Abstract
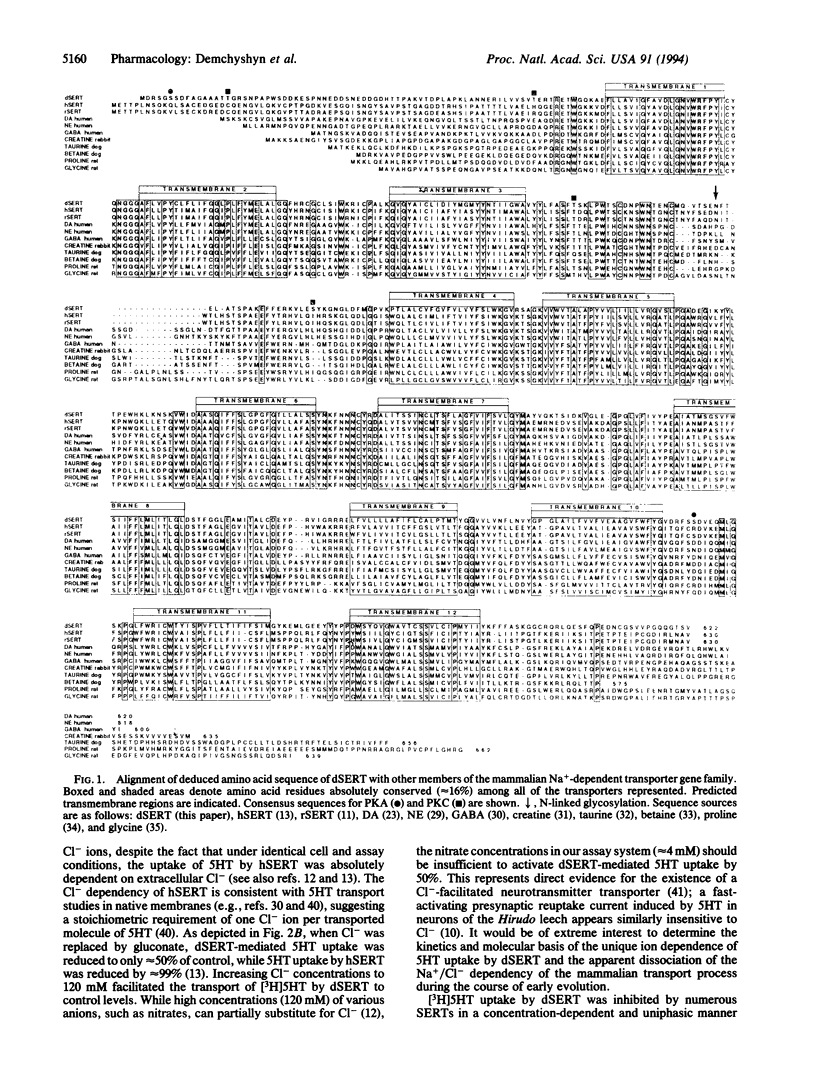
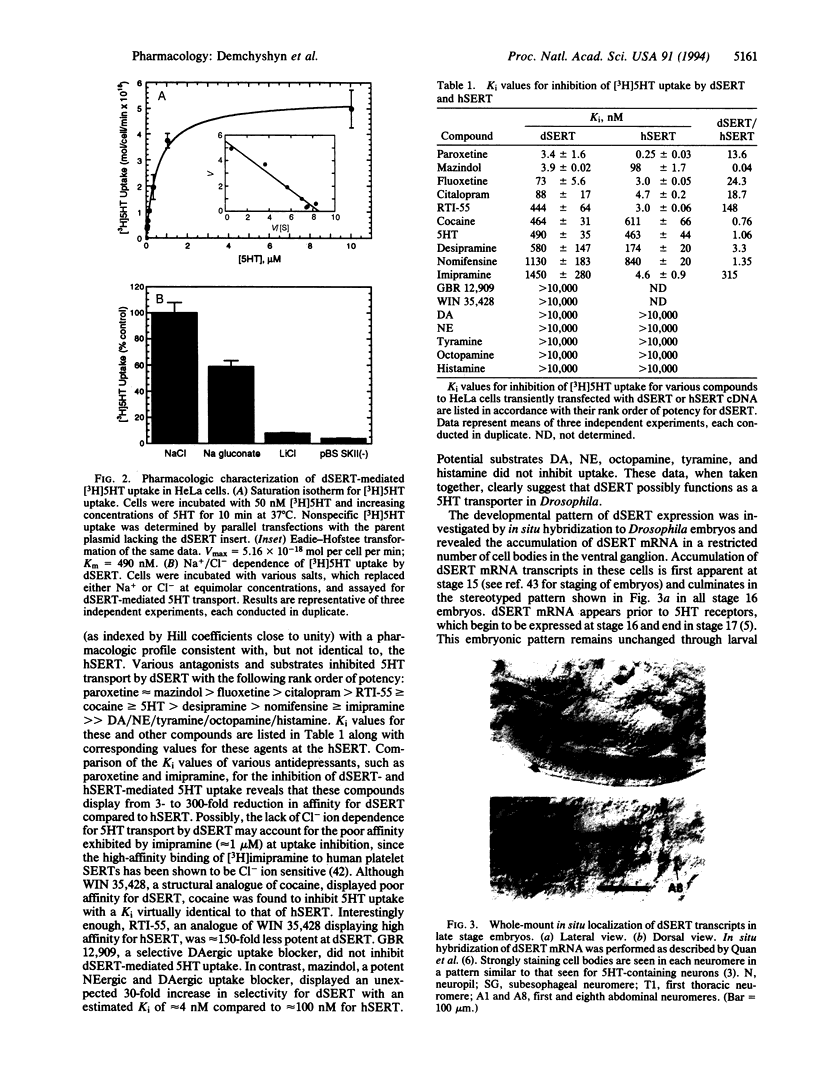
We report here on the isolation and characterization of a serotonin (5HT) transporter from Drosophila melanogaster. A 3.1-kb complementary DNA clone (dSERT) was found to encode a protein of 622 amino acid residues with a predicted molecular mass of approximately 69 kDa and a putative transmembrane topology characteristic of cloned members of the mammalian Na+/Cl- neurotransmitter cotransporter gene family. dSERT displays highest overall amino acid sequence identity with the mammalian 5HT (51%), norepinephrine (47%), and dopamine (47%) transporters and shares with all transporters 104 absolutely conserved amino acid residues. Upon transient expression in HeLa cells, dSERT exhibited saturable, high-affinity, and sodium-dependent [3H]5HT uptake with estimated Km and Vmax values of approximately 500 nM and 5.2 x 10(-18) mol per cell per min, respectively. In marked contrast to the human SERT (hSERT), 5HT-mediated transport by dSERT was not absolutely dependent on extracellular Cl-, while the sodium-dependent uptake of 5HT was facilitated by increased extracellular Cl- concentrations. dSERT displays a pharmacological profile and rank order of potency consistent with, but not identical to, mammalian 5HT transporters. Comparison of the affinities of various compounds for the inhibition of 5HT transport by both dSERT and hSERT revealed that antidepressants were 3- to 300-fold less potent on dSERT than on hSERT, while mazindol displayed approximately 30-fold greater potency for dSERT. Both cocaine and RTI-55 inhibited 5HT uptake by dSERT with estimated inhibition constants of approximately 500 nM, while high concentrations (> 10 microM) of dopamine, norepinephrine, octopamine, tyramine, and histamine failed to inhibit transport. In situ hybridization reveals the selective expression of dSERT mRNA to specific cell bodies in the ventral ganglion of the embryonic and larval Drosophila nervous system with a distribution pattern virtually identical to that of 5HT-containing neurons. The dSERT gene was mapped to position 60C on chromosome 2. The availability of the gene encoding the unique ion dependence and pharmacological characteristics of dSERT may allow for identification of those amino acid residues and structural motifs that confer the pharmacologic specificity and genetic regulation of the 5HT transport process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Kuhar M. J. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Blakely R. D., Berson H. E., Fremeau R. T., Jr, Caron M. G., Peek M. M., Prince H. K., Bradley C. C. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991 Nov 7;354(6348):66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Blakely R. D., Clark J. A., Rudnick G., Amara S. G. Vaccinia-T7 RNA polymerase expression system: evaluation for the expression cloning of plasma membrane transporters. Anal Biochem. 1991 May 1;194(2):302–308. doi: 10.1016/0003-2697(91)90233-j. [DOI] [PubMed] [Google Scholar]
