Abstract
It is often assumed that mating with close relatives reduces offspring fitness. In such cases, reduced offspring fitness may arise from inbreeding depression (i.e., genetic effects of elevated homozygosity) or from post-mating maternal investment. This can be due to a reduction in female investment after mating with genetically incompatible males (“differential allocation”) or compensation for incompatibility (“reproductive compensation”). Here, we looked at the effects of mating with relatives on offspring fitness in mosquitofish, Gambusia holbrooki. In this species, females are assumed to be nonplacental and to allocate resources to eggs before fertilization, limiting differential allocation. We looked at the effects of mating with a brother or with an unrelated male on brood size, offspring size, gestation period, and early offspring growth. Mating with a relative reduced the number of offspring at birth, but there was no difference in the likelihood of breeding, gestation time, nor in the size or growth of these offspring. We suggest that due to limited potential for maternal effects to influence these traits that any reduction in offspring fitness, or lack thereof, can be explained by inbreeding depression rather than by maternal effects. We highlight the importance of considering the potential role of maternal effects when studying inbreeding depression and encourage further studies in other Poeciliid species with different degrees of placentation to test whether maternal effects mask or amplify any genetic effects of mating with relatives.
Keywords: Lecithotrophic, maternal investment, offspring fitness, relatives
Introduction
Mating with close relatives often reduces offspring fitness (Keller and Waller 2002). This can take the form of a reduction in offspring birth weight, survival, or reproductive success, as well as resistance to disease, predation, and environmental stress (Keller and Waller 2002; Frommen et al. 2008). The decrease in offspring fitness resulting from mating with close relatives is often attributed to inbreeding depression (Charlesworth and Charlesworth 1987; Falconer and Mackay 1996). Inbreeding depression results from an increase in the levels of homozygosis (Keller and Waller 2002; Frommen et al. 2008) and has been explained by two main hypotheses. The overdominance hypothesis, where heterozygotes, which are assumed to be superior to homozygotes, decrease in frequency, and the partial dominance hypothesis where the unmasking of deleterious recessive alleles due to greater homozygosity reduces fitness (Charlesworth and Charlesworth 1987). However, inbreeding depression is not the only explanation for differences in offspring fitness when mating with close relatives rather than unrelated individuals.
Maternal investment in offspring in response to male traits is known to have important effects on offspring phenotypes (Kindsvater and Alonzo 2014). This means that variation in offspring traits, particularly those expressed early in life, may result from variation in maternal investment (i.e., maternal effects) rather than being solely attributable to offspring genotype. Mothers can differentially allocate resources into offspring to maximize their fitness (Sheldon 2000). This is widely associated with greater maternal investment into offspring sired by more attractive males, who possess generally preferred traits (e.g., large ornaments; Arct et al. 2010; Horvathova et al. 2012). It follows that differential allocation by females may also be influenced by the relatedness of their mating partner (Lihoreau et al. 2008) as genetically similar males are generally considered to be less attractive mates because of the potential costs of inbreeding (Tregenza and Wedell 2000). Females may therefore be expected to reduce investment in offspring that are sired by closely related males (e.g., Sardell and DuVal 2014). Alternatively, females could partially compensate for the lower quality of their offspring by providing more resources when mating to nonpreferred or genetically incompatible mates (Ratikainen and Kokko 2010). If present, maternal effects may enhance (for differential allocation) or mask (for reproductive compensation) the potentially negative genetic effects of mating with a relative.
Early life-history traits such as embryo survival, number, quality, and the viability of offspring (Bernasconi et al. 2004; Frommen et al. 2008) are closely related to fitness (DeRose and Roff 1999; Janicke et al. 2014) and, as such, often suffer from inbreeding depression (Roff 1998; DeRose and Roff 1999). However, these are the same traits that are most likely to be influenced by maternal effects (Wolf and Wade 2009; Kindsvater and Alonzo 2014). Consequently, it is important for studies investigating how mating with relatives influences offspring performance to consider, and ideally control for, maternal effects to avoid potentially inaccurate measures of inbreeding depression.
