Abstract
Introduction
Rifampicin has been used as adjunctive therapy in Staphylococcus aureus bacteraemia (SAB) with a deep infection focus. However, data for prognostic impact of rifampicin therapy is unestablished including the optimal initiation time point. We studied the impact of rifampicin therapy and the optimal initiation time for rifampicin treatment on prognosis in methicillin-sensitive S. aureus bacteraemia with a deep infection.
Methods
Retrospective, multicentre study in Finland including 357 SAB patients with a deep infection focus. Patients with alcoholism, liver disease or patients who died within 3 days were excluded. Patients were categorised according to duration of rifampicin therapy and according to whether rifampicin was initiated early (within 7 days) or late (7 days after) after the positive blood cultures. Primary end point was 90 days mortality.
Results
Twenty-seven percent of patients received no rifampicin therapy, 14% received rifampicin for 1-13 days whereas 59% received rifampicin ≥14 days. The 90 day mortality was; 26% for patients treated without rifampicin, 16% for rifampicin therapy of any length and 10% for early onset rifampicin therapy ≥14 days. Lack of rifampicin therapy increased (OR 1.89, p=0.026), rifampicin of any duration decreased (OR 0.53, p=0.026) and rifampicin therapy ≥14 days with early onset lowered the risk for a fatal outcome (OR 0.33, p<0.01) during 90 days follow-up.
Conclusion
Rifampicin adjunctive therapy for at least 14 days and initiated within 7 days of positive blood culture associated with improved outcome among SAB patients with a deep infection.
Introduction
Staphylococcus aureus is one of the most common pathogens in bacteraemic infections [1–3]. However, treatment results in S. aureus bacteraemia (SAB) have not been considerably improved despite the introduction of new antibiotics [1]. Complications and mortality in SAB have been related to methicillin-resistance [2,3] and haematogenous dissemination resulting in deep infection foci like endocarditis [3,4]. In recent studies, deep infection foci have been found in up to 80% of SAB patients [5,6]. Adjunctive rifampicin therapy has been explored as a way of improving treatment results in SAB with deep infections [5,7–15] whereas only few studies have evaluated the impact of adjunctive rifampicin therapy in SAB without a deep infection focus [8,9]. Rifampicin may be potentially valuable in S. aureus infections due to its bactericidal nature, high anti-staphylococcal activity and good biofilm penetrating capability [16–18]. Conventionally S. aureus has been regarded primarily as an extracellular bacterium although much data point towards possibilities of intracellular survival [19,20]. Intracellular survival has been suggested to enhance haematogenous dissemination and to result in subpopulation formation with reduced antibiotic susceptibility and relapse of S. aureus infection [19–23]. Recently it was proposed that the capability of rifampicin to achieve high intracellular concentrations may be essential for eradication of intra-leucocyte S. aureus infections [21].
In most studies, rifampicin has been combined to a staphylococcal penicillin [7–9,24], a glycopeptide (vancomycin) [8,9,25,26] or to a fluoroquinolone [10–15] in patients with chronic osteomyelitis, foreign body infections, endocarditis or other deep seated abscesses. These studies have been small in size including only from 14 to maximally 105 patients [7–15,24] and few studies only have been controlled and randomized [8–11,25]. Accordingly, statistically significantly higher cure rate with rifampicin treatment has been observed only in a few studies [5,8,11,14]. Recently, a stratified meta-analysis regarding Staphylococcus aureus bacteraemic patients only demonstrated a non-significant reduction in infection related deaths as a result of adjunctive rifampicin therapy [27].
Monotherapy with rifampicin results in rapid resistance development and combination therapy is a prerequisite for use of rifampicin in SAB [28,29]. Resistance development has been observed in studies with methicillin-resistant S. aureus (MRSA) or heteroresistant vancomycin-intermediate S. aureus (hVISA) [25,26,30,31]. In these studies, no positive prognostic effect of rifampicin was observed. In one study, 56% of patients developed rifampicin resistance and in each case the patient was bacteraemic at rifampicin initiation whereas none of the patients with negative blood cultures at rifampicin initiation developed resistance [26]. In this study, adjunctive rifampicin was associated with prolonged bacteraemia and poorer outcome which resulted in recommendations to commence rifampicin first after clearance of bacteraemia [26]. A large prospective study, primarily investigating the beneficial effect of a levofloxacin combination therapy in methicillin-sensitive S. aureus (MSSA) bacteraemia, produced a post hoc analysis with 331 patients on the effect of rifampicin on mortality. Rifampicin adjunctive therapy significantly improved outcome in patients with a deep infection focus [5]. This study was, however, not randomized, did not give an answer as to when rifampicin treatment should be started and did not identify the minimum duration of rifampicin therapy needed for a positive prognostic impact. Therefore, we found it interesting to study if rifampicin including combination therapy would affect the outcome in SAB patients with a deep infection focus and how the time point of rifampicin onset would affect the results in a larger patient material.
Materials and Methods
Patients and data collection
Altogether 617 adult patients with at least one positive blood culture for S. aureus were identified. When accounting for the exclusion and inclusion criteria a total of 357 patients with a deep infection focus were accepted for this retrospective study. Part of the patients had participated in our earlier prospective multicentre study including all five university and seven central hospitals in Finland during January 1999 to May 1999 and January 2000 to August 2002 [5]. The material was further extended with all SAB cases identified retrospectively who were not included into the previous prospective study between 1999–2002 [5] and all SAB patients between 2006–2007 from Helsinki University Central Hospital [6]. Two time-periods were included to exclude any unknown temporary differences in personnel or treatment practices. Data collection included age, gender, underlying diseases, McCabe’s classification on underlying diseases [32], medication, severe sepsis, septic shock and intensive care unit (ICU) treatment. Any infectious diseases specialist (IDS) consultation was documented. Infection foci documentation were based on clinical suspicion solely or verified by bacteriological, radiological or pathological findings. All antibiotic therapy including length and route of administration were documented. Patients were followed for 90 days and primary outcome was mortality at 90 days.
