Abstract
Low-density lipoprotein receptor-related protein 5 (LRP5) is a member of the LDLR family that orchestrates cholesterol homoeostasis. The role of LRP5 and the canonical Wnt pathway in the vascular wall of dyslipidaemic animals remains unknown. In this study, we analysed the role of LRP5 and the Wnt signalling pathway in mice fed a hypercholesterolaemic diet (HC) to trigger dyslipidaemia. We show that Lrp5−/− mice had larger aortic lipid infiltrations than wild-type mice, indicating a protective role for LRP5 in the vascular wall. Three members of the LDLR family, Lrp1, Vldlr and Lrp6, showed up-regulated gene expression levels in aortas of Lrp5−/− mice fed a hypercholesterolaemic diet. HC feeding in Lrp5−/− mice induced higher macrophage infiltration in the aortas and accumulation of inflammatory cytokines in blood. Wnt/β-CATENIN signalling proteins were down-regulated in HC Lrp5−/− mice indicating that LRP5 regulates the activation of Wnt signalling in the vascular wall. In conclusion, our findings show that LRP5 and the canonical Wnt pathway down-regulation regulate the dyslipidaemic profile by promoting lipid and macrophage retention in the vessel wall and increasing leucocyte-driven systemic inflammation.
Keywords: LRP5, atherosclerosis, plasma cholesterol, canonical Wnt signalling, macrophages
Introduction
Hypercholesterolaemia is a causal factor for atherosclerosis, which predisposes individuals to the development of clinical cardiovascular diseases 1. High levels of low-density lipoprotein (LDL) in plasma rapidly infiltrate the vessel wall triggering an inflammatory–immunomodulatory chain reaction 2,3. Members of the LDL receptor family are involved in lipoprotein transport and plasma LDL cholesterol clearance, modulating critical stages of atherosclerosis progression including inflammation, foam cell formation and endothelial activation 4,5. There is ongoing controversy on the role of LRP5 and the canonical Wnt/β-CATENIN pathway in lipid induced vascular damage and atherosclerosis. Indeed, activation of the β-CATENIN/lymphoid enhancer-binding factor 1 (LEF1) signalling in the endothelium occurs in response to atheroprone haemodynamic stimulations and precedes lesion development in ApoE−/− mice 6 and higher levels of active β-CATENIN are observed in disrupted atherosclerotic plaques compared to stable plaques from human carotid artery, suggesting a potential role for Wnt signalling in the evolution of atherosclerotic plaque 7. However, ApoE−/−Lrp5−/− mice fed a high-fat diet developed bigger atherosclerotic lesions that their ApoE−/− littermates 8, although the exceedingly high levels of cholesterol in these animals (almost 750 mg/dl) could have shadowed any effect of the canonical Wnt receptor, LRP5.
The canonical Wnt/β-CATENIN pathway has also been described to regulate inflammatory reactions although its role remains controversial. Indeed, Wnt/β-CATENIN pathway seems to inhibit inflammation as β-CATENIN inhibitors increase the expression of inflammatory genes in human aortic endothelial cells 9 and administration of GSK3β, an inhibitor of Wnt/β-CATENIN pathway in human monocytes triggers Toll-like receptor-mediated pro-inflammatory cytokine production 10. However, IL-1β and LPS induced nuclear β-CATENIN accumulation in human vascular endothelial cells 6 and activation of canonical Wnt genes have been found in endothelial cells of a rejected kidney model 11 suggesting that activation of the pathway triggers the inflammatory response.
We have recently reported that LRP5 is involved in monocyte to macrophage differentiation 12, it regulates macrophage motility and LRP5-expressing mononuclear cells are a fraction of the macrophages found in human advanced coronary atherosclerotic plaques 13. Still, the presence of LRP5-positive cells in these coronary plaques does not imply causality. Thus, to better understand the role of LRP5 and Wnt signalling in the early stages of lipid infiltration in the vessel wall, we studied the effects of a hyperlipidaemic diet inducing a mild increase in cholesterol serum levels in Lrp5−/− mice and in wild-type (WT) controls. We hypothesized that LRP5 and the Wnt signalling pathway have a role in the inflammatory process associated to atherosclerosis progression. Absence of LRP5, induced higher lipid infiltration in mouse thoracic aortas, increased the transcription of the LDLR family member Lrp6, induced higher macrophage infiltration and increased inflammatory cytokines secretion, supporting an anti-inflammatory role for LRP5 and the target genes of the Wnt/β-CATENIN pathway.