Here, we examine the effects of mating with relatives on offspring fitness in mosquitofish (Gambusia holbrooki), a species with limited opportunity for post-mating maternal effects. They are small fish that live in streams and ponds (Pyke 2005) with seasonally fluctuating water levels, so they are often exposed to stochastic reductions in population size, especially during dry seasons (Scribner et al. 1992; Griffiths and Magurran 1997). This makes them vulnerable to the risk of inbreeding. Furthermore, mosquitofish are lecithotrophic (i.e., allocate resources for embryo development to eggs before fertilization), which limits the opportunity for females to differentially allocate resources toward offspring after mating (i.e., matrotrophy; Ojanguren et al. 2005; Pollux et al. 2014). There is limited evidence of transfer of nutrients such as amino acids and metals in other species of mosquitofish (Marsh-Matthews et al. 2005, 2010; Cazan and Klerks 2014) that suggests post-fertilization transfer from mother to embryos. Although this means that there is the potential for maternal effects to confound those directly due to inbreeding depression, the lack of evidence for an increase in offspring mass between the egg and birth stage strongly suggests that transfer of nutrients does not generally occur in Gambusia holbrooki (Pollux et al. 2014).
We looked at the effects of mating with a sibling on several reproductive and early life-history traits. We examined offspring number, offspring size, gestation period, and early offspring growth. If we assume, based on the lack of evidence for matrotrophy, that eggs are fully provisioned prior to mating, we predicted that genetic effects of mating with relatives would most likely influence the number of offspring (via effects during fertilization or embryo development), as well as their size at birth and their growth after birth. On the other hand, we predicted that maternal effects are likely to influence the proportion of females breeding and gestation time (i.e., females can determine if and when to fertilize eggs).
Materials and Methods
Origin and maintenance of fish
Our laboratory stock of mosquitofish originated from 151 wild-caught females collected in Canberra, Australia in February and March 2013. F1 generation offspring were kept in single sex tanks under a 14:10 h photoperiod at 28°C and fed ad libitum with Artemia nauplii and commercial flakes.
Experimental design
To create our parental generation, we set up 150 unique male–female pairs that were randomly created from the F1 laboratory stock (described above). From these, we obtained 58 outbred F2 full-sib families that were used to examine the effects of mating with relatives on female reproductive effort and early life offspring performance. We used a fully balanced block design that involved mating individuals from two families (e.g., A and B). Brothers and sisters from full-sibling families were paired to create inbred offspring (AA and BB) and outbred offspring by the reciprocal crossings of males and females from each family (AB and BA; Fig.1). We set up multiple females (one to four full sisters) per cross-type (AA, AB, BA, BB). Within each block, the same potential number of females contributed to each cross-type. Only one male contributed to each cross-type so that within each block the offspring of each cross-type were either full siblings or paternal half siblings. Males and females were placed together for 1 week to allow mating. Females were then placed in individual 1-L tanks and allowed 6 weeks to give birth. They were checked for offspring twice daily. We set up 29 blocks yielding a maximum total of 58 inbred families and 58 outbred families. We recorded the age and size (standard length, SL in mm) of each female on the day she gave birth, the gestation time, the number of offspring, the size of offspring at birth, and their size 1 week later. To measure female size, females were anaesthetized by submersion in ice-cold water for a few seconds to reduce movement and then photographed alongside a microscopic ruler (0.1 mm gradation). To measure offspring size, fry were placed in a plastic dish (27 × 27 mm) with 2 mm depth of water to restrict movement and a scale at the bottom. All offspring were photographed within 18 h of birth.
Figure 1.
