improvement of contractile and hemodynamic function, the specificity of NRG-1/ErbB therapeutic treatment can be applied to an increasing diversity of developmental and disease conditions.
Conclusion
Because of the large number of time- and context-dependent effects of which NRG-1/ErbB signaling is capable, NRG-1 will continue to be studied as a tool for both tissue engineering and therapeutic purposes. Human PSCs have provided a platform for tissue engineering with clinical applications, and the advent of cardiomyocyte differentiation from human PSCs in fully defined conditions85,86 has set the stage for engineering functional cardiac tissue. Human PSC-derived cardiomyocytes, and thus the tissues made from them, display an immature phenotype,87 and NRG-1, whose signaling has been implicated in cardiac development, is a prime candidate for increasing maturation of engineered myocardium. By obtaining a fuller understanding of when and how NRG-1/ErbB signaling directs cardiac development, we can harness this pathway for stem cell therapies targeting the heart.
NRG-1/ErbB signaling also plays an important role in angiogenesis and cardiac homeostasis, as well as having cardioprotective and therapeutic effects pre- and postmyocardial injury. At least five clinical trials are currently under way in which rhNRG-1 is administered to CHF patients.88 The effects of NRG-1 in the clinical setting are yet to be fully uncovered, and the mechanisms of the observed homeostatic and cardioprotective functions must be untangled in order to fully utilize and tailor NRG-1/ErbB-based therapy. However, the ubiquity of NRG-1/ErbB signaling throughout life is an undeniably encouraging discovery with respect to NRG-1’s possible therapeutic targets. The vast therapeutic applications of NRG-1 in cardiac development and disease are an incredible motivation to more fully understand the context dependence of NRG-1/ErbB signaling.
Footnotes
ACADEMIC EDITOR: Karen Pulford, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the manuscript: CER. Contributed to the writing of the manuscript: KLKC. Agree with manuscript results and conclusions: CER, KLKC. Jointly developed the structure and arguments for the paper: CER, KLKC. Made critical revisions and approved final version: CER, KLKC. Both authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Falls D. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284(1):14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 2.Holmes WE, Sliwkowski MX, Akita RW, et al. Identification of heregulin, a specific activator of p185erbB2. Science. 1992;256(5060):1205–10. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 3.Peles E, Bacus SS, Koski RA, et al. Isolation of the NeuHER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992;69(1):205–16. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- 4.Wen D, Peles E, Cupples R, et al. Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992;69(3):559–72. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- 5.Goodearl AD, Davis JB, Mistry K, et al. Purification of multiple forms of glial growth factor. J Biol Chem. 1993;268(24):18095–102. [PubMed] [Google Scholar]
- 6.Marchionni MA, Goodearl AD, Chen MS, et al. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362(6418):312–8. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 7.Gassmann M, Casagranda F, Orioli D, et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378(6555):390–4. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 8.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378(6555):394–8. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 9.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378(6555):386–90. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 10.Mei L, Nave KA. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 2014;83(1):27–49. doi: 10.1016/j.neuron.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasti C, Hertig CM. Neuregulin-1/erbB activities with focus on the susceptibility of the heart to anthracyclines. World J Cardiol. 2014;6(7):653–62. doi: 10.4330/wjc.v6.i7.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wadugu B, Kühn B. The role of neuregulin/ErbB2/ErbB4 signaling in the heart with special focus on effects on cardiomyocyte proliferation. Am J Physiol Heart Circ Physiol. 2012;302(11):H2139–47. doi: 10.1152/ajpheart.00063.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar R, Yarmand-Bagheri R. The role of HER2 in angiogenesis. Semin Oncol. 2001;28(5 suppl 16):27–32. doi: 10.1016/s0093-7754(01)90279-9. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Z, Zhou M. Neuregulin signaling and heart failure. Curr Heart Fail Rep. 2010;7(1):42–7. doi: 10.1007/s11897-010-0003-y. [DOI] [PubMed] [Google Scholar]