Materials and methods
Animals and experimental design
Lrp5−/− mice, a kind gift from Dr. Bart Williams 14–16 were maintained in a C57BL/6 background. Mice were housed in cages under controlled temperature (21 ± 2°C) on a 12 hrs light/dark cycle with food and water ad libitum. Homozygous WT C57BL/6 mice (n = 22) and Lrp5−/− C57BL/6 mice (LRP5−/−; n = 22) were used for the protocols. The presence of Lrp5 alleles was assessed by PCR amplification from DNA extracted from tail biopsies in WT, heterozygous and homozygous littermates. Primers used were S17 (GGC TCG GAG GAC AGA CCT GAG), S23 (CTG TCA GTG CCT GTA TCT GTC C) and IRES31 (AGG GGC GGA ATT CGA TAG CT). Lrp5−/− and Wt mice were fed a normal chow diet (NC, Tekland diet, Harland Labs Berkeley, CA, USA) for 10 weeks. Animals were then divided into two groups to be fed NC or high cholesterol diet (HC, TD.88137, Harland Labs) for further 8 weeks (8–12 mice/group). Cardiac puncture was performed in mice under terminal anaesthesia (1 mg/kg Medetomidine and 75 mg/kg Ketamine, ip). The study protocol was conducted in conformity with the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals and approved by the local institutional animal research committee (ICCC051/5422).
Biochemical analysis and blood-derived mRNA
Blood samples were collected in serum separator gel tubes and PAX-tubes. Serum was obtained by centrifugation 1200g 20 min. at 4°C. Cholesterol, triglycerides and HDL levels were measured enzymatically by using commercially available kits (GERNON reagents) and read in a spectrophotometer (MC-15 SOFT; RAL). PAX-tubes were processed for preparation of blood-derived mRNA using PAXgene Blood RNA Kit (Qiagen Inc, Valencia, CA, USA). Real Time RT-PCR array was performed with RT2 Profiler PCR array PAMM-021 (SABiosciences, Qiagen).
Quantification of atherosclerotic lesions
Mice were anaesthetized and aortas were removed, carefully cleaned of adventitial fat under a stereoscopic microscope, and longitudinally cut with the luminal surface facing up (n = 6–8 mice/group). Aortas were fixed overnight in 4% paraformaldehyde, washed with ddH2O 1 hr in gentle shaking and stained with Oil-red-O (ORO) for 30 min. Aortas were rinsed with 70% ethanol and ddH2O; images were captured by Nikon Instruments, Melville, NY, USA Eclipse 80i microscope and digitized by Retiga 1300i QImaging, Surrey, BC, Canada Fast camera. ORO-stained area was quantified with Image J software and results are expressed as percentage of lipid area/total aortic area.
Thin layer chromatography
Aorta tissue (5 mg) was homogenized in NaOH 0.1 M. The organic solvent was removed under a N2 stream, the lipid extract was suspended in dichloromethane and separated by thin layer chromatography (TLC). TLC was performed on silica G-24 plates. Concentrations of standards (a mix of cholesterol, cholesterol palmitate, triglycerides, diglycerides and monoglycerides) were applied to each plate. The chromatographic developing solution was heptane/diethylether/acetic acid (74:21:4, vol/vol/vol). The spots corresponding to cholesteryl esters (CE) were quantified by densitometry.
Real time RT-PCR
Aortas were frozen in liquid nitrogen and aortic RNA was isolated with Trizol® Reagent (Invitrogen; Carlsbad, CA, USA n = 5–7 mice/group). Concentration was determined with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE, USA) and purity was checked by the A260/A280 ratio (ratios between 1.8 and 2.1 were considered acceptable). cDNA was synthesized from 0.5 μg RNA with cDNA Reverse transcription kit (Qiagen). The resulting cDNA samples were amplified with a RT-PCR thermal cycler (Applied Biosystems Carlsbad, CA, USA 7900HT) and the following specific probes from CONDA: Lrp5 (Mm.PT.49a.8045420), Lrp1 (Mm.PT.49a.7750137), Lrp2 (Mm.PT.49a.11916154), Lrp6 (Mm.PT.56a.6383636), Lrp8 (Mm.PT.49a.6553055), Ldlr (Mm.PT.49a.9930556) and Cd36 (Mm.PT.49a.12111555). Vldlr (Mm00443298_m1) was purchased from Applied Biosystems. Results were normalized with 18S probe from Applied Biosystems.
Immunohistochemistry
Immediately after surgical excision, aortas were immersed in fixative solution (4% paraformaldehyde) and embedded in paraffin, cut into 5 μm thick serial sections and placed on poly-L-lysine coated slides. Primary antibodies used were: Matrix Metalloproteinase-7, MMP-7 (Rabbit polyclonal; Abcam), Cambridge, UK β-CATENIN (Rabbit policlonal; Millipore) Bedford, MA, USA and HAM56 (Mouse monoclonal; Dako Glostrup, Denmark). Before incubation with primary antibodies, sections were washed and endogenous peroxidase activity was impressed with H2O2 and goat or horse serum block. Primary antibodies were detected using the avidin–biotin immunoperoxidase technique. Sections were incubated with an appropriate biotinylated secondary antibody (1:200; Vector Laboratories). Burlingame, CA, USA The chromogen used was 3,3′-diaminobenzidine. Haematoxylin was used for nuclear stain. Images were captured by Nikon Eclipse 80i microscope and digitized by Retiga 1300i Fast camera, magnification (×400).
Statistical analysis
Results are expressed as mean ± SEM. A Stat View statistical package was used for all the analysis. Comparisons among groups were performed by two-way anova analysis. Regression analyses were performed by applying Y = a + b*X lineal pattern selecting just highly adjusted equations. Slopes were compared by t-test. Statistical significance was considered when P < 0.05.
