Abstract
Delayed bone healing and non-union occur in approximately 10% of long bone fractures. Despite intense investigations and progress in understanding the processes governing bone healing, the specific pathophysiological characteristics of the local microenvironment leading to non-union remain obscure. The clinical findings and radiographic features remain the two important landmarks of diagnosing non-unions and even when the diagnosis is established there is debate on the ideal timing and mode of intervention. In an attempt to understand better the pathophysiological processes involved in the development of fracture non-union, a number of studies have endeavoured to investigate the biological profile of tissue obtained from the non-union site and analyse any differences or similarities of tissue obtained from different types of non-unions. In the herein study, we present the existing evidence of the biological and molecular profile of fracture non-union tissue.
Keywords: non-union(s), human tissue, bone morphogenic protein(s), mesenchymal stem cell(s)
Introduction
-
Materials and Methods
Eligibility Criteria
Information Sources
Study Selection
Extraction of Data
Data Analysis
-
Results
Literature Search
Studies Characteristics
Macroscopic Structure of non-union Tissue
Microscopic Structure of non-union Tissue
Bacteriology of the non-union
Evaluation of Tissue Sample
Cultures Characteristics
Discussion
Conclusion
Introduction
Bone healing is a complex but well-orchestrated physiological process which recapitulates aspects of the embryonic skeletal development in combination with the normal response to acute tissue injury 1,2. It encompasses multiple biological phenomena and is margined by the combination of osteoconduction (scaffold formation), osteoinduction (timed cellular recruitment controlled by multiple signalling molecules) and osteogenesis (new bone formation) 2–5. In contrast to the scar formation, which occurs in the majority of other tissue types in adults, bone has the innate capability to repair and regenerate, regaining its former biomechanical and biochemical properties 6–8.
During the bone healing process, a well-regulated series of overlapping processes take place in the cortical bone, the periosteum, the bone marrow and the undifferentiated fascial tissue surrounding the fracture 10,12,13. According to the histological appearance, two basic types of bone healing have been identified 6,7,11. The primary (direct) healing pattern occurs when anatomical reduction is achieved, along with almost absolute stability 3,15. The disrupted continuity of the bone in this type of healing is re-established with regeneration of the Harvesian system and the lamellar bone, with therefore no need of any remodelling 12,15. On the contrary, the secondary (indirect) healing pattern that occurs in the vast majority of clinical cases depends to the formation of fibrocartilaginous callus 3,6. This process can be broadly divided into five stages: that of inflammation, granulation tissue formation, soft callus formation (hyaline cartilage), hard callus formation (woven bone) and remodelling 6,9,11,14.
In more detail, following an injury the bone architecture is disrupted, as is the surrounding soft tissue continuity. Consequently, the local blood vessels are torn, a haematoma is formed and the coagulation cascade is activated 16. This fracture haematoma contains cells that originate from the peripheral and intramedullary blood, as well as from the bone marrow 15. They include inflammatory immune cells, neutrophils, monocytes and macrophages that are activated by the coagulation process; fibroblasts; and mesenchymal stem cells (MSCs) 6,16. Prostaglandins, cytokines and other proteins are abundant in this environment and contribute to the formation of a complex microenvironment which has different effect on each cell population 6. These mediators are known to increase cellular migration, proliferation, enhance osteogenesis, collagen synthesis and angiogenesis 6.
Subsequently, the necrotic or damaged pieces of bone are removed and the fracture haematoma is gradually replaced by granulation tissue 17. The osteoprogenitor cells then proliferate and differentiate, leading to deposition of collagen and formation of soft callus. An increased vascularity and intense cell proliferation in the cambium layer of the periosteum is evident in this stage 13,17. Bone formation then occurs by endochondral or intramembranous ossification. Initially, immature woven bone characterized by coarse collagen fibres arranged in a haphazard fashion is formed, but is then transformed to mature lamellar bone (remodelling) in a slow process 13,17. During remodelling that could last several months to years after fracture, both osteoblast and osteoclast activity is intense, with bone resorption followed by appositional production of new bone by osteoblasts 17.
In vitro investigations to evaluate osteogenic activity include measurements of a number of secreted substances (proteins) including: alkaline phosphatase (ALP), osteonectin, osteopontin, osteocalcin and bone sialoprotein. Alkaline phosphatase is a key protein secreted by osteoblasts in response to osteogenic activity and represents a marker of the earlier stage of osteoblast differentiation 18. Osteonectin, osteopontin and osteocalcin are non-collagenous bone matrix proteins, abundant in bone tissue 19. They are thought to be of great importance in bone development, growth, turnover and fracture repair; along with osterix, as essential factor for osteoblast differentiation and bone formation, they represent markers of the later stage of differentiation 18–20. Bone Sialoprotein, an extracellular matrix protein secreted by osteoblastic cells, has also been reported to modulate osteoblast differentiation and mineralization 21.
As already mentioned, the physiological sequence of fracture healing depends on numerous endogenous and exogenous factors 22,23. If this sensitive balance is altered in any way, complications may arise, such as delayed union or non-union. The criteria for defining a non-union are not yet standardized 24. FDA (Food and Drug Administration) defines a non-union as the incomplete fracture healing within 9 months following injury, along with absence of progressive signs of healing on serial radiographs over the course of three consecutive months 25. In the United States alone, it is estimated that 5–10% of all fractures are complicated by non-union or delayed union 26, posing an enormous economic burden to the healthcare system 27. The tibia and the femur are the most common long bones associated with the development of non-union 28,29.
According to the radiological and histological appearance, non-unions are characterized as: hypertrophic, usually resulting from insufficient fracture stabilization (extensive callus formation) 30; and atrophic, where the fracture stabilization is adequate but there is localized dysfunction in biological activity (little callus formation and presence of a fibrous tissue-filled fracture gap) 30,31. Synovial pseudarthrosis is considered as a different pathological entity, caused by inadequate immobilization with or without the presence of infection 32. Moreover, non-unions can be characterized according to the presence of bacteria at the fracture site, as septic or aseptic non-unions 33.
It is generally accepted that the progression to a non-union in most cases represents a multifactorial process. Various risk factors have been implicated with compromized fracture healing, including: patient dependent factors such as age, gender, medical comorbidities (i.e. anaemia, diabetes and hormone disorders), smoking and administration of pharmacological agents (i.e. steroids, non-steroidal anti-inflammatories, etc.); and patient independent factors such as the ‘personality’ of the fracture, presence of infection and adequacy of surgical technique 22,25,34.
The exact biological process leading to a non-union remains obscure and it is well accepted that any planned interventions to reverse this process should be well-timed and well-aimed to restore both biological and mechanical deficiencies 3,14,31,35. It can be postulated that by gaining a better understanding of the underlying mechanisms leading to a non-union, both clinicians and scientists would be allowed to target specific pathways independently, tailoring treatment to each patient's individual requirements 11. Therefore, the purpose of this review is to investigate the biological profile of tissue obtained from the non-union site and to analyse any differences or similarities of tissue obtained from different types of non-unions. Moreover, it aims to evaluate whether any interventions on the tissue obtained would influence in a positive aspect its biological characteristics and bone repair responses.
Materials and methods
This review was conducted in accordance to the PRISMA guidelines 36. Data were documented according to a standardized protocol, where objectives and inclusion criteria were specified in detail.
Eligibility criteria
Studies selected were original articles fulfilling the following inclusion criteria: (i) the tissue was obtained from a non-union site and examined or processed for defining its characteristics and properties; (ii) only tissue acquired from human subjects was included; (iii) articles were published in English language and (iv) the full text of each article was available. All studies that did not fulfil all eligibility criteria were excluded from further analysis, whereas no publication date restrictions were imposed.
Information sources
Studies were identified by searching the following resources/databases: PubMed Medline; Ovid Medline; Embase; Scopus; Google Scholar; and the Cochrane Library, to retrieve all available relevant articles. The terms used for the search included: non-union(s), nonunion(s), human, tissue, bone morphogenic protein(s) (BMP's) and MSCs. The identified articles and their bibliographies including any relevant reviews were manually searched for additional potential eligible studies.
Study selection
Two of the authors (M.P., I.P.) performed the eligibility assessment, in an independent, unblinded and standardized manner. Most citations were excluded on the basis of information provided by their respective title or abstract. In any other case, the complete manuscript was obtained, scrutinized by the two reviewers and included if fulfilling the eligibility criteria. Any disagreement between reviewers was resolved by consensus.
Extraction of data
Relevant information on author's name, publication year, patient demographics, site and duration of non-union, type of the non-union, characteristics and evaluation of tissue samples, culture properties, gene expression, protein expression and effect of additional interventions was carefully extracted.
Data analysis
All outcomes of interest were inserted in an electronic database and outcome of different studies were documented. The characteristics of tissue samples were then compared across different studies and the effect of any intervention was evaluated.
Results
Literature search
The electronic search of the literature retrieved 1532 citations, but only 21 of them met the selection criteria 14,18,19,22,30,35,37–51. Another three eligible papers 32,52,53 were obtained from the hand search of the references of the eligible studies and relevant review articles, yielding 24 eligible studies for the final analysis (Fig.1) 14,18,19,22,30,32,35,37–53.
Fig 1.
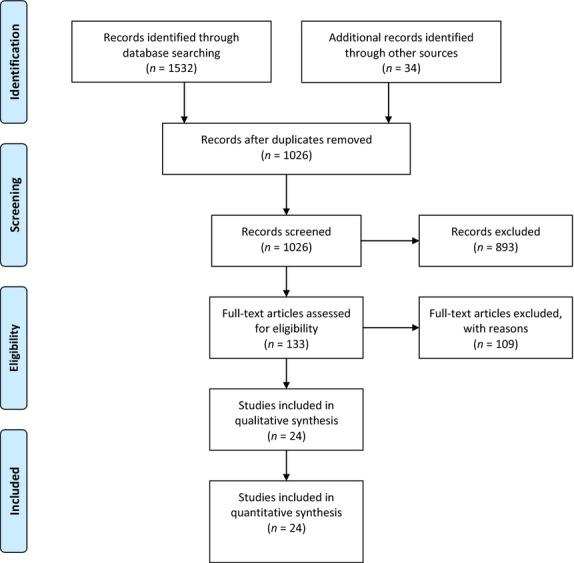
Flow chart of the study selection.
All studies were published from 1954 to 2013 and included 467 cases (Table1) 14,18,19,22,30,32,35,37–53. Some of the authors used the same tissue bank for their analysis, but as different investigations were performed in each study, they were included as different studies 14,19,35,39,47.
