Abstract
The Department of Energy’s Integrated Field-Scale Subsurface Research Challenge Site (IFRC) at Rifle, Colorado was created to address the gaps in knowledge on the mechanisms and rates of U(VI) bioreduction in alluvial sediments. Previous studies at the Rifle IFRC have linked microbial processes to uranium immobilization during acetate amendment. Several key bacteria believed to be involved in radionuclide containment have been described; however, most of the evidence implicating uranium reduction with specific microbiota has been indirect. Here, we report on the cultivation of a microorganism from the Rifle IFRC that reduces uranium and appears to utilize it as a terminal electron acceptor for respiration with acetate as electron donor. Furthermore, this bacterium constitutes a significant proportion of the subsurface sediment community prior to biostimulation based on TRFLP profiling of 16S rRNA genes. 16S rRNA gene sequence analysis indicates that the microorganism is a betaproteobacterium with a high similarity to Burkholderia fungorum. This is, to our knowledge, the first report of a betaproteobacterium capable of uranium respiration. Our results indicate that this microorganism occurs commonly in alluvial sediments located between 3-6 m below ground surface at Rifle and may play a role in the initial reduction of uranium at the site.
Introduction
The leaching of ore minerals from mining and mill sites has long been a serious problem, resulting in acid mine drainage and polluted run-off to surface and ground waters. One example of ore-processing contamination is subsurface uranium plumes that infiltrate groundwater [1, 2]. Natural attenuation of soluble, oxidized uranium can occur when hexavalent uranium is reduced and precipitated, but the process can be slow due to conditions where suitable endogenous electron donors are limiting. Therefore, recent research efforts have turned to field amendments of electron donors, such as acetate or ethanol, to stimulate microbial uranium (VI) reduction and bring the soluble concentration below safe drinking water standards in a timely manner [3–8]. A principal study site for uranium bioremediation research is the Integrated Field-Scale Subsurface Research Challenge Site (IFRC) in Rifle, Colorado (USA), which is situated near a historic vanadium/uranium mill that operated from the 1920‘s to the 1960’s (Fig 1). Extensive research into the in situ bioreduction of uranium has been conducted at the Rifle IFRC [1, 4, 9–14] with field experiments repeatedly demonstrating that groundwater uranium concentrations could be decreased below the U.S. Environmental Protection Agency’s (EPA) drinking water standard of 0.126 μM by adding acetate to the subsurface [1, 3, 15]. The initial decrease in aqueous uranium concentrations occurred concurrently with iron reduction, suggesting that an iron reducing bacterium may be involved in uranium reduction. Substantial shifts in the bacterial community have been documented at the Rifle site following acetate amendment, notably increases in Geobacter-like species [4, 9, 11, 12]. In addition, Geobacter uraniireducens and G. sulfurreducens have been isolated from the site or its near vicinity and have been shown to be capable of U(VI) reduction, although these bacteria do not utilize uranium as a terminal electron acceptor [16–17]. In contrast, laboratory experiments (including those using Rifle sediments) demonstrated uranium reduction associated with sulfate-reducing bacteria [18–19]. Likewise, stable isotope probing methods (SIP) have identified a variety of bacteria in the Rifle subsurface which utilize the acetate amended to the groundwater [1, 10, 13], but these active bacteria have not been directly linked to uranium reduction. Unfortunately, none of these studies have conclusively demonstrated growth by a bacterium present at the Rifle site using U(VI) as a terminal electron acceptor or that a particular bacterium capable of uranium reduction is widespread and abundant at the site prior to acetate amendment.
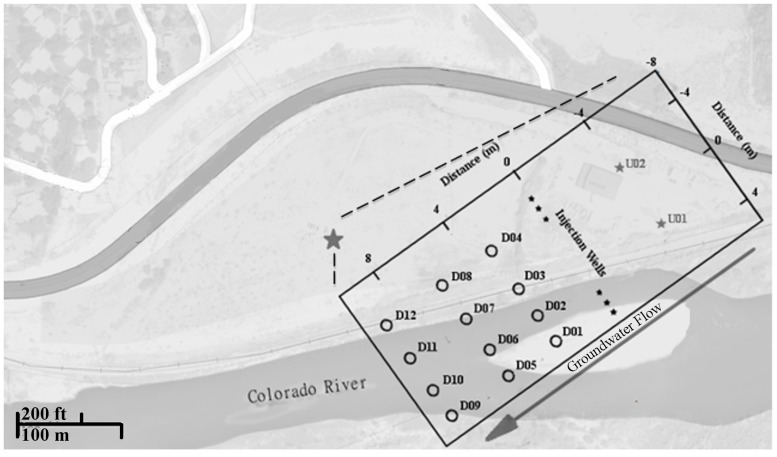
Fig 1. Map of the study area indicating sampling wells and groundwater flow.
This study was initiated to determine if a microorganism capable of growth on uranium could be isolated from Rifle samples. Using uranyl acetate as an electron acceptor at low concentrations (<10 μM) and acetate as an electron donor, a bacterium, designated strain Rifle, was isolated that could grow on U(VI). The growth of the culture was associated with the reduction of uranium, as evidenced by a partitioning from the soluble into the particulate phase. 16S ribosomal RNA gene analysis indicated the isolated strain was closely related to Burkholderia fungorum [20]. The spatial distribution of Burkholderia fungorum strain Rifle at the field site was assessed by TRFLP profiling of sediments collected during well installation by employing a diagnostic 4-bp cutter (MnlI) and a more definitive 6-bp cutter (EagI). The different terminal restriction fragments (TRF) associated with this strain were detected in subsurface sediments at the site prior to acetate amendment. The highest relative contribution to the overall profiles occurred at 3–5 meters depth. Since this microorganism is widely distributed at Rifle and capable of growth using uranium, it may be responsible for a portion of the U(VI) reduction observed during biostimulation with acetate. In addition, understanding the role this microorganism plays in the process of natural immobilization of uranium within the Rifle aquifer can help to elucidate processes inhibiting natural flushing of the aquifer and contributing to the problem of uranium plume persistence at this site and others like it [21, 22].
Material and Methods
Field Site
The Rifle IFRC experimental Plot C consists of a gallery of injection (4 cm diameter) /monitoring (10 cm diameter) wells installed in 2007 (Fig 1). Fifty cm thick core sections were collected at 3–6 m below ground surface, bagged, sparged with nitrogen in the field, and transferred to an anaerobic glove bag. Subsamples of sediment (10 g) from the various monitoring wells (D01-D12) were collected in the glove bag and immediately frozen at -80°C in the field prior to transport to the laboratory and processing. A detailed description of the site is found in Williams et al., 2010 [23].