Results
Serum cholesterol profile
We analysed the differences in serum cholesterol profiles of Wt and Lrp5−/− mice fed a normocholesterolaemic (NC) or hypercholesterolaemic (HC) diet. Figure1A shows an agarose gel with Wt, Lrp5−/− and Lrp5+/− alleles. Cholesterol serum levels of Lrp5−/− mice fed a NC diet containing 3.5% (w/w) fat and 0% cholesterol were lower than those of their Wt littermates (Fig.1B). When mice were fed a HC diet containing 21% (w/w) fat and 0.25% cholesterol, total cholesterol levels were significantly increased in both Lrp5−/− and Wt mice (Fig.1B). Interestingly, the overall increase in serum cholesterol levels in high-fat diet with respect to chow diet was double in Lrp5−/− (125.69 mg/dl) than in Wt mice (62.25 mg/dl, Fig.1B). Non-HDL cholesterol increased by 42 ± 8% and 300 ± 23% in HC Wt and HC Lrp5−/− mice with respect to their NC littermates (Fig.1C).
Fig 1.
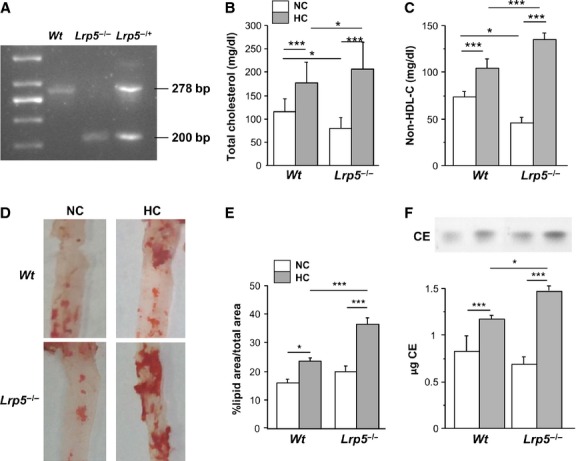
Hypercholesterolaemic (HC) Lrp5−/− mice model characterization. (A) Agarose gel showing Wt, Lrp5−/− and Lrp5−/+ alleles. (B) Serum cholesterol levels in Wt and Lrp5−/− mice fed a normocholesterolaemic (NC) or a HC diet. (C) Non-HDL-C in Wt and Lrp5−/− mice fed a NC or a HC diet. (D) Representative images of mouse thoracic aortas stained with ORO. (E) Quantification of lipid area in mice aortas. (F) Cholesteryl ester measurement by thin layer chromatography and bar graph showing CE quantification. *P < 0.05; ***P < 0.005.
Lipid deposition in aortas
The administration of a HC diet in Wt and Lrp5−/− mice led to a significant increase in early lesions in the aortic area with lipid infiltration in both groups. Indeed, the aortic lipid rich coverage increased by 7 ± 0.5% and by 17 ± 1% in Wt and Lrp5−/− mice respectively (Fig.1D and E). The increased lipid deposition in HC Lrp5−/− mice was over 70% higher than in HC Wt mice indicating a protective role for LRP5 in mice aortas. Furthermore, HC feeding induced higher levels of CE accumulation in Lrp5−/− (87 ± 8%) than in Wt (51 ± 6%) aortas with respect to NC mice (Fig.1F).
Positive correlation between plasma cholesterol levels and aortic lipid deposition in Wt and Lrp5−/− mice
To analyse the effect of total plasma cholesterol on aortic lipid coverage, regression analyses were performed for Wt and Lrp5−/− mice results. Significantly higher aortic lipid deposition was observed with higher plasma cholesterol levels (Fig.2A and B). The slope for this correlation was steeper for Lrp5−/− mice. Correlation analyses remained positive and significant between non-HDL cholesterol and aortic lipid coverage and slopes were significantly different with higher change in Lrp5−/− mice (Fig.2C and D).
Fig 2.
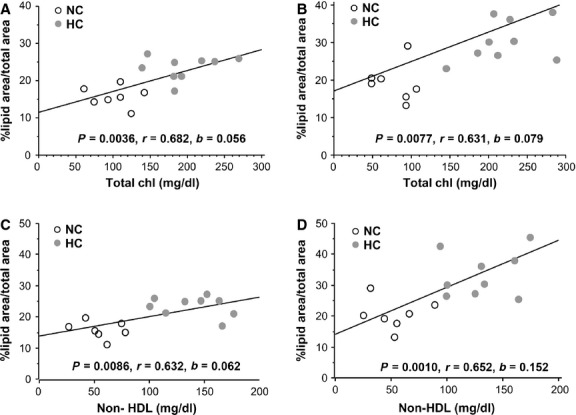
Regression analyses between total cholesterol and aortic lipid coverage in Wt (A) and Lrp5−/− mice (B) and between non-HDL cholesterol and aortic lipid coverage in Wt (C) and Lrp5−/− mice (D) with their statistical significances (p), correlation coefficients (r) and slopes (b).