Exclusion criteria
No MRSA cases were included. Patients suffering from alcoholism and acute or chronic liver diseases were excluded. These conditions may induce elevated liver enzymes and liver failure during rifampicin therapy. Thus these conditions carry a risk for a statistical bias as patients with these conditions are unlikely to receive adjunctive rifampicin therapy. The mean time-lapse between blood culture collection and positive blood culture results was 3 days and thus patients with a fatal outcome within 3 days of blood culture collection were excluded in order to allow for a fatal outcome before positive blood culture results and the possibility to initiate rifampicin therapy.
Ethics statement
The trial was approved by The institutional review board of Helsinki University Central Hospital and The Ethical committee of Helsinki University Central Hospital. A written informed consent was provided by each patient.
Definitions
Modified Duke criteria were used to define endocarditis [33]. Deep infection foci included pneumonia, endocarditis, purulent arthritis, osteomyelitis, deep-seated abscess and any foreign-body infection. IDS consultation within 7 days of positive blood cultures were categorized as informal telephone consultation or formal bedside consultation [6]. Severe sepsis and septic shock was classified as sepsis in combination with hypotension, hypoperfusion, or organ failure [34].
The length of antibiotic therapy
Patients were categorized according to duration of rifampicin therapy into four groups i) no rifampicin therapy, ii) short rifampicin therapy (1–13 days), iii) long rifampicin therapy (≥14 days) and iv) rifampicin therapy of any length (including all patients having received rifampicin therapy). Patients with rifampicin therapy for at least 14 days were further classified according to whether onset of rifampicin therapy took place within 7 days (early onset) or 7 days after (late onset) the positive blood culture for S. aureus. Rifampicin was administered 450 mg once daily for patients under 50 kg and 600 mg once daily for patients over 50 kg in weight. The standard antibiotic therapy was either cloxacillin, cefuroxime, ceftriaxone, vancomycin or clindamycin. Fluoroquinolone and aminoglycoside served as additional antibiotic therapy. Fluoroquinolone therapy included either levo-, moxi- or ciprofloxacin. Aminoglycoside therapy included either tobramycin or gentamicin.
Statistical analysis
Categorical variables were compared with Pearson’s X 2-test and odd ratios [OR] of 95% confidence intervals [CI] were calculated. Univariate factors with p<0.1 were entered into Cox regression model to estimate factors predicting 90-day mortality. All tests were two-tailed with p<0.05 as significant. SPSS version 12.0 [SPSS Inc., Chicago, IL, USA] was used.
Results
Patient characteristics
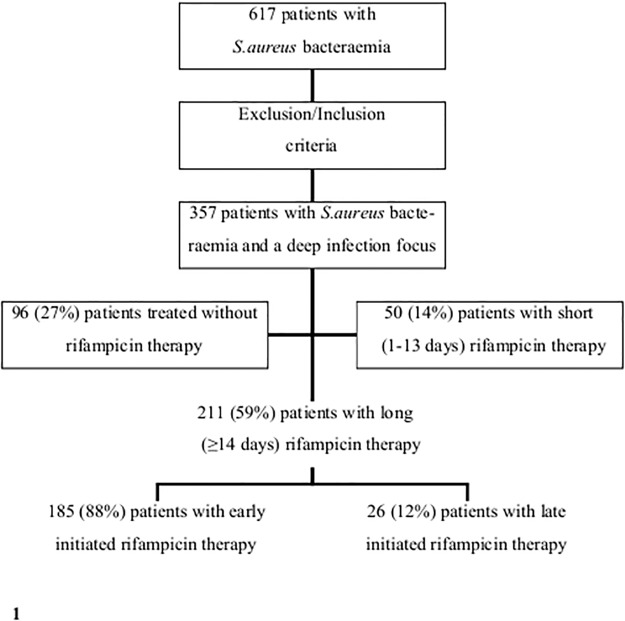
Altogether 617 SAB patients were identified. Patients with alcoholism or acute or chronic liver disease and fatal outcome within three days were excluded and only patients with a deep infection focus were included. Furthermore, patients with MRSA bacteraemia were not included in the study (n = 6). In total, 260 patients were excluded and altogether 357 patients were left for the analyses (Fig 1). Ninety-six (27%) of all 357 patients were treated without adjunctive rifampicin, 50 (14%) patients received adjunctive rifampicin for 1–13 days (short therapy) whereas 211 (59%) received adjunctive rifampicin for ≥14 days (long therapy) (Table 1). Table 1 present basic characteristics between the various treatment groups. Patients treated with short rifampicin therapy were more often males (OR 2.11, p<0.05) and had less often healthcare-associated SAB (OR 0.41, p<0.05) as compared to patients treated without rifampicin. No significant difference regarding age, chronic diseases according to McCabe′s classification and severity of illness at blood culture collection were observed between patients treated with short rifampicin therapy or no rifampicin therapy. The only difference between patients with short and long rifampicin treatment was the lower number of patients with septic shock at blood culture collection time point in the long rifampicin treatment group (1% vs. 6%, p<0.05). However, the total amount of patients with septic shock at blood culture collection time point was only 6 (1.7%) (Table 1).
Fig 1. Study profile. Originally 617 patients with methicillin-sensitive S. aureus bacteraemia were identified.
Patients with methicillin-resistant S. aureus bacteraemia were not included in the study (n = 6). The exclusion criteria were: patients with fatal outcome within 3 days of positive blood cultures, patients with alcoholism, acute or chronic liver diseases and patients without a deep infection foci. In total, 260 patients were excluded and altogether 357 patients were left for the analyses. Early initiation of rifampicin therapy was defined as an initiation within 7 days of positive blood cultures whereas late initiation of rifampicin therapy was defined as an initiation 7 days past positive blood cultures.