- Bruns D., Engert F., Lux H. D. A fast activating presynaptic reuptake current during serotonergic transmission in identified neurons of Hirudo. Neuron. 1993 Apr;10(4):559–572. doi: 10.1016/0896-6273(93)90159-o. [DOI] [PubMed] [Google Scholar]
- Budnik V., White K. Genetic dissection of dopamine and serotonin synthesis in the nervous system of Drosophila melanogaster. J Neurogenet. 1987 Dec;4(6):309–314. [PubMed] [Google Scholar]
- Budnik V., Wu C. F., White K. Altered branching of serotonin-containing neurons in Drosophila mutants unable to synthesize serotonin and dopamine. J Neurosci. 1989 Aug;9(8):2866–2877. doi: 10.1523/JNEUROSCI.09-08-02866.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Corey J. L., Quick M. W., Davidson N., Lester H. A., Guastella J. A cocaine-sensitive Drosophila serotonin transporter: cloning, expression, and electrophysiological characterization. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1188–1192. doi: 10.1073/pnas.91.3.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzel I. D., Gottmann K. Development of dopamine-containing neurons and dopamine uptake in embryos of Hirudo medicinalis. Dev Biol. 1988 Aug;128(2):277–283. doi: 10.1016/0012-1606(88)90290-4. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Neurogenetic dissection of learning and short-term memory in Drosophila. Annu Rev Neurosci. 1988;11:537–563. doi: 10.1146/annurev.ne.11.030188.002541. [DOI] [PubMed] [Google Scholar]
- Fremeau R. T., Jr, Caron M. G., Blakely R. D. Molecular cloning and expression of a high affinity L-proline transporter expressed in putative glutamatergic pathways of rat brain. Neuron. 1992 May;8(5):915–926. doi: 10.1016/0896-6273(92)90206-s. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B., Caron M. G. Molecular characterization of the dopamine transporter. Trends Pharmacol Sci. 1993 Feb;14(2):43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Giros B., el Mestikawy S., Godinot N., Zheng K., Han H., Yang-Feng T., Caron M. G. Cloning, pharmacological characterization, and chromosome assignment of the human dopamine transporter. Mol Pharmacol. 1992 Sep;42(3):383–390. [PubMed] [Google Scholar]
- Guimbal C., Kilimann M. W. A Na(+)-dependent creatine transporter in rabbit brain, muscle, heart, and kidney. cDNA cloning and functional expression. J Biol Chem. 1993 Apr 25;268(12):8418–8421. [PubMed] [Google Scholar]
- Hoffman B. J., Mezey E., Brownstein M. J. Cloning of a serotonin transporter affected by antidepressants. Science. 1991 Oct 25;254(5031):579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- Humphreys C. J., Beidler D., Rudnick G. Substrate and inhibitor binding and translocation by the platelet plasma membrane serotonin transporter. Biochem Soc Trans. 1991 Feb;19(1):95–98. doi: 10.1042/bst0190095. [DOI] [PubMed] [Google Scholar]