Increased macrophage infiltration in Lrp5−/− mice
Lrp5 mRNA expression in white blood cells showed a 77.5 ± 0.2% increase because of hypercholesterolaemia in Wt mice (Fig.3A). Lrp5 mRNA expression was negligible in white cells of Lrp5−/− mice. Immunostaining with HAM56 for macrophages (Fig.3B) showed a 47.6 ± 2% increase in macrophage infiltration to the intima in HC Lrp5−/− mice compared with HC Wt mice (Fig.3C). Monocytes–macrophages in aortic tissue were more abundant in Lrp5−/− mice.
Fig 3.
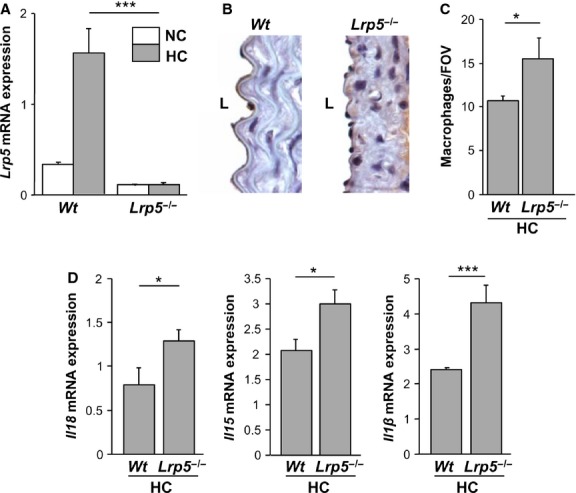
Hypercholesterolaemic (HC) Lrp5−/− mice show increased aortic macrophage infiltration. (A) Lrp5 expression levels in blood leucocytes in normocholesterolaemic (NC) or HC Wt and Lrp5−/− mice. (B) HC Wt and HC Lrp5−/− mice aortas labelled with HAM56, L, lumen. (C) Quantitative analysis of B expressed as number of macrophages/field of vision. (D) White blood cells gene expression of pro-inflammatory cytokines in HC Wt and HC Lrp5−/− mice. *P < 0.05; ***P < 0.005.
Expression of pro-inflammatory cytokines in white blood cells
We then analysed gene expression levels of pro-inflammatory cytokines in white blood cells from HC Wt and HC Lrp5−/− mice. Results show an increase of Il18, Il15 and Il1β mRNA expression by 62.5 ± 2%, 43 ± 1% and 91 ± 2% respectively in HC Lrp5−/− compared with HC Wt mice (Fig.3D). These results show increased inflammation in HC Lrp5−/− mice respect to their Wt littermates.
Wnt pathway activation in mice aortas
Levels of two proteins of the canonical Wnt pathway, β-CATENIN and MMP-7 were analysed in macroscopically ORO-stained areas of the aortas of Wt and Lrp5−/− mice. β-CATENIN staining was increased in aortas of HC Wt mice compared to NC Wt mice indicating an activation of the Wnt/β-CATENIN pathway by hypercholesterolaemia. As expected, there was no increase in β-CATENIN staining in HC Lrp5−/− mice (Fig.4A and B). Similarly MMP-7, another downstream protein of the pathway, was up-regulated in HC Wt compared with NC Wt mice, while in aortas from HC Lrp5−/− mice, the area covered by MMP-7 staining was smaller (Fig.4C and D). These results suggest that the canonical Wnt/β-CATENIN pathway is triggered by mild dyslipidaemia as a protective response and that this effect cannot be produced when LRP5 is absent.
Fig 4.
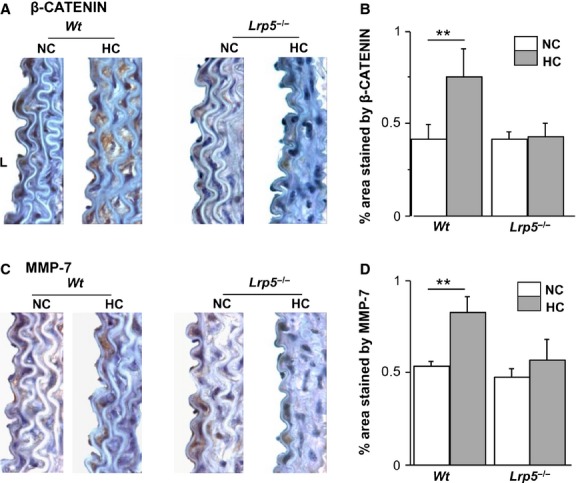
Wnt pathway modulation in aortas from Wt and Lrp5−/− mice. (A) Representative images of aortas sections immunostained with β-CATENIN in normocholesterolaemic (NC) or hypercholesterolaemic (HC) Wt and Lrp5−/− mice. (B) % area stained by β-CATENIN. (C) Representative images of aortas sections immunostained with MMP-7 in NC or a HC Wt and Lrp5−/− mice. (D) Quantitative analysis of aortas in C. **P < 0.01.
