Abstract
Background
Sudden sensorineural hearing loss (SSNHL) is a relatively common condition that is usually of unknown etiology. A number of individual studies have investigated the association between various serum lipids and SSNHL; however, the findings have been inconsistent. In an attempt to obtain more definitive information on the relationship between serum lipids and SSNHL, we carried out a systematic review and meta-analysis.
Methods
Medline, the Cochrane Library, and EMBASE were searched using the following key words: lipid, cholesterol, triglyceride, fat, serum, blood, sudden hearing loss, hearing loss, hearing disorders. Randomized controlled trials, prospective cohort studies, and retrospective case-control studies involving patients with SSNHL and healthy controls that examined the relationship (reported as odds ratios [OR]) between lipid profiles and SSNHL were included. Primary outcomes were total cholesterol and low-density lipoprotein cholesterol (LDL-C) concentrations. Secondary outcomes were triglyceride, high-density lipoprotein cholesterol, and lipoprotein(a) concentrations.
Results
A total of 6 case-control studies were included in this systematic review/meta-analysis. The total number of participants ranged from 30 to 250 in the case group and from 43 to 271 in the control group. Meta-analysis revealed no significant difference in total cholesterol levels between the case and control groups (pooled OR = 1.79, 95% confidence interval [CI] = 0.98 to 3.26, P = 0.057). Likewise, meta-analysis revealed no significant difference in LDL-C concentrations between the case and control groups (pooled OR = 1.15, 95% CI = 0.64 to 2.07, P = 0.639). Since there were an insufficient number of studies reporting data for the secondary outcomes, meta-analysis was not possible.
Conclusions
Our results do not provide evidence for serum lipids being associated with SSNHL, nor do they definitively rule out such an association. Additional studies are needed to ascertain the relationship, or lack thereof, between serum lipids and SSNHL.
Introduction
Sudden sensorineural hearing loss (SSNHL), or sudden deafness, is typically defined as the rapid hearing loss of at least 30 dB in 3 contiguous audiometric frequencies within 3 days [1]. The incidence of SSNHL has been estimated to range from 5 to 20 cases per 100,000 in general population and occurs most commonly among adults aged in their 50s and 60s [2,3]. The onset of SSNHL, typically first noticed on waking in the morning, may be sudden or develop over several days [4], and is unilateral in the vast majority of cases [2]. In addition to hearing loss, individuals with SSNHL may experience a range of other bothersome symptoms, including tinnitus, vertigo, and sensation of ear fullness [5].
Sudden sensorineural hearing loss is considered to be a medical emergency [5], and as such, requires prompt evaluation/treatment. Treatment for SSNHL is dependent on the underlying cause (if identifiable); in the case of idiopathic SSNHL whose cause is not known, a short course of corticosteroids is often prescribed [5,6]. A variety of factors are known to affect recovery from SSNHL, including the duration and severity of hearing loss, age, and the presence of other symptoms [6]. But impaired hearing has also been reported to gradually return to normal without any medical intervention in up to 65% of patients with idiopathic SSNHL [2,7]. So time to recovery in patients with hearing loss and a definitive underlying pathology may depend on the underlying cause(s) [6], but is otherwise difficult to gauge for patients with hearing loss of unknown etiology.
In most instances (approximately 70% of all cases), SSNHL is idiopathic in origin [4], and various etiologic mechanisms have also been proposed for SSNHL. These can be broadly classified as infectious, otologic, traumatic, vascular, and neoplastic, and have been reviewed in detail elsewhere [4–6]. There is some evidence that dyslipidemia may be a risk factor for SSNHL [8–11], and has been hypothesized to contribute to the initiation of an inflammatory or stressful response in the inner ear, leading to SSNHL [11]. There is also evidence that lipid-lowering therapy (specifically low-density lipoprotein cholesterol [LDL-C] apheresis) can be more effective than traditional treatment for patients with SSNHL who have elevated serum LDL-C concentrations [11]. But there is still no consensus as there are findings from other studies which do not support this assertion that dyslipidemia is a risk factor for SSNHL [12,13].