- Kitayama S., Shimada S., Xu H., Markham L., Donovan D. M., Uhl G. R. Dopamine transporter site-directed mutations differentially alter substrate transport and cocaine binding. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7782–7785. doi: 10.1073/pnas.89.16.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lesch K. P., Wolozin B. L., Estler H. C., Murphy D. L., Riederer P. Isolation of a cDNA encoding the human brain serotonin transporter. J Neural Transm Gen Sect. 1993;91(1):67–72. doi: 10.1007/BF01244919. [DOI] [PubMed] [Google Scholar]
- Lesch K. P., Wolozin B. L., Murphy D. L., Reiderer P. Primary structure of the human platelet serotonin uptake site: identity with the brain serotonin transporter. J Neurochem. 1993 Jun;60(6):2319–2322. doi: 10.1111/j.1471-4159.1993.tb03522.x. [DOI] [PubMed] [Google Scholar]
- Myers C. L., Lazo J. S., Pitt B. R. Translocation of protein kinase C is associated with inhibition of 5-HT uptake by cultured endothelial cells. Am J Physiol. 1989 Oct;257(4 Pt 1):L253–L258. doi: 10.1152/ajplung.1989.257.4.L253. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A., Hunnicutt E. J., Kantham L., Scavone C. Cocaine as a naturally occurring insecticide. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9645–9648. doi: 10.1073/pnas.90.20.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. J., Rudnick G. The role of chloride ion in platelet serotonin transport. J Biol Chem. 1982 Jun 10;257(11):6151–6155. [PubMed] [Google Scholar]
- Pacholczyk T., Blakely R. D., Amara S. G. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991 Mar 28;350(6316):350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Pristupa Z. B., Wilson J. M., Hoffman B. J., Kish S. J., Niznik H. B. Pharmacological heterogeneity of the cloned and native human dopamine transporter: disassociation of [3H]WIN 35,428 and [3H]GBR 12,935 binding. Mol Pharmacol. 1994 Jan;45(1):125–135. [PubMed] [Google Scholar]
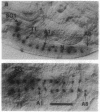
- Quan F., Wolfgang W. J., Forte M. A Drosophila G-protein alpha subunit, Gf alpha, expressed in a spatially and temporally restricted pattern during Drosophila development. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4236–4240. doi: 10.1073/pnas.90.9.4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S., Bauman A. L., Moore K. R., Han H., Yang-Feng T., Chang A. S., Ganapathy V., Blakely R. D. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith M. E., Zimanyi I., O'Reilly C. A. Role of ions and membrane potential in uptake of serotonin into plasma membrane vesicles from mouse brain. Biochem Pharmacol. 1989 Jul 1;38(13):2091–2097. doi: 10.1016/0006-2952(89)90062-2. [DOI] [PubMed] [Google Scholar]