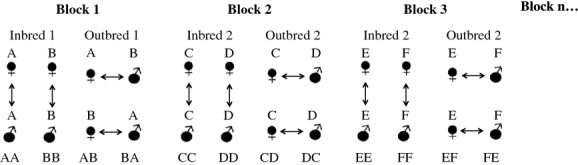
Block design for mating F2 families to create inbred vs outbred fish. Each block involved between one and four full sisters and one male per cross-type from two families (A and B in block 1, C and D in block 2, E and F in block 3, and so on). Arrows indicate matings.
Statistical analysis
We tested for a difference in reproductive success between females mating with a related or an unrelated male by comparing the proportion that gave birth within 6 weeks of the mating period using a chi-squared test. When testing for an effect of mating with relatives on gestation time and the number of offspring produced, we only included first broods by females that gave birth during the first 6 weeks. This avoids any confounding effect of a change in brood size with brood order (Larsen et al. 2011). These analyses were based on a single value per brood. To test for an inbreeding effect on size at birth and growth rates (size at 1 week of age – size at birth), we included the data from each individual offspring that the female gave birth to. Cross-types AA and BB were classified as inbreeding, while AB and BA were classified as outbreeding.
Female reproductive effort
We used generalized linear mixed-effect models (GLMM) with Gaussian error to test for fixed effects of treatment (related or unrelated male), female age, and female size on gestation time, number and size of offspring, and the growth rate of offspring with the lmer function using the lme4 package in R 3.0.2 software (R Development Core Team 2012). We included the female's family identity as a random effect when testing for effects on gestation time and number of offspring. We included the female's individual identity as a random factor when testing offspring size and growth (as we measured multiple fry per female). We treated maternal age and size as independent predictors because they were uncorrelated (r = −0.027, P = 0.716, N = 179; age range: 82–141 days, size: 22.76–31.25 mm).
Inbreeding coefficient
We calculated the standardized coefficient of inbreeding δ (Lande and Schemske 1985) as the percentage change with inbreeding: (outbred trait value – inbred trait value) / outbred trait value. A negative value indicates that inbred individuals had a larger value for the trait, interpretation of which depends on the direction of selection on the trait.
Results
There was no difference in the proportion of females producing broods when mated with either a related or unrelated male. From 162 females that mated with their brother, 79.6% gave birth, while from 147 females mated with an unrelated male, 77.5% gave birth (χ2 = 0.198, df = 1, P = 0.656). From 309 females that could have produced broods, 199 were used for analyses of first broods produced within 6 weeks of mating (112 mated with a brother; 87 with an unrelated male).
Female reproductive effort
The number of offspring a female gave birth to (range: 1- 15) was affected by whether or not she mated with a related male (Fig.2). Females mated to their brother gave birth to significantly fewer offspring than those mated to an unrelated male (an inbreeding coefficient of δ = 14.5%; Table1). The number of offspring in the brood was significantly negatively related to the female's age, but significantly positively related to her size (Table1).
Figure 2.
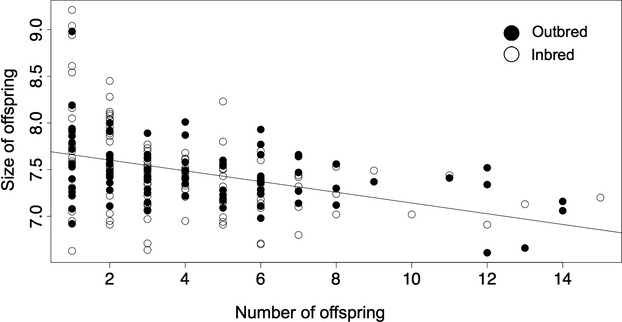
The association between number and size of outbred and inbred offspring.
Table 1.