Given the aforementioned lack of definitive information from individual studies, we carried out a systematic review and meta-analysis of the literature examining the relationship between serum lipids and SSNHL.
Materials and Methods
Selection criteria
Randomized controlled trials, prospective cohort studies, and retrospective case-control studies were considered for inclusion if they involved patients with SSNHL and healthy controls, and examined the relationship (determined as odds ratios [ORs]) between lipid profiles and SSNHL. Prospective cohort studies in which assessments were made before and after the onset of SSNHL were also considered for inclusion.
Studies published in the form of letters, comments, editorials, or case reports were excluded. Non-English language publications were also excluded.
Search strategy
Medline, the Cochrane Library, and EMBASE were searched on 14 April 2014 using combinations of the following key words: lipid, cholesterol, triglyceride, fat, serum, blood, sudden hearing loss, hearing loss, hearing disorders.
Reference lists of pertinent studies were also hand-searched to identify other potentially relevant studies not retrieved in the literature search.
Study selection and data extraction
Studies were identified by two independent reviewers using the search strategy already described. A third reviewer was consulted to resolve any disagreements between the two primary reviewers.
The following information and/or data were extracted from studies that met the eligibility criteria: name of the first author, year of publication, study design, number of participants in each treatment group, participants’ age and gender, serum cholesterol, triglyceride, LDL-C, high-density lipoprotein C, and lipoprotein(a) concentrations, and associations (ORs) between lipid parameters and SSNHL.
Quality assessment
The Newcastle-Ottawa Quality Assessment Scale was used to assess quality of the studies included in the systematic review/meta-analysis, and was performed by two reviewers with a third reviewer consulted in case of discrepancy. All studies received a score of 7 or 8, indicating good qualities.
Outcome measures
Comparisons were made between patients with SSNHL (case group) and control patients (control group). The primary outcomes were total cholesterol and LDL-C concentrations. The secondary outcomes were concentrations of triglyceride, HDL-C, and lipoprotein(a).
Statistical analysis
Pooled ORs were calculated for the primary outcomes (case vs control). Heterogeneity among the studies was assessed using the Cochran Q and the I2 statistic. For the Q statistic, P <0.10 indicates statistically significant heterogeneity. For the I2 statistic, which indicates the percentage of the observed between-study variability that is due to heterogeneity and not to chance, 0 to 25% indicates no heterogeneity, 25% to 50% indicates moderate heterogeneity, 50% to 75% indicates large heterogeneity, and 75% to 100% indicates extreme heterogeneity. If either Q statistic (P <0.1) or I2 statistic (>50%) indicated the existence of heterogeneity between studies, a random-effects model (DerSimonian–Laird method) of analysis would be used; otherwise, a fixed-effects model (Mantel-Haenszel method) would be used instead. A two-sided P value <0.05 was considered to be statistically significant. All statistical analyses were performed using the statistical software Comprehensive Meta-Analysis, version 2.0 (Biostat, Englewood, NJ).