Table 1. Characteristics of 357 patients with methicillin-sensitive S. aureus bacteraemia (SAB) and a deep infection focus categorized according to duration of rifampicin therapy.
| Rifampicin therapy | Short therapy vs. no therapy | Long therapy vs. short therapy | |||||
|---|---|---|---|---|---|---|---|
| Variables | No therapy n = 96 (27) | Short therapy n = 50 (14) | Long therapy n = 211 (59) | OR (95% CI) | p- value | OR (95% CI) | p- value |
| Male sex | 46 (48) | 33 (66) | 139 (66) | 2.11(1.04–4.29) | <0.05 | 0.99(0.52–1.91) | NS |
| Age >60 years | 60 (63) | 26 (52) | 105 (50) | 0.65(0.33–1.29) | NS | 0.91(0.49–1.69) | NS |
| Healthy-nonfatal disease A | 62 (65) | 34 (68) | 165 (78) | 1.17(0.56–2.41) | NS | 1.69(0.86–3.33) | NS |
| Ultimately-rapidly fatal disease A | 34 (35) | 16 (32) | 46 (22) | 0.86(0.42–1.78) | NS | 0.59(0.30–1.17) | NS |
| Healthcare-associated SAB | 68 (71) | 25 (50) | 107 (51) | 0.41(0.20–0.84) | <0.05 | 1.03(0.56–1.91) | NS |
| Intensive care unit B | 14 (15) | 8 (16) | 38 (18) | 1.12(0.43–2.87) | NS | 1.15(0.50–2.65) | NS |
| Severe sepsis B | 4 (4) | 5 (10) | 14 (7) | 2.56(0.65–9.98) | NS | 0.64(0.22–1.87) | NS |
| Septic shock B | 1 (1) | 3 (6) | 2 (1) | 5.71(0.58–56.6) | NS | 0.19(0.03–1.14) | <0.05 |
| Formal bedside IDS consultation C | 84 (88) | 49 (98) | 194 (92) | 7.00(0.88–55.5) | <0.05 | 0.23(0.03–1.79) | NS |
| Informal telephone IDS consultation C | 9 (9) | 0 | 14 (7) | — | — | — | — |
| Cloxacillin, standard antibiotic | 57 (59) | 35 (70) | 116 (55) | 1.59(0.77–3.31) | NS | 0.52(0.27–1.02) | NS |
| Cefuroxime, standard antibiotic | 24 (25) | 13 (26) | 61 (29) | 1.05(0.48–2.31) | NS | 1.16(0.58–2.33) | NS |
| Ceftriaxone, standard antibiotic | 8 (8) | 0 | 16 (8) | — | — | — | — |
| Vancomycin, standard antibiotic | 1 (1) | 1 (2) | 5 (2) | 1.94(0.12–31.7) | NS | 1.19(0.14–10.4) | NS |
| Clindamycin, standard antibiotic | 3 (3) | 0 | 3 (1) | — | — | — | — |
| Fluoroquinolone, additional antibiotic D | 59 (61) | 26 (52) | 101 (48) | 0.68(0.34–1.36) | NS | 0.85(0.46–1.57) | NS |
| Aminoglycoside, additional antibiotic E | 9 (9) | 10 (20) | 42 (20) | 2.42(0.91–6.41) | NS | 0.99(0.46–2.15) | NS |
| Endocarditis F | 8 (8) | 12 (24) | 42 (20) | 3.47(1.31–9.18) | <0.01 | 0.79(0.38–1.64) | NS |
| Deep-seated abscess F | 39(41) | 23 (46) | 117 (55) | 1.25(0.63–2.48) | NS | 1.46(0.79–2.71) | NS |
| Foreign body infection F | 29 (30) | 8 (16) | 48 (23) | 0.44(0.18–1.05) | NS | 1.55(0.68–3.52) | NS |
| Septic arthritis or osteomyelitis F | 39 (41) | 28 (56) | 98 (46) | 1.86(0.93–3.71) | NS | 0.69(0.37–1.28) | NS |
| SAB relapse G | 2 (2) | 0 | 2 (1) | — | — | — | — |
Patients with alcoholism, acute or chronic liver diseases, lack of deep infection foci, MRSA bacteraemia (n = 6) or a fatal outcome within 3 days have been excluded. Values are expressed as n (%). NS = non-significant. Short rifampicin therapy 1–13 days. Long rifampicin therapy ≥14 days.
A Classification according to McCabe and Jackson [32].
B Severity of illness at blood culture collection time point.
C Infectious diseases specialist (IDS) consultation.
D Fluoroquinolone: levo-, moxi- or ciprofloxacin.
E Aminoglycoside: tobramycin or gentamicin.
F Deep infection focus within 90 days follow-up.
G SAB relapse within 90 days of follow-up.
Antibiotic therapy
Altogether 99% of patients had intravenous antibiotic therapy that was effective in vitro against the S. aureus blood isolate. Vancomycin was given empirically in only 2% of cases. No difference was observed in the standard antibiotic therapy given after the blood culture results between the various rifampicin treatment groups (Table 1). Antistaphylococcal penicillin (cloxacillin) was given to 208 (58%) patients whereas a non cell-wall active agent (clindamycin) was used in only 1.7% of patients. Fluoroquinolone (levo-, moxi- or ciprofloxacin) as an additional antibiotic was provided to 186 (52%) of patients and no significant difference between the patients with no rifampicin therapy, short or long rifampicin therapy was observed. No significant difference in aminoglycoside treatment was observed between the various treatment groups (Table 1).