Table 1.
Patients' demographics
| Author | Year | Time frame | Number of specimens | Site | Patients' age (mean ± SD) | Amount of tissue |
|---|---|---|---|---|---|---|
| Palmer 37 | 2013 | Not mentioned | 34 (17 male) | Tibia: 19; femur: 12; humerus: 3 | 49 years (range, 18–71 years) | 1 mm3 biopsies |
| Koga 18 | 2013 | Not mentioned | 7 | Not mentioned | Not mentioned | “Small amount” |
| Zimmermann 22 | 2012 | March 2006 to May 2007 | 8 | Humerus: 3; femur: 3; tibia: 2 | 48.75 ± 9.63 years | 10 mm × 10 mm × 10 mm |
| Gille 38 | 2012 | November 2009 to March 2010 | 23 (15 male) | Tibial shaft | 47.4 years (range, 20–82 years) | At least 3, each measuring 1 cm3 |
| Fajardo* 14 | 2010 | August 2007 to March 2008 | 20 (14 male) | Femur: shaft – 2, subtrochanteric – 2, distal – 2; tibia: shaft – 2, proximal – 1, distal – 1; fibula: shaft – 3; clavicle: midshaft – 4; humerus: proximal – 1; ulna: shaft –2 | 46 years (range, 32–80 years) | Approximately 5 mg |
| Kwong† 35 | 2009 | Not mentioned | 7 (compared to 8 patients with uneventful healing) | Extra-articular fractures | Range, 18–87 years | Not mentioned |
| Iwakura 30 | 2009 | Not mentioned | 7 (6 male) | Femoral diaphysis: 3; tibial diaphysis: 2; humeral diaphysis: 1; ulnar diaphysis: 1 | 53.0 years (range, 37–74 years) | “Small amount” |
| Fajardo* 39 | 2009 | August 2007 to March 2008 | 15 (11 male) | Femur: shaft – 2, subtrochanteric – 2; tibia: shaft – 2, tibial plateau – 1, distal – 1; fibula: shaft – 2; clavicle: midshaft – 3; humerus: proximal – 1; ulna: shaft –1 | 46 years (range, 32–80 years); SD 14 years | Not mentioned |
| Bajada 40 | 2009 | Not mentioned | 8 (3 male) | Femur: 5; tibia: 3 | 55.6 years (range, 26–73 years) | Ranging in wet weight from 120 to 250 mg; mean 162.1 mg |
| Qu 41 | 2008 | Not mentioned | 15 (14 male) | Scaphoid bone | 29 years (range, 17–56 years) | >1 mm and up to 3 mm of abnormal bone on either side of the non-union |
| Hofmann 42 | 2008 | Not mentioned | 10 (4 male) compared to 10 (5 male) patients with uneventful healing | Femur: 5; humerus: 3; ulna: 1; pelvis: 1 | Non-unions: 59.3 ± 20.3 (range, 25–87 years); Controls: 55.3 ± 15.1 (range, 28–75 years) | Not mentioned |
| Bajada 43 | 2007 | 2004 | 1 (male) | Tibia | 34 years | Not mentioned |
| Kilian 52 | 2004 | Not mentioned | 7 (4 male) | Tibia: 4; humerus: 1; radius: 1; ulna: 1 | 37 years (range, 32–42 years) | Not mentioned |
| Reed 44 | 2002 | 1993–1999 | 11 (9 male) | Extra-articular fractures. Tibia: 7; femur: 2; fibula: 1; radius: 1 | 44 years (range, 14–74 years) | All biopsies >5 mm × 5 mm × 5 mm |
| Reed 44 | 2002 | 1993–1999 | 11 (8 male) | Extra-articular fractures. femur: 8; tibia: 3 | 51 years (range, 35–81 years) | All biopsies >5 mm × 5 mm × 5 mm |
| Kloen 45 | 2002 | Not mentioned | 17 non-unions; 4 delayed unions | Humerus: 12; femur: 5; tibia: 2; clavicle: 2 | 61 years (range, 30–85 years) | Not mentioned |
| Guerkov 46 | 2001 | Not mentioned | 7 (atrophic group: 1 male; hypertrophic group: 2 male) | Femur: 3; clavicle: 2; tibia: 1; iliac wing: 1 | 61 years (range, 30–85 years) | >0.5 cm3 |
| Lawton† 19 | 1999 | Not mentioned | 12 patients compared to 15 patients with uneventful healing | Not mentioned | Normal healing: range, 18–87 years | Not mentioned |
| Lawton† 47 | 1997 | Not mentioned | 12 patients compared to 15 patients with uneventful healing | Extra-articular long bone fractures | Normal healing: range, 18–87 years | Not mentioned |
| Santavirta 48 | 1992 | Not mentioned | 10 (7 male) | Tibia: 8; humerus: 2 | 48 years (range, 27–64 years) | Three parallel representative samples, each about 4 × 4 mm |
| Boyan 49 | 1992 | Not mentioned | 1 (male) | Tibia | 19 years | Fibrocartilage lying within the fracture gap and periosteal tissue stripped from the edges of the non-union |
| Quacci 50 | 1991 | Not mentioned | 2 (male) | Tibia | 18 and 23 years | 5 mm biopsy cannula |
| Milgram 51 | 1991 | Not mentioned | Extra-articular: 41; intra-articular: 54 | Extra-articular: tibia: 13; femur: 10; other: 18. Intra-articular: femur: 44; patella: 4; other: 6 | Not mentioned | Sample tissue included the whole fracture site (intact piece) |
| Heppenstall 32 | 1987 | 1970–1983 | 76 (39 males) | Humerus: 29; femur: 23; tibia: 18; clavicle: 3; metatarsal: 1; ulna: 1; radius: 1 | 39 ± 3 years | Not mentioned |
| Urist 53 | 1954 | 1948–1953 | 85 (19 biopsies between 2 and 7.5 years) | Tibia | Not mentioned | Not mentioned |
Both studies used the same samples for their analysis.
All three studies used the same samples for their analysis.
Studies characteristics
The studies characteristics are outlined in Table2 14,18,19,22,30,32,35,37–53. The definition of non-union varied between studies, but it was generally based on the radiographic appearance and clinical examination. Most of the samples were obtained during revision operations for the treatment of the non-unions.
Table 2.
Studies' characteristics
| Author | Duration of non-union | Classification | Definition of non-union | Isolation of tissue | Cells/material isolation |
|---|---|---|---|---|---|
| Palmer 37 | 10 months | Aseptic/Septic | Radiographic evidence of non-progression of healing for at least 3 months, or lack of healing by 9 months since the initial injury | Intra-operative specimens were collected from removed implants, surrounding tissue membrane and local soft tissue | Culture analysis; Ibis's second-generation molecular diagnostics; bacterial 16S rRNA-based fluorescence in situ hybridization (FISH) |
| Koga 18 | 11.0 months (range, 9–13 months) | Viable: 2 patients; Non-viable: 5 patients | >9 months had elapsed since the injury, and the fracture had shown no visible progressive signs of healing for 3 months | The non-union site was exposed by careful incision, and care was taken not to contaminate the bone and periosteum | Histological analysis; flow cytometry; cell proliferation; alkaline phosphatase activity assay; ALP mRNA; mRNA analysis; osterix expression; osteocalcin expression; mineralization assay |
| Zimmermann 22 | >9 months | Radiological appearance | >9 months from injury | Pseudarthrotic tissue was collected out of the fracture gap during regular surgical treatment | mRNA isolation; cDNA arrays |
| Gille 38 | 10.2 months (range, 6–34 months) | Aseptic | Absence of osseous healing >6 months from injury | Intra-operative biopsy samples | Cultures; PCR |
| Fajardo 14 | 16 months (range, 0.5–6 years) | Hypertrophic | Absence of osseous healing >6 months from injury | Multiple tissue samples from: (i) the non-union site and (ii) mineralized fracture callus from the surrounding region | RNA extraction; synthesis of cDNA; real-time quantitative PCR; western blot assay (only eight samples); immunohistochemistry (only eight samples) |
| Kwong 35 | Range, 1–48 months | Aseptic; only fractures with areas of cartilage were chosen | Absence of osseous healing >9 months after treatment | Fracture biopsies taken at surgery for treatment of malalignment or failure of fixation, as well as acute fractures that were operated upon in a delayed fashion | Immunohistochemical Analysis |
| Iwakura 30 | 11 months (range, 9–14 months) | Hypertrophic | >9 months from injury, no visible progressive signs of healing for 3 months | Samples were obtained during revision surgery | Histological analysis; immunophenotyping of non-union cells by flow cytometry; osteogenic induction; chondrogenic induction; adipogenic induction; total RNA extraction and RT-PCR |
| Fajardo 39 | 16 months (range, 0.5–6 years) | Hypertrophic | Absence of osseous healing >6 months from injury | Multiple tissue samples from: (i) the non-union site and (ii) mineralized fracture callus from the surrounding region | RNA extraction; synthesis of cDNA; real time quantitative PCR; western blot assay (only seven samples); immunohistochemistry (only seven samples): by standard technique |
| Bajada 40 | 3 years (range, 2–5 years) | Atrophic | Not mentioned | Tissue was excised from the site of non-union between the diaphyseal cortices and below the pseudocapsule | Histological analysis; CD immunoprofiling |
| Qu 41 | 36 months (range, 5–156 months) | Not mentioned | Not mentioned | Bone from either side of the non-union and the fibrocartilagenous central regions were harvested during reconstructive or salvage surgery | Immunocytochemical determination of osteocalcin; ALP enzyme assay |
| Hofmann 42 | Non-unions: 2.6 re-operations (range, 2–4 revisions); Controls: 0 re-operations | Hypertrophic | Not mentioned | Endosteal cancellous bone fragments were taken at sites proximal to non-unions during surgery. Control cultures were obtained from healthy individuals from endosteal sites during implant removal after uneventful fracture consolidation | Osteoblast cell viability; formation of alkaline phosphatase-positive (CFU-ALP) and mineralization-positive (CFU-M) colony forming units; global differences in gene expression |
| Bajada 43 | 9 years | Hypertrophic | Not mentioned | During operation for grafting | Histology |
| Kilian 52 | Not mentioned | Atrophic | Not mentioned | Patients surgically treated for resection of atrophic non-union and re-osteosynthesis | Immunohistochemistry; qualitative RT–PCR; LightCycler-based relative mRNA quantification |
| Reed 44 | 27 months (range, 11–62 months) | Hypertrophic | A fracture that had not healed within the expected time period, with no progression towards healing on successive radiographs | During surgery, biopsies taken of the material in the non-union gap (interfragmentary tissue) and the cortex immediately adjacent to the gap | Histology; immunohistochemistry; assessment of vascularization; assessment of vessel density |
| Reed 44 | 34 months (range, 12–60 months) | Atrophic | A fracture that had not healed within the expected time period, with no progression towards healing on successive radiographs | During surgery, biopsies taken of the material in the non-union gap (interfragmentary tissue) and the cortex immediately adjacent to the gap | Histology; immunohistochemistry; assessment of vascularization; assessment of vessel density |
| Kloen 45 | 22 months (range, 3.5–120 months) | Not mentioned | Absence of osseous healing >6 months from treatment | At the time of surgery | Histology; immunohistochemistry |
