Abstract
Introduction
Substance use is associated with common mental health disorders, but the causal effect of specific substances is uncertain. We investigate whether adolescent cannabis and cigarette use is associated with incident depression and anxiety, while attempting to account for confounding and reverse causation.
Methods
We used data from ALSPAC, a UK birth cohort study, to investigate associations between cannabis or cigarettes (measured at age 16) and depression or anxiety (measured at age 18), before and after adjustment for pre-birth, childhood and adolescent confounders. Our imputed sample size was 4561 participants.
Results
Both cannabis (unadjusted OR 1.50, 95% CI 1.26, 1.80) and cigarette use (OR 1.37, 95% CI 1.16, 1.61) increased the odds of developing depression. Adjustment for pre-birth and childhood confounders partly attenuated these relationships though strong evidence of association persisted for cannabis use. There was weak evidence of association for cannabis (fully adjusted OR 1.30, 95% CI 0.98, 1.72) and insufficient evidence for association for cigarette use (fully adjusted OR = 0.97, 95% CI 0.75, 1.24) after mutually adjusting for each other, or for alcohol or other substance use. Neither cannabis nor cigarette use were associated with anxiety after adjustment for pre-birth and childhood confounders.
Conclusions
Whilst evidence of association between cannabis use and depression persisted after adjusting for pre-term and childhood confounders, our results highlight the difficulties in trying to estimate and interpret independent effects of cannabis and tobacco on psychopathology. Complementary methods are required to robustly examine effects of cannabis and tobacco on psychopathology.
Background
A variety of mental health problems, including depression and anxiety, occur more commonly in substance users than non-users.[1–4] However, discerning causation from confounding, reverse causation, or selection or measurement bias in observational studies is problematic.[5] The cannabinoid system in the brain has been implicated in emotional regulation,[6] but biological mechanisms that might underlie the association between cannabis use and depression are not known, and indeed effects of cannabis may differ between the short and long term. For example, while people often report that cannabis acutely elevates their mood, long-term cannabis users show an increased risk of a number of adverse outcomes, including poorer education and social functioning,[7] which might be expected to increase risk of depression.
A recent systematic review and meta-analysis of longitudinal studies reported a 1.17-fold increase in odds of depression (95% CI 1.05, 1.30) in cannabis users, with a larger effect (OR = 1.62, 95% CI 1.21, 2.16) for heavy use,[8] and concluded that cannabis is associated with a modest increase in risk of developing depressive disorders. An earlier meta-analysis reported a similar association, but the authors argued that the majority of contributing studies did not adequately account for the possibility of reverse causation.[9] They also noted the wide variation in the number and type of confounders adjusted for. In particular, about half the studies included in their meta-analysis made no adjustment for alcohol or other drug use, which, given their potential depressogenic and anxiogenic effects during intoxication and withdrawal,[10, 11] strongly suggests the potential for residual confounding. Similar problems are likely to exist for determining the causal role of cannabis on anxiety.[9]
Tobacco use is also more common in people with depression and anxiety compared to the general population,[1, 3] and determining if these associations are causal is beset with the same difficulties as for cannabis. Whilst some studies indicate that smoking may be a self-medication behaviour,[12] other studies provide evidence in support of a biological causal mechanism for this association. For example, findings from animal studies suggest that nicotine effects on strengthening excitatory synapses on midbrain dopamine neurons might weaken coping mechanisms.[13]
The main aim of this study was to investigate the association between cannabis and cigarette use and incident depression and anxiety, whilst attempting to address issues relating to confounding, reverse causation and bias as robustly as we could. Furthermore, as cannabis and cigarette use are also associated with psychotic experiences (PEs), and we recently reported such associations within this sample,[14] we also examine the specificity of cannabis and cigarettes by comparing their effects on depression with those for PEs.
Methods
Participants
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a prospective, population-based birth cohort study that recruited 14,541 pregnant women resident in Avon, UK, with expected delivery dates April 1 1991 to December 31 1992 (http://www.alspac.bris.ac.uk). Information has been collected on the participants and their offspring from over 60 questionnaires and 9 clinic assessments.[15] (http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/). The current study is based on 4,561 individuals who completed the Revised Clinical Interview Schedule (CIS-R) at age 18. Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committee.
Measures
Exposures
Cannabis and cigarette use at age 16 were measured via self-report questionnaire (N = 5,068 & N = 5,074 respectively). Cumulative cannabis use was coded as a 4 level category variable: ‘0 times’, ‘1–20 times’, ‘21–60 times’ and ‘more than 60 times’ (see S1 File for further details). Frequency of smoking was coded as a 4 level category variable: ‘non-smokers’, ‘experimenters’, ‘weekly smokers’ and ‘daily smokers’.
Outcomes
Depression and anxiety were assessed at age 18 using the CIS-R [16] via a self-administered computerised interview. The primary outcomes were binary variables of depression (mild, moderate or severe) and of anxiety disorder (any of generalised anxiety disorder, social phobia, specific phobia, panic disorder, or agoraphobia) derived from algorithms based on ICD-10 criteria for unipolar depression and anxiety disorders respectively (see S1 File for further details).