Isolation of a bacterium capable of growth on uranium
Frozen sediment (1 g) from well D01 was thawed and spiked with 200 μM sodium acetate and 2 μM uranyl(VI) acetate as the sole electron acceptor in a modified minimal carbonate salts media under anaerobic conditions [24, 25]. Modifications included decreasing the NaCl concentration to 2.5 g/L, decreasing the bicarbonate to 1 g/L, and reducing the vitamin and trace salts concentrations by a factor of two. Enrichment cultures were incubated at room temperature for 14 days before transfer. Once slight turbidity was observed in the enrichment, an aliquot was diluted 1:10 with fresh media containing 200 μM sodium acetate and 2 μM uranyl acetate. With each transfer of the enrichment, 1 ml of liquid culture was filtered and extracted for TRFLP analysis [26]. The isolation transfers (7x) were repeated until the enrichment culture consisted of a single 16S rRNA gene TRFLP peak.
Testing of aerobic growth and identification of the isolated strain
The enrichment was screened for the potential of aerobic growth on Tryptic Soy Agar medium. After streaking onto plates, a large number of colonies with a uniform morphology were obtained. Following 5 rounds of colony purification, an isolate designated strain Rifle was screened by TRFLP again to ensure purity. The 16S rRNA gene from the strain was amplified via PCR using the primers 27F (5'-AGA GTT TGA TCC TGG CTC AG3’) and 1525R (5’-AAG GAG GTG WTC CAR CC-3’), following protocols described previously [27]. The amplicon was sequenced and analyzed via BLAST to detect closely related sequences (99% similarity: 1493/1503 bp identity-to B. fungorum strain KN-08). The isolate’s 16S rRNA gene (Genbank accession number-KP212894) was used to re-construct a phylogenetic tree with 1366 bp of unambiguously aligned sequence of strain Rifle and 24 closely matching taxa using Geneious software [28].
Verification of Growth on Uranium as an Electron Acceptor
In order to demonstrate that the isolate was capable of growth on acetate using U(VI) as a terminal electron acceptor, a dosage experiment was established to track changes in cell number associated with increasing concentrations of uranyl acetate. We began by inoculating strain Rifle grown on acetate and uranium (<5 mls) into 1-liter anaerobic minimal media with 200 μM sodium acetate. A schematic of the transfer apparatus that allowed for the anaerobic transfer of aliquots to replicate microcosms is shown in S1 Fig. This design allowed for the continuous sparging of all microcosms during the transfer procedure to minimize oxygen contamination. Initially, subsamples (9 ml) were anaerobically transferred under a headspace of N2 /CO2 [70:30] to triplicate 10 ml bottles. After these no-uranium amendment control cultures were established, the culture was supplemented with 1μM uranyl acetate and triplicate 9 ml cultures were established for the time course incubations (i.e. 0, 11, and 24 days; n = 9). The culture was then amended with an additional 1 μM uranyl acetate, bringing the concentration to 2 μM and dispensed as above. Further amendments of 3 μM, 5 μM, and 10 μM uranyl acetate were done to complete the experimental treatments. All cultures were incubated under anaerobic conditions (T0, T11, T24 days). Cell numbers were determined by collecting 1 ml of culture from each replicate microcosm at a given uranium amendment and time point (n = 3), preserving with 40 μL of 25% glutaraldehyde, staining with 1% SYBR gold for 15 min, and collected by filtration onto 0.22 μm GE polycarbonate black filters (GE Water & Process Technologies, Trevose, PA USA). Cells were enumerated in 10–15 microscopic fields via fluorescent microscopy using a BH2-RFCA microscope (Olympus, Japan).
Measurement of uranium by mass spectroscopy
For assessment of uranium concentration in the soluble and particulate fractions within the replicate microcosms, 2 ml of culture at each time point were filtered through 0.025 μm filters (Millipore Corp, Billerica, MA, USA) in an anaerobic chamber (Coy Laboratory Products, Grass Lakes, MI, USA). This soluble fraction (filtrate) was acidified by addition of 0.2 ml nitric acid (70%) and stored in glass vials until analysis. The particulate fraction (filter and the uranium adhering to the walls of the culture vials) were collected by dissolving the filter in 3 ml nitric acid and heating 250°C for 3 hours, or by rinsing the empty culture vials with 3 ml of nitric acid and vortexing for 2 minutes. The uranium concentration was determined by iCAPQ ICP-MS (Thermo Fisher Scientific Inc., Waltham, MA, USA) using Indium as an internal standard. Three iterations per sample were analyzed and RSD percentages averaged 3.6%. A commercial standard (High Purity Standards, Charleston, SC, USA) was used to generate a standard curve for uranium mass based on the instrument signal (counts per second; r2 = 0.9955).
Mapping of the Rifle strain at the study site
Bacterial community composition at the Plot C study site was assessed by 16S rRNA gene TRFLP analysis of DNA extracted from sediment samples collected in 2007. Triplicate nucleic acid extracts were generated from the subsampled sediment (0.25 g) from each core location (n = 12) at 3-6m below ground surface using a high EDTA/phenol/chloroform purification procedure [27, 29]. Amplification of 16S rRNA genes employed the bacterial forward primer 27F (6’-FAM-5'-AGA GTT TGA TCC TGG CTC AG-3’) and a universal reverse primer 1100R (5’-GGG TTG CGC TCG TTG-3’). Cycling parameters were 30 cycles of 94°C for 1 min, 55°C for 0.5 min, and 72°C for 1:10 min, followed by a final extension at 72°C for 10 min. Twenty ng of PCR product were digested for 6 hours with MnlI endonuclease and analyzed on an ABI 310 genetic analyzer to visualize the overall bacterial community profile of the sample, and to identify the presence/abundance of the Rifle strain [26]. Verification that samples positive for the strain Rifle MnlI-peak (166 bp) represented the Burkholderia fungorum strain Rifle isolate was done by digesting a subset of the amplicons using the endonuclease Eag1 (6-bp cutter) that yields a 215 bp terminal restriction fragment diagnostic of the strain.