Deep infection foci and consultations
The vast majority of deep infection foci were verified by bacteriological, radiological or pathological findings whereas only a few pneumonia cases (n = 5) were based on a clinical suspicion only. Patients treated with short rifampicin therapy received more often bedside IDS consultation (OR 7.00, p <0.05) and had more endocarditis diagnosed (OR 3.47, p<0.01) as compared to patients treated without rifampicin. When comparing patients with short rifampicin therapy to patients with long rifampicin therapy no significant difference in distribution of bedside IDS consultation or deep infection foci were observed. Altogether only 6% of the patients received informal telephone IDS (Table 1).
Outcome
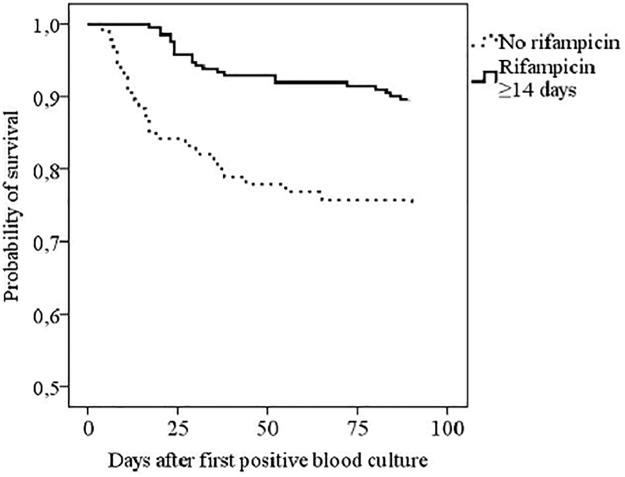
The total case fatality in 357 patients at 90 days was 18%. When comparing rifampicin therapy of any length to no rifampicin therapy, no significant difference in case fatality was seen at 28 days (11% vs. 17%, p = 0.130) whereas patients with rifampicin treatment had significantly lower mortality at 60 days (13% vs. 23%, p = 0.027) and at 90 days (16% vs. 26%, p = 0.026) (Table 2, Fig 2).
Table 2. Bacteraemic relapse and outcome of 357 patients with methicillin-sensitive S. aureus bacteraemia (SAB) and a deep infection focus categorized according to adjunctive rifampicin therapy.
| Rifampicin therapy | Any length of rifampicin therapy vs. no rifampicin therapy | |||
|---|---|---|---|---|
| Variables | No therapy n = 96 (27%) | Therapy of any length n = 261 (73%) | OR (95% CI) | p- value |
| SAB relapse A | 2 (2) | 2 (<1) | 0.40 (0.06–2.89) | 0.349 |
| Mortality, at 28 days | 16 (17) | 28 (11) | 0.60 (0.31–1.17) | 0.130 |
| Mortality, at 60 days | 22 (23) | 35 (13) | 0.51 (0.28–0.93) | 0.027 |
| Mortality, at 90 days | 25 (26) | 41 (16) | 0.53 (0.30–0.93) | 0.026 |
Patients with alcoholism, acute or chronic liver diseases, lack of deep infection foci, MRSA bacteraemia (n = 6) or a fatal outcome within 3 days have been excluded. Values are expressed as n (%).
A SAB relapse within 90 days follow-up
Fig 2. Kaplan-Meier analysis of 90 days survival in 307 Staphylococcus aureus bacteraemia patients with a deep infection focus according to rifampicin therapy (Log-Rank 0.000).
The exclusion criteria were: patients with fatal outcome within 3 days of positive blood cultures, patients with alcoholism, acute or chronic liver diseases and patients without a deep infection foci. Patients with methicillin-resistant S. aureus bacteraemia were not included in the study (n = 6).
The prognostic impact of rifampicin therapy and early or late onset of rifampicin therapy was evaluated by univariate analysis and Cox regression analysis (Table 3). In univariate analysis, parameters with positive prognostic impact were McCabes′s healthy-nonfatal underlying conditions (OR 0.18, p<0.001), rifampicin therapy of any duration (OR 0.53, p = 0.026), rifampicin therapy ≥14 days with early onset (OR 0.28, p<0.001) and formal bedside IDS consultation (OR 0.41, p = 0.029). Age >60 years (OR 3.02, p<0.001), McCabe′s ultimately-rapidly fatal underlying diseases (OR 5.45, p<0.001), healthcare-associated SAB (OR 2.23, p = 0.006), ICU treatment (OR 2.22, p = 0.013), severe sepsis (OR 3.12, p = 0.008), endocarditis (OR 2.11, p = 0.019), lack of rifampicin therapy (OR 1.89, p = 0.026) and informal IDS telephone consultation (OR 3.12, p = 0.008) were associated with negative prognosis. Gender, SAB relapse, fluoroquinolone therapy, aminoglycoside therapy and late onset of rifampicin therapy had no significant prognostic impact in univariate analysis (Table 3). In Cox regression analysis, the parameters age >60 years (OR 2.00, p = 0.017), severe sepsis (OR 3.64, p = 0.01), endocarditis (OR 2.68, p = 0.01) and informal telephone consultation (OR 2.11, p = 0.04) had a negative prognostic impact whereas McCabes′s healthy-nonfatal underlying condition (OR 0.23, p<0.01) and rifampicin therapy ≥14 days with early onset (OR 0.33, p<0.01) were markers of positive prognosis (Table 3). In order to investigate whether patients who died early were sicker, and thus more likely to be treated without rifampicin, the analyses of Table 3 were repeated by excluding patients with a fatal outcome within 21 days. The results of this Cox regression analysis resembled those of Table 3 with a positive prognostic impact of early onset of rifampicin therapy for ≥14 days (OR 0.48, p<0.01).