- Rudnick G., Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993 Oct 4;1144(3):249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- Saudou F., Boschert U., Amlaiky N., Plassat J. L., Hen R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 1992 Jan;11(1):7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S., Kitayama S., Lin C. L., Patel A., Nanthakumar E., Gregor P., Kuhar M., Uhl G. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science. 1991 Oct 25;254(5031):576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- Smith K. E., Borden L. A., Hartig P. R., Branchek T., Weinshank R. L. Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron. 1992 May;8(5):927–935. doi: 10.1016/0896-6273(92)90207-t. [DOI] [PubMed] [Google Scholar]
- Tempel B. L., Livingstone M. S., Quinn W. G. Mutations in the dopa decarboxylase gene affect learning in Drosophila. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3577–3581. doi: 10.1073/pnas.81.11.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Kwon H. M., Yamauchi A., Preston A. S., Marumo F., Handler J. S. Molecular cloning of the cDNA for an MDCK cell Na(+)- and Cl(-)-dependent taurine transporter that is regulated by hypertonicity. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8230–8234. doi: 10.1073/pnas.89.17.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallés A. M., White K. Development of serotonin-containing neurons in Drosophila mutants unable to synthesize serotonin. J Neurosci. 1986 May;6(5):1482–1491. doi: 10.1523/JNEUROSCI.06-05-01482.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallés A. M., White K. Serotonin-containing neurons in Drosophila melanogaster: development and distribution. J Comp Neurol. 1988 Feb 15;268(3):414–428. doi: 10.1002/cne.902680310. [DOI] [PubMed] [Google Scholar]
- Vandenbergh D. J., Persico A. M., Uhl G. R. A human dopamine transporter cDNA predicts reduced glycosylation, displays a novel repetitive element and provides racially-dimorphic TaqI RFLPs. Brain Res Mol Brain Res. 1992 Sep;15(1-2):161–166. doi: 10.1016/0169-328x(92)90165-8. [DOI] [PubMed] [Google Scholar]
- Veyhl M., Spangenberg J., Püschel B., Poppe R., Dekel C., Fritzsch G., Haase W., Koepsell H. Cloning of a membrane-associated protein which modifies activity and properties of the Na(+)-D-glucose cotransporter. J Biol Chem. 1993 Nov 25;268(33):25041–25053. [PubMed] [Google Scholar]
- Wierenga J. M., Hollingworth R. M. Octopamine uptake and metabolism in the insect nervous system. J Neurochem. 1990 Feb;54(2):479–489. doi: 10.1111/j.1471-4159.1990.tb01897.x. [DOI] [PubMed] [Google Scholar]
- Witz P., Amlaiky N., Plassat J. L., Maroteaux L., Borrelli E., Hen R. Cloning and characterization of a Drosophila serotonin receptor that activates adenylate cyclase. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8940–8944. doi: 10.1073/pnas.87.22.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi A., Uchida S., Kwon H. M., Preston A. S., Robey R. B., Garcia-Perez A., Burg M. B., Handler J. S. Cloning of a Na(+)- and Cl(-)-dependent betaine transporter that is regulated by hypertonicity. J Biol Chem. 1992 Jan 5;267(1):649–652. [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]