- 15.Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11(3):287–96. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 16.Yokozeki T, Wakatsuki S, Hatsuzawa K, Black RA, Wada I, Sehara-Fujisawa A. Meltrin beta (ADAM19) mediates ectodomain shedding of Neuregulin beta1 in the Golgi apparatus: fluorescence correlation spectroscopic observation of the dynamics of ectodomain shedding in living cells. Genes Cells. 2007;12(3):329–43. doi: 10.1111/j.1365-2443.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 17.Hu X, Hicks CW, He W, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9(12):1520–5. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 18.Montero JC, Yuste L, Díaz-Rodríguez E, Esparís-Ogando A, Pandiella A. Differential shedding of transmembrane neuregulin isoforms by the tumor necrosis factor-alpha-converting enzyme. Mol Cell Neurosci. 2000;16(5):631–48. doi: 10.1006/mcne.2000.0896. [DOI] [PubMed] [Google Scholar]
- 19.Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circ Res. 2012;111(10):1376–85. doi: 10.1161/CIRCRESAHA.112.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor AR, Taylor SB, Koenig JI. The involvement of type II neuregulin-1 in rat visuospatial learning and memory. Neurosci Lett. 2012;531(2):131–5. doi: 10.1016/j.neulet.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7(6):575–80. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 22.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 23.Jones JT, Akita RW, Sliwkowski MX. Binding specificities and affinities of egf domains for ErbB receptors. FEBS Lett. 1999;447(2–3):227–31. doi: 10.1016/s0014-5793(99)00283-5. [DOI] [PubMed] [Google Scholar]
- 24.Huertas-Vazquez A, Teodorescu C, Reinier K, et al. A common missense variant in the neuregulin 1 gene is associated with both schizophrenia and sudden cardiac death. Heart Rhythm. 2013;10(7):994–8. doi: 10.1016/j.hrthm.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBride KL, Zender GA, Fitzgerald-Butt SM, et al. Association of common variants in ERBB4 with congenital left ventricular outflow tract obstruction defects. Birth Defects Res A Clin Mol Teratol. 2011;91(3):162–8. doi: 10.1002/bdra.20764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudol M. Neuregulin 1-activated ERBB4 as a “dedicated” receptor for the Hippo-YAP pathway. Sci Signal. 2014;7:355, e29. doi: 10.1126/scisignal.aaa2710. [DOI] [PubMed] [Google Scholar]
- 27.Reischauer S, Arnaout R, Ramadass R, Stainier DYR. Actin binding GFP allows 4D in vivo imaging of myofilament dynamics in the zebrafish heart and the identification of Erbb2 signaling as a remodeling factor of myofibril architecture. Circ Res. 2014;115(10):845–56. doi: 10.1161/CIRCRESAHA.115.304356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hertig CM, Kubalak SW, Wang Y, Chien KR. Synergistic roles of neuregulin-1 and insulin-like growth factor-I in activation of the phosphatidylinositol 3-kinase pathway and cardiac chamber morphogenesis. J Biol Chem. 1999;274(52):37362–9. doi: 10.1074/jbc.274.52.37362. [DOI] [PubMed] [Google Scholar]
- 29.Baliga RR, Pimental DR, Zhao YY, et al. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70S6K, and MEK-MAPK-RSK. Am J Physiol Hear Circ Physiol. 1999;277(5):H2026–37. doi: 10.1152/ajpheart.1999.277.5.H2026. [DOI] [PubMed] [Google Scholar]
- 30.Rentschler S, Zander J, Meyers K, et al. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci U S A. 2002;99(16):10464–9. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Xu G, Wu Y, et al. Neuregulin-1 enhances differentiation of cardiomyocytes from embryonic stem cells. Med Biol Eng Comput. 2009;47(1):41–8. doi: 10.1007/s11517-008-0383-2. [DOI] [PubMed] [Google Scholar]