Results
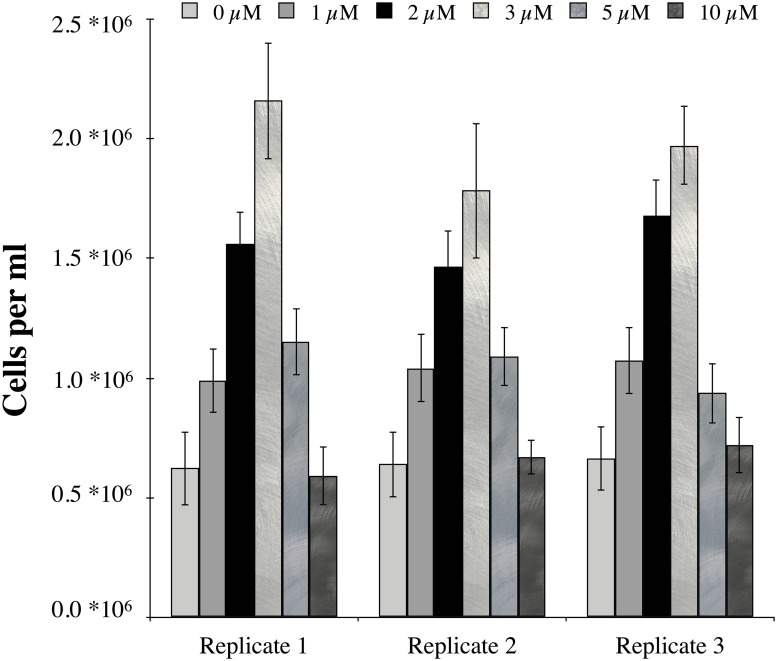
The Rifle IFRC experimental Plot C consists of a gallery of injection (4 cm diameter) /monitoring (10 cm diameter) wells installed in 2007 (Fig 1). Sediment samples from the well gallery were used to establish enrichment cultures on U(VI) minimal media. Colony purification on tryptic soy agar in air yielded a facultative anaerobic isolate with a TRF of 166 bp using MnlI. To verify whether this microorganism could grow on U(VI) as a terminal electron acceptor under anaerobic conditions, a dose-dependent experiment was established using increasing uranyl acetate concentrations (1–10 μM) with a uniform amount of acetate as electron donor (S1 Fig). The initial inoculum contained 0.45 ± 0.08 x 106 cells/ml. After 24 days in the uranium amendments, the control (0 μM, i.e. no uranium amendment) displayed a slight increase in cell numbers to 0.6 ±. 14 x 106 cells/ml (Fig 2). Each 1 μM uranium increment up to 3 μM yielded an increase of 0.5 ± .13 x 106 cells/ml. For the 5 and 10 μM amendments, lower cell numbers were observed, suggesting toxicity at the higher uranium dose.
Fig 2. Changes in cell number in replicate microcosms with the various uranyl acetate additions (0–10 μM) after a 24-day incubation.
Error bars indicate the variability (SD) in the cell counts for each replicate.
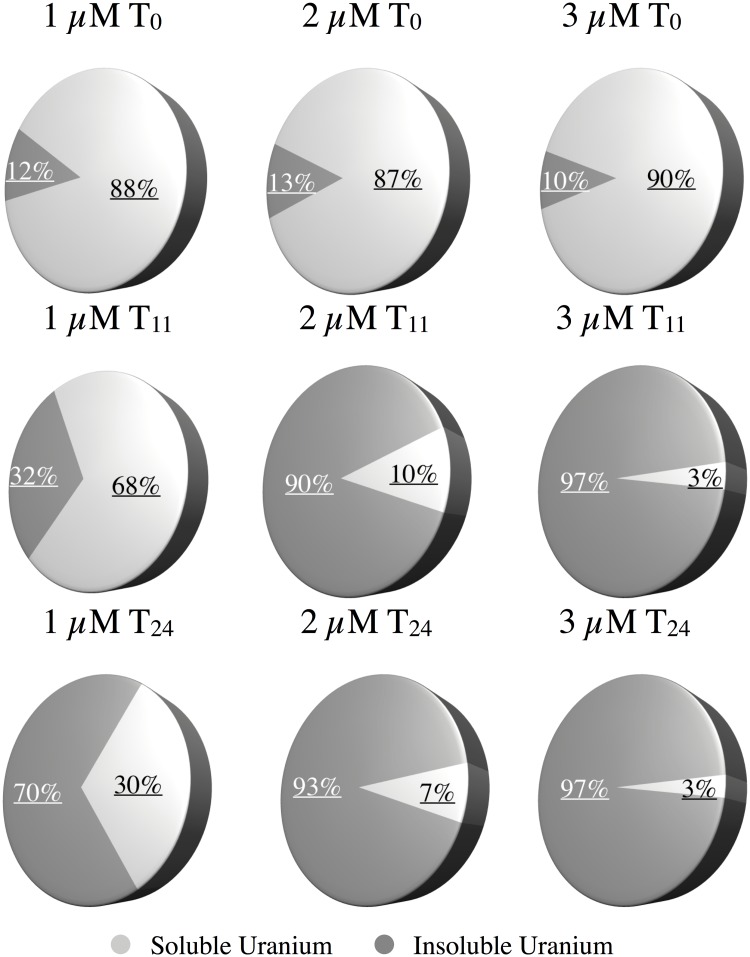
Verification that the increase in cell number of the pure culture was linked to the reduction of uranium was done by quantifying the fraction of uranium in both the soluble and insoluble pools. Uranium in the cultures spiked to 3 μM shifted from ~90% soluble at the T0 time point for all 3 concentrations to 70–97% insoluble by the end of the 24 day incubations (Fig 3). A mass balance indicated that 93–102% of the added uranium could be accounted for in the soluble/insoluble pools. Interestingly, the bacterium also reduced uranium as efficiently at 5 and 10 μM concentration (up to 97%-S2 Fig). However, the percent of soluble uranium at the T0 sampling time point was 78% for the 5 μM and 18% for the 10 μM treatments. Presumably, the bacteria were stimulated by the prior exposure to uranium during transfer and began reducing the radionuclide before the T0 samples could be collected.
Fig 3. Average proportion of soluble and insoluble uranium over time with uranyl acetate addition (0–3 μM) measured by ICP-mass spectrometry.