LDL receptors in WT and LRP5−/− mice aortas
LRP5 belongs to the LDL receptor superfamily of proteins involved in lipoprotein trafficking 5. To determine if other receptors were causing the lipid infiltration observed in aortas of Lrp5−/− mice, we analysed gene expression of receptors described to be involved in the initial stages of atherosclerosis lesions. Results show that Lrp2 gene expression was strongly down-regulated in Lrp5−/− mice independently of diet (Fig.5A), while Lrp8 gene expression was down-regulated in Wt and Lrp5−/− mice after HC diet (21.05 ± 2% and 57.14 ± 2% respectively, Fig.5B). Similarly, the classical Ldlr mRNA expression levels were reduced with HC feeding in both genotypes (21.4 ± 0.5% in HC Wt respect to NC Wt and 23.6 ± 1% in Lrp5−/−, Fig.5C). Cd36 increased its mRNA expression by 48.2 ± 1% in HC Wt mice with respect to NC Wt animals, but showed a 41.6 ± 0.5% decrease in HC Lrp5−/− mice with respect to NC Lrp5−/− mice (Fig.5D). On the contrary, Lrp1 significantly increased by 37 ± 2% in HC Wt mice and 21 ± 9% in HC Lrp5−/− mice aortas with respect to their NC littermates, but there was no significant effect because of HC in the Lrp5−/− mice (Fig.6A). The Vldlr mRNA levels were up-regulated by the HC diet in Wt and Lrp5−/− mice, although in a non-significant manner (Fig.6B). Finally, Lrp6 mRNA expression levels were increased by 31 ± 2% in HC Lrp5−/− mice with respect to NC Lrp5−/− mice (Fig.6C). Regression analyses revealed a significant positive correlation between Lrp6 gene expression levels and lipid rich coverage in aortas of Lrp5−/− mice, but not for Lrp1 (Fig.6D).
Fig 5.
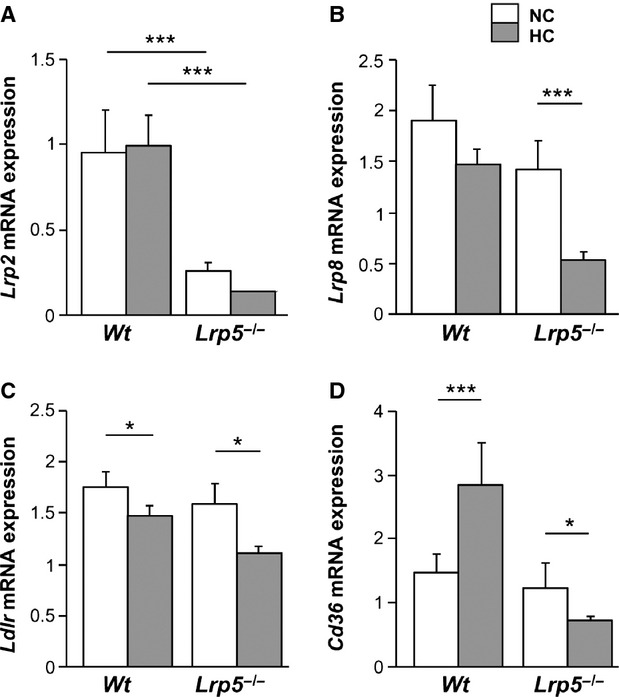
Receptors expression in mice aortas. mRNA expression levels of Lrp2 (A), Lrp8 (B), Ldlr (C) and Cd36 (D) in aortas from Wt and Lrp5−/− mice fed a normocholesterolaemic (NC) or a hypercholesterolaemic (HC) diet. *P < 0.05; ***P < 0.005.
Fig 6.
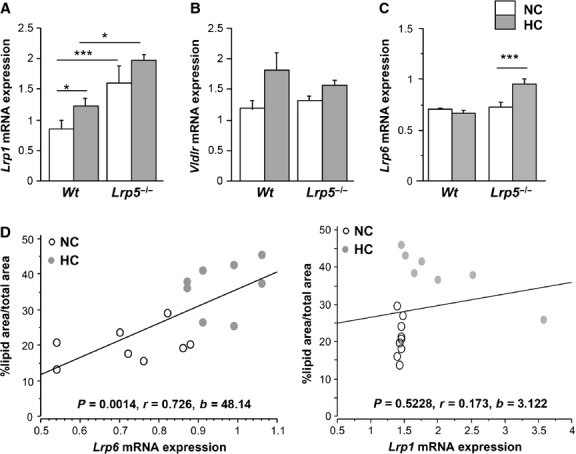
Receptors expression in mice aortas. mRNA expression levels of Lrp1 (A), Vldlr (B) and Lrp6 (C) in aortas from Wt and Lrp5−/− mice fed a normocholesterolaemic (NC) or a hypercholesterolaemic (HC) diet. *P < 0.05; ***P < 0.005. (D) Regression analyses between aortic lipid coverage and Lrp6 or Lrp1 gene expression levels in aortas of Lrp5−/− mice with their statistical significances (p), correlation coefficients (r) and slopes (b).