| Guerkov 46 | 20 months (range, 6–36 months) | Atrophic: 4; Hypertrophic: 3 | Not mentioned | At the time of revision surgery (central portion of the tissue) | Histology; cell proliferation; [3H]-thymidine incorporation; alkaline phosphatase specific activity; osteocalcin production; collagen production; local factor production |
| Lawton 19 | Range, 4–48 months | Not mentioned (presence of callus) | Not mentioned | Specimens of fracture callus from normally healing fractures (1–4 weeks after fracture) or non-unions (4–48 months after fracture) | In situ hybridization |
| Lawton 47 | Range, 4–48 months | Not mentioned (presence of callus) | Not mentioned | Specimens of fracture callus from normally healing fractures (1–4 weeks after fracture) or non-unions (4–48 months after fracture) | In situ hybridization |
| Santavirta 48 | Range, 4–25 months | 8 cases delayed union; 2 cases established non-unions | Not mentioned | Tissue from the area between the diaphyseal cortices below the pseudocapsule | Immunopathology (inflammatory-cell analysis, analysis of matrix metalloproteinases); neuroimmunology |
| Boyan 49 | 12 months | Not mentioned | Not mentioned | During surgical treatment | Histomicrograph; photomicrograph; alkaline phospatase activity; Elisa; densitometric analysis of the cytoplasmic dot blots |
| Quacci 50 | 8 months | Hypertrophic | Not mentioned | Through a 5 mm biopsy cannula | Light and electron microscopy |
| Milgram 51 | Not mentioned | Not mentioned | Not mentioned | Surgical resections, amputations and a small number of autopsy obtained specimens | Histological analysis |
| Heppenstall 32 | Humerus: 4.3 years, Tibia: 2.7 years | Synovial pseudarthrosis | Synovial pseudarthrosis | Biopsies | Light and electron microscopy |
| Urist 53 | >18 months | Not mentioned | X-rays >18 months showing: a bone defect; false motion; sclerosis of the bone ends; rounding, mushrooming, or moulding of the fracture surfaces; sealing of the medullary canal with compact bone to form functioning false bone surfaces and an apparent arrest of the process of osteogenesis in the fracture gap | During surgical interventions/autopsy | Histological analysis |
Macroscopic structure of non-union tissue
Urist et al. was the first to hypothesize the mechanism of non-union based on its macroscopic and microscopic characteristics 53. He reported that white soft tissue was interposed between the bone segments, a finding later supported by other authors 51, and explained this as fibrinoid degeneration of the connective tissue in the interior of the callus 53. With regards to synovial pseudarthrosis, a yellow frond-like material was found interposed between the bone fragments, with clear serous fluid filling this space in aseptic cases, whereas in septic cases murky fluid was present 32.
Microscopic structure of non-union tissue
Histology
The histological findings of non-union tissue are summarized in Table3 18,19,30,32,35,40,43–48,50,51,53. Where relevant information was available, a direct comparison of histological findings between atrophic and hypertrophic non-unions was attempted (Table4) 30,40,43,44,46,50.
Table 3.
Histology findings
| Author | Classification | Histology |
|---|---|---|
| Koga 18 | Viable: two patients; non-viable: five patients | Fibroblast-like morphologic characteristics |
| Kwong 35 | Aseptic non-unions, only fractures with areas of cartilage were chosen | Healing fractures: all consisted of areas of cartilage and significant woven bone formation. Non-healing fractures: in most, cartilaginous areas were accompanied by the presence of small amount of woven bone, but significant fibrous tissue. No notable differences in cellular morphology in the cartilaginous areas of the fractures between the two groups |
| Iwakura 30 | Hypertrophic | Mainly fibrous tissue and no ossicles. Non-union tissue contained various amounts of fibroblast-like cells. After a 21-day incubation under chondrogenic conditions, cell pellets had a spherical and glistening transparent appearance |
| Bajada 40 | Atrophic | Samples largely consisted of fibrocartilaginous tissue that contained occasional bony islands. In some areas, the excised non-union tissue was well populated by fibroblastic cells, but other areas were largely acellular and consisted mostly of a collagenous extracellular matrix. Areas of vascularization were seen consistently and the presence of osteoclasts within absorption pits was also occasionally notable. After enzymatic treatment to extract cells and their plating out into monolayer culture, the majority of the adherent cells present were stromal in appearance, i.e. bipolar and fibroblastic. Occasional multinucleated osteoclasts were also seen in the early cultures, as were cells with a stellate (possessed multiple cytoplasmic processes) or dendritic appearance |
| Bajada 43 | Hypertrophic | Fibrocartilaginous non-union with little evidence of new bone formation and no signs of infection |
| Reed 44 | Hypertrophic | Specimens contained fibrous tissue, fibrocartilage, hyaline cartilage and bony islands. Areas of new bone formation by both endochondral and intramembranous ossification. Morphologically samples appeared well vascularized |
| Reed 44 | Atrophic | Specimens contained fibrous tissue, fibrocartilage, hyaline cartilage and bony islands. Relatively few areas of new bone formation, predominantly via the endochondral route. Necrotic bone was more prevalent in the atrophic non-union group. Morphologically samples appeared well vascularized |
| Kloen 45 | Not mentioned | Delayed unions and non-unions: 11/21 specimens had foci of woven bone (having cuboid-shaped osteoblasts lining the osteoid, suggesting active bone formation) surrounded by large areas of fibrous tissue that was interspersed with areas of numerous blood vessels. Ten of 21 specimens had similar areas of fibrous tissue but lacked woven bone. Within the samples that contained woven bone, two patterns of bone formation were observed: (i) bone appeared to be forming directly from fibrous tissues; (ii) bone seemed to be forming from cartilage. Other observations included scattered lamellar bone fragments surrounded by osteoclasts and a paucity of lining osteoblasts. Some specimens also showed villous projections resembling synovial pseudarthroses with lining cells resembling synoviocytes |
| Guerkov 46 | Atrophic: 4; hypertrophic: 3 | Mainly fibrous tissue with organized collagen bundles. No ossicles were seen in any of the sections examined. All sections from atrophic non-unions were oligocellular and contained few vessels, whereas those from hypertrophic non-unions were more cellular, with little evidence of cartilaginous tissue |
| Lawton 19 | Not mentioned (had callus) | Human fracture callus: heterogeneous appearance with several of the elements of normal fracture healing (haematoma, fibrous tissue, woven and compact lamellar bone, and cartilage) being present in close proximity in any one section. Non-union gap: tissues consisted largely of vascularized fibrous tissue or avascular cartilage |
| Lawton 47 | Not mentioned (presence of callus) | Areas of old bone, new bone formation, non-union gap (either fibrous, cartilaginous or both), and an interface between the gap and bony material |
| Santavirta 48 | 8 cases delayed union; 2 cases established non-unions | The morphology of the samples was not dependent on the duration of delayed union/non-union. All samples contained connective tissue of varying density, in which tissue fibroblast-like mononuclear cells seemed to predominate. The cellularity varied inside each sample from poorly cellular, tight connective tissue areas to highly cellular strangs with occasional cartilage or bony islets |
| Quacci 50 | Hypertrophic | Light microscopy: non-union tissue was composed of connective tissue, cartilage (had a hypertrophic aspect and frequently presented degenerative aspects) and fragmented osteoid-like trabeculae |
| Milgram 51 | Not mentioned | Extra-articular locations: presence of non-mineralized fibrous or fibrocartilaginous tissue between the ends of the bone at the old fracture site. Also demonstrated a spectrum of clefts at the site of non-union ranging from tiny microscopic spaces within the soft tissue of the non-union to dominant clefts that completely separated the ends of the fracture (i.e. frank pseudarthrosis). Intra-articular locations: demonstrated the same sequence of changes occurring in 24 of the cases. However, 30 of them demonstrated no tissues of a fibrous non-union |
| Heppenstall 32 | Synovial pseudarthrosis | Light microscopy (62 patients): hyaline cartilage, synovial-like lining cells, or synovium and fibrous tissue was present |
| Urist 53 | Not mentioned | When healing does not occur <18 months, the interior of the callus is more likely to show: inflammatory and fibrous connective tissue; failure of fibrous tissue to regress; fibrinoid and hyaline degeneration |
Table 4.
Comparison of histological findings between atrophic – hypertrophic non-unions
| Type of tissue | Atrophic | Hypertrophic |
|---|---|---|
| Fibrocartilaginous tissue | 40,44 | 43,44 |
| Fibrous tissue | 44,46 | 30,44 |
| Cartilaginous tissue | – | 44,46,50 |
| Collagenous extracellular matrix/connective tissue | 40,46 | 40,46,50 |
| Bone tissue | No ossicles 46; occasional bony islands 40,44 | No ossicles 30,46; bony islands 43,44,50 |
| Necrotic bone | More prevalent 44 | – |
| Bone production | Predominantly via the endochondral route 44 | Bone formation by both endochondral and intramembranous ossification 44 |
| Cells | Generally oligocellular 46; some areas acellular 40 | More cellular 46 |
| Fibroblastic: majority of cells 40 | Fibroblast-like 30 | |
| Osteoclasts: occasionally 40 | ||
| Bipolar cells: majority of cells 40 | ||
| Cells with a stellate (possessed multiple cytoplasmic processes) or dendritic appearance 40 | ||
| Vascularization | Well vascularized 40,44; few vessels 46 | Well vascularized 44 |
Immunohistochemistry
The immunohistochemical findings of non-union tissue are summarized in Table5 14,19,35,39,44,45,47,48,52. Interestingly, BMP's were present in the non-union tissue, although their expression was reduced 35,39,45. Moreover, matrix metalloproteinases (MMP's) were also reported to be present in the non-union tissue, not localized in a particular cell type or cellular component 14,48.