To ensure that the U(VI) was reduced (rather than adhering to cells or abiotically precipitating with phosphates in the media) a separate growth experiment with 2 μM uranyl acetate was conducted as described above. However, subsamples from this time course were filtered through 0.025 μm filters in air and not in the anaerobic chamber. The results demonstrate the uranium is largely in the soluble fraction (>90%) when exposed to oxygen (S3 Fig) and verify that the U(VI) is reduced to U(IV) during growth by strain Rifle.
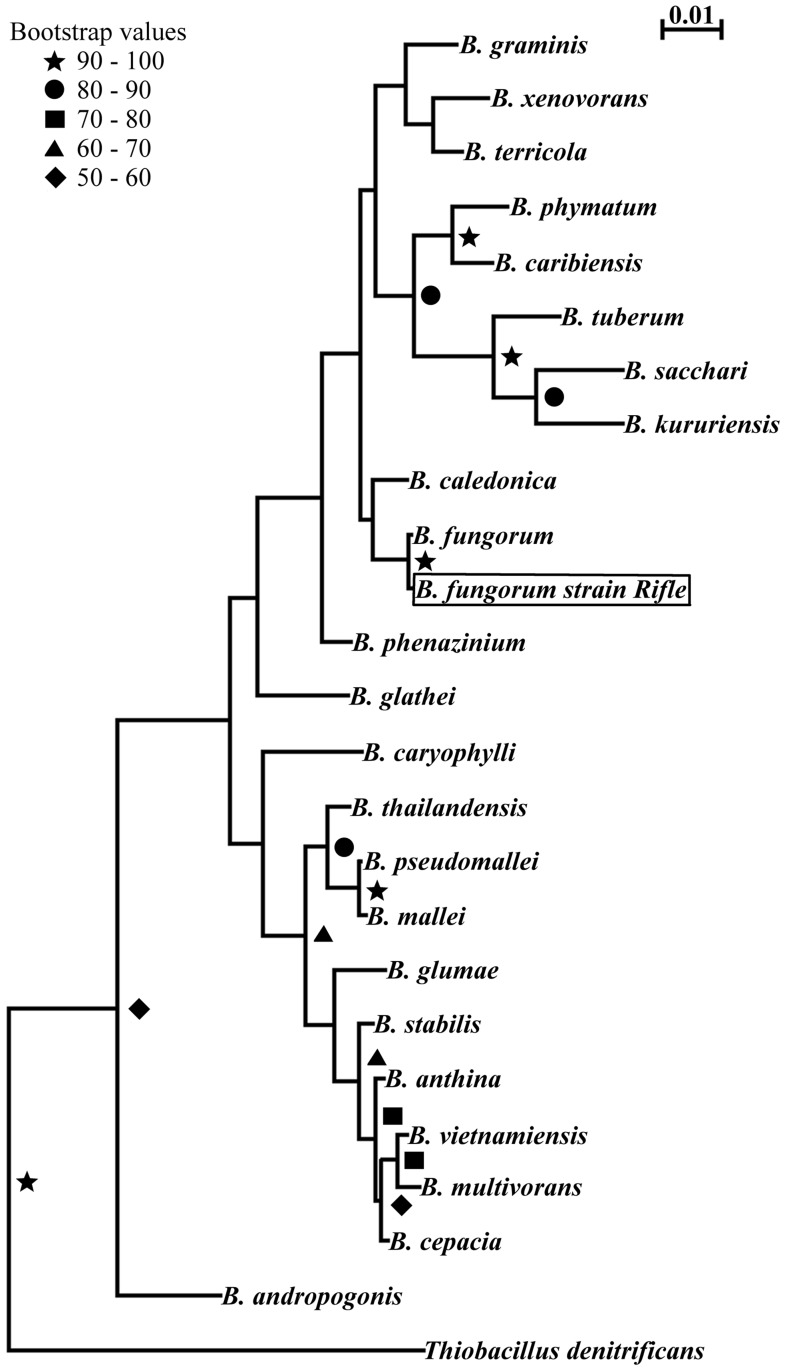
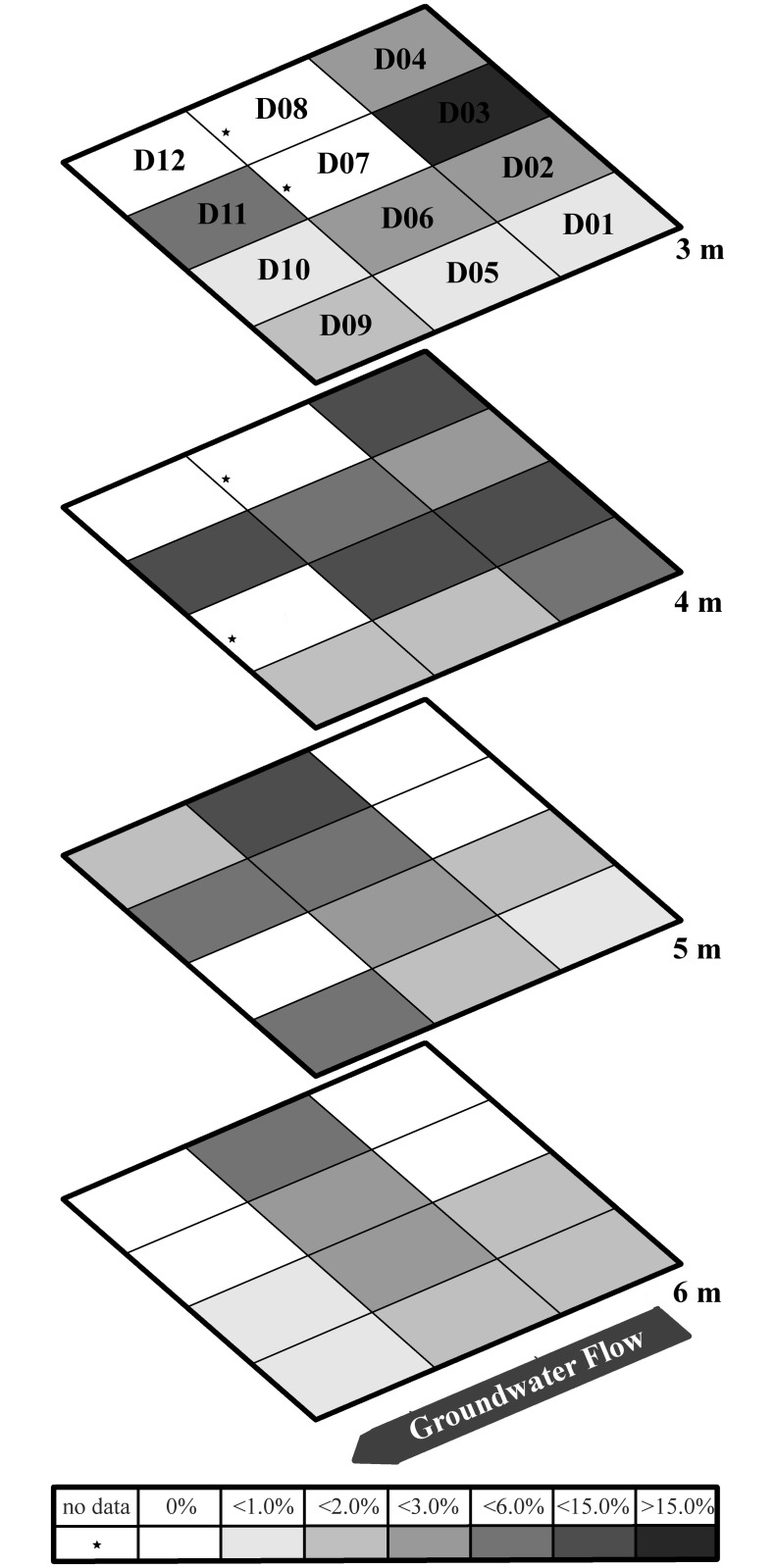
To determine the identity of strain Rifle, 16S rRNA sequence analysis was performed. BLAST results indicated the Rifle isolate was closely related to members of the Burkholderia genus. A phylogenetic tree of strain Rifle and other bacterial taxa places the Rifle strain within the Burkholderia cluster, closest to B. fungorum (Fig 4). The spatial distribution of Burkholderia fungorum strain Rifle within the field gallery was determined by TRFLP analysis of 16S rRNA genes in sediments recovered during well installation. These sediments were not field amended with acetate and represent the microbial community prior to perturbation to stimulate uranium reduction through organic carbon injection. A 166 bp TRFLP peak in 16S rRNA gene profiles from the site using MnlI was considered diagnostic of Burkholderia fungorum strain Rifle in the various samples. The contribution of OTU 166 to the overall bacterial community is presented in Fig 5. The relative abundance of Burkholderia fungorum strain Rifle ranged from 0–15% in the community profiles within these sediments. The 166 peak was most abundant in the upper part of the soil column (3 and 4 m depth) and the signal generally diminished at lower depths (6 m). The highest contribution of the 166 bp peak to the overall community (>15%) was in well D03 at 3 m. The patchy nature of the Burkholderia fungorum strain Rifle distribution can be seen in many wells where the relative contribution to the microbial community could vary from <15% to <1% within 1 to 2 m. To test whether the 166 bp peak might represent more than one 16S rRNA gene, a second set of enzyme digests of the fluorescent amplicons was performed using a 6-bp cutter (EagI), yielding a 215bp TFLP peak for B. fungorum strain Rifle. This analysis produced the appropriate sized TRF for all samples analyzed and the EagI 215 bp peak area was 1–50% of the original MnlI 166 peak area.
Fig 4. Maximum likelihood phylogenetic tree re-construction of Burkholderia type strains using 1366 bp of unambiguously aligned sequence of the 16S rRNA gene.
Burkholderia fungorum strain Rifle is indicated.
Fig 5. Spatial distribution of Burkholderia fungorum strain Rifle based on percent contribution to overall microbial community using the 166 bp MnlI peak in the bacterial TRFLP profile.
Discussion
The reduction of soluble U(VI) to less soluble U(IV) species is an essential step in the redox immobilization of this toxic radionuclide in contaminated groundwater. Initially, uranium reduction was believed to be entirely abiotic in nature, with organic and inorganic compounds acting as reducing agents. This notion changed in the early 1990’s when biotic uranium reduction was described for a dissimilatory Fe(III)-reducing bacterium [30]. Since then, numerous bacteria have been found which are capable of reducing uranium [31]. Of these bacteria, only Anaeromyxobacter dehalogenans; Carboxydothermus