Results
Literature search
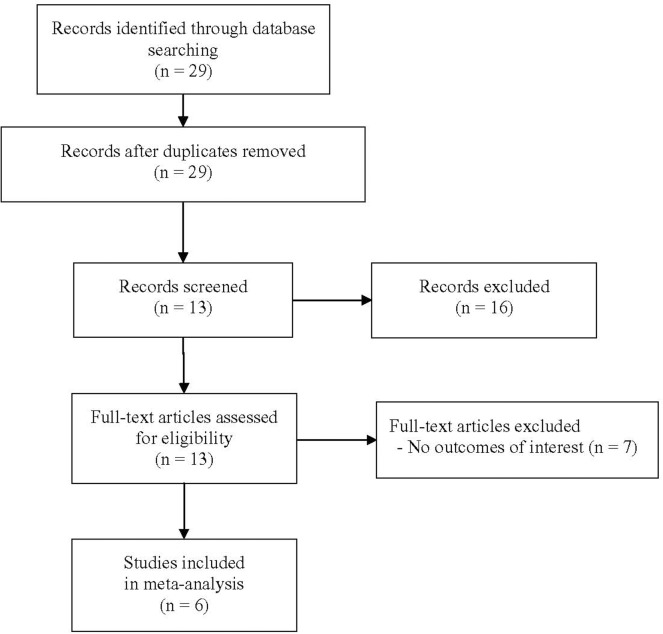
A total of 29 unique articles were identified in the literature search (Fig 1). Of these, 16 did not meet the eligibility criteria on title/abstract review and were excluded. The remaining 13 articles underwent full-text review. Among those 13 articles, 7 [14–20] were subsequently excluded because they did not report outcomes of interest (specifically, the values of odd ratio and/or 95% confidence limits), so only 6 studies [9–12,21,22] were eligible for inclusion in the meta-analysis.
Fig 1. Flow chart of study selection.
Study characteristics
All of the 6 studies included in the meta-analysis were case-control studies, of which the key characteristics are summarized in Table 1. The total number of participants ranged from 30 to 250 in the case group and from 43 to 271 in the control group. Participants were generally around 50 years of age, with the mean age ranging from 45.5 to 56 years in the case group and from 43 to 55 years in the control group. There was a slightly higher proportion of male participants in 4 of the 6 studies [9,11,12,22]. Overall, the proportion of males in the studies ranged from 43.3% to 54.8% in the case group and from 43.2% to 60.5% in the control group. In 4 of the 5 studies [9–11,22], total cholesterol concentrations were higher in the case group (range: 200 to 227 mg/dL) than in the control group (range: 175 to 227 mg/dL). Likewise, in 3 of the 4 studies [10,11,22], LDL-C concentrations were higher in the case group (range: 114 to 131 mg/dL) than in the control group (range: 111 to 124 mg/dL). Only 2 studies each reported participants’ triglyceride [9,22], HDL-C [11,22], and lipoprotein(a) [21,22] concentrations. Triglyceride concentrations ranged from 117.0 to 124.4 mg/dL in the case group and from 128.2 to 128.4 mg/dL in the control group. HDL-C concentrations ranged from 55.3 to 61 mg/dL in the case group and from 54 to 56.3 mg/dL in the control group. Lipoprotein(a) concentrations were reported as mean (standard deviation) in 1 study [22] and median (range) in the other [21] study.
Table 1. Summary of basic characteristics of studies included in meta-analysis.
| Author(Year) | Comparison | Subjects (N) | Age † (years) | Male(Year) | TC † (mg/dL) | TG†(mg/dL) | LDL-C † (mg/dL) | HDL-C † (mg/dL) | LP(a) † (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| Weng (2013) | Case | 250 | 56.41 | 54.8 | 182.9 (39.4) | 117.0 (85.6) | 103.4 (33.1) | 55.3 (15.5) | 5434 (5151) |
| Control | 250 | (15, 84) # | 169.0 (36.2) | 128.2 (287.5) | 91.9 (27.9) | 56.3 (16.2) | 6201 (7285) | ||
| Aimoni (2010) | Case | 141 | 54.6 | 53.2 | 227.2 (40.0) | 124.4 (64.9) | NA | NA | NA |
| (15.8) | |||||||||
| Control | 271 | 55.0 | 52.4 | 214.4 (40.8) | 128.4 (101.9) | NA | NA | NA | |
| (15.8) | |||||||||
| Cadoni (2010) | Case | 43 | 50.0 | 44.2 | 213 (44) | NA | 131 (32.40) | NA | NA |
| (14) | |||||||||
| Control | 43 | 43 | 60.5 | 175 (21.43) | NA | 110.82 (22.66) | NA | NA | |
| (11) | |||||||||
| Cadoni (2007) | Case | 30 | 45.5 | 43.3 | 200 (38.95) | NA | 128 (35.89) | NA | NA |
| (23, 72) # | |||||||||
| Control | 60 | 49.5 | 43.3 | 175 (26.51) | NA | 110.7 (31.34) | NA | NA | |