Confounders
Based on the literature for associations between both cannabis and cigarette use with depression and anxiety, we considered the following as potential confounders: a) pre-birth confounders (family history of depression[17] [binary measure assessed via maternal questionnaire], maternal education[18] [a 5-level categorical variable assessed via maternal questionnaire], urban living [urban/town/village/hamlet, obtained from postcode of residence], and gender[19]); and b) childhood confounders (IQ at age 8[20] [assessed via Wechsler Intelligence Scale for Children[21]], borderline personality traits[22] [an 8-level measure assessed via interview at age 11], victimisation[23] [a binary measure assessed at age 8 via interview], peer problems[24] [a 10-point scale assessed via the strengths and difficulties interview at age 8[25]], and conduct disorder trajectory group ages 4–13[26] [membership of one of 4 trajectory paths (early onset persistent/childhood-limited/adolescent onset/low) as described in[27]]). We also examined alcohol use[28] [using the Alcohol Use Disorders Identification Test (AUDIT) score ranging from 0 to 40, assessed via questionnaire[29]] and other illicit drug use[30] [a 3 level measure none/other drugs/stimulants assessed via questionnaire] at age 16 as potential confounders. Cigarette and cannabis use were also considered as confounders for each other.
Statistical analysis
We carried out the analyses in STATA version 13 (Stata Corp LP, College Station, TX USA) and Mplus. We assessed exposure-outcome relationships before and after adjustment for confounders using logistic regression. We excluded individuals with depression (cut-off score of 11 on moods and feelings questionnaire (MFQ)[31, 32]) or anxiety (from DAWBA[33]) at age 16 from the relevant analyses, in order to minimise reverse causation. We examined the impact of confounding by comparing unadjusted estimates (model 1) with those adjusting for pre-birth confounders (model 2), and those further adjusted for childhood confounders (model 3). Further adjustment was made separately for age 16 cigarette use or cannabis use as appropriate (model 4a), alcohol use (model 4b), and other illicit drug use (model 4c). Finally, we also ran a fully adjusted analysis (model 5). This step-wise approach was used to elucidate the impact of different confounders on the associations.
We used a bivariate probit regression model in Mplus to investigate whether cannabis (or cigarettes) had similar (or different) effects on depression as they did on PEs, whilst taking into account their comorbidity. Using Wald tests, we compared a model where effect estimates for depression and PEs were allowed to differ with one where they were constrained to be the same. Probit estimates were converted to approximate odds ratios (ORs) to allow for easier interpretation. A binary measure of PEs (suspected or definite versus none) at age 18 was derived following a semi-structured interview conducted by trained psychologists.[34]
Analytical sample
The complete sample with data on outcomes, exposures and confounders was 1791 (Fig 1). To address the potential bias introduced by attrition in our sample we conducted multiple imputation, creating 100 imputed datasets using additional information from over 50 variables associated with our observed measures and with missingness to make the assumption that data were ‘missing at random’ more plausible. The measures included related to pre-birth factors, childhood and adolescent behaviours, and earlier measures of psychopathology and substance use at various ages. Our primary results are presented using this sample with imputed confounder and exposure data (imputed sample N = 4561). There were 4345 people who had information on both depression and PEs at age 18, for the imputed bivariate analysis. Omitting those with previous depression or anxiety from imputed data is not trivial,[35] therefore the same estimates were obtained using a series of appropriately parameterized interaction models. Pre-existing depression or anxiety was included as a moderator variable in both the imputation and the analyses that followed. Given the number of interaction terms required, a separate imputation model was derived for each exposure/outcome pair in turn.
Fig 1. Study Participant Flow Diagram.
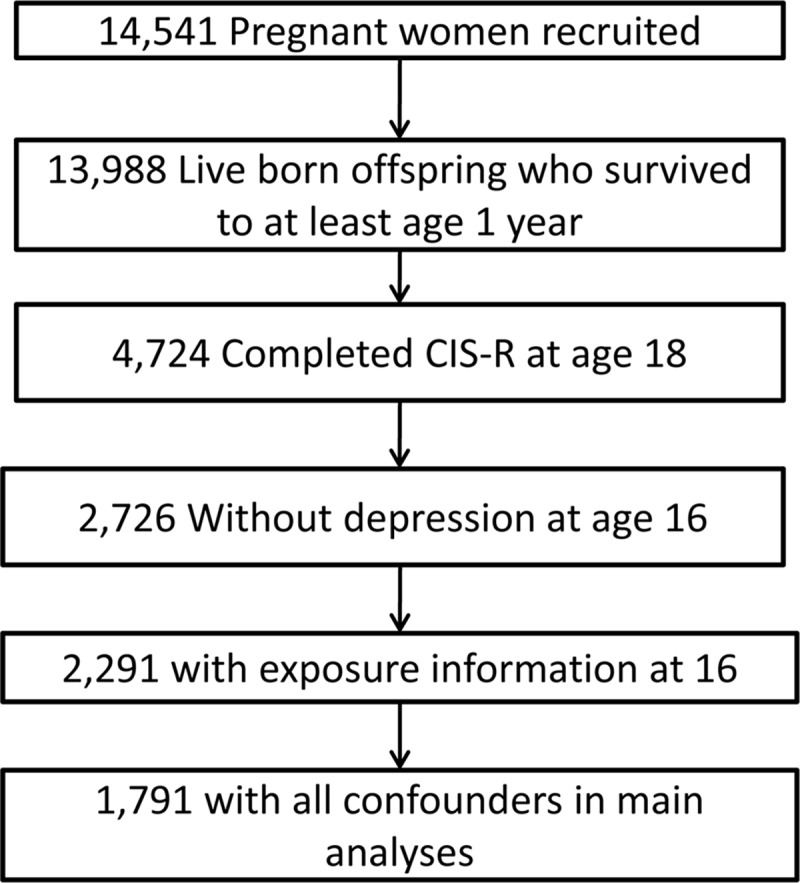
As a sensitivity analysis we assessed associations in the whole sample (N = 4561), without excluding those with depression at age 16. We also repeated the main analyses in the complete case dataset (N = 1512).