ferrireducens, Desulfotomaculum reducens, Geobacter metallireducens, and Shewanella putrefaciens have been reported to grow using U(VI) as a terminal electron acceptor [30–35]. More often, a resting cell assay at 200–10,000 μM is used to assess uranium reducing potential and over 30 bacteria have been described that reduce U(VI) to U(IV) but are not necessarily capable of growth on uranium [31, 36]. In addition, mixed cultures have demonstrated uranium reduction under co-metabolic conditions with terminal electron acceptors including sulfate [37] or iron [16, 30, 38–40].
Because so few of the known bacteria capable of growth on uranium have been detected at the Rifle IFRC site, much of the effort has focused on the role of Geobacter-like organisms during the course of acetate amendment and bioremediation. Yet, stable isotope probing experiments indicated that a variety of bacterial species are stimulated by the amendment of acetate at Rifle [1, 10, 13]. Interestingly, one of the major bacteria discovered at Rifle that incorporates 13C-acetate also displayed a 166 bp peak (using Mnl I) in the active community profiles [13]. This earlier report identified the 166 bp peak as an alphaproteobacterium from clone libraries. Here, we suggest that the 166 bp peak in the unamended sediment could also be Burkholderia fungorum strain Rifle. This result is consistent with our digestion of the TRFLP amplicons using EagI, suggesting that 1–50% of the Mnl I 166 bp peak area as some other microorganisms (such as the previously described alphaproteobacterium).
Furthermore, a next generation 16S rRNA gene analysis of samples taken from the Rifle site indicated that Burkholderiales comprised nearly 30% of the subsurface microbial community prior to field amendment [41]. Burkholderia-like microorganisms have also been shown to be a substantial proportion of the microbial community in uranium reducing enrichments from the Oak Ridge, TN IFRC site [6, 42, 43]. As with Rifle, the beta-proteobacteria detected after enrichment at Oak Ridge were not thought to play a role in uranium reduction. However, the fact that Burkholderia fungorum. strain Rifle grows on U(VI) is not entirely surprising as this genus is well documented for its wide variety of metabolic capabilities. For example, Burkholderia species have been found to be capable of anaerobic growth on nitrate, including B. fungorum [44, 45], B. xenovorans [46] and B. pseudomallei [47]. In addition, many members of the genus have been isolated based on the ability to degrade a broad range of carbon compounds [48–54]. This metabolic flexibility by Burkholderia species has been attributed to the large differences in their multiple genomes, varying as much as 2 Mbp between strains [55, 56]. Interestingly, a preliminary study of the terminal electron acceptor preferences by B. fungorum strain Rifle indicated only oxygen and uranium are respired on M9 media with dextrose as an electron donor (S4 Fig). This study suggested B. fungorum strain Rifle does not grow on iron, in contrast to all other known bacteria capable of growth on uranium which are also capable of utilizing iron as a terminal electron acceptor. These results imply a genomic comparison of our isolate with other related Burkholderia species that cannot grow on uranium should provide additional insight into the genes and mechanisms of biotic uranium reduction in this genus and may represent a novel respiratory pathway for radionuclide reduction.