| (23, 77) # | |||||||||
| Rudack (2006) | Case | 142 | 51.2 | 54.2 | 215 (32) | NA | 114 (29) | 61 (16) | NA |
| (17.2) | |||||||||
| Control | 84 | 49.8 | 54.2 | 227 (38) | NA | 124 (29) | 54 (14) | NA | |
| (13.6) | |||||||||
| Marcucci (2005) | Case | 155 | 54 | 43.2 | NA | NA | NA | NA | 111 |
| (19, 79 # | (1, 1146)* | ||||||||
| Control | 155 | 54 | 43.2 | NA | NA | NA | NA | 103 | |
| (19, 78) # | (11, 695)* |
Data expressed as:
† mean (standard deviation)
# mean (range)
* median (range)
Abbreviations: TC, total cholesterol; TG, triglyceride; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; LP(a), Lipoprotein(a); NA, no data available.
Assessment of serum lipids as risk factors for SSNHL
Table 2 summarizes the findings from the studies included in the meta-analysis regarding the assessment of serum lipids as risk factors for SSNHL. All but 1 study [22] provided results for multivariate regression analyses in the determination of ORs. All 6 studies reported serum total cholesterol as a risk factor for SSNHL. Various cut-off concentrations were used in this assessment both within and between studies; however, higher concentrations of total cholesterol were associated with an increased risk of SSNHL in 5 of 6 studies [9–11,21,22]. The strength of the relationship was highly variable between studies. Four studies [10–12,22] reported serum LDL-C as a risk factor for SSNHL, with all the findings showing that the risk of SSNHL was decreased with lower LDL-C concentrations. Three studies [9,21,22] reported serum triglycerides as a risk factor for SSNHL. The results varied between studies and depending on the cut-off concentrations used. Only 2 studies each reported serum HDL-C and lipoprotein(a) concentrations as risk factors for SSNHL; the findings were inconsistent between these studies.
Table 2. Summary of the assessment of serum lipids as risk factors (determined as odds ratios) for sudden sensorineural hearing loss for studies included in meta-analysis.
| Odds Ratio | |||||
|---|---|---|---|---|---|
| Author(Year) | TC | TG | LDL-C | HDL-C | LP(a) |
| Weng (2013) | 1.459 (1.211–1.758) c | 0.976 (0.897–1.062) c | 1.628 (1.287, 2.061) c | 0.847 (0.552, 1.300) c | 0.999 (0.999–1.000) c |
| Aimoni (2010) | >235 vs ≤200: | >140 vs ≤86: | NA | NA | NA |
| 2.25 (1.25, 4.03) a ; 201–235 vs ≤200: 1.87 (1.07, 3.24) a | 1.19 (0.67, 2.11) a ; | ||||
| 87–139 vs ≤86: | |||||
| 1.00 (0.58, 1.74) a | |||||
| Cadoni (2010) | >200 vs ≤200: | NA | <130 vs >a130: | NA | NA |
| 33.76 (1.96, 562.31) a | 0.74 (0.02, 25.41) a | ||||
| Cadoni (2007) | >200 vs ≤200: | NA | <130 vs >130: | NA | NA |
| 36.7 (3.25, 414.52) a | 0.17 (0.01, 1.98) a | ||||
| Rudack (2006) | 0.780 (0.433, 1.405) c | NA | 0.934 (0.519, 1.680) | 1.937 (1.068, 3.513) c | NA |
| Marcucci (2005) | >190 vs <147: | >98 vs <82: | NA | NA | >163 vs <67: |
| 19 (7, 50.1) a ; | 3.0 (0.3, 42) a ; | 1.8 (0.5, 4.1) a ; | |||
| 148–190 vs <147: 4.8 (1.9, 12.6) a | 82–98 vs <82: | 68–163 vs <67: | |||
| 0.6 (0.4, 1.3) a | 1.2 (0.5, 2.53) a |
Abbreviations: TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; LP(a), lipoprotein(a); NA, no data available.