- 32.Chen M, Bi L-L, Wang Z-Q, Zhao F, Gan X-D, Wang YG. Time-dependent regulation of neuregulin-1β/ErbB/ERK pathways in cardiac differentiation of mouse embryonic stem cells. Mol Cell Biochem. 2013;380(1–2):67–72. doi: 10.1007/s11010-013-1658-y. [DOI] [PubMed] [Google Scholar]
- 33.Patel R, Kos L. Endothelin-1 and neuregulin-1 convert embryonic cardiomyocytes into cells of the conduction system in the mouse. Dev Dyn. 2005;233(1):20–8. doi: 10.1002/dvdy.20284. [DOI] [PubMed] [Google Scholar]
- 34.Sun M, Yan X, Bian Y, Caggiano AO, Morgan JP. Improving murine embryonic stem cell differentiation into cardiomyocytes with neuregulin-1: differential expression of microRNA. Am J Physiol Cell Physiol. 2011;301(1):C21–30. doi: 10.1152/ajpcell.00141.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao J, Galindo CL, Tran T-L, Sawyer DB. Neuregulin-1β induces embryonic stem cell cardiomyogenesis via ErbB3/ErbB2 receptors. Biochem J. 2014;458(2):335–41. doi: 10.1042/BJ20130818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao YY, Sawyer DR, Baliga RR, et al. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273(17):10261–9. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 37.Zhu W-Z, Xie Y, Moyes KW, Gold JD, Askari B, Laflamme MA. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res. 2010;107(6):776–86. doi: 10.1161/CIRCRESAHA.110.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montaigne D, Hurt C, Neviere R. Mitochondria death/survival signaling pathways in cardiotoxicity induced by anthracyclines and anticancer-targeted therapies. Biochem Res Int. 2012;2012:951539. doi: 10.1155/2012/951539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56(2):185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 40.Vejpongsa P, Yeh ETH. Prevention of anthracycline-induced cardiotoxicity. J Am Coll Cardiol. 2014;64(9):938–45. doi: 10.1016/j.jacc.2014.06.1167. [DOI] [PubMed] [Google Scholar]
- 41.Horie T, Ono K, Nishi H, et al. Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc Res. 2010;87(4):656–64. doi: 10.1093/cvr/cvq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawyer DB. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105(13):1551–4. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 43.Giraud M-N, Flück M, Zuppinger C, Suter TM. Expressional reprogramming of survival pathways in rat cardiocytes by neuregulin-1beta. J Appl Physiol. 2005;99(1):313–22. doi: 10.1152/japplphysiol.00609.2004. [DOI] [PubMed] [Google Scholar]
- 44.Guo Yong-fang WX. NRG-1 attenuates mitochondrial dysfunction in a rat model of heart failure. Chin Med J. 2012;125(5):807–14. [PubMed] [Google Scholar]
- 45.Crone SA, Zhao YY, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8(5):459–65. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 46.Pentassuglia L, Sawyer DB. ErbB/integrin signaling interactions in regulation of myocardial cell-cell and cell-matrix interactions. Biochim Biophys Acta. 2013;1833(4):909–16. doi: 10.1016/j.bbamcr.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2-dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J Mol Cell Cardiol. 2006;41(2):228–35. doi: 10.1016/j.yjmcc.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cote GM, Miller TA, Lebrasseur NK, Kuramochi Y, Sawyer DB. Neuregulin-1alpha and beta isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp Cell Res. 2005;311(1):135–46. doi: 10.1016/j.yexcr.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Cantó C, Pich S, Paz JC, et al. Neuregulins increase mitochondrial oxidative capacity and insulin sensitivity in skeletal muscle cells. Diabetes. 2007;56(9):2185–93. doi: 10.2337/db06-1726. [DOI] [PubMed] [Google Scholar]
- 50.Goldshmit Y, Erlich S, Pinkas-Kramarski R. Neuregulin rescues PC12-ErbB4 cells from cell death induced by H(2)O(2). Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. J Biol Chem. 2001;276(49):46379–85. doi: 10.1074/jbc.M105637200. [DOI] [PubMed] [Google Scholar]