In conclusion, Burkholderia fungorum strain Rifle is the first microorganism isolated specifically from the Rifle IFRC site that is capable of growth on U(VI), and exhibits an identical growth yield to uranium as Anaeromyxobacter dehalogenans [35]. Previously, members of the Burkholderiaceae were not suspected to play a role in uranium reduction and immobilization. Given the long period of uranium contamination at the site (>60-years), even slow rates of natural uranium reduction by Burkholderia fungorum strain Rifle and similar microorganisms could be expected to immobilize a potentially significant pool of uranium within the aquifer and can lead to uranium plume persistence. Such immobilization has the potential to impede the groundwater compliance strategy for Rifle and similar mill tailings impacted sites, which rely on natural flushing of the aquifer by low uranium groundwater to remove residual contamination. This study suggests that the ability to respire uranium could be widespread among facultative anaerobes and testing to date is often performed under conditions that could be toxic to many microorganisms. Furthermore, our findings highlight how integrated research at DOE field sites can lead to the discovery of novel metabolic capabilities in different microorganisms and to new ideas for promoting biostimulation and uranium reduction at these contaminated sites.
Supporting Information
(TIFF)
(TIFF)
(TIFF)
Cells were grown in M9 media with 0.4% dextrose as an electron donor and the terminal electron acceptor indicated. Error bars indicate the variability (SD) in the cell counts for each microcosm. The inset is the anaerobic incubations on a different scale.
(TIFF)
Acknowledgments
The Rifle IFRC was initially managed by the Pacific Northwest National Laboratory (operated by Battelle Memorial Institute for the U.S. Department of Energy) and later by the Lawrence Berkeley National Laboratory and the University of California. The authors wish to thank Dick Dayvault, Mike Wilkins, and Aaron D. Peacock for their assistance in the field.
Data Availability
All relevant data are within the paper and its Supporting Information files or accessible through Genbank (Accession: KP212894).
Funding Statement
The Rifle IFC is funded by the Subsurface Biogeochemical Research (SBR) program, Biological and Environmental Research, Office of Science, U.S. Department of Energy (Contract Number DE-AC06-76RLO-1830; managed by the Pacific Northwest National Laboratory and Battelle Memorial Institute). Currently, the Rifle IFC is managed by the Lawrence Berkeley National Laboratory under Contract No. DE-AC02-05CH11231 to the University of California. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Williams KH, Long PE, Davis JA, Wilkins MJ, N’Guessan AL, Steefel CI, et al. Acetate availability and its influence on sustainable bioremediation of uranium-contaminated groundwater. Geomicrobiol J. 2011; 28: 519–539. [Google Scholar]
- 2. Newsome L. The biogeochemistry and bioremediation of uranium and other priority radionuclides. Chem Geol. 2014; 363: 164–184. [Google Scholar]
- 3. Senko JM, Istok JD, Suflita JM, Krumholz LR. In-situ evidence for uranium immobilization and remobilization. Environ Sci Technol. 2002; 36: 1491–1496. [DOI] [PubMed] [Google Scholar]
- 4. Anderson RT, Vrionis HA, Ortiz-Bernad I, Resch CT, Long PE, Dayvault R, et al. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl Environ Microbiol. 2003; 69: 5884–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Istok JD, Senko JM, Krumholz LR, Watson D, Bogle MA, Peacock A, et al. In situ bioreduction of technetium and uranium in a nitrate-contaminated aquifer. Environ Sci Technol. 2004; 38: 468–475. [DOI] [PubMed] [Google Scholar]
- 6. North NN, Dolhopf SL, Petrie L, Istok JD, Balkwill DL, Kostka JE. Change in bacterial community structure during in situ biostimulation of subsurface sediment cocontaminated with uranium and nitrate. Appl Environ Microbiol. 2004; 70: 4911–4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peacock AD, Chang YJ, Istok JD, Krumholz L, Geyer R, Kinsall B, et al. Utilization of microbial biofilms as monitors of bioremediation. Microb Ecol. 2004; 47: 284–292. [DOI] [PubMed] [Google Scholar]
- 8. Long PE, Williams KH, Davis JA, Fox PM, Wilkins MJ, Yabusaki SB, et al. Bicarbonate Impact on U(VI) Bioreduction in a Shallow Alluvial Aquifer. Geochim Cosmochim Acta. 2015; 10.1016/j.gca.2014.11.013 25684790 [DOI] [Google Scholar]
- 9. Holmes DE, Finneran KT, O’Neil RA, Lovley DR. Enrichment of members of the family Geobacteraceae associated with stimulation of dissimilatory metal reduction in uranium-contaminated aquifer sediments. Appl Environ Microbiol. 2002; 68: 2300–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang YJ, Long PE, Geyer R, Peacock AD, Resch CT, Sublette K, et al. Microbial incorporation of C-13-labeled acetate at the field scale: Detection of microbes responsible for reduction of U(VI). Environ Sci Technol. 2005; 39: 9039–9048. [DOI] [PubMed] [Google Scholar]
- 11. Vrionis HA, Anderson RT, Ortiz-Bernad I, O’Neill KR, Resch CT, Peacock AD, et al. Microbiological and geochemical heterogeneity in an in situ uranium bioremediation field site. Appl Environ Microbiol. 2005; 71: 6308–6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilkins MJ, VerBerkmoes NC, Williams KH, Callister SJ, Mouser PJ, Elifantz H, et al. Proteogenomic monitoring of Geobacter physiology during stimulated uranium bioremediation. Appl Environ Microbiol. 2009; 75: 6591–6599. 10.1128/AEM.01064-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kerkhof LJ, Williams KH, Long PE, McGuinness LR. Phase preference by active, acetate-utilizing bacteria at the Rifle, CO integrated field research challenge site. Environ Sci Technol. 2011; 45: 1250–1256. 10.1021/es102893r [DOI] [PubMed] [Google Scholar]