Table 5.
Immunohistochemistry findings
| Author | Classification | Immunohistochemistry |
|---|---|---|
| Fajardo 14 | Hypertrophic | MMP-7 and MMP-12 were found to be stained within the substance of the non-union tissue and not localized within a particular cell type or cellular component. Both enzymes were likewise not visualized in the bone callus specimens |
| Kwong 35 | Aseptic non-unions, only fractures with areas of cartilage were chosen | There was a significant reduction in BMP-2 and BMP-14 expression in cartilaginous areas of non-healing fractures compared to healing fractures, but no statistical differences in the endogenous expression of noggin and chordin (BMP inhibitors) |
| Fajardo 39 | Hypertrophic | BMP-7: absent in the non-union specimens but present in the fracture callus specimens. BMP-2: positive immunostaining was restricted consistently to the fibrous tissue of the non-union tissue |
| Kilian 52 | Atrophic | Immunostaining appeared in close vicinity to immature osteoid trabeculae. EDB+ fibronectin immunostaining was negative for scFvL19 antibody |
| Reed 44 | Hypertrophic | No statistically significant difference in median vessel counts between atrophic, hypertrophic and normal unions |
| Reed 44 | Atrophic | No statistically significant difference in median vessel counts between atrophic, hypertrophic and normal unions |
| Kloen 45 | Not mentioned | The most consistent expression was that of BMP-2, BMP-4, and BMP-7 in the osteoblasts lining the newly formed osteoid. The staining was cytoplasmic and, in certain specimens, was specifically located in the Golgi apparatus, illustrating local production of BMP. No correlation between the location of the delayed union or non-union and staining. In the areas of dense fibrous tissue the presence of staining for all BMP isoforms tested was the same as or less than that in the areas close to bone at all time-points after the fracture. Expression of Type IA, Type IB, and Type II BMP Receptors: positive staining was observed in the osteoblasts lining the ossified tissue, in the areas near the ossification sites, and in the fibrous tissue. As observed for the BMP antibodies, there was a trend towards decreased staining in areas remote from bone formation. There was no clear trend between a decreased percentage of positive staining and an increased duration of the non-union. Expression of pSmad1: in the osteoblasts lining the areas of reactive bone formation as well as in osteoclasts, fibroblast-like cells and chondroblast-type cells |
| Lawton 19 | Not mentioned (had callus) | In normally healing fractures, mature osteoblasts on woven bone were negative for MGP mRNA, but positive for osteonectin, osteopontin and osteocalcin mRNA molecules. In non-unions, osteoblasts displayed a novel phenotype: they were positive for MGP mRNA, in addition to osteonectin, osteopontin and osteocalcin mRNA molecules |
| Lawton 47 | Not mentioned (had callus) | In areas of new bone covered by plump osteoblasts, the matrix was either stained uniformly or in a superficial zone, indicating the presence of collagen type III. Fibrous tissue in the fracture gap was also immunostained positively |
| Santavirta 48 | Eight cases delayed union; two cases established non-unions | Most inflammatory cells were CD4 T lymphocytes and their number was always twice that of the CD8 positive cells. Staining for CD11b positive monocyte/macrophages showed in all samples positive cells scattered in the connective tissue stroma with perivascular enrichments. Mast cells were absent or very rare. Almost all resident cells seem to be involved in tissue remodelling as suggested by their content of fibroblast-type MMP-1 and its proteolytic activator MMP-3 or stromelysin, whereas MMP-8 was rare or absent |
Neuroimmunohistochemistry
Only one study performed neuroimmunohistochemical analysis revealing paucity or total lack of peripheral innervation in the non-union tissue 48.
Analysis of vessel density
Blood vessels were present in cases of hypertrophic non-unions, with a varying density (Table6) 44,48,50. When comparing however atrophic and hypertrophic non-union tissue, an interesting finding was that the number of fields containing no blood vessels, some blood vessels and hot-spots, was very similar 44. This was also confirmed with immunohistochemistry studies, where no significant difference was evident in the median vessel count between atrophic/hypertrophic non-unions and normal unions 44. Finally, histological findings confirmed the presence of vascular tissue in both types of non-unions (Table3) 19,40,44,46.
Table 6.
Tissue examination
| Author | Analysis of vessel density | Electron microscopy (Ultrastructural Examination) |
|---|---|---|
| Reed 44 | The number of fields containing no blood vessels, some blood vessels and hot-spots was very similar in the atrophic and hypertrophic non-union groups | Not applicable |
| Santavirta 48 | Samples mostly consisted of vascularized connective tissue of varying density | Not applicable |
| Quacci 50 | A lot of blood vessels were present in the tissue, often appearing free of blood and occluded by thrombi at different organization stages | Fibroblasts and chondrocytes found in the non-union tissue seemed normal, with a good secretion apparatus. The cell membranes were able to produce matrix vesicles. Hydroxyapatite crystals could be observed in the cell matrix or inside matrix vesicles |
| Heppenstall 32 | Not applicable | (5 patients) Large amounts of surface fibrin. Some cells had profuse rough endoplasmic reticulum and resembled fibrocytes or Type B synovial lining cells. Some of these cells contained prominent lipid droplets and intermediate filaments. There were also phagocytic cells with vacuoles containing granular and cellular debris, resembling to Type A lining cells or monocyte-macrophages. Surrounding the cells were some necrotic cells, clusters of apatite crystals and occasional clumps of collagen fibres infiltrated with more fibrin-like material. Deeper was more densely packed collagen |
Electron microscopy
Two studies performed ultrastructural examination of the non-union tissue by the means of electron microscopy (Table6) 32,50. In a study by Quacci et al., it was found that the non-union tissue contained normal fibroblasts and chondrocytes 50. In addition, Heppenstall et al. who examined synovial pseudarthrosis reported large amounts of surface fibrin and densely packed collagen 32.
Bacteriology of the non-union
Palmer et al. analysed 34 samples obtained from patients with non-unions 37. Although eight samples had a positive conventional culture, only four of 34 cases were negative following analysis of bacterial DNA using a combination of Ibis molecular diagnostics and fluorescence in situ hybridization techniques. Similarly, Gille et al. examined culture negative samples of 23 patients and reported the presence of bacterial RNA following analysis with PCR in two patients (8.7%) 38.
Evaluation of tissue sample
Cell surface protein expression
Three studies performed flow cytometry to determine the presence of specific proteins on the cell surface (Table7) 18,30,40. The non-union tissue was found to be positive for MSC's related markers CD13 30, CD29 18,30, CD44 18,30, CD90 30, CD105 18,30,40 and CD166 18,30, but negative for haematopoietic markers CD14 18,30, CD34 18, CD45 18,30,40 and CD143 18,30.
Table 7.
Cell surface protein expression
| Author | Cell surface protein expression (flow Cytometry) |
|---|---|
| Koga 18 | Strongly positive for the MSC's related markers CD29, CD44, CD105 and CD166 but negative for the hematopoietic markers CD14, CD34, CD45 and CD133 |
| Iwakura 30 | Positive for MSC's related markers CD13, CD29, CD44, CD90, CD105 and CD166, but negative for hematopoietic markers CD14, CD34, CD45 and CD133 |
| Bajada 40 | Less than 1% of NUSC and BMSC were immunopositive for CD34 and CD45, while 78% ± 14% (mean ± SD) of NUSC and 92% ± 7% (mean ± SD) of BMSC were immunopositive for CD105 |
MSC: mesenchymal stem cells; NUSC: non-union stromal cells; BMSC: bone marrow stromal cells.
Cell senescence
Bajada et al. was the only author to report on the cell senescence of non-union stromal cells 40. According to his findings, from passage I onwards, many of the cells developed an appearance that was less bipolar and more spread along with the development of prominent stress fibres. Further passages lead to prolonged culture doubling times (phenotypic changes are consistent with the onset of cell senescence). When examining the proportion of SA-β gal positive cells, that was significantly greater in the non-union stromal cells when compared to the bone marrow stromal cells, but that did not correlate with the patient's age, number of previous operative procedures or time between original fracture and operative management.
Cultures characteristics
Properties
Cell morphology, viability and proliferation are outlined in Table8 18,30,40–42,46,49.
Table 8.
Cell culture characteristics
| Author | Classification | Intervention | Cell morphology | Cell viability (MTT-Test) | Cell proliferation |
|---|---|---|---|---|---|
| Koga 18 | Viable: two patients; non-viable: five patients | Group A: BMP-7 alone; Group B: BMP-7+ low-intensity pulsed Ultra Sound | Not applicable | Not applicable | No significant difference in the DNA concentration between the two groups on days 3, 5 and 7 |
| Iwakura 30 | Hypertrophic | Not applicable | Not applicable | Not applicable | Proliferation capacity of non-union cells was significantly inferior to that of fracture haematoma cells |
| Bajada 40 | Atrophic | Not applicable | Not applicable | Not applicable | Both non-union and bone marrow stromal cells differentiated along each mesenchymal lineage, forming alkaline phosphatase-positive cells (i.e. osteoblastic differentiation), oil red O positive cells (adipocytic differentiation) and depositing an extracellular matrix in pellet culture that stained metachromatically with toluidine blue and was immunopositive for type II collagen (chondrogenic differentiation) |
| Qu 41 | Not mentioned | rhBMP-2 | Not applicable | Not applicable | Osteoblastic cell populations isolated from bone harvested from the ilium and the three regions of the scaphoid non-unions had similar proliferative capacity |
| Hofmann 42 | Hypertrophic | Not applicable | Although the morphology of confluent cells did not differ between controls and non-unions, there were significantly more bone nodules in the controls group | At day 4 the mitochondrial succinyldehydrogenase enzyme activity was significantly higher in human osteoblast cultures (compared to human non-union osteoblasts), indicating that the number of metabolically active (viable) cells was higher in this group | At 4 weeks, all cultures in both groups were confluent monolayers, and there was no significant difference in cell numbers between the groups |
| Guerkov 46 | Atrophic: 4; hypertrophic: 3 | Pulsed electromagnetic field stimulation | Atrophic non-unions: cells formed a uniform monolayer of elongated cells that had few cellular extensions. Hypertrophic non-unions: also consisted of elongated cells, but the cells were more cuboidal, having cellular extensions in a multilayer. After the cells were treated with pulsed electromagnetic field stimulation for 4 days, cells from the atrophic non-unions were small, elongated, or cuboidal, whereas cells from the hypertrophic non-unions were multi-layered, mostly cuboidal and had cellular extensions connecting with adjacent cells. Cells that were not stimulated remained elongated and fibroblastic | Not applicable | Pulsed electromagnetic field stimulation had no significant effect on the proliferation of hypertrophic and atrophic non-union cultures, at any of the times examined |
| Boyan 49 | Not mentioned | BMP (bovine or dog) | Following BMP treatment cells became elongated and more fibroblast like, with no distinct foci of aggregated cells | Not applicable | Incubation with BMP resulted in an inhibition in cell proliferation in periosteal (significant at 2 mg/ml BMP); 3.7-fold inhibition and fibrocartilage cells (significant at 1 mg/ml BMP; fourfold inhibition) |
BMP: bone morphogenic protein.