Discussion
Over the last decades, LRP5 has been involved in several pathways, including bone development, impaired fat tolerance and glucose metabolism 8,17–19. Impaired plasma clearance of chylomicron remnants and reduced glucose tolerance was observed in LRP5-deficient mice fed a high-fat diet compared with their Wt littermates 17. Here, we have studied the role of LRP5 and the canonical Wnt pathway in mildly HC Lrp5−/− mice. Total cholesterol and non-HDL plasma levels increased after HC feeding in Wt and Lrp5−/− mice. However, total cholesterol levels in HC Lrp5−/− mice that had a significantly higher aortic lipid infiltration were higher than those in HC Wt mice, suggesting an involvement for LRP5 during dyslipidaemia, at the initial stages of atherosclerosis development. Similarly in humans, LPR5-rs3736228T alleles that cause loss-of-function in LRP5 protein show increased plasmatic cholesterol levels in Chinese Han population 20 and is considered an independent risk factor for hypercholesterolaemia in the male Japanese population 21.
Hypercholesterolaemic Lrp5−/− mice show larger lipid infiltration in the thoracic aorta compared with HC Wt animals. Accordingly, Immunohistochemistry analyses revealed an increase in macrophage staining in the intima layer of HC Lrp5−/− mice aortas confirming our previous in vitro results showing that monocytes that overexpress LRP5 show a down-regulation of the differentiation processes by the sequestration of β-CATENIN to the cell membrane 12. Consequently, here we show that mice lacking LRP5 have more macrophages in the vascular wall. Activated monocytes/macrophages stimulate cytokine secretion promoting pro-inflammatory chronic stimulation 1,22,23. Therefore, we analysed the expression of pro-inflammatory and pro-atherogenic cytokines (Il18, Il15 and Il1β) in white blood cells from HC Wt and HC Lrp5−/− mice 24–27. Wnt/β-CATENIN activation inhibits the inflammatory response in endothelial cells 9 and enhances injury repair and healing responses in other inflammatory diseases including rheumatoid arthritis and colitis 28. We found increased inflammatory cytokines gene expression in white blood cells from HC Lrp5−/− mice indicating increased inflammation.
IHC analyses revealed a down-regulation of the Wnt canonical pathway members, β-CATENIN and MMP7, in the absence of LRP5 evidencing a down-regulation of the canonical Wnt signalling pathway in HC Lrp5−/− mice aortas. This supports our previous in vitro findings showing that LRP5 silencing in human macrophages abrogates Wnt/β-CATENIN pathway activation 13.
Aortic Lrp6 expression increased in Lrp5−/− animals fed the HC diet and directly correlated with aortic lesion area. LRP6 has been found overexpressed in human atherosclerotic lesions 29 and regulates LDLR-mediated LDL uptake as LDLR internalization is severely diminished in Lrp6−/− cells 30. We analysed the expression of other receptors that have been described to participate in lipid internalization. Indeed, LRP2 contributes to HDL metabolism by internalizing ApoA-I and ApoA-II, which are structural components of HDLs 31; LRP8 is a component of the interactions between the endothelium and monocytes and leucocyte transendothelial migration, foam cell formation and activation of platelet aggregation 32; and the classical LDLR is known for its involvement in lipoprotein transport and plasmatic LDL cholesterol clearance 33. Lrp2, Lrp8 and Ldlr expression levels were down-regulated in aortas from HC Lrp5−/− indicating that they were not contributing to the observed lipid deposition in mice aortas. The atherogenicity of CD36 remains unclear, CD36 deficiency has been associated with enhanced atherosclerotic cardiovascular diseases 34, but cultured macrophages from these patients present a reduced uptake of oxidized LDL 35. Our results do not support the contribution of Cd36 to the lipid-rich phenotype of Lrp5−/− mice aortas as its expression is increased in HC Wt mice but reduced in HC Lrp5−/− mice. Lrp1 is up-regulated in aortas from Lrp5−/− mice. LRP1 has been found up-regulated in advanced human atherosclerotic plaques where its up-regulation is induced by extracellular lipids in human smooth muscle cells and human macrophages 36,37. Up-regulated LRP1 expression is also found in the aorta of rabbits and pigs after HC diets suggesting a pro-atherogenic role for LRP1 overexpression 36,38. Consistently, here we show enhanced Lrp1 expression levels in HC conditions in Wt and Lrp5−/− mice. Vldlr, a multiligand receptor that binds VLDL and chylomicron remnants 39 is not highly modified by mild hypercholesterolaemia or LRP5−/−.
Interestingly, there was an increase in Lrp6 gene expression levels in aortas of Lrp5−/− animals after HC feeding; however, Lrp6 did not trigger the canonical Wnt pathway. Lrp5−/− and Lrp6−/− mice have different phenotypes suggesting that these receptors cannot compensate each other's function 40–42. Also, mammary stem cells require LRP5 to trigger Wnt signalling despite of LRP6 co-expression 42. In view of our results, we suggest that Lrp1 and Lrp6 contribute to the increased aortic lipid deposition observed in HC Lrp5−/− mice, but further analysis on the LRP1-LRP5 and LRP6-LRP5 interactions are needed to better characterize this hypothesis.