Results
Cannabis and depression
There were 128 people (7.2%, 95% CI 6.0, 8.4) who had depression at age 18, 94 females and 34 males. Of our sample with complete data, 491 (27.4%) had ever used cannabis by age 16, and 59 (3.3%) had used more than 60 times. Higher levels of cannabis use were more common in participants whose mothers had not undertaken higher education, in those with conduct disorder and who used other substances, and in those with a higher childhood IQ (Table 1).
Table 1. Descriptives of confounders by cumulative cannabis use at age 16% (N).
| Cumulative cannabis age 16 | 0 times 72.4 (1300) | <20 times 20.3 (367) | 21–60 times 3.8 (65) | >60 times 3.6 (59) | P value |
|---|---|---|---|---|---|
| FH depression | 26.2 (341) | 29.4 (108) | 36.9 (24) | 32.2 (19) | 0.039 |
| FH schizophrenia | 0.8 (10) | 1.1 (4) | 0 | 1.7 (1) | 0.622 |
| Low Maternal education 1 | 77.7 (1010) | 70.3 (258) | 61.5 (40) | 71.2 (42) | 0.001 |
| Urban dwelling | 89.2 (1159) | 87.5 (321) | 89.2 (58) | 88.1 (52) | 0.573 |
| Gender (female) | 57.4 (746) | 65.1 (239) | 53.9 (35) | 45.8 (27) | 0.787 |
| Borderline personality 2 | 1.3 (17) | 1.1 (4) | 1 | 1.7 (1) | 0.700 |
| IQ at age 8 m(sd) | 109.1 (15.2) | 111.9 (15.6) | 115.7 (14.9) | 111.9 (13.9) | <0.001 |
| Psychotic experiences age 12 | 3.4 (44) | 4.6 (17) | 0 | 5.1 (3) | 0.732 |
| Conduct disorder 3 | 5.2 (68) | 9.3 (34) | 7.7 (5) | 15.3 (9) | <0.001 |
| Peer problems (4 or more) | 7.1 (93) | 5.2 (19) | 10.8 (7) | 8.5 (5) | 0.225 |
| Bullied | 30.3 (394) | 29.7 (109) | 29.2 (19) | 32.2 (19) | 0.976 |
| Cigarette smoking age 16 4 | 2.3 (30) | 21.3 (78) | 40.0 (26) | 71.2 (42) | <0.001 |
| Illicit drugs age 16 | 6.2 (80) | 32.7 (120) | 63.1 (41) | 81.4 (48) | <0.001 |
| Alcohol use age 16 5 | 20.9 (272) | 62.9 (231) | 61.5 (40) | 84.8 (50) | <0.001 |
FH- family history; IQ- intelligence quotient.
1No higher education. Categorised like this for purposes of presentation in this table only.
2Defined as score of 4 or over, for the purposes of presentation in this table only.
3Early onset persistent group membership. Categorised like this for purposes of presentation in this table only.
4Weekly or daily smoking. Categorised like this for purposes of presentation in this table only.
5Hazardous alcohol use. Categorised like this for purposes of presentation in this table only.
In the unadjusted analysis there was a 1.5-fold increase in the odds of depression per category increase of cumulative cannabis use (95% CI 1.26, 1.80). Adjustment for childhood confounders attenuated the association by approximately 20% though strong evidence of an association remained (Table 2). Further adjustment for adolescent substance use led to further attenuation in the size of the association (fully adjusted OR 1.30, 95% CI 0.98, 1.72). This implies an increased risk of depression of 2.2 in the highest category of self-reported cannabis use (> 60 times) versus never users.
Table 2. Logistic regression of intensity of cannabis or cigarette use at age 16 and Depression at age 18 using imputed data (100 imputations), accounting for those with depression at age 16* (N = 4561).
| Model | Cannabis | Cigarettes | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| 1 | 1.50 | 1.26, 1.80 | <0.001 | 1.37 | 1.16, 1.61 | <0.001 |
| 2 | 1.52 | 1.26, 1.82 | <0.001 | 1.29 | 1.09, 1.53 | 0.003 |
| 3 | 1.41 | 1.17, 1.71 | <0.001 | 1.20 | 1.01, 1.43 | 0.033 |
| 4a | 1.32 | 1.03, 1.68 | 0.027 | 1.11 | 0.90, 1.38 | 0.331 |
| 4b | 1.35 | 1.09, 1.67 | 0.006 | 1.15 | 0.95, 1.40 | 0.155 |
| 4c | 1.38 | 1.09, 1.75 | 0.007 | 1.15 | 0.95, 1.40 | 0.153 |
| 5 | 1.30 | 0.98, 1.72 | 0.065 | 1.09 | 0.87, 1.36 | 0.460 |
Model 1—Case depression at 18 by unit increase of 4-level categorical cumulative cannabis or frequency of cigarette use at 16.