Results of GLMs (Gaussian error) for the response variables: gestation time, number of offspring, size of offspring, and growth of offspring of females mated to related and unrelated males. Inbreeding coefficient (% change with inbreeding). Bold values represent significant values
| Response | Predictor | β | SE | df | t | P | Mean ± SE (N) | δ | |
|---|---|---|---|---|---|---|---|---|---|
| Inbred | Outbred | ||||||||
| Gestation time (days) | Intercept | 28.756 | 17.224 | 101.150 | 1.669 | 0.098 | 33.67 ± 0.794 (112) | 32.33 ± 0.883 (87) | −4.145 |
| Treatment | 1.228 | 1.204 | 159.180 | 1.020 | 0.309 | ||||
| Female size | 0.230 | 0.532 | 132.770 | 0.432 | 0.667 | ||||
| Female age | −0.028 | 0.083 | 70.880 | −0.343 | 0.733 | ||||
| Number of offspring | Intercept | −4.941 | 6.125 | 112.740 | −0.807 | 0.422 | 3.83 ± 0.256 (112) | 4.48 ± 0.344 (87) | 14.509 |
| Treatment | −1.003 | 0.404 | 154.160 | −2.481 | 0.014 | ||||
| Female size | 0.671 | 0.186 | 143.070 | 3.599 | <0.001 | ||||
| Female age | −0.065 | 0.030 | 77.000 | −2.180 | 0.032 | ||||
| Size of offspring (mm) | Intercept | 7.030 | 0.783 | 101.800 | 8.975 | <0.001 | 7.352 ± 0.029 (212) | 7.368 ± 0.016 (590) | 0.217 |
| Treatment | 0.021 | 0.069 | 135.200 | 0.310 | 0.757 | ||||
| Female size | 0.003 | 0.024 | 121.700 | 0.138 | 0.890 | ||||
| Female age | 0.003 | 0.004 | 85.940 | 0.763 | 0.448 | ||||
| Growth of offspring (mm in first week) | Intercept | 3.104 | 1.164 | 99.150 | 2.666 | 0.009 | 3.633 ± 0.045 (172) | 3.560 ± 0.028 (501) | −2.050 |
| Treatment | 0.106 | 0.094 | 140.540 | 1.128 | 0.261 | ||||
| Female size | 0.024 | 0.036 | 114.280 | 0.680 | 0.498 | ||||
| Female age | −0.003 | 0.005 | 83.500 | −0.589 | 0.557 | ||||
In contrast, we found no evidence that mating with related males affected the gestation time of females, the size of offspring at birth (range: 6.61–9.21 mm), or early offspring growth. Nor did we find any effect of female size or age on any of these traits. Further, we found no repeatable difference in gestation time among families (Table1).
Discussion
Variation in traits expressed in offspring can be attributable to both parental effects and offspring genotype. For example, life-history traits related to female reproductive effort are a maternal character but they can also affect offspring fitness (Bernardo 1996; Fischer et al. 2006). When assessing whether mating with relatives causes inbreeding depression, maternal effects from differential allocation or reproductive compensation could exacerbate or mask potential genetic effects. In the present study, we found that mating with a relative (full sibling) in Gambusia holbrooki significantly reduced the number of offspring at birth (δ = 14.5%). There was, however, no significant decrease in the likelihood of breeding, no increase in gestation time (δ = 4.2%), and no reduction in the size (δ = 0.2%) or growth (δ = 2.1%) of the resultant offspring. Given the reproductive physiology of G. holbrooki (fully yolked eggs are produced prior to mating), there is no obvious mechanism for post-mating maternal effects on offspring size or growth, and maternal effects on offspring number and gestation time seem unlikely. It has, however, been suggested that mosquitofish are incipient matrotrophic rather than lecithotrophic organisms based on transfer of metals from mothers to offspring (Cazan and Klerks 2014), so we cannot definitively exclude the possibility that there are subtle maternal effects. Nonetheless, the decline in offspring dry weight from the egg to birth stage in G. holbrooki suggests that there is no transfer of nutrients to offspring (Pollux et al. 2014).
