Abstract
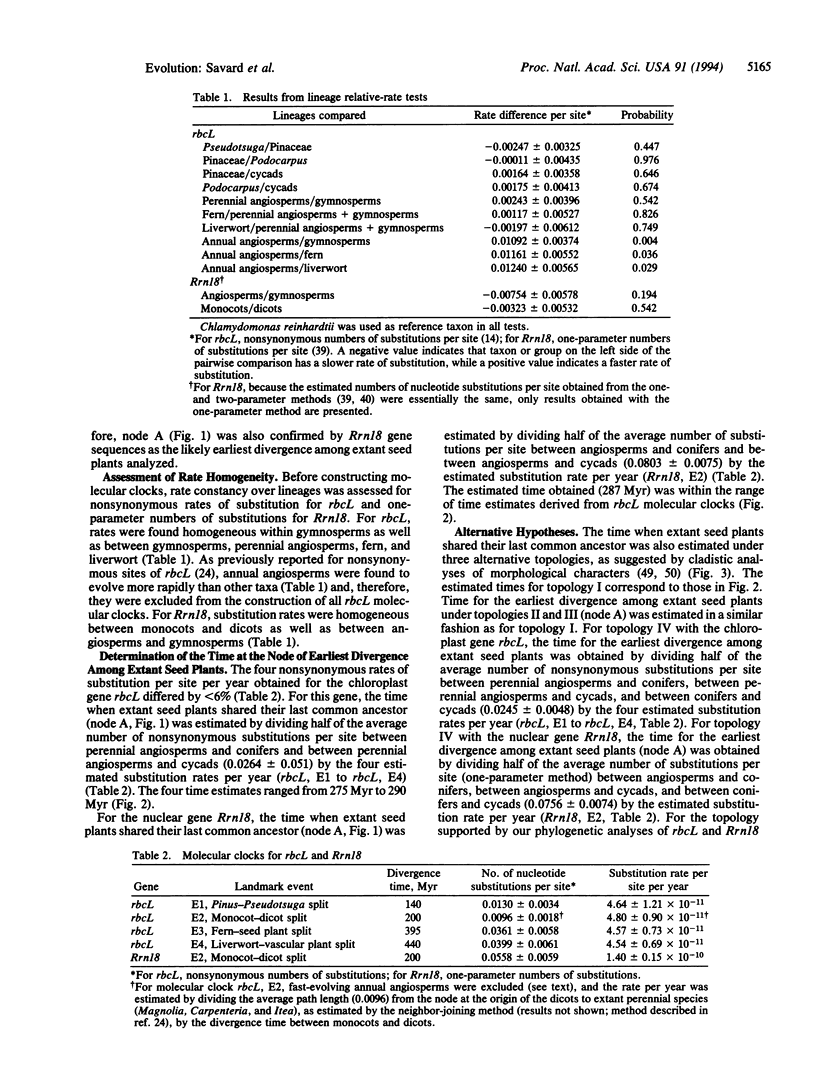
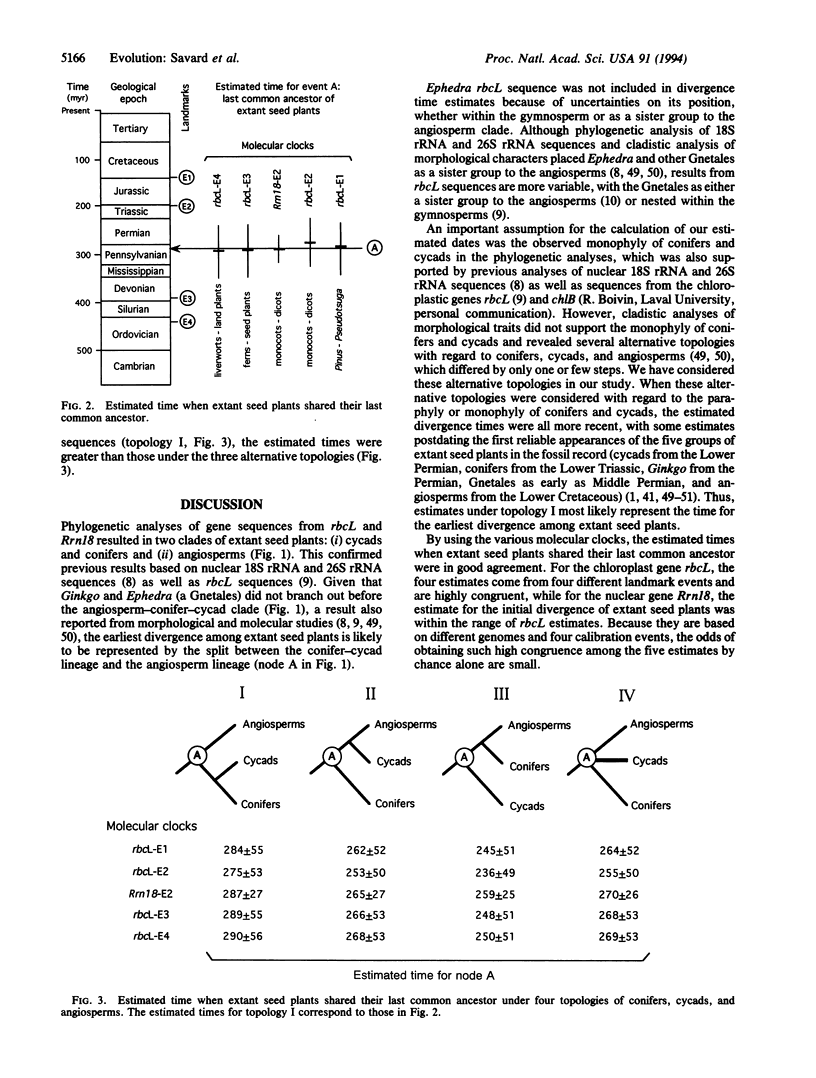
We have estimated the time for the last common ancestor of extant seed plants by using molecular clocks constructed from the sequences of the chloroplastic gene coding for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase (rbcL) and the nuclear gene coding for the small subunit of rRNA (Rrn18). Phylogenetic analyses of nucleotide sequences indicated that the earliest divergence of extant seed plants is likely represented by a split between conifer-cycad and angiosperm lineages. Relative-rate tests were used to assess homogeneity of substitution rates among lineages, and annual angiosperms were found to evolve at a faster rate than other taxa for rbcL and, thus, these sequences were excluded from construction of molecular clocks. Five distinct molecular clocks were calibrated using substitution rates for the two genes and four divergence times based on fossil and published molecular clock estimates. The five estimated times for the last common ancestor of extant seed plants were in agreement with one another, with an average of 285 million years and a range of 275-290 million years. This implies a substantially more recent ancestor of all extant seed plants than suggested by some theories of plant evolution.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert V. A., Williams S. E., Chase M. W. Carnivorous plants: phylogeny and structural evolution. Science. 1992 Sep 11;257(5076):1491–1495. doi: 10.1126/science.1523408. [DOI] [PubMed] [Google Scholar]
- Bousquet J., Strauss S. H., Doerksen A. H., Price R. A. Extensive variation in evolutionary rate of rbcL gene sequences among seed plants. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7844–7848. doi: 10.1073/pnas.89.16.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg M. T. Chloroplast gene sequences and the study of plant evolution. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):363–367. doi: 10.1073/pnas.90.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dron M., Rahire M., Rochaix J. D. Sequence of the chloroplast DNA region of Chlamydomonas reinhardii containing the gene of the large subunit of ribulose bisphosphate carboxylase and parts of its flanking genes. J Mol Biol. 1982 Dec 25;162(4):775–793. doi: 10.1016/0022-2836(82)90547-2. [DOI] [PubMed] [Google Scholar]
- Golenberg E. M., Giannasi D. E., Clegg M. T., Smiley C. J., Durbin M., Henderson D., Zurawski G. Chloroplast DNA sequence from a miocene Magnolia species. Nature. 1990 Apr 12;344(6267):656–658. doi: 10.1038/344656a0. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkins V. D., Tsai C. H., Strauss S. H. Sequence of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from a gymnosperm, Douglas fir. Plant Mol Biol. 1990 Sep;15(3):505–507. doi: 10.1007/BF00019168. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980 Dec;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kimura M. Evolutionary rate at the molecular level. Nature. 1968 Feb 17;217(5129):624–626. doi: 10.1038/217624a0. [DOI] [PubMed] [Google Scholar]