Model 2—as model 1 with additional adjustment for pre birth confounders (family history of depression, gender, urban dwelling, maternal education).
Model 3—as model 2 with additional adjustment for childhood confounders (borderline personality, IQ at age 8, PEs at age 12, conduct disorder trajectory group membership, peer problems, bullied).
Model 4a —as model 3 with additional adjustment for cigarette use/cannabis use (as appropriate).
Model 4b —as model 3 with additional adjustment for alcohol use.
Model 4c —as model 3 with additional adjustment for illicit drug use (other than cannabis).
Model 5—as model 3 with additional adjustment for cigarette (or cannabis), alcohol, other illicit drug use.
* The imputed interaction model allowed us to account for 15% of participants who reported depression at age 16, without exclusions.
Cigarette use and depression
Of those with complete data, 803 (44.8%) had ever smoked a cigarette, with 99 (5.5%) smoking every day. Higher frequency of cigarette use occurred in females, and in those with conduct disorder and those who used other substances (Table 3).
Table 3. Descriptives of confounders by frequency of cigarette use at age 16% (N).
| Cigarette frequency age 16 | Never 55.2 (988) | Experimenter 35.0 (627) | Weekly 4.3 (77) | Daily 5.5 (99) | P value |
|---|---|---|---|---|---|
| FH depression | 25.8 (255) | 28.9 (181) | 28.6 (22) | 34.4 (34) | 0.044 |
| FH schizophrenia | 0.9 (9) | 0.8 (5) | 0 | 1.0 (1) | 0.742 |
| Maternal education 1 | 74.8 (739) | 75.0 (470) | 79.2 (61) | 80.8 (80) | 0.189 |
| Urban dwelling | 88.7 (876) | 89.0 (558) | 90.9 (70) | 86.9 (86) | 0.925 |
| Gender (female) | 51.0 (504) | 67.5 (423) | 72.7 (56) | 64.7 (64) | <0.001 |
| Borderline personality 2 | 1.0 (10) | 1.6 (10) | 0 | 2.0 (2) | 0.467 |
| IQ at age 8 m(sd) | 109.9 (15.2) | 110.8 (15.3) | 108.6 (14.2) | 106.6 (16.7) | 0.199 |
| Psychotic experiences age 12 | 3.0 (30) | 4.2 (26) | 2.6 (2) | 6.1 (6) | 0.138 |
| Conduct disorder 3 | 5.3 (52) | 7.0 (44) | 9.1 (7) | 13.1 (13) | 0.001 |
| Peer problems | 7.6 (75) | 5.7 (36) | 1.3 (1) | 12.1 (12) | 0.661 |
| Bullied | 30.5 (301) | 30.0 (188) | 27.3 (21) | 31.3 (31) | 0.868 |
| Cannabis age 16 4 | 5.0 (49) | 47.2 (296) | 73.3 (58) | 88.9 (88) | <0.001 |
| Illicit drugs age 16 | 5.2 (51) | 22.5 (141) | 44.2 (34) | 63.6 (63) | <0.001 |
| Alcohol use age 16 5 | 15.3 (151) | 50.7 (318) | 67.5 (52) | 72.7 (73) | <0.001 |
1No higher education. Categorised like this for purposes of presentation in this table only.
2Defined as score of 4 or over, for the purposes of presentation in this table only.
3Early onset persistent group membership. Categorised like this for purposes of presentation in this table only.
4Ever used cannabis. Categorised like this for purposes of presentation in this table only.
5Hazardous alcohol use. Categorised like this for purposes of presentation in this table only.
Cigarette use at age 16 was associated with an increased odds of depression at age 18 (unadjusted OR per category of cigarette use = 1.37, 95% CI 1.16, 1.61). Adjusting for pre-birth and childhood confounders attenuated the association by about 50%, though some evidence of an association persisted (Table 2). Further adjusting for cannabis use eliminated evidence of association between cigarette use and depression, with adjustment for alcohol, or illicit drug use, resulting in similar (though less strongly attenuated) results (fully adjusted OR = 0.97, 95% CI 0.75, 1.24). Cigarette use and cannabis use were correlated (polychoric rho = 0.78, SE 0.01).
Bivariate Analysis
Adjustment for childhood confounders and substance use also attenuated the association between cannabis or tobacco and psychotic experiences. There was no evidence (Table 4) of differences between i) the association between cannabis and depression, and that between cannabis and PEs (Model 5 Wald test -0.010, p = 0.913), or ii) the association between cigarette use and depression, and that between cigarettes and PEs (Model 5 Wald test -0.043, p = 0.523).