The smaller brood size of females mated to a related rather than an unrelated male has several potential explanations. First, sperm allocation toward related and unrelated females might differ (Firman and Simmons 2008; Lewis and Wedell 2009). However, it is unlikely that this explains our findings because males did not choose between females, and previous studies on mosquitofish (Head et al., in press) and more generally (Barry and Kokko 2010) show that males are rarely choosy when encountering females sequentially. Further, even very low sperm transfer is still likely to provide sufficient sperm to fertilize a full clutch (Bisazza and Marin 1991; Johnson et al. 2010). Second, females might decide not to fertilize all their eggs when mating with males of low compatibility (e.g., Olsson et al. 1996; Birkhead 1998). This is unlikely for several reasons: (1) Our experimental design reduced the potential for choice – females were virgins and previous work on Poeciliids has shown that virgins are not choosy with respect to mate quality (Pitcher et al. 2003), (2) There is little evidence of mate choice for unrelated males in Poeciliids (e.g., Pitcher et al. 2008; Ala-Honkola et al. 2010), but see (Kelley et al. 1999; Zajitschek and Brooks 2008) for studies showing male mate preferences based on familiarity and (Hain and Neff 2007) showing kin recognition in Poeciliids), and (3) If females differentially used sperm, this should increase their gestation time, and/or affect the proportion of females breeding. This did not occur. Females cannot provision eggs after fertilization, and lack superfetation (Ojanguren et al. 2005; Pollux et al. 2014), so there is no immediate benefit of discriminating against a related male's sperm (e.g., Pitcher et al. 2008). In short, there is no obvious adaptive explanation why females would partially fertilize a clutch.
Third, the most plausible explanation for females having fewer offspring when mated with related males is reduced fertilization success (i.e., low sperm survival due to sperm–female tract or egg interactions) and/or inbreeding depression lowering embryo survival (Pitcher et al. 2008; Johnson et al. 2010). In general, the evidence for a negative effect of mating with a related male-on-female reproductive effort is inconclusive: some studies report fewer offspring or eggs (e.g., Pitcher et al. 2008; Johnson et al. 2010), but others do not (e.g., Simmons et al. 2006; Ala-Honkola et al. 2009). However, based on studies of other Poeciliids, inbreeding depression for embryo viability is most likely to explain why G. holbrooki had fewer offspring after a full-sib mating (Pitcher et al. 2008; Johnson et al. 2010).
Offspring size at birth is under directional selection as larger offspring tend to be more competitive and survive better in stressful environments (Smith and Fretwell 1974; Simmons and Garcia-Gonzalez 2007). Larger offspring also tend to become adults with above average reproductive success (e.g., Czesak and Fox 2003). We did not, however, find any evidence of inbreeding reducing offspring size at birth or post birth growth, even though this should occur if higher homozygosity reduces the physiological efficiency with which offspring convert resources (i.e., egg yolk then Artemia) into body mass. One explanation for a lack of inbreeding depression is that offspring with bad genetic combinations died before birth. This explanation is also consistent with fewer offspring being born to females who mated with a brother.
In our experiment, males and females were allowed 1 week to interact and mate. We predicted that if females avoid mating with related males that those paired with their brother would take longer to mate and/or refrain from fertilizing their eggs and therefore would take longer to give birth. This did not occur. There is conflicting evidence for effects of mating with relatives on gestation time in Poeciliids: Some studies show that it increases (e.g., Pitcher et al. 2008), while others show no difference in gestation time (e.g., Ala-Honkola et al. 2009). Further experiments measuring egg fertilization following artificial insemination might yield more information about the mechanism, if any, by which females reduce the likelihood of inbreeding.