Alkaline phosphatase activity assay – messenger RNA evaluation
Alkaline phosphatase activity and messenger RNA (mRNA) evaluation is outlined in Table9 18,19,30,40–42,46,49,50.
Table 9.
ALP activity and mRNA examination
| Author | Classification | Intervention | ALP activity assay | ALP mRNA | mRNA |
|---|---|---|---|---|---|
| Koga 18 | Viable: two patients; non-viable: five patients | Group A: BMP-7 alone; Group B: BMP-7+ low-intensity pulsed Ultra Sound | The ALP activity of the non-union tissue-derived cells in Group B was significantly higher by 57% and 32% than that in Group A group on days 7 and 14 respectively | In Group B, the expression level of ALP mRNA was significantly up-regulated by 55%, 24%, 50% and 49% compared with the BMP-7-alone group on days 3, 7, 10, and 14 respectively | The expression level of Runx2 mRNA in Group B was significantly higher by 49% and 134% compared with the BMP-7-alone group on days 10 and 14 respectively |
| Iwakura 30 | Hypertrophic | Not applicable | The level of ALP activity under osteogenic conditions was significantly higher than under control conditions on day 21, and ALP activity of non-union cells was significantly higher than that of fracture haematoma cells under differentiated conditions | The expression of ALP under osteogenic conditions was higher than under undifferentiated conditions in the control group | Not applicable |
| Bajada 40 | Atrophic | Not applicable | The ALP activity of the non-union stromal cells cultures appeared markedly lower than that for bone marrow stromal cells cultures | Not applicable | Not applicable |
| Qu 41 | Not mentioned | rhBMP-2 | Baseline ALP activity was similar among cell populations isolated from all regions of the scaphoid non-unions and the ilium after 14 days of culture. rhBMP-2 treatment resulted in a significant increase in ALP activity in all groups (proximal: 1.7-fold; central: 2.1-fold; distal: 1.9-fold; iliac: 1.5-fold) | Not applicable | Not applicable |
| Hofmann 42 | Hypertrophic | Not applicable | The comparison of CFU-ALP as an early marker for osteoblast differentiation at day 7 did not show significant differences compared to controls | Not applicable | Not applicable |
| Guerkov 46 | Atrophic: 4; hypertrophic: 3 | Pulsed electromagnetic field stimulation | There was a time-dependent increase in ALP specific activity in all cultures that was significant in the cell layers and in isolated cells at 4 days after confluence. Exposure of the cultures to pulsed electromagnetic field stimulation had no effect on the enzyme activity in either the cell layers or isolated cells. At Day 4, enzyme specific activity in the cell layer had increased in pulsed electromagnetic field treated and control cultures by 99% and 90% respectively. The time-dependent increases in the isolated cells were comparable. In addition, no differences between cultures from atrophic or hypertrophic non-unions were observed | Not applicable | Not applicable |
| Lawton 19 | Not mentioned (presence of callus) | Not applicable | Not applicable | Not applicable | Osteoblasts in non-unions: positive for MGP mRNA signal (in the zone of new bone formation and in the interface zone; old bone zone: almost always negative; gap zone: rarely contained osteoblasts). Small and large chondrocytes in non-unions: negative. Small and large chondrocytes in normal fractures: positive for MGP mRNA. Osteoblasts in normal fractures: never detected |
| Boyan 49 | Not mentioned | BMP (bovine or dog) | There was significant reduction in ALP specific activity in matrix vesicles and plasma membranes from human fibrocartilage and periosteal cells incubated with 2 mg/ml BMP (not at 1 mg/ml BMP). As with connective tissue cells, ALP activity in the plasma membrane did not differ from that of the matrix vesicle membranes, before or after the exposure to BMP. Baseline ALP activity in cultures of human periosteal cells was comparable to fibrocartilage cells delivered from human non-union tissue | Incubation with BMP resulted in dose-dependent increase in transcription of ALP | The relative amounts of each type of mRNA differed (ALP, Collagen Type I and II) |
| Quacci 50 | Hypertrophic | Not applicable | Some matrix vesicles presented ALPase activity inside them, but the main enzymatic activity was present outside and strictly connected to the vesicle membrane | Not applicable | Not applicable |
BMP: bone morphogenic protein; ALP: alkaline phosphatase; mRNA: messenger RNA; CFU: colony forming units.
Osterix
Koga et al. has studied the effect of low-intensity pulsed ultrasound on non-union cells cultured with the presence of BMP-7 and reported no significant difference in the expression of osterix 18.
Osteocalcin
Osteocalcin expression is outlined in Table10 18,19,30,40–42,46.
Table 10.
Osteocalcin expression and mineralization assay
| Author | Classification | Intervention | Osteocalcin | Mineralization assay |
|---|---|---|---|---|
| Koga 18 | Viable: two patients; non-viable: five patients | Group A: BMP-7 alone; Group B: BMP-7+ low-intensity pulsed Ultra Sound | No significant differences | The intensity of Alizarin Red S staining in the Group B was significantly higher by 30% than in Group A at day 2 |
| Iwakura 30 | Hypertrophic | Not applicable | The expression of osteocalcin under osteogenic conditions was higher than under undifferentiated conditions in the control group | After a 21-day incubation under osteogenic conditions, induced non-union cells formed a mineralized matrix (mineralization significantly higher than that of fracture haematoma cells), contrasting with an absence of mineralized matrix under undifferentiated conditions after the same duration |
| Bajada 40 | Atrophic | Not applicable | Not applicable | Although non-union stromal cells elevated their expression of these markers in response to osteogenic stimuli, there was a marked and significant reduction in their capacity to differentiate along an osteoblastic lineage compared to bone marrow stromal cells |
| Qu 41 | Not mentioned | rhBMP-2 | All populations had low numbers of osteocalcin-positive cells (7–9%) when grown in the presence of standard medium. There was no statistical difference in the number of osteoblasts between any of the three regions of the scaphoid and the ilium among cells grown under standard conditions, nor was there any correlation between the number of osteoblasts and the duration of the non-union. Cell populations originating from the central fibrocartilagenous part of the non-union had the greatest variability in osteocalcin staining. Significant increases in osteocalcin expression were observed in all groups in response to treatment with rhBMP-2 (ilium: 2.9-fold increase; proximal and distal: 2.3-fold increase; central: 2.0-fold increase) | Cell populations derived from scaphoid non-unions formed an extracellular matrix that showed very little bone nodule formation when maintained in culture for 28 days. Treatment with rhBMP also resulted in a significant increase in the number of bone nodules for all groups (proximal: 3.5-fold; central: 10.5-fold; distal: 4.9-fold; iliac: 3.4-fold) |
| Hofmann 42 | Hypertrophic | Not applicable | Not applicable | The mineralization of extracellular matrix (CFU-M) was very low in human non-union osteoblast cultures that were cultured under the same culture conditions and was significantly less than that in human osteoblast cultures |
| Guerkov 46 | Atrophic: 4; hypertrophic: 3 | Pulsed electromagnetic field stimulation | Osteocalcin was expressed at very low levels by the cultures, indicating the fourth passage cultures contained few, if any, committed osteoblasts. Pulsed electromagnetic field stimulation did not affect production of osteocalcin by non-union cells. | Not applicable |
| Lawton 19 | Not mentioned (presence of callus) | Not applicable | Weakly positive in flattened lining cells on lamellar bone. Positive in multinucleate resorptive cells. Consistently negative in endothelial cells. | Not applicable |
Osteonectin
Osteonectin expression was investigated by Lawton et al. 19. Osteonectin was found to be strongly positive in non-cuboidal and induced osteoblasts of early woven bone, as well as cuboidal osteoblasts of later woven bone. Included osteoblasts and flattened lining cells on lamellar bone were only weakly positive, whereas endothelial cells were consistently negative.
Osteopontin
Lawton et al. investigated osteopontin expression during the different stages of repair 19. Osteopontin was found to be weakly positive in non-cuboidal osteoblasts on early woven bone, and moderately positive in cuboidal osteoblasts on the surface of woven bone later in repair. Multinucleate resorptive cells were associated with a strong signal, in comparison with most flattened cells on the surface of lamellar bone and endothelial cells that were negative.
Bone Sialoprotein
Iwakura et al. studied the expression of Bone Sialoprotein under osteogenic conditions and found it to be higher in the non-union cells than under undifferentiated conditions in the human dermal fibroblasts (controls) 30.
Mineralization assay
Mineralization assay outcomes are outlined in Table10 18,19,30,40–42,46.
Dickkopf-1 expression
The expression of Dickkopf-1 (Dkk-1) was studied by Bajada et al. 40. According to his findings, both non-union and bone marrow stromal cells secreted Dkk-1 into conditioned medium at comparable levels under control (i.e. non stimulated) conditions. However, Dkk-1 levels detected in stimulated non-union stromal cells conditioned medium were markedly and significantly greater than those found in stimulated bone marrow stromal cells cultures.
Gene expression
Several authors have examined the expression of different genes in the non-union tissue. A summary of their results is outlined in Table11 14,22,30,42,52 and Table12 47,49.
Table 11.