Table 4. Bivariate probit regression analysis of intensity of cannabis or cigarette use at 16 and depression and PEs at age 18 in the imputed dataset.
| N = 4345 | Depression | Psychotic Experiences | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | Wald test (p value) | |
| Cannabis | |||||||
| 1 | 1.34 | 1.18, 1.51 | <0.001 | 1.38 | 1.23, 1.55 | 0.001 | -0.023 (0.637) |
| 3 | 1.29 | 1.13, 1.47 | <0.001 | 1.41 | 1.24, 1.59 | <0.001 | -0.056 (0.292) |
| 5 | 1.11 | 0.89, 1.38 | 0.343 | 1.12 | 0.91, 1.39 | 0.260 | -0.010 (0.913) |
| Cigarettes | |||||||
| 1 | 1.37 | 1.23, 1.53 | <0.001 | 1.42 | 1.28, 1.57 | <0.001 | -0.018 (0.654) |
| 3 | 1.24 | 1.10, 1.39 | <0.001 | 1.33 | 1.19, 1.49 | <0.001 | -0.046 (0.307) |
| 5 | 1.08 | 0.91, 1.28 | 0.367 | 1.15 | 0.98, 1.36 | 0.069 | -0.043 (0.523) |
Model 1—Case depression at 18 and suspected/definite PEs at 18 by unit increase of 4-level categorical cumulative cannabis use or frequency of cigarette use at 16.
Model 3—as model 2 with additional adjustment for childhood confounders (borderline personality, IQ at age 8, PEs at age 12, conduct disorder trajectory group membership, peer problems, bullied).
Model 5—as model 3 with additional adjustment for cigarette (or cannabis), alcohol and other illicit drug use.
Anxiety
There were 162 (9.0%, 95% CI 7.8, 10.5) participants in our sample with anxiety at age 18. There was weak evidence of an association between cannabis use and anxiety disorder at age 18, but no evidence after adjustment for pre-birth and childhood confounders (S1 Table), and further attenuation still after adjusting for substance use (unadjusted OR = 1.13, 95% CI 0.98, 1.31; fully adjusted OR = 0.96, 95% CI 0.75, 1.24). The same pattern of association was seen between cigarette use and anxiety (unadjusted OR = 1.14, 95% CI 1.00, 1.30; fully adjusted OR = 0.85, 95% CI 0.69, 1.04).
Sensitivity analyses
In the complete case analysis, associations between cannabis and depression were of a larger magnitude than in the imputed sample (S2 Table). Adjustment for cigarette use increased the estimate size, and the fully adjusted OR was larger than the unadjusted. Associations between cigarette use and depression were broadly similar to the imputed sample. Associations between cannabis and anxiety and between cigarettes and anxiety were also broadly similar to the imputed sample (S3 Table).
Discussion
Intensity of cannabis use at age 16 was associated with an increased incidence of depression at age 18. Adjusting for confounders assessed pre-birth and during childhood partly attenuated the association with depression, although strong evidence for an association with depression persisted. Further adjustment for cigarette, alcohol, or other drug use weakened the evidence of association with depression, but also widened the confidence intervals around the effect estimates. The strong correlation between cannabis use and other substances, particularly cigarette use, may have led to collinearity problems with the statistical model, as evinced by the increase in the size of the standard errors, and makes it difficult to interpret the results of cannabis independently from those for other substance use.
In addition, investigation of cannabis users who claimed not to smoke cigarettes in our study revealed that the vast majority of them (44 out of 47) used tobacco with their cannabis and were thus still exposed to tobacco.[14] Therefore, our study (and almost certainly other studies in countries where cannabis is smoked with tobacco that have adjusted for cigarette smoking) will not have adequately adjusted for tobacco use. A further difficulty in interpreting results adjusted for cigarette or other substance use is that it is feasible that use of these substances occurred secondary to the use of cannabis, for example through exposure to situations where tobacco, alcohol or other drugs became more readily available. This is an example of “collider bias”[36] and could have biased estimates of the association between cannabis and depression.[37]
Cigarette use was also associated with an increased incidence of depression. However, adjusting for confounders measured pre-birth and during childhood attenuated the association with depression to a greater extent than they did for cannabis and depression. Further adjustment for cannabis or other substance use eliminated this association, though the same problem of potential collider bias exists here too.
In contrast to our findings for depression, there was only weak evidence of associations between cannabis or cigarettes and anxiety, and these associations were eliminated after adjusting for pre-birth and childhood confounders.
Whilst most results were similar in the sensitivity analysis using our smaller, non-imputed (complete case) sample, one exception was that the association between cannabis and depression increased after adjustment for cigarette use. One explanation for this difference is that our complete case analysis is biased as a result of non-differential attrition. Indeed the direction of change in the estimate after adjusting for cigarette use is opposite to that expected given the relationship between cigarette use and depression, and that between cigarette use and cannabis. Our multiple imputation analysis is likely to be correcting for this to some extent as our imputation model was carefully designed to try and accurately predict the missingness in our variables.