a, adjusted odds ratio (95% confidence interval)
c, crude odds ratio (95% confidence interval);
Meta-analysis
Meta-analysis was performed for the total cholesterol and LDL-C concentrations. As only 2 studies reported useable results for triglyceride, HDL-C, and lipoprotein(a) concentrations, meta-analysis was not performed for these variables.
Total cholesterol
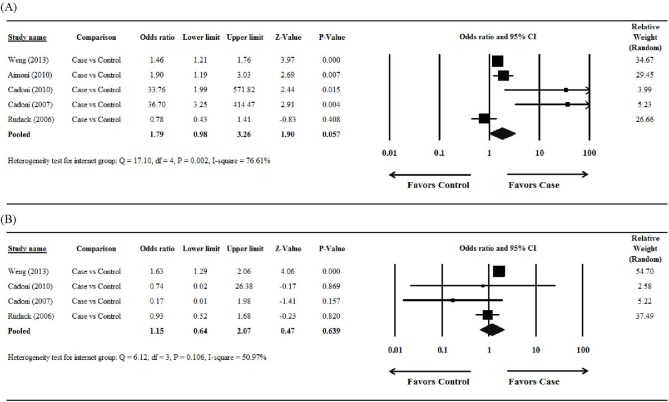
The study by Marcucci et al [21] did not report useable data and was excluded from this analysis. There was significant heterogeneity when data from the 5 studies were pooled (Q = 17.10, df = 4, P = 0.002, I2 = 76.61%); therefore, a random-effects model of analysis was used (Fig 2A). The overall analysis revealed that there was no significant difference in total cholesterol levels between the case and control groups (pooled OR = 1.79, 95% confidence interval [CI] = 0.98 to 3.26, P = 0.057).
Fig 2. Forest plots showing the results of the meta-analysis of (A) total cholesterol and (B) low-density lipoprotein cholesterol for the case/sudden sensorineural hearing loss group vs the control group.
Abbreviation: CI, confidence interval.
Low-density lipoprotein cholesterol
The studies reported by Aimoni et al [9] and Marcucci et al [21] did not report useable data and were excluded from this analysis. There was significant heterogeneity when data from the 4 studies were pooled (Q = 6.12, df = 3, P = 0.106, I2 = 50.97%); therefore, a random-effects model of analysis was used (Fig 2B). The overall analysis revealed that there was no significant difference in LDL-C concentrations between the case and control groups (pooled OR = 1.15, 95% CI = 0.64 to 2.07, P = 0.639).
Sensitivity analysis
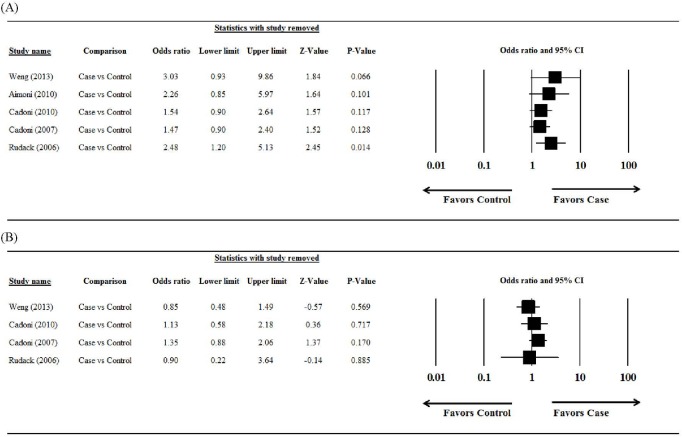
The results of the sensitivity analysis (leave-one-out approach to) are summarized in Fig 2. For total cholesterol, the pooled estimate was different when the leave-one-out approach was used, indicating that the meta-analysis had poor reliability (Fig 3A). For LDL-C, the direction and magnitude of pooled estimates did not vary considerably when the leave-one-out approach was used, indicating that the meta-analysis had good reliability (Fig 3B).