- 51.Patel N, Barrientos A, Landgraf R. The growth factor receptor ERBB2 regulates mitochondrial activity on a signaling time scale. J Biol Chem. 2013;288(49):35253–65. doi: 10.1074/jbc.M113.478271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waring CD, Vicinanza C, Papalamprou A, et al. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J. 2012;35(39):2722–31. doi: 10.1093/eurheartj/ehs338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naqvi N, Li M, Calvert JW, et al. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell. 2014;157(4):795–807. doi: 10.1016/j.cell.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lemmens K, Doggen K, De Keulenaer GW. Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am J Physiol Heart Circ Physiol. 2011;300(3):H931–42. doi: 10.1152/ajpheart.00385.2010. [DOI] [PubMed] [Google Scholar]
- 55.Okoshi K, Nakayama M, Yan X, et al. Neuregulins regulate cardiac parasympathetic activity: muscarinic modulation of beta-adrenergic activity in myocytes from mice with neuregulin-1 gene deletion. Circulation. 2004;110(6):713–7. doi: 10.1161/01.CIR.0000138109.32748.80. [DOI] [PubMed] [Google Scholar]
- 56.Lemmens K, Fransen P, Sys SU, Brutsaert DL, De Keulenaer GW. Neuregulin-1 induces a negative inotropic effect in cardiac muscle: role of nitric oxide synthase. Circulation. 2004;109(3):324–6. doi: 10.1161/01.CIR.0000114521.88547.5E. [DOI] [PubMed] [Google Scholar]
- 57.Kuramochi Y, Lim CC, Guo X, Colucci WS, Liao R, Sawyer DB. Myocyte contractile activity modulates norepinephrine cytotoxicity and survival effects of neuregulin-1beta. Am J Physiol Cell Physiol. 2004;286(2):C222–9. doi: 10.1152/ajpcell.00312.2003. [DOI] [PubMed] [Google Scholar]
- 58.Noireaud J, Andriantsitohaina R. Recent insights in the paracrine modulation of cardiomyocyte contractility by cardiac endothelial cells. Biomed Res Int. 2014;2014:923805. doi: 10.1155/2014/923805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hedhli N, Russell KS. Cytostatic drugs, neuregulin activation of erbB receptors, and angiogenesis. Curr Hypertens Rep. 2010;12(6):411–7. doi: 10.1007/s11906-010-0148-9. [DOI] [PubMed] [Google Scholar]
- 60.Kuramochi Y, Cote GM, Guo X, et al. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem. 2004;279(49):51141–7. doi: 10.1074/jbc.M408662200. [DOI] [PubMed] [Google Scholar]
- 61.Lemmens K, Segers VFM, Demolder M, De Keulenaer GW. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J Biol Chem. 2006;281(28):19469–77. doi: 10.1074/jbc.M600399200. [DOI] [PubMed] [Google Scholar]
- 62.Kalinowski A, Plowes NJR, Huang Q, Berdejo-Izquierdo C, Russell RR, Russell KS. Metalloproteinase-dependent cleavage of neuregulin and autocrine stimulation of vascular endothelial cells. FASEB J. 2010;24(7):2567–75. doi: 10.1096/fj.08-129072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iivanainen E, Paatero I, Heikkinen SM, et al. Intra- and extracellular signaling by endothelial neuregulin-1. Exp Cell Res. 2007;313(13):2896–909. doi: 10.1016/j.yexcr.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 64.Szmit S, Jank M, Maciejewski H, et al. Relationship between clinical data and gene expression in the HER2/ErbB2-dependent signaling pathway in patients with acute heart failure. J Appl Genet. 2013;54(4):447–53. doi: 10.1007/s13353-013-0164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fang SJ, Wu XS, Han ZH, et al. Neuregulin-1 preconditioning protects the heart against ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Chin Med J (Engl) 2010;123(24):3597–604. [PubMed] [Google Scholar]
- 66.Hedhli N, Huang Q, Kalinowski A, et al. Endothelium-derived neuregulin protects the heart against ischemic injury. Circulation. 2011;123(20):2254–62. doi: 10.1161/CIRCULATIONAHA.110.991125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao J, Li B, Zheng Z, et al. Therapeutic effects of neuregulin-1 gene transduction in rats with myocardial infarction. Coron Artery Dis. 2012;23(7):460–8. doi: 10.1097/MCA.0b013e32835877da. [DOI] [PubMed] [Google Scholar]