Conclusions
Studies often report reduced reproductive performance of females mating with related males and attribute this to inbreeding depression (i.e., genetic effects). These studies, however, almost always ignore the potential role of postmating maternal effects in response to the identity of their mating partner. Here, we show a reduction in the number of offspring produced when females mated with a full sibling in the mosquitofish, a species that has limited opportunity to influence this trait via maternal effects. Furthermore, there was no difference between females mated to related or unrelated males in traits that we expected to be influenced by maternal effects (gestation time and whether they breed) or in traits that are unlikely to be affected by maternal affects (offspring birth size and growth). A comparative study measuring inbreeding effects in species that vary in their ability to alter offspring traits via post-mating maternal effects is needed. We suggest that Poeciliids are an ideal group in which to conduct the requisite empirical studies because: (1) closely related species vary substantially in their level of placentation (Pollux et al. 2014), hence ability to adjust provisioning of nutrients to offspring, depending on the relatedness of their mate; (2) the risk of inbreeding seems to have played a role in mate choice in some Poeciliids (e.g., Zajitschek and Brooks 2008) so an adaptive phenotypically plastic maternal response based on relatedness to males with whom they mate is plausible.
Acknowledgments
We thank James Davies and the ANU Animal Services team for fish maintenance. This work was supported by the Australian Research Council (DP120100339). Animal use permit: ANU AEEC animal ethics protocol A2011/64. R.V.-T. is supported by fellowships from Consejo Nacional de Ciencia y Tecnología-México and the Research School of Biology.
Data Accessibility
Data will be deposited in Dryad upon acceptance.
Conflict of Interest
None declared.
References
- Ala-Honkola O, Uddstrom A, Diaz Pauli B. Lindstrom K. Strong inbreeding depression in male mating behaviour in a poeciliid fish. J. Evol. Biol. 2009;22:1396–1406. doi: 10.1111/j.1420-9101.2009.01765.x. [DOI] [PubMed] [Google Scholar]
- Ala-Honkola O, Tuominen L. Lindstrom K. Inbreeding avoidance in a poeciliid fish (Heterandria formosa. Behav. Ecol. Sociobiol. 2010;64:1403–1414. [Google Scholar]
- Arct A, Rutkowska J, Martyka R, Drobniak SM. Cichon M. Kin recognition and adjustment of reproductive effort in zebra finches. Biol. Lett. 2010;6:762–764. doi: 10.1098/rsbl.2010.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry KL. Kokko H. Male mate choice: why sequential choice can make its evolution difficult. Anim. Behav. 2010;80:163–169. [Google Scholar]
- Bernardo J. The particular maternal effect of propagule size, especially egg size: patterns, models, quality of evidence and interpretations. Am. Zool. 1996;36:216–236. [Google Scholar]
- Bernasconi G, Ashman TL, Birkhead TR, Bishop JD, Grossniklaus U, Kubli E, Marshall DL, Schmid B, Skogsmyr I, Snook RR, Taylor D, Till-Botttraud I, Ward PI, Zeh DW. Hellriegel B. Evolutionary ecology of the prezygotic stage. Science. 2004;303:971–975. doi: 10.1126/science.1092180. [DOI] [PubMed] [Google Scholar]
- Birkhead TR. Cryptic female choice: criteria for establishing female sperm choice. Evolution. 1998;52:1212–1218. doi: 10.1111/j.1558-5646.1998.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Bisazza A. Marin G. Male size and female mate choice in the eastern mosquitofish. Copeia. 1991;1991:728–733. [Google Scholar]
- Cazan AM. Klerks PL. Evidence of maternal copper and cadmium transfer in two live-bearing fish species. Ecotoxicology. 2014;23:1774–1783. doi: 10.1007/s10646-014-1342-3. [DOI] [PubMed] [Google Scholar]