Table 3. Cox regression analysis for prognostic factors according to 90-day mortality in 357 patients with methicillin-sensitive S. aureus bacteraemia (SAB) with at least one deep infection focus.
| Univariate analysis | Cox regression analysis 1 | |||||
|---|---|---|---|---|---|---|
| Survived N = 291 (82) | Died N = 66 (18) | OR (95% CI) | p- value | OR (95% CI) | p- value | |
| Male sex | 181 (62) | 37 (56) | 0.78(0.45–1.33) | NS | — | — |
| Age >60 years | 142 (49) | 49 (74) | 3.02(1.66–5.49) | <0.001 | 2.00(1.13–3.54) | 0.017 |
| Healthy-nonfatal disease A | 233 (80) | 28 (42) | 0.18(0.10–0.32) | <0.001 | 0.23(0.14–0.39) | <0.01 |
| Ultimately-rapidly fatal disease A | 58 (20) | 38 (58) | 5.45(3.09–9.61) | <0.001 | — | — |
| Healthcare associated SAB | 153 (53) | 47 (71) | 2.23(1.25–3.99) | 0.006 | — | — |
| Intensive care unit B | 42 (14) | 18 (27) | 2.22(1.18–4.19) | 0.013 | — | — |
| Severe sepsis B | 14 (5) | 9 (14) | 3.12(1.29–7.57) | 0.008 | 3.64(1.76–7.53) | 0.01 |
| Endocarditis C | 44 (15) | 18 (27) | 2.11(1.12–3.95) | 0.019 | 2.68(1.53–4.71) | 0.01 |
| Fluoroquinolone therapy D | 156 (54) | 30 (45) | 0.72(0.42–1.23) | NS | — | — |
| Aminoglucoside therapy | 45 (15) | 16 (24) | 1.75(0.92–3.34) | NS | — | — |
| Lack of rifampicin therapy | 71 (24) | 25 (38) | 1.89(1.07–3.32) | 0.026 | — | — |
| Rifampicin therapy of any duration E | 220 (76) | 41 (62) | 0.53(0.30–0.93) | 0.026 | — | — |
| Rifampicin therapy ≥14 days, early onset F | 167 (57) | 18 (27) | 0.28(0.15–0.50) | <0.001 | 0.33(0.19–0.57) | <0.01 |
| Rifampicin therapy ≥14 days, late onset G | 22 (8) | 4 (6) | 0.79(0.26–2.37) | NS | — | — |
| Bedside IDS consultation H | 271 (93) | 56 (84) | 0.41(0.18–0.93) | 0.029 | — | — |
| Telephone IDS consultation H | 14 (5) | 9 (14) | 3.12(1.29–7.57) | 0.008 | 2.11(1.01–4.42) | 0.04 |
Patients with alcoholism, acute or chronic liver diseases, lack of deep infection foci, MRSA bacteraemia (n = 6) or a fatal outcome within 3 days have been excluded. Values are expressed as N (%) and odds ratio (OR) for fatal outcome within 90 days. NS = non-significant.
1 Cox regression analysis results are shown only for parameters with significant prognostic impact (p<0.05).
A Classification according to McCabe and Jackson [32].
B Severity of illness at blood culture collection time point
C Endocarditis diagnosed within 90 days follow-up.
D Fluoroquinolone: levo-, moxi- or ciprofloxacin.
E Including all patients with rifampicin therapy of any length.
F Early onset i.e. onset within 7 days of positive blood culture.
G Late onset i.e. onset 7 days after positive blood culture.
H Infectious diseases specialist (IDS) consultation
Discussion
The main finding of this study was a potential positive prognostic impact due to adjunctive rifampicin therapy in patients with a deep infection focus in methicillin-sensitive SAB. During the follow-up time period of 90 days, the mortality among patients treated with adjunctive rifampicin therapy for at least 14 days started during the first week of the positive blood culture was ten percent whereas the mortality for patients treated without rifampicin therapy during the corresponding time period was twenty-six percent. Early onset adjunctive rifampicin therapy for at least 14 days decreased the risk of fatal outcome to one third in Cox regression analysis.
Our study demonstrating significantly improved treatment results with rifampicin combination therapy in SAB with a deep infection focus is in line with previous studies [8,11,14] and studies showing a non-significant tendency towards better treatment result with rifampicin [7,9,10,15]. In common to most of these studies has been a low MRSA prevalence. In contrast to many previous studies, we only analysed mortality as the main outcome parameter. Rifampicin adjunctive therapy did not influence SAB relapse although this conclusion has to be made with a great caution due to the low occurrence of SAB relapses in our material. Early mortality, i.e. mortality during the 3 first days, was excluded from the analysis to be able to see the unbiased effect of rifampicin therapy on outcome.
Studies of rifampicin combination therapy in SAB with high MRSA prevalence (76–100%) have reported poorer clinical outcome due to rifampicin combination [25,26,30,31]. In a prospective report of MRSA native valve endocarditis, rifampicin-vancomycin therapy resulted in non-significantly prolonged bacteraemia as compared to vancomycin only [25]. A retrospective study investigated the benefit of adjunctive rifampicin therapy in native valve endocarditis with a high MRSA prevalence (76%) and showed prolonged bacteraemia and a significant negative prognostic impact for patients receiving rifampicin therapy [26]. Delay in effective antibiotic therapy may result in worse outcome in SAB [35]. MRSA has been associated with delayed effective antibiotic therapy and worse prognosis as compared to MSSA [2]. The present study included only MSSA cases and 99% of patients received effective intravenous antibiotic therapy from the first day of the blood culture collection. Invasive and bacteraemic infections due to MRSA are rare in Finland with MRSA prevalence remaining near 3% [36]. Vancomycin has been shown to increase the tendency for bacteraemia persistence and recurrence as compared to antistaphylococcal penicillin cloxacillin [37]. Vancomycin was provided to only 2% of our patients. Thus, the impact of rifampicin therapy on outcome could be evaluated without disturbance from delayed empirical antibiotic therapy. However, our study gives no answer on the value of rifampicin combination therapy in MRSA bacteraemia.