The effect sizes we observed are consistent with previous meta-analyses that have reported associations between cannabis use and later depression of a moderate-sized effect.[8, 9] Furthermore, whilst we do not find evidence to support an association between cannabis use and incident anxiety we cannot exclude a small effect compatible with confidence limits from a recent meta-analysis of association between cannabis use and anxiety.[38] Although authors of that study attempted to assess the quality of included studies, estimating causal effect sizes from meta-analyses of observational studies are inherently limited by inclusion of studies with sub-optimal adjustment for confounding. For example, in Moore and colleagues’ meta-analysis attenuation of unadjusted estimates was greater in studies that adjusted for the most comprehensive sets of confounders.[9]
Whilst research from post-mortem human brain,[39] neuroimaging,[40] and animal[41] studies provide some evidence of plausible mechanisms by which cannabis might cause depression, this body of evidence is by no means compelling. For example, evidence from animal models suggests that tetrahydrocannabinol (THC: a cannabinoid receptor type 1 (CB1) agonist) during adolescence increases adult anhedonia and other models of depression and anxiety,[42, 43] but there is also evidence that rimonabant (a CB1 antagonist) increases anxiety, depression and suicidality.[44] Differences between acute and longer term effects of cannabis on the brain have yet to be clearly elucidated, and have to incorporate evidence of euphoria during acute intoxication with long-term adverse outcomes in cannabis users such as impaired occupational and social function that might be expected to increase depression (though studies of these outcomes are subject to the same problems of residual confounding as studies of depression). Furthermore, whilst studies comparing the effects of cannabis strains with different concentrations of THC, or different ratios of THC to cannabidiol (a cannabinoid that acts as a CB1 antagonist) on depression and anxiety can potentially help with understanding of the causal role of cannabis on these disorders, there are few such studies to date.[45]
Despite a common impression that the evidence supporting a causal effect of cannabis on psychosis is stronger than that for depression we found no evidence that associations between cannabis or cigarette use and depression were different to those between cannabis or cigarette use and PEs in our bivariate analysis. Whilst it is possible that we did not have adequate power to detect a difference, nevertheless our results suggest that associations between substance use and mental health, if causal, seem to be non-specific, both in terms of substances and outcomes.
There are a number of important limitations to our study. First, wave non-response and attrition (almost inevitable in a longitudinal study of this nature) means that our sample was small compared to the original ALSPAC sample, and power to detect small effects was limited; nevertheless our sample was still larger than most other cohorts that have examined the research questions we address here,[9] and we used multiple imputation to reduce attrition bias. It is possible that our imputation did not adequately address the missing at random assumption and that bias from selective attrition persisted, or that higher-order confounding was present and inadequately represented in the imputed models. However, imputed models are still likely to be less biased than use of complete case data. Our exposure measures were based on self-report data, which might be a limitation particularly for illicit drug use. If, for example, proneness to depression made individuals more reluctant to admit to illegal drug use this may have led to us underestimating the association between cannabis use and depression, though we have no reasons to suspect this occurred. Our outcome measures were assessed via computerised interview, therefore may not fully have captured depression or anxiety in the participants. However, prevalence was equivalent to that expected from previous literature [46, 47]. Finally, the problem of co-occurrence of cannabis and tobacco in this and other studies to date has already been discussed above, and would not be improved substantially by increasing sample size or having more accurate measures of substance use. Identifying populations where individuals use cannabis without mixing it with tobacco may help address this limitation, though we are not aware of any longitudinal studies in such populations currently. Utilising genetic variants associated with tobacco or cannabis use as instrumental variables in Mendelian randomisation analyses offers a potential approach for determining whether cannabis or tobacco have causal effects on psychopathology, but is limited by requirement of samples larger than currently available,[48] and by the absence of genetic proxies for cannabis use.[49]
Conclusions
Whilst most associations we examined were explained to a substantial degree by confounders assessed pre-term or during childhood, strong evidence of association between cannabis use and depression persisted. However, interpreting results for cannabis or cigarette use independent of each other is problematic, and our study highlights the limitations inherent in observational studies that aim to determine whether cannabis or tobacco use have causal effects on psychopathology. Novel approaches are required to determine whether associations between cannabis or tobacco and psychopathology are causal, and to help inform public knowledge and health prevention policy.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. SHG and SZ had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis
Data Availability
Data used for this submission will be made available on request to the ALSPAC executive committee (alspac-exec@bristol.ac.uk). The ALSPAC data management plan (available here: http://www.bristol.ac.uk/alspac/researchers/data-access/) describes in detail the policy regarding data sharing, which is through a system of managed open access.
Funding Statement
The UK Medical Research Council (Grant: 74882) the Wellcome Trust (Grant: 076467) and the University of Bristol provide core support for ALSPAC. Suzanne Gage and Marcus Munafo are members of the UK Centre for Tobacco and Alcohol Studies. This publication is the work of the authors and Suzanne Gage, Matt Hickman, and Stan Zammit serve as guarantors for the contents of this paper. JH is supported by the UK Medical Research Council (Grants G0800612 and G0802736) and this work was also supported by MRC grant G0701503 and Wellcome Trust grant 084268/Z/07/Z. The funders were not involved in the design, data collection, analysis or write up of the study. All authors are independent of the funders.