Fig 3. Results of the sensitivity analysis examining the influence of individual studies on pooled estimates, as determined using the leave-one-out approach, for (A) total cholesterol and (B) low-density lipoprotein cholesterol (case/sudden sensorineural hearing loss group vs control group).
Abbreviation: CI, confidence interval.
Publication bias
Publication bias could not be assessed for the meta-analysis outcomes because there were fewer than the minimum of 5 studies required to detect funnel plot asymmetry [23].
Discussion
In this systematic review and meta-analysis, we examined the relationship between serum lipid concentrations and SSNHL. A total of 6 case-control studies met the criteria for inclusion in our study. Overall, our results do not support a relationship between serum lipids and SSNHL. However, given the small number of studies included and other limitations in this systematic review and meta-analysis, it is reasonable to assume our results are less than definitive and further research is warranted.
Meta-analysis was performed for two outcomes assessed in our study, the concentrations of total cholesterol and LDL-C. In both instances, there was no significant difference between the case (SSNHL) and control groups, suggesting that these variables are not associated with SSNHL. Interestingly, most studies individually reported that total cholesterol concentrations were higher in patients with SSNHL compared with control patients. Our findings suggest that factors other than SSNHL such as diet, lifestyle, and/or comorbidities may account for the higher concentrations of total cholesterol in the individual studies. There was considerable variability among the individual studies concerning the relationship between total serum cholesterol concentrations and SSNHL. Cadoni et al [10,11] reported a remarkably strong association relative to all other studies, but their studies included relatively small numbers of participants and thus carried less weight in our meta-analysis. The reasons underlying the striking findings reported by Cadoni et al may reflect different participant characteristics and/or factors accounted for in the analyses.
In addition to the concentrations of serum total cholesterol and LDL-C, we also retrieved information on several secondary outcomes, including the concentrations of serum triglyceride, HDL-C, and lipoprotein(a). Unfortunately, there were few data available for these outcomes so we were unable to perform meta-analysis for them. As a result, we cannot draw any conclusion on the relationship between these other types of lipids and SSNHL.
A number of studies [14–16,18,19] were excluded from our analysis for not providing outcomes of interest (the values of odd ratios) after full-text review, but they do deserve some discussion. Findings from these studies were inconsistent, with some suggesting that there may be an association between serum lipids and SSNHL, and others disputing such an association. Specifically, Lu et al [14] found that the concentrations of total cholesterol, triglyceride, and lipoprotein(a) were significantly higher in patients with SSNHL compared with controls, while Oreskovic et al [19] reported only higher concentrations of cholesterol and LDL-C were found in patients with SSNHL. Chang et al [16] suggested that hypercholesterolemia may be an independent risk factor for SSNHL. In contrast, Mosnier et al [15] found no difference in prevalence of hyperlipidemia between patients with SSNHL and controls, while Ullrich et al [18] reported that patients with SSNHL had serum lipid profiles similar to those of normal individuals. These studies are a reflection of broader coverage of this topic in literature, showing a lack of consensus concerning the relationship between serum lipids and SSNHL. One previous systematic review and meta-analysis on SSNHL in adults examined the association of acquired and inherited cardiovascular risk factors, including medical history (hypertension, diabetes mellitus, stroke and ischemic cardiopathy) and life style habits such as smoking and alcohol consumption, with SSNHL [24]. Interestingly, the authors found that history of smoking and alcohol consumption were associated with an increased risk of developing SSNHL, but hypertension and diabetes mellitus were not. However, they did not include dyslipidemia in their analysis, and our study filled this void. Given that dyslipidemia is also an established risk factor for cardiovascular disease, a link between dyslipidemia and SSNHL would not be unexpected.