No data is available on the optimal timing of adjunctive rifampicin therapy initiation alongside standard antibiotic treatment. In MRSA bacteraemia, early rifampicin treatment was associated with emergence of resistance in 37–56% of cases when rifampicin was started during the bacteraemic phase [26,30,31]. In one study, each case of rifampicin resistance was due to therapy started during the bacteraemic phase whereas no resistance developed when rifampicin was initiated subsequent to bacteraemia clearance [26]. One report compared MRSA and hVISA bacteraemic patients who were receiving rifampicin-vancomycin therapy and demonstrated prolonged bacteraemia and higher rifampicin-resistance development for hVISA cases. Due to hVISA, the vancomycin serum concentration was below the required hVISA MIC and the authors interpreted this as rifampicin monotherapy resulting in the development of rifampicin resistance [30]. Hence, the development of rifampicin resistance in MRSA bacteraemia might be related to relative weak anti-staphylococcal effect or problems in tissue penetration of vancomycin or to longer persistence of bacteraemia during vancomycin therapy rather than due to methicillin-resistance of the staphylococcal strain [37,38]. Follow-up blood cultures were not routinely taken in our patient cohort and the present study does not give an answer on the question of possibility of rifampicin resistant development in MSSA bacteraemia.
In the present study, the median time from blood culture to clinical awareness of S. aureus being the causative pathogen was 3 days in average. In practice, rifampicin treatment was commenced earliest at this time point i.e. 3 days subsequent to effective empirical antimicrobial therapy, mostly a beta-lactam. Our results clearly point to a positive prognostic impact when rifampicin treatment is started during the first week of positive blood culture. Altogether the present study included 26 patients with a deep infection focus in which rifampicin was started later than 7 days past positive blood culture and continued for longer than 14 days. Among these patients rifampicin treatment showed no signs of improved prognosis. However, these results have to be interpreted with great caution due to the small patient number (n = 26) and the retrospective nature of this study. Once started, rifampicin therapy is usually continued for several weeks [38]. The patient group who received rifampicin therapy shorter that 14 days was also too small (n = 50) for rigorous and detailed analysis. Thus, no firm conclusions and no recommendations concerning a lack of prognostic influence of short rifampicin therapy or late started rifampicin therapy (≥14 days) can be made. Future randomized controlled trials with higher patient numbers are needed both to confirm our findings and to give an answer to whether later onset or shorter rifampicin treatment will have any prognostic impact.
The present study did not investigate the impact of different rifampicin dosages or the impact of administration routes on prognosis. Rifampicin was administered 450 mg once daily for patients under 50 kg and 600 mg once daily for patients over 50 kg in weight. This dosage, however, is far lower than that used in some studies e.g. 450 mg every 12 hours [11] or 20mg/kg given in divided doses twice a day without exceeding daily doses of 1800 mg [14]. The potential positive prognostic impact associated with adjunctive rifampicin therapy for at least 14 days observed in the present study might have been stronger with higher rifampicin doses.
The present study excluded patients with a fatal outcome within 3 days in order to allow for death before positive blood culture results and the possibility to receive rifampicin therapy. It could be argued that patients who died early were more ill and thus more likely to be treated without rifampicin. However, as a subanalysis the Cox regression was performed by excluding patients with a fatal outcome within 21 days and the results were very similar to the main Cox regression analysis with early onset of rifampicin therapy for at least 14 days associating to positive prognosis.
Also other attempts were made to control reasons for the difference in outcome between the groups. Most of the factors with prognostic impact in this study have been identified earlier e.g. age [3,39,40], McCabe′s classification [3], endocarditis [3,39] and IDS consultation [3,6]. Patients treated without rifampicin therapy were less often males and had more often healthcare-associated SAB. The present study can not provide any clear explanation for this gender distribution. Healthcare-associated SAB has been associated to poorer SAB outcome [39], however, when controlling for all of these factors, early onset rifampicin therapy for at least 14 days still remained a favourable prognostic factor (OR 0.33, p<0.01). Case fatality in our material was only 18% at 90 days follow-up which is at the lower end compared to many other SAB studies [3,40,41,42]. The low mortality has most probably decreased the power to detect a positive prognostic effect of rifampicin.
SAB patients with alcoholism and acute or chronic liver diseases were excluded from our main patient cohort. The risk for liver failure as a complication for rifampicin therapy is strongly accentuated in patients with alcoholism or liver diseases and these conditions are viewed as contraindications for rifampicin [43]. Alcoholism and liver diseases unavoidably creates a statistical bias as patients with these conditions are very unlikely to receive rifampicin therapy.
In conclusion, despite the retrospective nature of the present study the results indicated that in MSSA bacteraemia patients with a deep infection focus may gain from adjunctive rifampicin therapy of at least 14 days of length when rifampicin is initiated within 7 days of positive blood culture. Recommendations on rifampicin combination with other antimicrobial regimens in MRSA bacteraemia cannot be made based on the results of the present study and prospective randomized studies on the effect of rifampicin combination in MSSA and MRSA bacteraemia are needed.
Data Availability
Both The Institutional Review Board of Helsinki University Central Hospital and The Ethical Committee of Helsinki University Central Hospital strictly forbid any sharing of clinical patient data. The clinical patient data in this study can under no circumstances be made public. We are by law not allowed to share any of the clinical patient information. The clinical patient data of this study can be stored only within the database of Helsinki University Central Hospital. However, regarding further research (e.g. meta-analyses by other research groups) the data needed to make the final analyses of PONE-D-14-3852OR1 i.e. the raw data needed for Table 3 Cox regression analyses can be shared upon request.Requests for data (e.g. regarding future meta-analyses) will be received by corresponding author Dr. Erik Forsblom, e-mail: erik.forsblom@helsinki.fi or the chairman of The ethical commitee of Helsinki University Central Hospital Dr. Anneli Lauhio, e-mail: anneli.lauhio@hus.fi. Researchers can send requests for data to the corresponding author Dr. Erik Forsblom, e-mail: erik.forsblom@helsinki.fi or the chairman of The ethical commitee of Helsinki University Central Hospital Dr. Anneli Lauhio, e-mail: anneli.lauhio@hus.fi.