- 14. Bargar JR, Williams KH, Campbell KM, Long PE, Stubbs JE, Suvorova EI, et al. Uranium redox transition pathways in acetate-amended sediments. Proc Natl Acad Sci USA. 2013; 110: 4506–4511. [Google Scholar]
- 15. Finneran KT, Housewright ME, Lovley DR. Multiple influences of nitrate on uranium solubility during bioremediation of uranium-contaminated subsurface sediments. Environ Microbiol. 2002; 4: 510–516. [DOI] [PubMed] [Google Scholar]
- 16. Shelobolina ES, Coppi MV, Korenevsky AA, DiDonato LN, Sullivan SA, Konishi H, et al. Importance of c-type cytochromes for U(VI) reduction by Geobacter sulfurreducens . BMC Microbiol. 2007; 7: 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shelobolina ES, Vrionis HA, Findlay RH, Lovley DR. Geobacter uraniireducens sp nov., isolated from subsurface sediment undergoing uranium bioremediation. Int J Syst Evol Microbiol. 2008; 58: 1075–1078. 10.1099/ijs.0.65377-0 [DOI] [PubMed] [Google Scholar]
- 18. Spear JR, Figueroa LA, Honeyman BD Modeling reduction of uranium U(VI) under variable sulfate concentrations by sulfate-reducing bacteria. Appl Environ Microbiol. 2000; 66: 3711–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moon HS, McGuinness L, Kukkadapu RK, Peacock AD, Komlos J, Kerkhof LJ, et al. Microbial reduction of uranium under iron- and sulfate-reducing conditions: Effect of amended goethite on microbial community composition and dynamics. Water Res. 2010; 44: 4015–4028. 10.1016/j.watres.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 20. Coenye T, Laevens S, Willems A, Ohlen M, Hannant W, Govan JRW, et al. Burkholderia fungorum sp nov, and Burkholderia caledonica sp nov., two new species isolated from the environment, animals and human clinical samples. Int J Syst Evol Microbiol. 2001; 51: 1099–1107. [DOI] [PubMed] [Google Scholar]
- 21. Campbell KM, Davis J, Bargar J, Giammar D, Bernier-Latmani R, Kukkadapu R, et al. Composition, stability, and measurement of reduced uranium phases for groundwater bioremediation at Old Rifle, CO. Appl Geochem. 2011; 26: S167–S169. [Google Scholar]
- 22. Qafoku NP, Gartman BN, Kukkadapu RK, Arey BW, Williams KH, Mouser PJ, et al. Geochemical and mineralogical investigation of uranium in multi-element contaminated, organic-rich subsurface sediment. Appl Geochem. 2014; 42: 77–85. [Google Scholar]
- 23. Williams KH, Nevin KP, Franks A, Englert A, Long PE, Lovley DR. Electrode-Based Approach for Monitoring In Situ Microbial Activity During Subsurface Bioremediation. Environ Sci Technol. 2010; 44: 47–54. 10.1021/es9017464 [DOI] [PubMed] [Google Scholar]
- 24. Knight VK, Nijenhuis I, Kerkhof LJ, Häggblom MM. Degradation of aromatic compounds coupled to selenate reduction. Geomicrobiol J. 2002; 19: 77–86. [Google Scholar]
- 25. Fennell DE, Rhee SK, Ahn YB, Häggblom MM, Kerkhof LJ. Detection and characterization of a dehalogenating microorganism by terminal restriction fragment length polymorphism fingerprinting of 16S rRNA in a sulfidogenic, 2-bromophenol-utilizing enrichment. Appl Environ Microbiol. 2004; 70: 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGuinness LM, Salganik M, Vega L, Pickering KD, Kerkhof LJ. Replicability of bacterial communities in denitrifying bioreactors as measured by PCR/T-RFLP analysis. Environ Sci Technol. 2006; 40: 509–515. [DOI] [PubMed] [Google Scholar]
- 27. Sakano Y, Kerkhof L. Assessment of changes in microbial community structure during operation of an ammonia biofilter with molecular tools. Appl Environ Microbiol. 1998; 64: 4877–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003; 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 29. Corredor JE, Wawrik B, Paul JH, Tran H, Kerkhof L, Lopez JM, et al. Geochemical Rate-RNA integration study: Ribulose-1,5-bisphosphate carboxylase/oxygenase gene transcription and photosynthetic capacity of planktonic photoautotrophs. Appl Environ Microbiol. 2004; 70: 5559–5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lovley DR, Phillips EJP, Gorby YA, Landa ER. Microbial reduction of uranium. Nature. 1991; 350: 413–416. [Google Scholar]
- 31. Wall JD, Krumholz LR. Uranium reduction. Ann Rev Microbiol. 2006; 60: 149–166. [DOI] [PubMed] [Google Scholar]
- 32. Tebo BM, Obraztsova AY.Sulfate-reducing bacterium grows with Cr(VI), U(VI), Mn(IV), and Fe(III) as electron acceptors. FEMS Microbiol Let. 1998; 162:193–198. [Google Scholar]
- 33. Blakeney MD, Moulaei T, DiChristina TJ. Fe(III) reduction activity and cytochrome content of Shewanella putrefaciens grown on ten compounds as sole terminal electron acceptor. Microbiol Res. 2000; 155: 87–94. [DOI] [PubMed] [Google Scholar]
- 34. Khijniak TV, Slobodkin AI, Coker V, Renshaw JC, Livens FR, Bonch-Osmolovskaya EA, et al. Reduction of uranium(VI) phosphate during growth of the thermophilic bacterium Thermoterrabacterium ferrireducens . Appl Environ Microbiol. 2005; 71: 6423–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanford RA, Wu Q, Sung Y, Thomas SH, Amos BK, Prince EK, et al. Hexavalent uranium supports growth of Anaeromyxobacter dehalogenans and Geobacter spp. with lower than predicted biomass yields. Env Micriobiol. 2007; 9: 2885–2893 [DOI] [PubMed] [Google Scholar]