References
- 1. Farrell M, Howes S, Bebbington P, Brugha T, Jenkins R, Lewis G, et al. Nicotine, alcohol and drug dependence and psychiatric comorbidity. Results of a national household survey. Br J Psychiatry. 2001;179:432–7. [DOI] [PubMed] [Google Scholar]
- 2. Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of general psychiatry. 2004;61(8):807–16. [DOI] [PubMed] [Google Scholar]
- 3. Rao U. Links between depression and substance abuse in adolescents: neurobiological mechanisms. American journal of preventive medicine. 2006;31(6 Suppl 1):S161–74. [DOI] [PubMed] [Google Scholar]
- 4. Rubino T, Zamberletti E, Parolaro D. Adolescent exposure to cannabis as a risk factor for psychiatric disorders. J Psychopharmacol. 2012;26(1):177–88. 10.1177/0269881111405362 [DOI] [PubMed] [Google Scholar]
- 5. Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98(11):1493–504. [DOI] [PubMed] [Google Scholar]
- 6. Marco EM, Laviola G. The endocannabinoid system in the regulation of emotions throughout lifespan: a discussion on therapeutic perspectives. J Psychopharmacol. 2012;26(1):150–63. 10.1177/0269881111408459 [DOI] [PubMed] [Google Scholar]
- 7. Rossler W, Hengartner MP, Angst J, Ajdacic-Gross V. Linking substance use with symptoms of subclinical psychosis in a community cohort over 30 years. Addiction. 2012;107(6):1174–84. 10.1111/j.1360-0443.2011.03760.x [DOI] [PubMed] [Google Scholar]
- 8. Lev-Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, Rehm J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychological medicine. 2013;44(4):797–810. 10.1017/S0033291713001438 [DOI] [PubMed] [Google Scholar]
- 9. Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319–28. [DOI] [PubMed] [Google Scholar]
- 10. Edwards G, Gross MM. Alcohol dependence: provisional description of a clinical syndrome. Br Med J. 1976;1(6017):1058–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leventhal AM, Ameringer KJ, Osborn E, Zvolensky MJ, Langdon KJ. Anxiety and depressive symptoms and affective patterns of tobacco withdrawal. Drug Alcohol Depend. 2013;133(2):324–9. 10.1016/j.drugalcdep.2013.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laje RP, Berman JA, Glassman AH. Depression and nicotine: preclinical and clinical evidence for common mechanisms. Current psychiatry reports. 2001;3(6):470–4. [DOI] [PubMed] [Google Scholar]
- 13. Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37(4):577–82. [DOI] [PubMed] [Google Scholar]
- 14. Gage SH, Hickman M, Heron J, Munafo M, Lewis G, Macleod J, et al. Associations of cannabis and cigarette use with psychotic experiences at age 18: findings from the Avon Longitudinal Study of Parents and Children. Psychological medicine. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort Profile: The 'Children of the 90s'—the index offspring of the Avon Longitudinal Study of Parents and Children. International journal of epidemiology. 2013;42(1):111–27. 10.1093/ije/dys064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewis G, Pelosi AJ, Araya R, Dunn G. Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers. Psychological medicine. 1992;22(2):465–86. [DOI] [PubMed] [Google Scholar]
- 17. Hamet P, Tremblay J. Genetics and genomics of depression. Metabolism: clinical and experimental. 2005;54(5 Suppl 1):10–5. [DOI] [PubMed] [Google Scholar]
- 18. Barrera M, Prelow HM, Dumka LE, Gonzales NA, Knight GP, Michaels ML, et al. Pathways from family economic conditions to adolescents' distress: Supportive parenting, stressors outside the family, and deviant peers. J Community Psychol. 2002;30(2):135–52. [Google Scholar]
- 19. Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Frontiers in neuroendocrinology. 2014;35(3):320–30. 10.1016/j.yfrne.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loeber R, Farrington DP, Stouthamer-Loeber M, Moffitt TE, Caspi A, Lynam D. Male mental health problems, psychopathy, and personality traits: key findings from the first 14 years of the Pittsburgh Youth Study. Clinical child and family psychology review. 2001;4(4):273–97. [DOI] [PubMed] [Google Scholar]
- 21. Wechsler D, Golombok J, Rust J. Wechsler Intelligence Scale for Children Sidcup, United Kingdom: The Psychological Corporation; 1992. [Google Scholar]
- 22. Baer RA, Peters JR, Eisenlohr-Moul TA, Geiger PJ, Sauer SE. Emotion-related cognitive processes in borderline personality disorder: a review of the empirical literature. Clinical psychology review. 2012;32(5):359–69. 10.1016/j.cpr.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 23. Kaltiala-Heino R, Frojd S. Correlation between bullying and clinical depression in adolescent patients. Adolescent health, medicine and therapeutics. 2011;2:37–44. 10.2147/AHMT.S11554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mesman J, Koot HM. Child-reported depression and anxiety in preadolescence: I. Associations with parent- and teacher-reported problems. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(11):1371–8. [DOI] [PubMed] [Google Scholar]
- 25. Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38(5):581–6. [DOI] [PubMed] [Google Scholar]
- 26. Mazza JJ, Abbott RD, Fleming CB, Harachi TW, Cortes RC, Park J, et al. Early Predictors of Adolescent Depression A 7-Year Longitudinal Study. J Early Adolescence. 2009;29(5):664–92. [Google Scholar]