Funding Statement
This study was financially supported by grants from: The Medical Society of Finland, The Medicinska Understödsföreningen Liv och Hälsa, The SSAC-foundation, The Maud Kuistila Memorial Foundation, The Suomen Infektiolääkärit r.y. foundation and The Swedish Cultural Foundation in Finland. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, Kirby A, Tilley R, Török ME, et al. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis 2011; 11: 208–222. 10.1016/S1473-3099(10)70285-1 [DOI] [PubMed] [Google Scholar]
- 2. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteraemia: 470 a meta-analysis. Clin Infect Dis 2003; 36: 53–59. [DOI] [PubMed] [Google Scholar]
- 3. Rieg S, Peyerl-Hoffmann G, de With K, Theilacker C, Wagner D, Hübner J, et al. Mortality of Staphylococcus aureus bacteraemia and infectious diseases specialist consultation—a study of 521 patients in Germany. J Infect 2009; 59: 232–239. 10.1016/j.jinf.2009.07.015 [DOI] [PubMed] [Google Scholar]
- 4. Fowler VG Jr, Olsen MK, Corey GR, Woods CW, Cabell CH, Reller LB, et al. Clinical identifiers of complicated Staphylococcus aureus bacteraemia. Arch Intern Med 2003; 163: 2066–2072. [DOI] [PubMed] [Google Scholar]
- 5. Ruotsalainen E, Järvinen A, Koivula I, Kauma H, Rintala E, Lumio J, et al. Levofloxacin does not decrease mortality in Staphylococcus aureus bacteraemia when added to the standard treatment: a prospective and randomized clinical trial of 381 patients. J Intern Med 2006; 259: 179–190. [DOI] [PubMed] [Google Scholar]
- 6. Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone Consultation Cannot Replace Bedside Infectious Disease Consultation in the Management of Staphylococcus aureus Bacteraemia. Clin Infect Dis 2012; 56: 527–535. 10.1093/cid/cis889 [DOI] [PubMed] [Google Scholar]
- 7. Norden CW, Fierer J, Bryant RE. Chronic staphylococcal osteomyelitis: treatment with regimens containing rifampicin. Rev Infect Dis 1983; 5: 495–501. [DOI] [PubMed] [Google Scholar]
- 8. Van der Auwera P, Meunier-Carpentier F, Klastersky J. Clinical study of combination therapy with oxacillin and rifampicin for staphylococcal infections. Rev Infect Dis 1983; 5: 515–521. [DOI] [PubMed] [Google Scholar]
- 9. Van der Auwera P, Klastersky J, Thys JP, Meunier-Carpentier F, Legrand JC. Doubleblind, placebo-controlled study of oxacillin combined with rifampicin in the treatment of staphylococcal infections. Antimicrob Agents Chemother 1985; 28: 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heldman AW, Hartert TV, Ray SC, Daoud EG, Kowalski TE, Pompili VJ, et al. Oral antibiotic treatment of right-sided staphylococcal endocarditis in injection drug users: prospective randomized comparison with parenteral therapy. Am J Med 1996; 101: 68–76. [DOI] [PubMed] [Google Scholar]
- 11. Zimmerli W, Widmer AF, Blatter M, Frei R, Oschner PE. Role of rifampicin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection [FBI] Study Group. JAMA 1998; 279: 1537–1541. [DOI] [PubMed] [Google Scholar]
- 12. Dworkin RJ, Lee BL, Sande MA, Chambers HF. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet 1989; 4: 1071–1073. [DOI] [PubMed] [Google Scholar]
- 13. Berdal JE, Skråmm I, Mowinckel P, Gulbrandsen P, Bjørnholt JV. Use of rifampicin and ciprofloxacin combination therapy after surgical debridement in the treatment of early manifestation prosthetic joint infections. Clin Microbiol Infect 2005; 11: 843–845. [DOI] [PubMed] [Google Scholar]
- 14. Senneville E, Joulie D, Legout L, Valette M, Dezèque H, Beltrand E, et al. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus . Clin Inf Dis 2011; 53: 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schrenzel J, Harbarth S, Schockmel G, Genné D, Bregenzer T, Flueckiger U, et al. A randomized clinical trial to compare fleroxacin-rifampicin with flucloxacillin or vancomycin for the treatment of staphylococcal infection. Clin Infect Dis 2004; 39: 1285–1292. [DOI] [PubMed] [Google Scholar]
- 16. Zimmerli W, Frei R, Widmer AF, Rajacic Z. Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus . J Antimicrob Chemother 1994; 33: 959–967. [DOI] [PubMed] [Google Scholar]
- 17. Calfee DP. Rifamycins In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 6th ed. Vol. 1 Philadelphia: Churchill Livingstone; 2005. pp. 374–387. [Google Scholar]
- 18. Craig WA. Rifampicin and related drugs In: Gorbach SL, Bartlett JG, Blacklow NR, eds. Infectious Diseases. 3rd ed. Lippincott Williams & Wilkins: Philadelphia; 2004. pp. 277–280. [Google Scholar]
- 19. Alexander EH, Hudson MC. Factors influencing the internalization of Staphylococcus aureus and impacts on the course of infections in humans. Appl Microbiol Biotechnol 2001; 56: 361–366. [DOI] [PubMed] [Google Scholar]
- 20. Garzoni C, Kelley WL. Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol 2009; 17: 59–65. 10.1016/j.tim.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 21. Thwaites GE, Gant V. Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nat Rev Microbiol 2011; 9: 215–222. 10.1038/nrmicro2508 [DOI] [PubMed] [Google Scholar]
- 22. Lowy FD. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol 2000; 8: 341–343. [DOI] [PubMed] [Google Scholar]