- Charlesworth D. Charlesworth B. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 1987;18:237–268. [Google Scholar]
- Czesak ME. Fox CW. Evolutionary ecology of egg size and number in a seed beetle: genetic trade-off differs between environments. Evolution. 2003;57:1121–1132. doi: 10.1111/j.0014-3820.2003.tb00321.x. [DOI] [PubMed] [Google Scholar]
- DeRose MA. Roff DA. A comparison of inbreeding depression in life-history and morphological traits in animals. Evolution. 1999;53:1288–1292. doi: 10.1111/j.1558-5646.1999.tb04541.x. [DOI] [PubMed] [Google Scholar]
- Falconer DS. Mackay TFC. Introduction to quantitative genetics. Harlow, UK: Longman; 1996. [Google Scholar]
- Firman RC. Simmons LW. Polyandry facilitates postcopulatory inbreeding avoidance in house mice. Evolution. 2008;62:603–611. doi: 10.1111/j.1558-5646.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- Fischer K, Bot ANM, Brakefield PM. Zwaan BJ. Do mothers producing large offspring have to sacrifice fecundity? J. Evol. Biol. 2006;19:380–391. doi: 10.1111/j.1420-9101.2005.01046.x. [DOI] [PubMed] [Google Scholar]
- Frommen JG, Luz C, Mazzi D. Bakker TCM. Inbreeding depression affects fertilization success and survival but not breeding coloration in threespine sticklebacks. Behaviour. 2008;145:425–441. [Google Scholar]
- Griffiths SW. Magurran AE. Schooling preferences for familiar fish vary with group size in a wild guppy population. Proc. R. Soc. Lond. B Biol. Sci. 1997;264:547–551. [Google Scholar]
- Hain TJA. Neff BD. Multiple paternity and kin recognition mechanisms in a guppy population. Mol. Ecol. 2007;16:3938–3946. doi: 10.1111/j.1365-294X.2007.03443.x. [DOI] [PubMed] [Google Scholar]
- Head ML, Jacomb F, Vega-Trejo R, Jennions MD. Male mate choice and mating success under simultaneous versus sequential choice conditions. Animal Behaviour (in press) 2015.
- Horvathova T, Nakagawa S. Uller T. Strategic female reproductive investment in response to male attractiveness in birds. Proc. R. Soc. Lond. B Biol. Sci. 2012;279:163–170. doi: 10.1098/rspb.2011.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke T, Vellnow N, Lamy T, Chapuis E. David P. Inbreeding depression of mating behavior and its reproductive consequences in a freshwater snail. Behav. Ecol. 2014;25:288–299. [Google Scholar]
- Johnson AM, Chappell G, Price AC, Rodd FH, Olendorf R. Hughes KA. Inbreeding depression and inbreeding avoidance in a natural population of guppies (Poecilia reticulata. Ethology. 2010;116:448–457. [Google Scholar]
- Keller LF. Waller DM. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002;17:230–241. [Google Scholar]
- Kelley JL, Graves JA. Magurran AE. Familiarity breeds contempt in guppies. Nature. 1999;401:661–662. doi: 10.1038/44314. [DOI] [PubMed] [Google Scholar]
- Kindsvater HK. Alonzo SH. Females allocate differentially to offspring size and number in response to male effects on female and offspring fitness. P. Roy. Soc. Lond. B Biol. Sci. 2014;281:20131981. doi: 10.1098/rspb.2013.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R. Schemske DW. The evolution of self-fertilization and inbreeding depression in plants.1. Genetic models. Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Larsen LK, Pelabon C, Bolstad GH, Viken A, Fleming IA. Rosenqvist G. Temporal change in inbreeding depression in life-history traits in captive populations of guppy (Poecilia reticulata): evidence for purging? J. Evol. Biol. 2011;24:823–834. doi: 10.1111/j.1420-9101.2010.02224.x. [DOI] [PubMed] [Google Scholar]
- Lewis Z. Wedell N. Male moths reduce sperm investment in relatives. Anim. Behav. 2009;77:1547–1550. [Google Scholar]