Gene expression
| Author | General gene expression | Real-time PCR |
|---|---|---|
| Zimmermann 22 | Genes expressed more than two times than in normal tissue: CDO1; PDE4DIP; COMP; FMOD; CLU; FN1; ACTA2; TSC22D1 | Not applicable |
| Fajardo 14 | MMP-7 and MMP-12 mRNAs were significantly elevated in the non-union tissue when compared with local mineralized callus from the same site | MMP-7 and MMP-12 were the only enzymes (of 53 examined) significantly elevated in non-union tissue when compared with local mineralized callus from the same site |
| Iwakura 30 | Not applicable | It showed the expression of mRNA of Col II, Col X, SOX9 and aggrecan chondrogenic conditions after a 21-day induction. Under adipogenic conditions after a 21-day culture period, it showed the expression of LPL and PPAR-g2 (higher than under undifferentiated conditions in the control group) |
| Fajardo 39 | BMP gene expression in healing bone displayed several up-regulated genes between the two tissues | BMP antagonist genes (DRM, follistatin, noggin): increased in non-union tissue when compared to fracture callus tissue. BMP receptors (R1A, R1B, R2): expressed but did not demonstrate any significant differences. BMP-4: up-regulated in non-union tissue when compared to the fracture callus tissue. RNA levels of the BMP antagonists Drm/Gremlin, follistatin and Noggin: up-regulated in the non-union tissues. BMP-7: increased in the fracture callus tissue |
| Hofmann 42 | Gene terms significantly overrepresented in human non-union osteoblast cultures: skeletal development; response to wounding; organ morphogenesis; vasculature development; proteinaceous extracellular matrix; extracellular space; cytokine activity; glycosaminoglycan binding; growth factor activity; insulin-like growth factor binding. Genes significantly down-regulated in human non-union osteoblast cultures: IGF-2, FGF-1, FGF-receptor 2 (FGF-R2), BMP-4, TGF-β2, PDGF, Wnt-induced proteins (WISP2 and 3), β-catenin and prostaglandin E2 receptor EP4 | Confirmed the results of the microarray, especially regarding the down-regulation of some genes involved in osteoblast differentiation and bone metabolism |
| Kilian 52 | Not applicable | In qualitative and quantitative RT–PCR, EDA+ fibronectin mRNA was detectable at low levels. in none of the seven non-union samples, EDB+ fibronectin mRNA transcription was detected by qualitative and quantitative PCR |
Table 12.
Collagen gene expression
| Author | Intervention | Type I | Type II | Type III |
|---|---|---|---|---|
| Lawton 47 | Not applicable | Signal for procollagen type I mRNA over fibroblasts and over osteoblasts on woven bone was uniformly strong in most non-unions and normal fractures | Not applicable | Non-unions: in the zone of new bone formation and the interface zone, a population of surface and included osteoblasts was strongly positive for the procollagen type III mRNA signal; osteoblasts in the old zone were usually negative, while the gap zone contained osteoblasts only rarely; fibroblasts were frequently positive in the gap zone and interface. Normal fractures: procollagen type III mRNA was seen in the very early granulation tissue, where most of the positive cells were mesenchymal spindle cells (a cell population that includes osteoblast precursors; osteoblasts were in the vast majority negative; small areas of fibrous tissue in which fibroblasts were either negative or weakly positive |
| Boyan 49 | BMP (bovine or dog) | There was no stimulation of Type I collagen message in the non-union fibrocartilage cells. Non-union periosteal cells were found to be more strongly activated by BMP | The increase in mRNA levels of Type II collagen was not significant compared to controls | Not applicable |
Western blot assay
Western blot assay was used to detect the presence of specific proteins in the tissue under examination. Fajardo et al. investigated the presence of MMP's and reported that MMP-7 and MMP-12 were present in both non-union and mineralized callus tissue; however, the signal intensity of both enzymes was stronger in the non-union tissue 14. In another study, he and his team examined the presence of BMP's 39. His finding included: BMP-2 was present in both non-union and mineralized callus tissue; BMP-4 was detected in non-union samples but decreased in healing bone samples; BMP-7 was detected in the healing bone but was absent in the non-union samples.
Comparison between atrophic and hypertrophic non-union tissue
Table4 30,40,43,44,46,50 and Table13 30,40,42,44,46 compare the characteristics of tissue obtained from atrophic and hypertrophic non-unions.
Table 13.
Comparison between atrophic/hypertrophic non-union tissue
| Type of analysis | Atrophic | Hypertrophic |
|---|---|---|
| Histology | Table4 | |
| Immunohistochemistry/vessel density | No difference in the median vessel count between atrophic/hypertrophic non-unions 44 | |
| Cell surface antigen profile | CD 105 40 | CD13, CD29, CD44, CD90, CD105, and CD166 30 |
| Cells formed a uniform monolayer of elongated cells that had few cellular extensions 46 | Also consisted of elongated cells, but the cells were more cuboidal, having cellular extensions in a multilayer 46 | |
| Cell proliferation | No significant effect of pulsed electromagnetic field stimulation 46 | |
| ALP activity | No differences between cultures from atrophic or hypertrophic non-unions 46 | |
| Osteocalcin | Low levels 46 | Low levels 46; higher than in human dermal fibroblasts 30 |
| Mineralization assay | Reduced compared to bone marrow stromal cells 40 | Higher than haematoma cells 30; lower than human osteoblasts (normal healing) 42 |
Effect of interventions to the non-union tissue
Table14 18,41,46,49 outlines the effects of either pulsed electromagnetic field stimulation or BMP's on the non-union tissue.
Table 14.
Effect of interventions
| Author | Koga 18 | Qu 41 | Guerkov 46 | Boyan 49 |
| Type of intervention | Group A: BMP-7 alone; Group B: BMP-7+ low-intensity pulsed Ultra Sound | rhBMP-2 | Pulsed electromagnetic field stimulation | BMP (bovine or dog) |
| Cell morphology | Not applicable | Not applicable | Changed (Table8) | Changed (Table8) |
| Cell proliferation | No effect | No effect | No effect | Inhibition in periosteal and fibrocartilage cells |
| [3H]-Thymidine incorporation | Not applicable | Not applicable | No effect | Not applicable |
| Collagen synthesis | Not applicable | Not applicable | No effect | Not applicable |
| Transforming growth factor-β1 | Not applicable | Not applicable | Effect in a time-dependent manner | Not applicable |
| Prostaglandin E2 | Not applicable | Not applicable | No effect | Not applicable |
| Alkaline phosphatase activity assay | The ALP activity higher in Group B | Significant increase in all regions | No effect: cell layers or isolated cells. At Day 4, enzyme specific activity in the cell layer had increased in pulsed electromagnetic field treated and control cultures by 99% and 90% respectively (comparable increase) | Reduction: matrix vesicles and plasma membranes from human fibrocartilage and periosteal cells incubated with 2 mg/ml BMP (not at 1 mg/ml BMP). No effect: connective tissue cells, plasma membrane, matrix vesicle membranes |
| ALP messenger RNA | Up-regulated by 55%, 24%, 50% and 49% compared with the Group A on days 3, 7, 10 and 14 respectively | Not applicable | Not applicable | Dose-dependent increase |
| mRNA | The expression level of Runx2 mRNA in Group B was significantly higher by 49% and 134% compared with Group A on days 10 and 14 respectively | Not applicable | Not applicable | The relative amounts of each type of mRNA differed (alkaline phosphatase, Collagen Type I and II) |
| Osterix | No effect | Not applicable | Not applicable | Not applicable |
| Osteocalcin | No effect | Significant increases in osteocalcin expression in all groups | No effect | Not applicable |
| Mineralization Assay | Significantly higher by 30% than in Group A at day 2 | Significant increase in the number of bone nodules for all groups (proximal: 3.5-fold; central: 10.5-fold; distal: 4.9-fold; iliac: 3.4-fold) | Not applicable | Not applicable |
| Type I collagen expression | Not applicable | Not applicable | Not applicable | No effect in non-union fibrocartilage cells but increase in periosteal cells |
| Type II collagen expression | Not applicable | Not applicable | Not applicable | No effect |
| Glycosaminoglycan | Not applicable | Not applicable | Not applicable | Increase |
Genetic predisposition to fracture non-union
Several authors have investigated the theory of genetic predisposition to fracture non-union by analysing samples from peripheral venous blood 33,54 or bone callus 55, and comparing them with uneventful healing fractures. Numerous polymorphisms such as those of two specific SNPs (rs1372857, genotype GG and rs2053423, genotype TT) were identified to be associated with an increased risk of developing non-union 33,55,56.
Discussion
Non-unions represent a significant public health problem and have been associated with devastating consequences for the patients, their family and the society as a whole 57. The mechanism behind the progression of a fracture to a non-union state is multifactorial and as a consequence the treatment can be very challenging. The treatment of non-unions has evolved over the years from prolonged immobilization 53 to the use of biological stimulation and polytherapy. Such a strategy attempts to address all the elements of a compromized fracture healing response 3,31.
With regard to the macroscopic appearance of non-unions, a common finding is the interposition of soft tissue between the bone fragments 51,53. In aseptic non-unions, this tissue is whiter in colour, occasionally surrounded by clear fluid, compared to infected non-unions where this tissue becomes more yellowish and frequently surrounded by murky fluid 32. The experience of the authors confirms the above findings and in fact the macroscopic appearance of the non-union tissue is used as an additional marker for confirming/suspecting an underlying septic process.
Regarding the culture characteristics of the non-union tissue, there was an inconsistency in the reported findings. This may be because of the different types of non-union tissue examined (i.e. atrophic and hypertrophic), as well as because of the different topography of the non-unions from where samples were obtained. Finally, the expression of several genes was reported to be different in non-union tissue and controls 14,22,30,39,42,52, a finding suggesting that such differences may contribute to the pathogenesis of non-unions.
Several similarities were reported in the histological analysis of atrophic and hypertrophic non-unions. The main types of tissues involved include fibrous, cartilaginous and connective tissue in varying degree 30,40,43,44,46,50. In atrophic non-unions, bony islands were not always present 30,40,43,44,46,50, whereas necrotic bone was more prevalent 44. Generally, the cellular density of atrophic non-unions was lower compared to hypertrophic non-unions, while some areas were completely acellular 40,46. This suggests a different cellular background, which may correspond to the higher failure rate following revision surgery of atrophic non-unions 31.
More importantly, Iwakura et al. showed that tissue derived from hypertrophic non-unions contains MSC's 30, a finding later confirmed by Koga et al. 18. Similarly, Bajada et al. reported the presence of biologically active cells in atrophic non-union tissue, largely CD34/CD45-negative, CD105-positive, with the potential to differentiate to osteoblastic, adipogenic and chondrocytic lineages 40.
In contrast to the common preconception that atrophic non-unions are relatively avascular and inert 44,58, several authors have confirmed the vascularity of the atrophic non-union tissue 19,32,40,44,46,48,50. In addition, Reed et al. reported no significant difference in the vessel density between atrophic non-unions, hypertrophic non-unions and healing fractures 44. This biological finding may be of importance, as it suggests that treatments targeting to the enrichment and restoration of local angiogenesis could be applied as an effective treatment modality in the clinical setting.