- 68.Formiga FR, Pelacho B, Garbayo E, et al. Controlled delivery of fibroblast growth factor-1 and neuregulin-1 from biodegradable microparticles promotes cardiac repair in a rat myocardial infarction model through activation of endogenous regeneration. J Control Release. 2014;173:132–9. doi: 10.1016/j.jconrel.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 69.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138(2):257–70. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 70.Gu X, Liu X, Xu D, et al. Cardiac functional improvement in rats with myocardial infarction by up-regulating cardiac myosin light chain kinase with neuregulin. Cardiovasc Res. 2010;88(2):334–43. doi: 10.1093/cvr/cvq223. [DOI] [PubMed] [Google Scholar]
- 71.Galindo CL, Kasasbeh E, Murphy A, et al. Anti-remodeling and anti-fibrotic effects of the neuregulin-1β glial growth factor 2 in a large animal model of heart failure. J Am Heart Assoc. 2014;3(5):e000773. doi: 10.1161/JAHA.113.000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hill MF, Patel AV, Murphy A, et al. Intravenous glial growth factor 2 (GGF2) isoform of neuregulin-1β improves left ventricular function, gene and protein expression in rats after myocardial infarction. PLoS One. 2013;8(2):e55741. doi: 10.1371/journal.pone.0055741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu X, Gu X, Li Z, et al. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol. 2006;48(7):1438–47. doi: 10.1016/j.jacc.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 74.Ky B, Kimmel SE, Safa RN, et al. Neuregulin-1 beta is associated with disease severity and adverse outcomes in chronic heart failure. Circulation. 2009;120(4):310–7. doi: 10.1161/CIRCULATIONAHA.109.856310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rohrbach S, Niemann B, Silber R-E, Holtz J. Neuregulin receptors erbB2 and erbB4 in failing human myocardium – depressed expression and attenuated activation. Basic Res Cardiol. 2005;100(3):240–9. doi: 10.1007/s00395-005-0514-4. [DOI] [PubMed] [Google Scholar]
- 76.Uray IP, Connelly JH, Thomázy V, et al. Left ventricular unloading alters receptor tyrosine kinase expression in the failing human heart. J Hear Lung Transplant. 2002;21(7):771–82. doi: 10.1016/s1053-2498(02)00390-x. [DOI] [PubMed] [Google Scholar]
- 77.Rohrbach S, Yan X, Weinberg EO, et al. Neuregulin in cardiac hypertrophy in rats with aortic stenosis: differential expression of erbB2 and erbB4 Receptors. Circulation. 1999;100(4):407–12. doi: 10.1161/01.cir.100.4.407. [DOI] [PubMed] [Google Scholar]
- 78.Li B, Zheng Z, Wei Y, et al. Therapeutic effects of neuregulin-1 in diabetic cardiomyopathy rats. Cardiovasc Diabetol. 2011;10:69. doi: 10.1186/1475-2840-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao R. Study of efficacy on cardiac remodeling of recombinant human neuregulin-1 in stable chronic systoic heart failure patients. 2012 Home – ClinicalTrials.gov. 2015. [Accessed February 26, 2015]. Available at: https://clinicaltrials.gov/
- 80.Gao R. Study of efficacy on NT-proBNP of recombinant human neuregulin-1 in chronic heart failure patients. Home – ClinicalTrials.gov. 2015. [Accessed February 26, 2015]. Available at: https://clinicaltrials.gov/
- 81.Gao R. Clinical trial to evaluate the efficacy and safety of recombinant human neuregulin-1. Home – ClinicalTrials.gov. 2015. [Accessed February 26, 2015]. Available at: https://clinicaltrials.gov/
- 82.Mendes-Ferreira P, De Keulenaer GW, Leite-Moreira AF, Brás-Silva C. Therapeutic potential of neuregulin-1 in cardiovascular disease. Drug Discov Today. 2013;18(17–8):836–42. doi: 10.1016/j.drudis.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 83.Gao R, Zhang J, Cheng L, et al. A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol. 2010;55(18):1907–14. doi: 10.1016/j.jacc.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 84.Jabbour A, Hayward CS, Keogh AM, et al. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail. 2011;13(1):83–92. doi: 10.1093/eurjhf/hfq152. [DOI] [PubMed] [Google Scholar]
- 85.Lian X, Hsiao C, Wilson G, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109(27):E1848–57. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burridge PW, Matsa E, Shukla P, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11(8):855–60. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473(7347):326–35. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Home – ClinicalTrials.gov. 2015. [Accessed February 26, 2015]. Available at: https://clinicaltrials.gov/