In conclusion, our findings show that lipid depositions in the aorta are larger in HC Lrp5−/− mice with mildly increased levels of blood cholesterol demonstrating a protector role for LRP5 in the vascular wall. LRP5 and proteins from the Wnt/β-CATENIN pathway are up-regulated in HC Wt aortas and this increase is lost when LRP5 is absent. The absence of LRP5 regulates the dyslipidaemic profile by promoting lipid and macrophage retention in the vessel wall and increasing leucocyte driven systemic inflammation.
Acknowledgments
We thank S Change Huerta for Huertas, M Amado and S Florit for technical assistance, Dr. Camino for help with genes and proteins nomenclature and Dr. Juan for the IHC analyses. This work was supported by grants from the Spanish Ministry of Economy and Competitiveness (SAF 2010-16549 to LB), Institute of Health Carlos III, ISCIII (TERCEL RD12/0019/0026 and RIC RD12/0042/0027 to LB); I3 Contract (to MBP) and a Danone Institute Fellowship (to Change CRS for JCR). MBP designed the research, performed experiments, analysed, interpreted data and co-wrote the manuscript, CRS performed experiments, collected data and co-wrote the manuscript, LB co-wrote the manuscript and provided resources for the study.
Conflicts of interest
The authors confirm that there are no conflicts of interest.
References
- Badimon L, Storey RF, Vilahur G. Update on lipids, inflammation and atherothrombosis. Thromb Haemost. 2011;105:S34–42. doi: 10.1160/THS10-11-0717. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- Badimon L, Vilahur G. LDL-cholesterol versus HDL-cholesterol in the atherosclerotic plaque: inflammatory resolution versus thrombotic chaos. Ann N Y Acad Sci. 2012;1254:18–32. doi: 10.1111/j.1749-6632.2012.06480.x. [DOI] [PubMed] [Google Scholar]
- Jeon H, Blacklow SC. Structure and physiologic function of the low-density lipoprotein receptor. Annu Rev Biochem. 2005;74:535–62. doi: 10.1146/annurev.biochem.74.082803.133354. [DOI] [PubMed] [Google Scholar]
- Go G-W, Mani A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J Biol Med. 2012;85:19–28. [PMC free article] [PubMed] [Google Scholar]
- Gelfand BD, Meller J, Pryor AW, et al. Hemodynamic activation of beta-catenin and T-cell-specific transcription factor signaling in vascular endothelium regulates fibronectin expression. Arterioscler Thromb Vasc Biol. 2011;31:1625–33. doi: 10.1161/ATVBAHA.111.227827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedel A, Nègre-Salvayre A, Heeneman S, et al. E-cadherin/beta-catenin/T-cell factor pathway is involved in smooth muscle cell proliferation elicited by oxidized low-density lipoprotein. Circ Res. 2008;103:694–701. doi: 10.1161/CIRCRESAHA.107.166405. [DOI] [PubMed] [Google Scholar]
- Magoori K, Kang M-J, Ito MR, et al. Severe hypercholesterolemia, impaired fat tolerance, and advanced atherosclerosis in mice lacking both low density lipoprotein receptor-related protein 5 and apolipoprotein E. J Biol Chem. 2003;278:11331–6. doi: 10.1074/jbc.M211987200. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim J, Kim DW, et al. Wnt5a induces endothelial inflammation via beta-catenin-independent signaling. J Immunol. 2010;185:1274–82. doi: 10.4049/jimmunol.1000181. [DOI] [PubMed] [Google Scholar]
- Martin M, Rehani K, Jope RS, et al. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–84. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Toerne C, Schmidt C, Adams J, et al. Wnt pathway regulation in chronic renal allograft damage. Am J Transplant. 2009;9:2223–39. doi: 10.1111/j.1600-6143.2009.02762.x. [DOI] [PubMed] [Google Scholar]
- Borrell-Pagès M, Romero JC, Badimon L. LRP5 negatively regulates differentiation of monocytes through abrogation of Wnt signalling. J Cell Mol Med. 2014;18:314–25. doi: 10.1111/jcmm.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell-Pagès M, Romero JC, Juan-Babot O, et al. Wnt pathway activation, cell migration, and lipid uptake is regulated by low-density lipoprotein receptor-related protein 5 in human macrophages. Eur Heart J. 2011;32:2841–50. doi: 10.1093/eurheartj/ehr062. [DOI] [PubMed] [Google Scholar]
- Holmen SL, Giambernardi TA, Zylstra CR, et al. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res. 2004;19:2033–40. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- Lindvall C, Evans NC, Zylstra CR, et al. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J Biol Chem. 2006;281:35081–7. doi: 10.1074/jbc.M607571200. [DOI] [PubMed] [Google Scholar]
- Cui Y, Niziolek PJ, MacDonald BT, et al. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17:684–91. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino T, Asaba H, Kang M-J, et al. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc Natl Acad Sci USA. 2003;100:229–34. doi: 10.1073/pnas.0133792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–23. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–9. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X-Y, Chen Y, Xu L, et al. Association of LPR5 polymorphism with bone mass density and cholesterol level in population of Chinese Han. Exp Clin Endocrinol Diabetes. 2010;118:388–91. doi: 10.1055/s-0029-1225613. [DOI] [PubMed] [Google Scholar]