- 36. Prakash OM, Gihring TM, Dalton DD, Chin K, Green SJ, Akob DM, et al. Geobacter daltonii sp. nov., an Fe(III)- and uranium(VI)-reducing bacterium isolated from a shallow subsurface exposed to mixed heavy metal and hydrocarbon contamination. Int J Syst Evol Microbiol. 2010; 60: 546–553. 10.1099/ijs.0.010843-0 [DOI] [PubMed] [Google Scholar]
- 37. Boonchayaanant B, Kitanidis PK, Criddle CS. Growth and cometabolic reduction kinetics of a uranium- and sulfate-reducing Desulfovibrio Clostridia mixed culture: Temperature effects. Biotech Bioeng. 2008; 99: 1107–1119. [DOI] [PubMed] [Google Scholar]
- 38. Lovley DR, Phillips EJP. Reduction of Uranium by Desulfovibrio desulfuricans . Appl Environ Microbiol. 1992; 58:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brooks SC, Frederickson JK, Carroll SL, Kennedy DW, Zachara JM, Plymale AE, et al. Inhibition of bacterial U(VI) reduction by calcium. Environ Sci Technol. 2003; 37: 1850–1858. [DOI] [PubMed] [Google Scholar]
- 40. Nevin KP, Holmes DE, Woodard TL, Hinlein ES, Ostendorf DW, Lovley DR. Geobacter bemidjiensis sp nov and Geobacter psychrophilus sp nov., two novel Fe(III)-reducing subsurface isolates. Int J Syst Evol Microbiol. 2005; 55: 1667–1674. [DOI] [PubMed] [Google Scholar]
- 41. Miller CS, Handley KM, Wrighton KC, Frischkorn KR, Thomas BC, Banfield JF. Short-read assembly of full-length 16S amplicons reveals bacterial diversity in subsurface sediments. Plos One. 2013; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moreels D, Crosson G, Garafola C, Monteleone D, Taghavi S, Fitts JP, van der Lelie D, Microbial community dynamics in uranium contaminated subsurface sediments under biostimulated conditions with high nitrate and nickel pressure. Environ Sci Pol Res. 2008; 15: 481–491. 10.1007/s11356-008-0034-z [DOI] [PubMed] [Google Scholar]
- 43. Vishnivetskaya TA, Brandt CC, Madden AS, Drake MM, Kostka JE, Akob DM, et al. Microbial community changes in response to ethanol or methanol amendments for U(VI) reduction. Appl Environ Microbiol. 2010; 76: 5728–5735. 10.1128/AEM.00308-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Andreolli M, Lampis S, Zenaro E, Salkinoja-Salonen M, Vallini G. Burkholderia fungorum DBT1: a promising bacterial strain for bioremediation of PAHs-contaminated soils. FEMS Microbiol Let. 2011; 319: 11–18. 10.1111/j.1574-6968.2011.02259.x [DOI] [PubMed] [Google Scholar]
- 45. Chaudhary HJ, Peng GX,Hu M, He YM, Yang LJ, Luo Y, et al. Genetic diversity of endophytic diazotrophs of the wild rice, Oryza alta and identification of the new diazotroph, Acinetobacter oryzae sp nov . Micro Ecol. 2012; 63: 813–821. 10.1007/s00248-011-9978-5 [DOI] [PubMed] [Google Scholar]
- 46. King GM. Nitrate-dependent anaerobic carbon monoxide oxidation by aerobic CO-oxidizing bacteria. FEMS Microbiol Ecol. 2006; 56: 1–7. [DOI] [PubMed] [Google Scholar]
- 47. Hamad MA, Austin CR, Stewart AL, Higgins M, Vazquez-Torres A, Voskuil MI. Adaptation and antibiotic tolerance of anaerobic Burkholderia pseudomallei . Antimicrob Agents and Chemother. 2011; 55: 3313–3323. 10.1128/AAC.00953-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mueller JG, Devereaux R, Santavy DL, Lantz SE, Willis SG, Pritchard PH. Phylogenetic and physiological comparisons of PAH-degrading bacteria from geographically diverse soils. Antonie Van Leeuwenhoek. 1997; 71: 329–343. [DOI] [PubMed] [Google Scholar]
- 49. Wang JL, Han LP, Shi HC, Qian Y. Biodegradation of quinoline by gel immobilized Burkholderia sp . Chemosphere. 2001; 44: 1041–1046. [DOI] [PubMed] [Google Scholar]
- 50. Denef VJ, Park J, Tsoi TV, Rouillard JM, Zhang H, Wibbenmeyer JA, et al. Biphenyl and benzoate metabolism in a genomic context: Outlining genome-wide metabolic networks in Burkholderia xenovorans LB400. Appl Environ Microbiol. 2004; 70: 4961–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ali SW, Yu FB, Li LT, Li XH, Gu LF, Jiang JD, et al. Studies revealing bioremediation potential of the strain Burkholderia sp. GB-01 for abamectin contaminated soils. World J Microbiol Biotechnol. 2012; 28: 39–45. 10.1007/s11274-011-0790-7 [DOI] [PubMed] [Google Scholar]
- 52. Tanase AM, Ionescu R, Chiciudean I, Vassu T, Stoica I. Characterization of hydrocarbon-degrading bacterial strains isolated from oil-polluted soil. Int Biodeterior Biodegradation. 2013; 84: 150–154. 43. [Google Scholar]
- 53. Kotik M, Davidova A, Voriskova J, Baldrian P. Bacterial communities in tetrachloroethene-polluted groundwaters: A case study. Sci Tot Environ. 2013; 454: 517–527. [DOI] [PubMed] [Google Scholar]
- 54. Plangklang P, Reungsang A. Biodegradation of carbofuran in sequencing batch reactor augmented with immobilised Burkholderia cepacia PCL3 on corncob. Chem Ecol. 2013; 29: 44–57. [Google Scholar]
- 55. Konstantinidis KT, Tiedje JM. Trends between gene content and genome size in prokaryotic species with larger genomes. Proc Natl Acad Sci USA. 2004; 101: 3160–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chain PSG, Denef VJ, Konstnatinidis KT, Vergez LM, Aguillo L, Reyes VL, et al. Burkholderia xenovorans LB400 harbors a multi-replicon, 9.73 Mbp genome shaped for versatility. Proc Natl Acad Sci USA. 2006; 103: 15280–15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIFF)
(TIFF)
(TIFF)
Cells were grown in M9 media with 0.4% dextrose as an electron donor and the terminal electron acceptor indicated. Error bars indicate the variability (SD) in the cell counts for each microcosm. The inset is the anaerobic incubations on a different scale.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files or accessible through Genbank (Accession: KP212894).