Our systematic review and meta-analysis has a number of limitations that should be acknowledged. First, only a small number of studies were eligible for inclusion, which reduced the power of our meta-analysis. Second, as already mentioned, there were insufficient data available to perform meta-analysis for the association between SSNHL and concentrations of other lipids such as triglyceride, HDL-C and lipoprotein(a). Third, sensitivity analysis for total cholesterol indicated that the meta-analysis for this variable had poor reliability (reflecting the considerable variability between 2 of the studies included and the remaining studies). Hence, these results must be interpreted with caution. Finally, because of the small number of studies found to be eligible for inclusion in the meta-analyses, we were not able to perform an assessment of publication bias.
In summary, the results of our systematic review and meta-analysis do not provide sufficient evidence for the association between serum lipids and SSNHL, and the information currently available in the literature on this subject is by no means definitive. Hence, additional studies are warranted to fully ascertain the relationship between serum lipids and SSNHL, in order to enhance our understanding of this enigmatic condition.
Supporting Information
(DOC)
(DOC)
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Wilson WR, Byl FM, Laird N (1980) The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol 106: 772–776. [DOI] [PubMed] [Google Scholar]
- 2. Byl FM Jr. (1984) Sudden hearing loss: eight years' experience and suggested prognostic table. Laryngoscope 94: 647–661. [PubMed] [Google Scholar]
- 3. Nakashima T, Itoh A, Misawa H, Ohno Y (2000) Clinicoepidemiologic features of sudden deafness diagnosed and treated at university hospitals in Japan. Otolaryngol Head Neck Surg 123: 593–597. [DOI] [PubMed] [Google Scholar]
- 4. Chau JK, Lin JR, Atashband S, Irvine RA, Westerberg BD (2010) Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope 120: 1011–1021. 10.1002/lary.20873 [DOI] [PubMed] [Google Scholar]
- 5. Stew BT, Fishpool SJ, Williams H (2012) Sudden sensorineural hearing loss. Br J Hosp Med (Lond) 73: 86–89. [DOI] [PubMed] [Google Scholar]
- 6. Kuhn M, Heman-Ackah SE, Shaikh JA, Roehm PC (2011) Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends Amplif 15: 91–105. 10.1177/1084713811408349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mattox DE, Simmons FB (1977) Natural history of sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol 86: 463–480. [DOI] [PubMed] [Google Scholar]
- 8. Capaccio P, Ottaviani F, Cuccarini V, Bottero A, Schindler A, Cesana BM, et al. (2007) Genetic and acquired prothrombotic risk factors and sudden hearing loss. Laryngoscope 117: 547–551. [DOI] [PubMed] [Google Scholar]
- 9. Aimoni C, Bianchini C, Borin M, Ciorba A, Fellin R, Martini A, et al. (2010) Diabetes, cardiovascular risk factors and idiopathic sudden sensorineural hearing loss: a case-control study. Audiol Neurootol 15: 111–115. 10.1159/000231636 [DOI] [PubMed] [Google Scholar]
- 10. Cadoni G, Scipione S, Agostino S, Addolorato G, Cianfrone F, Leggio L, et al. (2007) Coenzyme Q 10 and cardiovascular risk factors in idiopathic sudden sensorineural hearing loss patients. Otol Neurotol 28: 878–883. [DOI] [PubMed] [Google Scholar]