- Urano T, Shiraki M, Usui T, et al. A1330V variant of the low-density lipoprotein receptor-related protein 5 (LRP5) gene decreases Wnt signaling and affects the total body bone mineral density in Japanese women. Endocr J. 2009;56:625–31. doi: 10.1507/endocrj.k09e-133. [DOI] [PubMed] [Google Scholar]
- Shalhoub J, Falck-Hansen MA, Davies AH, et al. Innate immunity and monocyte-macrophage activation in atherosclerosis. J Inflamm (Lond) 2011;8:9. doi: 10.1186/1476-9255-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badimon L. Interleukin-18: a potent pro-inflammatory cytokine in atherosclerosis. Cardiovasc Res. 2012;96:172–5. doi: 10.1093/cvr/cvs226. ; discussion 176–180. [DOI] [PubMed] [Google Scholar]
- Kirii H, Niwa T, Yamada Y, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–60. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- Houtkamp MA, van Der Wal AC, de Boer OJ, et al. Interleukin-15 expression in atherosclerotic plaques: an alternative pathway for T-cell activation in atherosclerosis? Arterioscler Thromb Vasc Biol. 2001;21:1208–13. doi: 10.1161/hq0701.092162. [DOI] [PubMed] [Google Scholar]
- Wuttge DM, Eriksson P, Sirsjö A, et al. Expression of interleukin-15 in mouse and human atherosclerotic lesions. Am J Pathol. 2001;159:417–23. doi: 10.1016/S0002-9440(10)61712-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte JL, Smith AA, Helms JA. Wnt signaling and injury repair. Cold Spring Harb Perspect Biol. 2012;4:a008078. doi: 10.1101/cshperspect.a008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramati AR, Singh R, Lin A, et al. Wild-type LRP6 inhibits, whereas atherosclerosis-linked LRP6R611C increases PDGF-dependent vascular smooth muscle cell proliferation. Proc Natl Acad Sci USA. 2011;108:1914–8. doi: 10.1073/pnas.1019443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Go G-W, Singh R, et al. LRP6 protein regulates low density lipoprotein (LDL) receptor-mediated LDL uptake. J Biol Chem. 2012;287:1335–44. doi: 10.1074/jbc.M111.295287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugué-Pujol S, Rousset X, Château D, et al. Apolipoprotein A-II is catabolized in the kidney as a function of its plasma concentration. J Lipid Res. 2007;48:2151–61. doi: 10.1194/jlr.M700089-JLR200. [DOI] [PubMed] [Google Scholar]
- Quinn KL, Henriques M, Tabuchi A, et al. Human neutrophil peptides mediate endothelial-monocyte interaction, foam cell formation, and platelet activation. Arterioscler Thromb Vasc Biol. 2011;31:2070–9. doi: 10.1161/ATVBAHA.111.227116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Receptor-mediated control of cholesterol metabolism. Science. 1976;191:150–4. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- Yuasa-Kawase M, Masuda D, Yamashita T, et al. Patients with CD36 deficiency are associated with enhanced atherosclerotic cardiovascular diseases. J Atheroscler Thromb. 2012;19:263–75. doi: 10.5551/jat.10603. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Kashiwagi H, Yamashita S, et al. Reduced uptake of oxidized low density lipoproteins in monocyte-derived macrophages from CD36-deficient subjects. J Clin Invest. 1995;96:1859–65. doi: 10.1172/JCI118231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente-Cortés V, Otero-Viñas M, Sánchez S, et al. Low-density lipoprotein upregulates low-density lipoprotein receptor-related protein expression in vascular smooth muscle cells: possible involvement of sterol regulatory element binding protein-2-dependent mechanism. Circulation. 2002;106:3104–10. doi: 10.1161/01.cir.0000041434.28573.0b. [DOI] [PubMed] [Google Scholar]
- Llorente-Cortés V, Badimon L. LDL receptor-related protein and the vascular wall: implications for atherothrombosis. Arterioscler Thromb Vasc Biol. 2005;25:497–504. doi: 10.1161/01.ATV.0000154280.62072.fd. [DOI] [PubMed] [Google Scholar]
- Llorente-Cortes V, Casani L, Cal R, et al. Cholesterol-lowering strategies reduce vascular LRP1 overexpression induced by hypercholesterolaemia. Eur J Clin Invest. 2011;41:1087–97. doi: 10.1111/j.1365-2362.2011.02513.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Suzuki J, Kohno M, et al. Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase. J Biol Chem. 1995;270:15747–54. doi: 10.1074/jbc.270.26.15747. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, et al. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–8. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Kato M, Patel MS, Levasseur R, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–14. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Chin EN, Fakhraldeen SA, et al. Both LRP5 and LRP6 receptors are required to respond to physiological Wnt ligands in mammary epithelial cells and fibroblasts. J Biol Chem. 2012;287:16454–66. doi: 10.1074/jbc.M112.362137. [DOI] [PMC free article] [PubMed] [Google Scholar]