- Lihoreau M, Zimmer C. Rivault C. Mutual mate choice: when it pays both sexes to avoid inbreeding. PLoS ONE. 2008;3:e3365. doi: 10.1371/journal.pone.0003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh-Matthews E, Brooks M, Deaton R. Tan H. Effects of maternal and embryo characteristics on post-fertilization provisioning in fishes of the genus Gambusia. Oecologia. 2005;144:12–24. doi: 10.1007/s00442-005-0030-7. [DOI] [PubMed] [Google Scholar]
- Marsh-Matthews E, Deaton R. Brooks M. Survey of matrotrophy in lecithotrophic poeciliids. In: Uribe MC, Grier HJ, editors. Viviparous Fishes II, the Proceedings of the III International Symposium on Viviparous Fishes. Homestead, FL: New Life Publications; 2010. pp. 255–258. , eds., and. [Google Scholar]
- Ojanguren AF, Evans JP. Magurran AE. Multiple mating influences offspring size in guppies. J. Fish Biol. 2005;67:1184–1188. [Google Scholar]
- Olsson M, Shine R, Madsen T, Gullberg A. Tegelstrom H. Sperm selection by females. Nature. 1996;383:585–585. [Google Scholar]
- Pitcher TE, Neff BD, Rodd FH. Rowe L. Multiple mating and sequential mate choice in guppies: females trade up. Proc. R. Soc. Biol. Sci. Ser. B. 2003;270:1623–1629. doi: 10.1098/rspb.2002.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher TE, Rodd FH. Rowe L. Female choice and the relatedness of mates in the guppy (Poecilia reticulata. Genetica. 2008;134:137–146. doi: 10.1007/s10709-008-9246-x. [DOI] [PubMed] [Google Scholar]
- Pollux BJA, Meredith RW, Springer MS. Reznick DN. The evolution of the placenta drives a shift in sexual selection in live bearing fish. Nature. 2014;513:233–236. doi: 10.1038/nature13451. [DOI] [PubMed] [Google Scholar]
- Pyke GH. A review of the biology of Gambusia affinis and G-holbrooki. Rev. Fish Biol. Fish. 2005;15:339–365. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- Ratikainen II. Kokko H. Differential allocation and compensation: who deserves the silver spoon? Behav. Ecol. 2010;21:195–200. [Google Scholar]
- Roff DA. Effects of inbreeding on morphological and life history traits of the sand cricket, Gryllus firmus. Heredity. 1998;81:28–37. [Google Scholar]
- Sardell RJ. DuVal EH. Differential allocation in a lekking bird: females lay larger eggs and are more likely to have male chicks when they mate with less related males. P. Roy. Soc. Lond. B Biol. Sci. 2014;281:20132356. doi: 10.1098/rspb.2013.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner KT, Wooten MC, Smith MH, Kennedy PK. Rhodes OE. Variation in life-history and genetic-traits of Hawaiian mosquitofish populations. J. Evol. Biol. 1992;5:267–288. [Google Scholar]
- Sheldon BC. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 2000;15:397–402. doi: 10.1016/s0169-5347(00)01953-4. [DOI] [PubMed] [Google Scholar]
- Simmons LW. Garcia-Gonzalez F. Female crickets trade offspring viability for fecundity. J. Evol. Biol. 2007;20:1617–1623. doi: 10.1111/j.1420-9101.2007.01320.x. [DOI] [PubMed] [Google Scholar]
- Simmons LW, Beveridge M, Wedell N. Tregenza T. Postcopulatory inbreeding avoidance by female crickets only revealed by molecular markers. Mol. Ecol. 2006;15:3817–3824. doi: 10.1111/j.1365-294X.2006.03035.x. [DOI] [PubMed] [Google Scholar]
- Smith CC. Fretwell SD. Optimal balance between size and number of offspring. Am. Nat. 1974;108:499–506. [Google Scholar]
- Tregenza T. Wedell N. Genetic compatibility, mate choice and patterns of parentage: invited review. Mol. Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- Wolf JB. Wade MJ. What are maternal effects (and what are they not)? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:1107–1115. doi: 10.1098/rstb.2008.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajitschek SRK. Brooks RC. Distinguishing the effects of familiarity, relatedness, and color pattern rarity on attractiveness and measuring their effects on sexual selection in guppies (Poecilia reticulata. Am. Nat. 2008;172:843–854. doi: 10.1086/593001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be deposited in Dryad upon acceptance.