- 27. Barker ED, Maughan B. Differentiating early-onset persistent versus childhood-limited conduct problem youth. The American journal of psychiatry. 2009;166(8):900–8. 10.1176/appi.ajp.2009.08121770 [DOI] [PubMed] [Google Scholar]
- 28. Degenhardt L, Hall W, Lynskey M. Alcohol, cannabis and tobacco use among Australians: a comparison of their associations with other drug use and use disorders, affective and anxiety disorders, and psychosis. Addiction. 2001;96(11):1603–14. [DOI] [PubMed] [Google Scholar]
- 29. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction. 1993;88(6):791–804. [DOI] [PubMed] [Google Scholar]
- 30. Meyer JS. 3,4-methylenedioxymethamphetamine (MDMA): current perspectives. Substance abuse and rehabilitation. 2013;4:83–99. 10.2147/SAR.S37258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Angold A, Costello EJ, Messer SC, Pickles A, Winder F, Silver D. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. International journal of methods in psychiatric research. 1995;5(4):237–49. [Google Scholar]
- 32. Messer SC, Angold A, Costello EJ, Loeber R, VanKammen W, StouthamerLoeber M. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents: Factor composition and structure across development. International journal of methods in psychiatric research. 1995;5(4):251–62. [Google Scholar]
- 33. Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41(5):645–55. [PubMed] [Google Scholar]
- 34. Zammit S, Kounali D, Cannon M, Gunnell DD, Heron J, Jones PB, et al. Psychotic experiences and psychotic disorder at age 18 in relation to psychotic experiences at age 12 in a longitudinal, population-based cohort study. Am J Psychiat. 2013. [DOI] [PubMed] [Google Scholar]
- 35. Mars B, Heron J, Crane C, Hawton K, Lewis G, Macleod J, et al. Clinical and Social Outcomes of Adolescent self-harm: Population based birth cohort study. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cole SR, Platt RW, Schisterman EF, Chu H, Westreich D, Richardson D, et al. Illustrating bias due to conditioning on a collider. International journal of epidemiology. 2010;39(2):417–20. 10.1093/ije/dyp334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–25. [DOI] [PubMed] [Google Scholar]
- 38. Kedzior KK, Laeber LT. A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population- a meta-analysis of 31 studies. BMC psychiatry. 2014;14(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koethe D, Llenos IC, Dulay JR, Hoyer C, Torrey EF, Leweke FM, et al. Expression of CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. Journal of neural transmission. 2007;114(8):1055–63. [DOI] [PubMed] [Google Scholar]
- 40. Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: an FMRI study. Drug Alcohol Depend. 2009;105(1–2):139–53. 10.1016/j.drugalcdep.2009.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hill MN, Sun JC, Tse MT, Gorzalka BB. Altered responsiveness of serotonin receptor subtypes following long-term cannabinoid treatment. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2006;9(3):277–86. [DOI] [PubMed] [Google Scholar]
- 42. Bambico FR, Nguyen NT, Katz N, Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiology of disease. 2010;37(3):641–55. 10.1016/j.nbd.2009.11.020 [DOI] [PubMed] [Google Scholar]
- 43. Rubino T, Vigano D, Realini N, Guidali C, Braida D, Capurro V, et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33(11):2760–71. 10.1038/sj.npp.1301664 [DOI] [PubMed] [Google Scholar]
- 44. Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology. 2009;205(1):171–4. 10.1007/s00213-009-1506-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morgan CJ, Gardener C, Schafer G, Swan S, Demarchi C, Freeman TP, et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychological medicine. 2012;42(2):391–400. 10.1017/S0033291711001322 [DOI] [PubMed] [Google Scholar]
- 46. Costello EJ, Egger H, Angold A. 10-year research update review: the epidemiology of child and adolescent psychiatric disorders: I. Methods and public health burden. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(10):972–86. [DOI] [PubMed] [Google Scholar]
- 47. Goldman S. Developmental epidemiology of depressive disorders. Child and adolescent psychiatric clinics of North America. 2012;21(2):217–35, vii. 10.1016/j.chc.2011.12.002 [DOI] [PubMed] [Google Scholar]
- 48. Lewis SJ, Araya R, Smith GD, Freathy R, Gunnell D, Palmer T, et al. Smoking is associated with, but does not cause, depressed mood in pregnancy—a mendelian randomization study. PLoS One. 2011;6(7):e21689 10.1371/journal.pone.0021689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gage SH, Davey Smith G, Zammit S, Hickman M, Munafo MR. Using mendelian randomisation to infer causality in depression and anxiety research. Depression and anxiety. 2013;30(12):1185–93. 10.1002/da.22150 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Data used for this submission will be made available on request to the ALSPAC executive committee (alspac-exec@bristol.ac.uk). The ALSPAC data management plan (available here: http://www.bristol.ac.uk/alspac/researchers/data-access/) describes in detail the policy regarding data sharing, which is through a system of managed open access.