- Li W. H., Wu C. I., Luo C. C. A new method for estimating synonymous and nonsynonymous rates of nucleotide substitution considering the relative likelihood of nucleotide and codon changes. Mol Biol Evol. 1985 Mar;2(2):150–174. doi: 10.1093/oxfordjournals.molbev.a040343. [DOI] [PubMed] [Google Scholar]
- Martin W., Lydiate D., Brinkmann H., Forkmann G., Saedler H., Cerff R. Molecular phylogenies in angiosperm evolution. Mol Biol Evol. 1993 Jan;10(1):140–162. doi: 10.1093/oxfordjournals.molbev.a039989. [DOI] [PubMed] [Google Scholar]
- Muse S. V., Weir B. S. Testing for equality of evolutionary rates. Genetics. 1992 Sep;132(1):269–276. doi: 10.1093/genetics/132.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefs J. M., Van de Peer Y., De Rijk P., Goris A., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):1987–2015. doi: 10.1093/nar/19.suppl.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Savard L., Lalonde M. Sequence of 18S rDNA of actinorhizal Alnus glutinosa (Betulaceae). Plant Mol Biol. 1991 Apr;16(4):725–728. doi: 10.1007/BF00023436. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The nucleotide sequence of the tobacco chloroplast gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Gene. 1982 Nov;20(1):91–102. doi: 10.1016/0378-1119(82)90090-7. [DOI] [PubMed] [Google Scholar]
- Soltis D. E., Soltis P. S., Clegg M. T., Durbin M. rbcL sequence divergence and phylogenetic relationships in Saxifragaceae sensu lato. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4640–4644. doi: 10.1073/pnas.87.12.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe K. H., Gouy M., Yang Y. W., Sharp P. M., Li W. H. Date of the monocot-dicot divergence estimated from chloroplast DNA sequence data. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6201–6205. doi: 10.1073/pnas.86.16.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. I., Li W. H. Evidence for higher rates of nucleotide substitution in rodents than in man. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1741–1745. doi: 10.1073/pnas.82.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga K., Kubota Y., Ishii T., Wada K. Nucleotide sequence of atpB, rbcL, trnR, dedB and psaI chloroplast genes from a fern Angiopteris lygodiifolia: a possible emergence of Spermatophyta lineage before the separation of Bryophyta and Pteridophyta. Plant Mol Biol. 1992 Jan;18(1):79–82. doi: 10.1007/BF00018458. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Perrot B., Bottomley W., Whitfeld P. R. The structure of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acids Res. 1981 Jul 24;9(14):3251–3270. doi: 10.1093/nar/9.14.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Whitfeld P. R., Bottomley W. Sequence of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from pea chloroplasts. Nucleic Acids Res. 1986 May 12;14(9):3975–3975. doi: 10.1093/nar/14.9.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Kate L. P. Carrier screening in CF. Nature. 1989 Nov 9;342(6246):131–131. doi: 10.1038/342131a0. [DOI] [PubMed] [Google Scholar]