Low-grade infection represents a challenge for the treating surgeon, as laboratory markers (such as C-Reactive Protein, erythrocyte sedimentation rate, white blood count) and conventional cultures of intra-operative samples can be negative 37,38. A possible explanation for this phenomenon could be the presence of biofilms (bacteria adhere on implants and tissues around the fracture site, forming matrix-enclosed communities), which are resistant to “normal” concentrations of systemic antibiotics 37. Palmer et al. and Gille et al. have reported the benefit of utilizing molecular based techniques to identify these infections 37,38. This can be very important, as distinguishing between septic and aseptic non-union is essential for determining the course of treatment. However, limitations of their use in clinical practice include: the fact that single-primer PCR can only detect one target organism 37; concerns for oversensitivity with regard to clinical relevance 37,59 and associated cost implications.
Cell senescence is known to play an important role in healing and tissue regeneration 60. In essence, the senescence of adult stem cells or more differentiated cells present in the non-union tissue may represent one of the main mechanisms of the loss of the regenerative potential, leading to healing impairment 60. As already mentioned, Bajada et al. reported that an increased proportion of non-union stromal cells were senescent when compared to bone marrow stromal cells, which did not correlate with the patient's age 40. However, the pathways leading to this genomic damage and the contribution of several factors (such as repeated cellular replication and the consequent cell stress 40) are yet to be determined.
Bone morphogenic proteins are some of the major signalling molecules, promoting the differentiation of MSC's into chondrocytes or osteoblasts 12,13. Kloen et al. reported evidence of ongoing BMP signalling in the non-union tissue, where endogenous BMP's, their receptors and molecules involved in their signal transduction were present in the tissue 45. Moreover, others have suggested that imbalance in the expression of BMP's and their inhibitors Drm (gremlin), follistatin, noggin and chordin, might account for the impaired bone forming ability 35,39. When the non-union tissue was cultured in the presence of exogenous BMP, the MSC's differentiated into functional osteoblasts, with an increased bone nodule formation 41,49. Treatments regulating concentrations of BMP's have already been used in clinical practice with encouraging results (such as BMP-2 and BMP-7 31). Future research is needed to investigate the effects of similar agonist molecules or their inhibitors.
Matrix metalloproteinases are proteases that play an important role in bone remodelling and bone repair. When the MMP's or their inhibitors are disrupted, disorders of fracture healing may occur 14. In a study by Fajardo et al., MMP-7 and MMP-12 genes were reported to be significantly up-regulated within the tissue of hypertrophic non-unions 14. When the hypertrophic non-union tissue was examined in vitro, it was found that the same proteins directly bounded to and degraded BMP-2, a highly osteoinductive agent 14. This action of the MMP's may be responsible for the impaired fracture healing in the case of hypertrophic non-unions, even though the same finding may not correlate to atrophic fracture non-unions.
Several reports suggest that low-intensity pulsed ultrasound treatment stimulates bone healing, although the mechanism behind this remains obscure 61,62. When applying low-intensity pulsed ultrasound in non-union cells cultures, it was found that there was a significant effect on the osteogenic differentiation rather than proliferation of non-union tissue cells 18. In addition, growth factor synthesis and release was stimulated 46. The use of low-intensity pulsed ultrasound can therefore improve union rates and accelerate the healing process.
Dickkopf-1 is a secreted protein acting as an antagonist of the Wnt signalling pathway, suppressing fracture repair by inhibiting osteogenic differentiation 40,63. Bajada et al. has compared the levels of Dkk-1 in atrophic non-union stromal cells and bone marrow stromal cells, reporting an increased secretion by the non-union cells, associated with reduced osteoblastic differentiation 40. When they treated the bone marrow stromal cells with recombinant human Dkk-1 or conditioned medium from the non-union cells, the effect on osteogenic differentiation remained inhibitory 40. This finding suggests that Dkk-1 may play an important role in the development of non-unions, however further research is needed to shed more light on the underlying mechanism of an increased Dkk-1 production by non-union cells.
Another important element of progression to non-union that needs to be discussed is genetic predisposition. Several authors have investigated this theory by analysing samples from peripheral venous blood 33,54, and bone callus 55 and comparing them with uneventful healing fractures. Numerous polymorphisms such as those of two specific SNPs (rs1372857, genotype GG and rs2053423, genotype TT) were identified to be associated with an increased risk of developing non-union 33,55,56.
The herein study has some limitations. First, it excludes studies involving experimental animal models. However, the outcome of such studies should be treated with caution, as they cannot be translated directly to the clinical scenarios. Second, there is an inherent inconsistency in defining non-union, and as such the timing of tissue harvesting would be slightly different, which might be responsible for some of the differences reported among similar studies. Moreover, as the term MSC's is fairly recent, studies performed in earlier years used a different terminology for the same cells, such as osteoprogenitors, skeletal stem cells, etc. As a result, their findings could not be compared to those of more recent studies.
Strengths of the study include the systematic approach of analysing the results and the detailed careful analysis of the data obtained. Collectively, this manuscript presents our current understanding of the molecular and cellular pathways that can be involved in the development of non-union. Direct recommendations to be applied in the clinical setting cannot be safely made with the available evidence. We deem essential that a widely accepted definition of the timeframe for non-unions should be set allowing an earlier intervention in such cases. The conceptual frame of the “diamond concept” for a successful fracture healing response should be considered in cases where bone repair is desirable 5. Cellular therapies and inductive molecules with scaffolds have a role to play in future treatment strategies, as would do tissue engineering approaches 64. Although still under intense investigation genetic therapy could be another treatment option in the foreseeable future.
Conclusion
In conclusion, failure of fracture healing and progression to non-union represents a not uncommon clinical complication carrying devastating consequences. The histopathological appearance of non-union tissue between atrophic and hypertrophic non-union indicates that both types of non-unions are not avascular and contain a potentially active population of MSC's. Pathways believed to be involved in their pathogenesis include an imbalance in the expression of BMP's and their inhibitors, and an up-regulated expression of several substances such as that of the MMP's and Dkk-1which can block the BMP and Wnt pathways respectively. Immerging evidence also support a genetic predisposition in this patient group.
Acknowledgments
MP and IP performed the research. MP and PVG designed the research study. MP, EJ and PVG analysed the data. MP wrote the paper. All authors contributed to the preparation of the manuscript.
Funding
No funding was received for the completion of this project.
Conflicts of interest
All authors declare no conflict of interest.
References
- Ferguson C, Alpern E, Miclau T, et al. Does adult fracture repair recapitulate embryonic skeletal formation? Mech Dev. 1999;87:57–66. doi: 10.1016/s0925-4773(99)00142-2. [DOI] [PubMed] [Google Scholar]
- Komatsu DE, Warden SJ. The control of fracture healing and its therapeutic targeting: improving upon nature. J Cell Biochem. 2010;109:302–11. doi: 10.1002/jcb.22418. [DOI] [PubMed] [Google Scholar]
- Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury. 2007;38:S3–6. doi: 10.1016/s0020-1383(08)70003-2. [DOI] [PubMed] [Google Scholar]
- Schroeder JE, Mosheiff R. Tissue engineering approaches for bone repair: concepts and evidence. Injury. 2011;42:609–13. doi: 10.1016/j.injury.2011.03.029. [DOI] [PubMed] [Google Scholar]
- Giannoudis PV, Panteli M, Calori GM. Bone healing: the diamond concept. In: Bentley G, editor. European Instructional Lectures. Berlin, Heidelberg: Springer; 2014. pp. 3–16. [Google Scholar]
- Kolar P, Schmidt-Bleek K, Schell H, et al. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev. 2010;16:427–34. doi: 10.1089/ten.TEB.2009.0687. [DOI] [PubMed] [Google Scholar]
- Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Henle P, Zimmermann G, Weiss S. Matrix metalloproteinases and failed fracture healing. Bone. 2005;37:791–8. doi: 10.1016/j.bone.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Tsiridis E, Upadhyay N, Giannoudis P. Molecular aspects of fracture healing: which are the important molecules? Injury. 2007;38:S11–25. doi: 10.1016/j.injury.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Sarahrudi K, Thomas A, Mousavi M, et al. Elevated transforming growth factor-beta 1 (TGF-beta1) levels in human fracture healing. Injury. 2011;42:833–7. doi: 10.1016/j.injury.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoudis PV, Ahmad MA, Mineo GV, et al. Subtrochanteric fracture non-unions with implant failure managed with the “Diamond” concept. Injury. 2013;44(Suppl. 1):S76–81. doi: 10.1016/S0020-1383(13)70017-2. [DOI] [PubMed] [Google Scholar]
- Einhorn TA. The science of fracture healing. J Orthop Trauma. 2005;19:S4–6. doi: 10.1097/00005131-200511101-00002. [DOI] [PubMed] [Google Scholar]
- Phillips AM. Overview of the fracture healing cascade. Injury. 2005;36:S5–7. doi: 10.1016/j.injury.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Fajardo M, Liu CJ, Ilalov K, et al. Matrix metalloproteinases that associate with and cleave bone morphogenetic protein-2 in vitro are elevated in hypertrophic fracture nonunion tissue. J Orthop Trauma. 2010;24:557–63. doi: 10.1097/BOT.0b013e3181ed294c. [DOI] [PubMed] [Google Scholar]
- Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–5. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carano RA, Filvaroff EH. Angiogenesis and bone repair. Drug Discovery Today. 2003;8:980–9. doi: 10.1016/s1359-6446(03)02866-6. [DOI] [PubMed] [Google Scholar]
- Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998;355 Suppl:S7–21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- Koga T, Lee SY, Niikura T, et al. Effect of low-intensity pulsed ultrasound on bone morphogenetic protein 7-induced osteogenic differentiation of human nonunion tissue-derived cells in vitro. J Ultrasound Med. 2013;32:915–22. doi: 10.7863/ultra.32.6.915. [DOI] [PubMed] [Google Scholar]
- Lawton DM, Andrew JG, Marsh DR, et al. Expression of the gene encoding the matrix gla protein by mature osteoblasts in human fracture non-unions. Molecular pathology. 1999;52:92–6. doi: 10.1136/mp.52.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi G, Perego S, Luzi L, et al. A four-season molecule: osteocalcin. Updates in its physiological roles. Endocrine. 2015;48:394–404. doi: 10.1007/s12020-014-0401-0. [DOI] [PubMed] [Google Scholar]
- Monfoulet L, Malaval L, Aubin JE, et al. Bone sialoprotein, but not osteopontin, deficiency impairs the mineralization of regenerating bone during cortical defect healing. Bone. 2010;46:447–52. doi: 10.1016/j.bone.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Zimmermann G, Schmeckenbecher KH, Boeuf S, et al. Differential gene expression analysis in fracture callus of patients with regular and failed bone healing. Injury. 2012;43:347–56. doi: 10.1016/j.injury.2011.10.031. [DOI] [PubMed] [Google Scholar]
- Pountos I, Panteli M, Panagiotopoulos E, et al. Can we enhance fracture vascularity: what is the evidence? Injury. 2014;45:S49–57. doi: 10.1016/j.injury.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Bhandari M, Guyatt GH, Swiontkowski MF, et al. A lack of consensus in the assessment of fracture healing among orthopaedic surgeons. J Orthop Trauma. 2002;16:562–6. doi: 10.1097/00005131-200209000-00004. [DOI] [PubMed] [Google Scholar]
- Bishop JA, Palanca AA, Bellino MJ, et al. Assessment of compromised fracture healing. J Am Acad Orthop Surg. 2012;20:273–82. doi: 10.5435/JAAOS-20-05-273. [DOI] [PubMed] [Google Scholar]
- Nauth A, Miclau T, 3rd, Li R, et al. Gene therapy for fracture healing. J Orthop Trauma. 2010;24:S17–24. doi: 10.1097/BOT.0b013e3181cec6fb. [DOI] [PubMed] [Google Scholar]
- Kanakaris NK, Giannoudis PV. The health economics of the treatment of long-bone non-unions. Injury. 2007;38:S77–84. doi: 10.1016/s0020-1383(07)80012-x. [DOI] [PubMed] [Google Scholar]
- Sarmiento A, Sharpe FE, Ebramzadeh E, et al. Factors influencing the outcome of closed tibial fractures treated with functional bracing. Clin Orthop Relat Res. 1995;315:8–24. [PubMed] [Google Scholar]
- Oni OO, Hui A, Gregg PJ. The healing of closed tibial shaft fractures. The natural history of union with closed treatment. J Bone Joint Surg Br. 1988;70:787–90. doi: 10.1302/0301-620X.70B5.3192581. [DOI] [PubMed] [Google Scholar]
- Iwakura T, Miwa M, Sakai Y, et al. Human hypertrophic nonunion tissue contains mesenchymal progenitor cells with multilineage capacity in vitro. J Orthop Res. 2009;27:208–15. doi: 10.1002/jor.20739. [DOI] [PubMed] [Google Scholar]
- Pneumaticos SG, Panteli M, Triantafyllopoulos GK, et al. Management and outcome of diaphyseal aseptic non-unions of the lower limb: a systematic review. Surgeon. 2014;12(3):166–75. doi: 10.1016/j.surge.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Heppenstall RB, Brighton CT, Esterhai JL. Synovial pseudarthrosis: a clinical, roentgenographic-scintigraphic, and pathologic study. J Trauma. 1987;27:463–70. [PubMed] [Google Scholar]
- Zeckey C, Hildebrand F, Glaubitz LM, et al. Are polymorphisms of molecules involved in bone healing correlated to aseptic femoral and tibial shaft non-unions? J Orthop Res. 2011;29:1724–31. doi: 10.1002/jor.21443. [DOI] [PubMed] [Google Scholar]
- Einhorn TA. Enhancement of fracture healing. Instr Course Lect. 1996;45:401–16. [PubMed] [Google Scholar]
- Kwong FN, Hoyland JA, Freemont AJ, et al. Altered relative expression of BMPs and BMP inhibitors in cartilaginous areas of human fractures progressing towards nonunion. J Orthop Res. 2009;27:752–7. doi: 10.1002/jor.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MP, Altman DT, Altman GT, et al. Can we trust intraoperative culture results in nonunions? J Orthop Trauma. 2014;28:384–90. doi: 10.1097/BOT.0000000000000043. [DOI] [PubMed] [Google Scholar]
- Gille J, Wallstabe S, Schulz AP, et al. Is non-union of tibial shaft fractures due to nonculturable bacterial pathogens? A clinical investigation using PCR and culture techniques. J Orthop Surg Res. 2012;7:20. doi: 10.1186/1749-799X-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo M, Liu CJ, Egol K. Levels of expression for BMP-7 and several BMP antagonists may play an integral role in a fracture nonunion: a pilot study. Clin Orthop Relat Res. 2009;467:3071–8. doi: 10.1007/s11999-009-0981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajada S, Marshall MJ, Wright KT, et al. Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone. 2009;45:726–35. doi: 10.1016/j.bone.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Qu G, von Schroeder HP. The osteogenic potential of pseudoarthrosis tissue and bone from human scaphoid non-unions. J Hand Surg Eur Vol. 2008;33:449–56. doi: 10.1177/1753193408090122. [DOI] [PubMed] [Google Scholar]
- Hofmann A, Ritz U, Hessmann MH, et al. Cell viability, osteoblast differentiation, and gene expression are altered in human osteoblasts from hypertrophic fracture non-unions. Bone. 2008;42:894–906. doi: 10.1016/j.bone.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Bajada S, Harrison PE, Ashton BA, et al. Successful treatment of refractory tibial nonunion using calcium sulphate and bone marrow stromal cell implantation. J Bone Joint Surg Br. 2007;89:1382–6. doi: 10.1302/0301-620X.89B10.19103. [DOI] [PubMed] [Google Scholar]
- Reed AA, Joyner CJ, Brownlow HC, et al. Human atrophic fracture non-unions are not avascular. J Orthop Res. 2002;20:593–9. doi: 10.1016/S0736-0266(01)00142-5. [DOI] [PubMed] [Google Scholar]
- Kloen P, Doty SB, Gordon E, et al. Expression and activation of the BMP-signaling components in human fracture nonunions. J Bone Joint Surg Am. 2002;84-A:1909–18. doi: 10.2106/00004623-200211000-00001. [DOI] [PubMed] [Google Scholar]
- Guerkov HH, Lohmann CH, Liu Y, et al. Pulsed electromagnetic fields increase growth factor release by nonunion cells. Clin Orthop Relat Res. 2001;384:265–79. doi: 10.1097/00003086-200103000-00031. [DOI] [PubMed] [Google Scholar]
- Lawton DM, Andrew JG, Marsh DR, et al. Mature osteoblasts in human non-union fractures express collagen type III. Mol Pathol. 1997;50:194–7. doi: 10.1136/mp.50.4.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santavirta S, Konttinen YT, Nordstrom D, et al. Immunologic studies of nonunited fractures. Acta Orthop Scand. 1992;63:579–86. [PubMed] [Google Scholar]
- Boyan BD, Schwartz Z, Swain LD, et al. Initial effects of partially purified bone morphogenetic protein on the expression of glycosaminoglycan, collagen, and alkaline phosphatase in nonunion cell cultures. Clin Orthop Relat Res. 1992;278:286–304. [PubMed] [Google Scholar]
- Quacci D, Dell'Orbo C, Salvi M, et al. Ultrastructural aspects of human nonunion. Histol Histopathol. 1991;6:87–93. [PubMed] [Google Scholar]
- Milgram JW. Nonunion and pseudarthrosis of fracture healing. A histopathologic study of 95 human specimens. Clin Orthop Relat Res. 1991;268:203–13. [PubMed] [Google Scholar]
- Kilian O, Dahse R, Alt V, et al. Expression of EDA+ and EDB+ fibronectin splice variants in bone. Bone. 2004;35:1334–45. doi: 10.1016/j.bone.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Urist MR, Mazet R, Jr, McLean FC. The pathogenesis and treatment of delayed union and non-union; a survey of eighty-five ununited fractures of the shaft of the tibia and one hundred control cases with similar injuries. J Bone Joint Surg Am. 1954;36-A:931–80. ; passim. [PubMed] [Google Scholar]
- Dimitriou R, Carr IM, West RM, et al. Genetic predisposition to fracture non-union: a case control study of a preliminary single nucleotide polymorphisms analysis of the BMP pathway. BMC Musculoskelet Disord. 2011;12:44. doi: 10.1186/1471-2474-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong DH, Liu XG, Guo YF, et al. Genome-wide association and follow-up replication studies identified ADAMTS18 and TGFBR3 as bone mass candidate genes in different ethnic groups. Am J Hum Genet. 2009;84:388–98. doi: 10.1016/j.ajhg.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriou R, Kanakaris N, Soucacos PN, et al. Genetic predisposition to non-union: evidence today. Injury. 2013;44:S50–3. doi: 10.1016/S0020-1383(13)70012-3. [DOI] [PubMed] [Google Scholar]
- Fernandez-Bances I, Perez-Basterrechea M, Perez-Lopez S, et al. Repair of long-bone pseudoarthrosis with autologous bone marrow mononuclear cells combined with allogenic bone graft. Cytotherapy. 2013;15:571–7. doi: 10.1016/j.jcyt.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Paley D, Catagni MA, Argnani F, et al. Ilizarov treatment of tibial nonunions with bone loss. Clin Orthop Relat Res. 1989;241:146–65. [PubMed] [Google Scholar]
- Panousis K, Grigoris P, Butcher I, et al. Poor predictive value of broad-range PCR for the detection of arthroplasty infection in 92 cases. Acta Orthop. 2005;76:341–6. [PubMed] [Google Scholar]
- Behrens A, van Deursen JM, Rudolph KL, et al. Impact of genomic damage and ageing on stem cell function. Nat Cell Biol. 2014;16:201–7. doi: 10.1038/ncb2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man J, Shelton RM, Cooper PR, et al. Low intensity ultrasound stimulates osteoblast migration at different frequencies. J Bone Miner Metab. 2012;30:602–7. doi: 10.1007/s00774-012-0368-y. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Arai Y, Takenaka N, et al. Three key factors affecting treatment results of low-intensity pulsed ultrasound for delayed unions and nonunions: instability, gap size, and atrophic nonunion. J Orthop Sci. 2013;18:803–10. doi: 10.1007/s00776-013-0415-0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Whetstone HC, Lin AC, et al. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007;4:e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pountos I, Georgouli T, Kontakis G, et al. Efficacy of minimally invasive techniques for enhancement of fracture healing: evidence today. Int Orthop. 2010;34:3–12. doi: 10.1007/s00264-009-0892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