- 23. Sendi P, Proctor RA. Staphylococcus aureus as an intracellular pathogen: the role of small colony variants. Trends Microbiol 2009; 17: 54–58. 10.1016/j.tim.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 24. Norden CW, Bryant R, Palmer D, Montgomerie JZ, Wheat J. Chronic osteomyelitis caused by Staphylococcus aureus: controlled clinical trial of nafcillin therapy and nafcillin-rifampicin therapy. South Med J 1986; 79: 947–951. [DOI] [PubMed] [Google Scholar]
- 25. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampicin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 1991; 115: 674–680. [DOI] [PubMed] [Google Scholar]
- 26. Riedel DJ, Weekes E, Forrest GN. Addition of rifampicin to standard therapy for treatment of native valve infective endocarditis caused by Staphylococcus aureus . Antimicrob Agents Chemother 2008; 52: 2463–2467. 10.1128/AAC.00300-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thwaites G, Auckland C, Barlow G, Cunningham R, Davies G, Edgeworth J, et al. Adjunctive rifampicin to reduce early mortality from Staphylococcus aureus bacteraemia (ARREST): study protocol for a randomised controlled trial. Trials 2012; 13: 241 10.1186/1745-6215-13-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spratt BG. Resistance to antibiotics mediated by target alterations. Science 1994; 264: 388–393. [DOI] [PubMed] [Google Scholar]
- 29. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52: 285–292. 10.1093/cid/cir034 [DOI] [PubMed] [Google Scholar]
- 30. Maor Y, Hagin M, Belausov N, Keller N, Ben-David D, Rahav G. Clinical features of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteraemia versus those of methicillin-resistant S. aureus bacteraemia. J Infect Dis 2009; 199: 619–624. 10.1086/596629 [DOI] [PubMed] [Google Scholar]
- 31. Lai CC, Tan CK, Lin SH, Liao CH, Huang YT, Hsueh PR. Emergence of rifampicin resistance during rifampicin-containing treatment in elderly patients with persistent methicillin-resistant Staphylococcus aureus bacteraemia. J Am Geriatr Soc 2010; 58: 1001–1003. 10.1111/j.1532-5415.2010.02842.x [DOI] [PubMed] [Google Scholar]
- 32. McCabe WR, Jackson GG. Gram negative bacteraemia 1. Etiology and ecology. Arch Intern Med 1962; 110: 847–855. [Google Scholar]
- 33. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30: 633–638. [DOI] [PubMed] [Google Scholar]
- 34. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001. SCCM/ ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 35. Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. Outcomes analysis 465 of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteraemia. Clin Infect Dis 2003; 36: 1418–1423. [DOI] [PubMed] [Google Scholar]
- 36.Finnish National Institute for Health and Welfare. Report 17/2011, page 28. http://www.thl.fi/thl-client/pdfs/1d73f597-8188-4ff5-b33c-101d7e1c3e90.
- 37. Siegman-Igra Y, Reich P, Orni-Wasserlauf R, Schwartz D, Giladi M. The role of vancomycin in the persistence or recurrence of Staphylococcus aureus bacteraemia. Scand J Infect Dis 2005; 37: 572–578. [DOI] [PubMed] [Google Scholar]
- 38. Tice AD, Hoaglund PA, Shoultz DA. Risk factors and treatment outcomes in osteomyelitis. J Antimicrob Chemother 2003; 51: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 39. Forsblom E, Ruotsalainen E, Mölkänen T, Ollgren J, Lyytikäinen O, Järvinen A. Predisposing factors, disease progression and outcome in 430 prospectively followed patients of healthcare- and community-associated Staphylococcus aureus bacteraemia. J Hosp Infect 2011; 78: 102–107. 10.1016/j.jhin.2011.03.010 [DOI] [PubMed] [Google Scholar]
- 40. Mylotte JM, Tayara A. Staphylococcus aureus bacteraemia: predictors of 30-day mortality in a large cohort. Clin Infect Dis 2000; 31: 1170–1174. [DOI] [PubMed] [Google Scholar]
- 41. Jensen AG, Wachmann CH, Espersen F, Scheibel J, Skinhoj P, Frimodt-Møller N. Treatment and outcome of Staphylococcus aureus bacteraemia: a prospective study of 278 cases. Arch Intern Med 2002; 162: 25–32. [DOI] [PubMed] [Google Scholar]
- 42. Ringberg H, Thorén A, Lilja B. Metastatic complications of Staphylococcus aureus septicaemia. To seek is to find. Infection 2000; 28: 132–136. [DOI] [PubMed] [Google Scholar]
- 43. Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 2006; 174: 935–952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Both The Institutional Review Board of Helsinki University Central Hospital and The Ethical Committee of Helsinki University Central Hospital strictly forbid any sharing of clinical patient data. The clinical patient data in this study can under no circumstances be made public. We are by law not allowed to share any of the clinical patient information. The clinical patient data of this study can be stored only within the database of Helsinki University Central Hospital. However, regarding further research (e.g. meta-analyses by other research groups) the data needed to make the final analyses of PONE-D-14-3852OR1 i.e. the raw data needed for Table 3 Cox regression analyses can be shared upon request.Requests for data (e.g. regarding future meta-analyses) will be received by corresponding author Dr. Erik Forsblom, e-mail: erik.forsblom@helsinki.fi or the chairman of The ethical commitee of Helsinki University Central Hospital Dr. Anneli Lauhio, e-mail: anneli.lauhio@hus.fi. Researchers can send requests for data to the corresponding author Dr. Erik Forsblom, e-mail: erik.forsblom@helsinki.fi or the chairman of The ethical commitee of Helsinki University Central Hospital Dr. Anneli Lauhio, e-mail: anneli.lauhio@hus.fi.