- 11. Cadoni G, Scorpecci A, Cianfrone F, Giannantonio S, Paludetti G, Lippa S. (2010) Serum fatty acids and cardiovascular risk factors in sudden sensorineural hearing loss: a case-control study. Ann Otol Rhinol Laryngol 119: 82–88. [DOI] [PubMed] [Google Scholar]
- 12. Rudack C, Langer C, Stoll W, Rust S, Walter M (2006) Vascular risk factors in sudden hearing loss. Thromb Haemost 95: 454–461. [DOI] [PubMed] [Google Scholar]
- 13. Quaranta N, Ramunni A, Brescia P, D'Elia A, Vacca A, Ria R. (2008) Soluble intercellular adhesion molecule 1 and soluble vascular cell adhesion molecule 1 in sudden hearing loss. Otol Neurotol 29: 470–474. 10.1097/MAO.0b013e318170b650 [DOI] [PubMed] [Google Scholar]
- 14. Lu YY, Jin Z, Tong BS, Yang JM, Liu YH, Duan M. (2008) A clinical study of microcirculatory disturbance in Chinese patients with sudden deafness. Acta Otolaryngol 128: 1168–1172. 10.1080/00016480801901626 [DOI] [PubMed] [Google Scholar]
- 15. Mosnier I, Stepanian A, Baron G, Bodenez C, Robier A, Meyer B, et al. (2011) Cardiovascular and thromboembolic risk factors in idiopathic sudden sensorineural hearing loss: a case-control study. Audiol Neurootol 16: 55–66. 10.1159/000312640 [DOI] [PubMed] [Google Scholar]
- 16. Chang SL, Hsieh CC, Tseng KS, Weng SF, Lin YS (2014) Hypercholesterolemia is correlated with an increased risk of idiopathic sudden sensorineural hearing loss: a historical prospective cohort study. Ear Hear 35: 256–261. 10.1097/AUD.0b013e3182a76637 [DOI] [PubMed] [Google Scholar]
- 17. Orita S, Fukushima K, Orita Y, Nishizaki K (2007) Sudden hearing impairment combined with diabetes mellitus or hyperlipidemia. Eur Arch Otorhinolaryngol 264: 359–362. [DOI] [PubMed] [Google Scholar]
- 18. Ullrich D, Aurbach G, Drobik C (1992) A prospective study of hyperlipidemia as a pathogenic factor in sudden hearing loss. Eur Arch Otorhinolaryngol 249: 273–276. [DOI] [PubMed] [Google Scholar]
- 19. Oreskovic Z, Shejbal D, Bicanic G, Kekic B (2011) Influence of lipoproteins and fibrinogen on pathogenesis of sudden sensorineural hearing loss. J Laryngol Otol 125: 258–261. 10.1017/S0022215110002252 [DOI] [PubMed] [Google Scholar]
- 20. Nagaoka J, Anjos MF, Takata TT, Chaim RM, Barros F, Penido Nde O. (2010) Idiopathic sudden sensorineural hearing loss: evolution in the presence of hypertension, diabetes mellitus and dyslipidemias. Braz J Otorhinolaryngol 76: 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marcucci R, Alessandrello Liotta A, Cellai AP, Rogolino A, Berloco P, Leprini E, et al. (2005) Cardiovascular and thrombophilic risk factors for idiopathic sudden sensorineural hearing loss. J Thromb Haemost 3: 929–934. [DOI] [PubMed] [Google Scholar]
- 22. Weng T, Devine EE, Xu H, Yan Z, Dong P (2013) A clinical study of serum lipid disturbance in Chinese patients with sudden deafness. Lipids Health Dis 12: 95 10.1186/1476-511X-12-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR (2000) Empirical assessment of effect of publication bias on meta-analyses. BMJ 320: 1574–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin RJ, Krall R, Westerberg BD, Chadha NK, Chau JK (2012) Systematic review and meta-analysis of the risk factors for sudden sensorineural hearing loss in adults. Laryngoscope 122: 624–635. 10.1002/lary.22480 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper.