Abstract
Background
Cigarette smoking is reported to decrease survival and induce chemotherapy resistance in patients with various cancers. However, the impact of cigarette smoking on patients with esophageal squamous cell carcinoma (ESCC) remains unknown.
Methods
A total of 1,084 ESCC patients were retrospectively enrolled from a southern Chinese institution. Patients were divided into two groups according to their treatment modalities: the SC group (surgery with chemotherapy) (n = 306) and the S group (surgery without chemotherapy) (n = 778). Smoking status was quantified as smoking history (non-smoker, ex-smoker, and current smoker) and cumulative smoking (0, between 0 and 20, and greater than 20 pack-years). The association between cigarette smoking and overall survival (OS) was evaluated using the Kaplan-Meier method and univariate/multivariate regression analysis.
Results
Among 1,084 patients, 702 (64.8%) reported a cigarette smoking history, and the 5-year OS for non-smokers and smokers was 45.8% and 37.3%, respectively. In the SC group, compared with non-smoker, the adjusted HRs of ex-smoker and current smoker were 1.540 (95% CI, 1.1–2.2) and 2.110 (95% CI, 1.4–3.1), respectively; there is a correlative trend of decreased OS with increased cigarette smoking (P trend = 0.001). These associations were insignificant in the S group. In subgroup analysis of the SC group, the lower OS conferred by smoking was not significantly modified by age, gender, body mass index, alcohol drinking, or chemotherapy method (chemotherapy and chemoradiotherapy).
Conclusion
Our results suggest that smoking may affect treatment outcome in patients with resected ESCC who received chemotherapy.
Introduction
Esophageal cancer is a frequent cause of death worldwide. Despite great improvements in preoperative examination, surgical technology, and multidisciplinary treatment over the past decades, the survival rate is still unsatisfactory [1–3]. Therefore, it is necessary to identify novel prognostic factors to recognize high-risk patients.
It is well documented that cigarette smoking promotes tumorigenesis [4–7]. Recently, the association between cigarette smoking and survival have been evaluated in patients with colorectal cancer [8], head and neck cancer [9], breast cancer [10], gastric cancer [11], and lung cancer [12]. However, controversy still exists regarding its role in esophageal cancer. Some studies indicated that smoking might decrease survival [13, 14], but other studies revealed no significant association [15].
Previous studies indicated that the effect of cigarette smoking on survival may be partially attributed to its interaction with chemotherapy [16–19]. Experiments in vitro also revealed that the exposure of cancer cell lines to nicotine and other metabolites of cigarette smoke can lead to chemoresistance [20–25]. However, this association is still unknown for patients with esophageal cancer.
We performed this study to evaluate the effect of cigarette smoking on long-term survival and to clarify whether it is associated with chemotherapy resistance among patients with established esophageal squamous cell carcinoma (ESCC).
Materials and Methods
Ethics Statement
Study approval was obtained from the independent ethics in committees at the Cancer Center of Sun Yat-sen University. Written informed consent was given by all participants for their clinical records to be used in this study. All patient data were anonymized and de-identified in a confidential manner.
Selection of patients
Between January 2000 and December 2008, 1773 patients with pathologically confirmed ESCC were treated in the Sun Yat-sen University Cancer Center. Among these patients, 1185 (66.8%) patients who received surgical resection at the thoracic surgery department were reviewed. Excluding 69 patients without complete resection, 14 patients have distant metastases (M1), 13 patients with excessively advanced tumor status (T4b), and 5 patients with preexisting/concurrent malignant disease, 1084 patients finally constituted our study cohort. An incomplete resection included microscopically positive resection (R1), macroscopically positive resection (R2), and surgical exploration without esophagectomy. January 2000 was the date after which multidisciplinary treatment was widely applied for esophageal cancer.
Analysis of clinical stage was performed using barium esophagography, computed tomography scan of the chest, abdomen, and cervical regions, electronic gastroscopy, ultrasound gastroscopy and bronchoscopy. PET/CT was not routinely carried out. Pathologic staging was performed based on the 7th American Joint Committee on Cancer (AJCC) staging system [26]. Patients who presented pathologically complete response after neoadjuvant treatment were diagnosed as stage 0.
Smoking status
Smoking status was translated and quantified into smoking history and cumulative smoking. In our hospital, we defined patients who had smoked more than 100 cigarettes in their lifetime as "smokers", which is similar to metrics used in previous studies [27]. Smoking history was enrolled as non-smoker, ex-smoker, or current smoker based on the medical record. Cumulative smoking was defined as pack-years (PY), which was calculated as: (the average number of cigarette smoked per day/20) × smoking years.
Treatments
Treatment options were determined based on tumor stage, the doctor's opinion, and the patient's desires. Surgical procedure applied in this study included primary tumor resection and lymph nodes dissection. The details of these common surgical procedures are described in prior studies [3]. Chemotherapy was typically applied as a two-drug regimen of platinum-based drugs for 4–6 cycles. Preoperative radiotherapy was mainly delivered to the primary tumor with a 3 cm cranio-caudal margin, and was also applied to metastatic lymph nodes and regional lymph nodes. The total dose used was 40–50 Gy. Postoperative radiotherapy was delivered to the anastomosis, supraclavicular, and mediastinal lymphatics, with a total dose of 46–64 Gy.
Follow-up
After primary treatment, most patients were asked to participate in outpatient follow up every three months for the first two years, every six months for years 3–5, and every 12 months thereafter. Regular assessment included physical examination, blood test, endoscopy, chest X-ray, and ultrasound test. Computed tomography scan of the chest, abdomen, and cervical region was performed at least once a year. For those who could not afford regular follow up visits, a telephone follow-up was performed instead. Survival status was reverified using the best available method in March 2014. The median time from the date of surgery to the last censoring was 68.5 months.
Statistics
The statistical analysis was performed using the SPSS 19.0 software package (SPSS, Inc., Chicago, IL). Overall survival (OS) was defined from the date of surgery to the date of death or final follow-up. Censored cases were defined as patients (1) who were lost in contact during the follow up, and (2) who were still alive at the end of the study. The survival rate was calculated using the Kaplan-Meier method, and the differences between curves were assessed by the log-rank test. Statistical significance was assumed at a two-sided probability value < 0.05.
In this study, analysis was performed in three groups (entire cohort, patients treated by surgery with chemotherapy [SC group], and patients treated by surgery without chemotherapy [S group]). As the major exposure of interest, smoking status was analyzed in univariate analysis with other confounders, including age (as a continuous variable), gender, body mass index (BMI) (as a continuous variable), alcohol drinking (non-drinker, drinker), tumor diameter (less than or equal to 3, between 3 and 5, or greater than 5 cm), and AJCC stage (0–I, II, or III). Factors proved with statistical significance (P < 0.1) in univariate analysis would be introduced into multivariate analysis.
Results
Patient Characteristics
A total of 1084 patients were enrolled as the target population. Of these, 35.2% (382/1084) were non-smokers and 64.8% (702/1084) were smokers. The median value of cumulative smoking was 20 PY.
In this study, 63.7% (690/1084) of patients were treated by surgery alone, 8.1% (88/1084) by surgery and radiotherapy, 23.4% (254/1084) by surgery and chemotherapy, and 4.8% (52/1084) by surgery and chemoradiotherapy. Therefore, 778 patients did not received chemotherapy were divided into S group and other 306 patients into SC group. The patient characteristics are listed in Table 1.
Table 1. Patient clinicopathological characteristics.
| Characteristics | Number (percentage) |
|---|---|
| Median age (range) (year) | 57 (30–82) |
| Gender | |
| Male | 851 (78.5) |
| Female | 233 (21.5) |
| Body mass index (kg/m 2) | 21.7±3.1 |
| Smoking history | |
| Non-smoker | 382 (35.2) |
| Smoker | 702 (64.8) |
| Ex-smoker | 411 (37.9) |
| Current smoker | 291 (26.9) |
| Cumulative smoking (pack-year) | |
| 0 | 382 (35.2) |
| >0&≤20 | 247 (22.8) |
| >20 | 455 (42.0) |
| Alcohol drinking | |
| Non-drinker | 724 (66.8) |
| Drinker | 360 (33.2) |
| Tumor diameter (cm) | |
| ≤3 | 212 (19.6) |
| >3&≤5 | 462 (42.6) |
| >5 | 410 (37.8) |
| AJCC stage | |
| 0-I | 98 (9.0) |
| II | 499 (46.0) |
| III | 487 (44.9) |
| Treatment | |
| SC group | 306 (28.2) |
| S group | 778 (71.8) |
SC group, surgery with chemotherapy group; S group, surgery without chemotherapy group.
Prognostic Significance of Cigarette smoking for the Entire Cohort (n = 1084)
The 5-year OS rate was 40.0% for the entire cohort, with a median survival time of 36.0 months. In univariate analysis, we observed that smoking history (P trend = 0.002) and cumulative smoking (P trend = 0.001) correlated with the OS rate. After multivariate analysis, factors proven to have independent prognostic significance were smoking history, cumulative smoking, age, BMI, alcohol drinking, and AJCC stage (Table 2). The OS curves of entire cohort stratified by smoking history and cumulative smoking are presented in S1 Fig.
Table 2. Univariate and multivariate analysis of cigarette smoking for the entire cohort (n = 1084).
| Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline and clinical features | uHR | 95% CI | P-value | aHR | 95% CI | P-value | aHR | 95% CI | P-value |
| Smoking history | |||||||||
| Non-smoker | 1 | 1 | |||||||
| Ex-smoker | 1.214 | 1.0–1.5 | 0.039 | 1.160 | 1.0–1.4 | 0.117 | |||
| Current smoker | 1.416 | 1.2–1.7 | 0.001 | 1.315 | 1.1–1.6 | 0.007 | |||
| P trend | 0.002 | 0.025 | |||||||
| Cumulative smoking (pack-year) | |||||||||
| 0 | 1 | 1 | |||||||
| >0&ati | 1.131 | 0.9–1.4 | 0.251 | 1.176 | 1.0–1.5 | 0.135 | |||
| >20 | 1.413 | 1.2–1.7 | <0.001 | 1.382 | 1.2–1.7 | <0.001 | |||
| P trend | 0.001 | 0.002 | |||||||
| Age (year) | 1.014 | 1.0–1.0 | 0.001 | 1.019 | 1.0–1.0 | <0.001 | 1.013 | 1.0–1.0 | 0.004 |
| Gender (male/female) | 0.739 | 0.6–0.9 | 0.003 | 0.966 | 0.7–1.2 | 0.790 | 0.952 | 0.7–1.2 | 0.709 |
| Body mass index (kg m -2) | 0.969 | 0.9–1.0 | 0.015 | 0.970 | 0.9–1.0 | 0.023 | 0.973 | 0.9–1.0 | 0.044 |
| Alcohol drinking (non-drinker/drinker) | 1.460 | 1.2–1.7 | <0.001 | 1.348 | 1.1–1.6 | <0.001 | 1.372 | 1.2–1.6 | <0.001 |
| Tumor diameter (cm) (≤3/>3&≤5/>5) | 1.165 | 1.1–1.3 | 0.004 | 1.106 | 1.0–1.2 | 0.064 | 1.099 | 1.0–1.2 | 0.080 |
| AJCC stage (0-I/II/III) | 1.885 | 1.7–2.2 | <0.001 | 1.919 | 1.7–2.2 | <0.001 | 1.851 | 1.6–2.1 | <0.001 |
uHR, unadjusted hazard ratio; CI, confidence interval; aHR, adjusted hazard ratio.
Prognostic Significance of Cigarette Smoking for Patients with Different Treatment Modalities
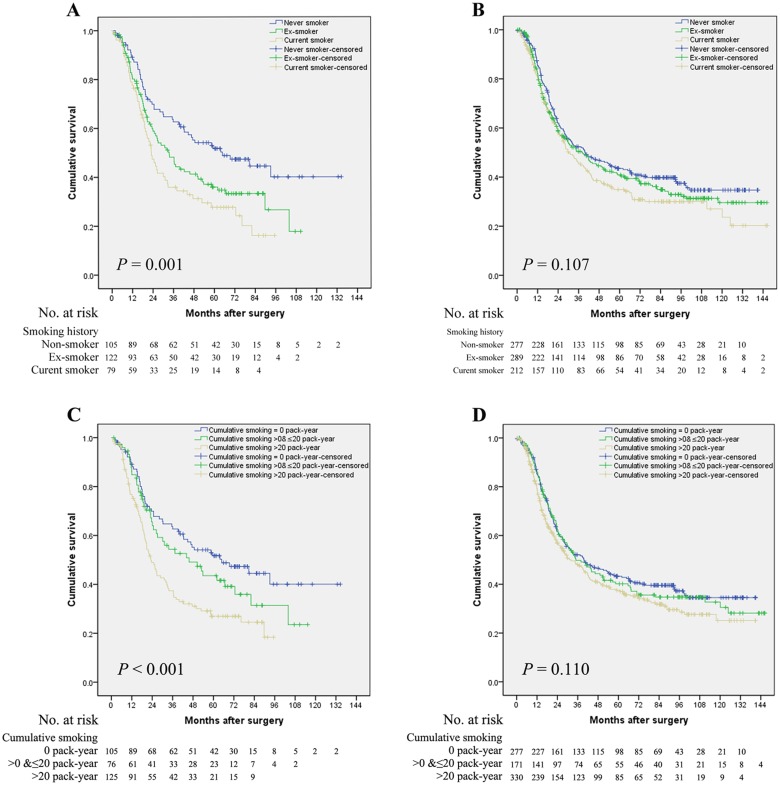
In the SC group, we observed a significant association between smoking history and OS in univariate analysis (P trend = 0.001) (Fig 1A). Compared with non-smoker, the unadjusted HR for ex-smoker and current smoker was 1.528 (95% confidence interval [CI], 1.1–2.2) and 2.014 (95% CI, 1.4–3.0). This effect was further confirmed in multivariate analysis (P trend = 0.001); the adjusted HRs of ex-smoker and current smoker were 1.540 (95% CI, 1.1–2.2) and 2.110 (95% CI, 1.4–3.1), respectively. However, no significant association between OS and smoking history was observed in the S group (Fig 1B) (Table 3).
Fig 1. Overall survival curves stratified by cigarette smoking for esophageal squamous cell carcinoma patients with different treatment modalities.
(A) In the SC group, the median survival time of non-smoker, ex-smoker, and current smoker was 64.9, 33.6, and 23.2 months, respectively (P = 0.001). (B) In the S group, the median survival time of non-smoker, ex-smoker, and current smoker was 39.7, 38.3, and 30.4 months, respectively (P = 0.107). (C) In the SC group, the median survival time of patients with cumulative smoking of 0, >0&≤20, and >20 pack-year were 64.9, 45.7, and 23.2 months, respectively (P < 0.001). (D) In the S group, the median survival time of patients with cumulative smoking of 0, >0&≤20, and >20 pack-year were 39.7, 34.9, and 32.1 months, respectively (P = 0.110). Abbreviations: SC group, surgery with chemotherapy group; S group, surgery without chemotherapy group.
Table 3. Prognostic analysis of cigarette smoking for patients with different treatment modalities.
| Smoking history | Cumulative smoking (pack-year) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Non-smoker | Ex-smoker | Current smoker | P trend | 0 | >0&≤20 | >20 | P trend | |||
| In SC group (n = 306) | |||||||||||
| No. of events | 52 | 73 | 55 | 52 | 42 | 86 | |||||
| No. at risk | 105 | 121 | 80 | 105 | 76 | 125 | |||||
| uHR (95% CI) | 1 | 1.528 (1.1–2.2) | 2.014 (1.4–3.0) | 1 | 1.344 (0.9–2.0) | 2.016 (1.4–2.9) | |||||
| P | 0.020 | <0.001 | 0.001 | 0.159 | <0.001 | <0.001 | |||||
| aHR (95% CI) | 1 | 1.540 (1.1–2.2) | 2.110 (1.4–3.1) | 1 | 1.398 (0.9–2.1) | 1.944 (1.4–2.8) | |||||
| P | 0.018 | <0.001 | 0.001 | 0.109 | <0.001 | 0.001 | |||||
| In S group (n = 778) * | |||||||||||
| No. of events | 163 | 167 | 133 | 163 | 104 | 196 | |||||
| No. at risk | 277 | 289 | 212 | 277 | 171 | 330 | |||||
| uHR (95% CI) | 1 | 1.128 (0.9–1.4) | 1.280 (1.0–1.6) | 1 | 1.066 (0.8–1.4) | 1.242 (1.0–1.5) | |||||
| P | 0.276 | 0.035 | 0.107 | 0.608 | 0.041 | 0.110 | |||||
SC group, surgery with chemotherapy group; S group, surgery without chemotherapy group; uHR, unadjusted hazard ratio; CI, confidence interval, aHR adjusted hazard ratio.
* Not assessed in multivariate model due to insignificant results of the univariate analysis (P > 0.10).
We next examined the effect of cumulative smoking on OS. In the SC group, patients with a higher consumption of tobacco had a lower OS rate in univariate analysis (P trend < 0.001) (Fig 1C). Compared with non-smokers, the unadjusted HR for smokers with cumulative smoking of ≤20 PY and >20 PY were 1.344 (95% CI, 0.9–2.0) and 2.016 (95% CI, 1.4–2.9), respectively. In addition, the higher risk of death for patients with higher cumulative smoking remained significant in multivariate analysis (P trend <0.001), with adjusted HRs of 1.398 (95% CI, 0.9–2.1) and 1.944 (95% CI, 1.4–2.8) for smokers with cumulative smoking of ≤20 PY and >20 PY, respectively. Nevertheless, no significant prognostic impact was observed in the S group in terms of cumulative smoking after univariate analysis (P trend = 0.110) (Fig 1D) (Table 3).
Subgroup Analysis for the SC group (n = 306)
The above results showed that the smoking status played an independent role in the prognosis of the SC group. We therefore broke down the SC group into subgroups stratified by baseline and clinical features to further assess the association between smoking history and OS. As shown in Table 4, the increased risk of death conferred by smoking was not significantly modified by age (≤56 and >56 years), gender, BMI (≤22 and >22 kg/m2), alcohol drinking, and chemotherapy method (chemotherapy and chemoradiotherapy). Additionally, this association was not significant among patients with short tumor diameter (<3 cm) and patients with early AJCC stage (0–II).
Table 4. Impact of cigarette smoking on overall survival based on patient characteristics for the SC group (n = 306).
| 5-year OS rate (%) | No. of events/No. at risk | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Factor | Non-smoker | Smoker | aHR | 95% CI | P | |
| Age (year) | ||||||
| ≤56* | 46.9 | 22/48 | 58/106 | 1.620 | 1.0–2.5 | 0.045 |
| >56 | 32.9 | 30/57 | 70/95 | 1.894 | 1.2–2.9 | 0.003 |
| Gender | ||||||
| Male | 36.5 | 31/54 | 126/199 | 1.496 | 1.0–2.2 | 0.045 |
| Female | 56.1 | 21/51 | 2/2 | 4.967 | 1.1–22.6 | 0.038 |
| Body mass index (kg m -2) | ||||||
| ≤22.0* | 37.0 | 27/45 | 71/113 | 1.570 | 1.0–2.5 | 0.047 |
| >22.0 | 42.8 | 25/60 | 57/88 | 2.016 | 1.2–3.3 | 0.004 |
| Alcohol drinking | ||||||
| Non-drinker | 41.4 | 46/92 | 68/112 | 1.487 | 1.0–2.2 | 0.039 |
| Drinker | 17.9 | 6/13 | 60/89 | 2.605 | 1.0–6.5 | 0.040 |
| Tumor diameter (cm) | ||||||
| ≤3 | 37.0 | 13/25 | 27/41 | - | - | -** |
| >3&≤5 | 39.8 | 25/49 | 43/69 | 1.732 | 1.0–2.9 | 0.039 |
| >5 | 43.3 | 14/31 | 58/91 | 2.009 | 1.1–3.6 | 0.020 |
| AJCC stage | ||||||
| 0-II | 53.1 | 18/44 | 42/80 | 1.129 | 0.5–2.5 | 0.760 |
| III | 29.9 | 34/61 | 86/121 | 1.726 | 1.2–2.6 | 0.008 |
| Chemotherapy method | ||||||
| Chemotherapy | 41.1 | 42/88 | 100/166 | 1.578 | 1.1–2.3 | 0.014 |
| Chemoradiotherapy | 34.8 | 10/17 | 28/35 | 2.683 | 1.3–5.7 | 0.011 |
SC group, surgery with chemotherapy group; aHR, adjusted hazard ratio; CI confidence interval.
* Median;
** Not assessed due to an insignificant result in the univariate analysis (P > 0.10).
Discussion
Our study observed adverse association between cigarette smoking and long-term survival of resected ESCC. Furthermore, in subgroup analysis, the effect of cigarette smoking was restricted to patients who had received chemotherapy.
Over the past decades, the role of cigarette smoking in etiology has been well established for patients with esophageal cancer, irrespective of pathological type [4, 5]. However, little research has been done on its impact on survival. A case-control study from China observed a significantly higher esophageal cancer death rates in smokers compared with nonsmokers in all geographical groups [13]. Recently, Yaegashi et al. provided clear evidence that the outcome of esophageal cancer was adversely associated with the cumulative amount of cigarette smoking [14]. However, in another study for ESCC patients who had been treated by primary radiotherapy, there is no association between smoking status and 2-year mortality and recurrence [15]. These conflicting results were similar to our results. Although we observed significant detrimental effects of cigarette smoking in entire cohort, this association was not significant in the vast majority of patients who had been treated by surgery without chemotherapy (S group).
To our knowledge, the effect of cigarette smoking on chemotherapy has been evaluated in various human cancers [16–19]. There is only one study in esophageal cancer, and the results indicated that heavy cigarette smoking (cumulative smoking >20 PY) is a poor prognostic factor in patients with ESCC who had been treated by chemoradiotherapy [28]. However, in that study, the author combined non-smokers and light smokers into a group of non-heavy smokers (cumulative smoking ≤20 PY), which may be biased and make it difficult to clearly explain the effect of smoking behavior. Therefore, in this study, we quantified the smoking status as smoking history and cumulative smoking and proved that cigarette smoking was harmful for the entire cohort. Next, we stratified the population into two groups based on treatment modalities and observed that the detrimental impact of cigarette smoking was limited to patients who had received chemotherapy; patients with current smoking behavior and patients with higher cigarette consumption would suffer higher mortality than those with ex-smoking behavior and those with less cigarette consumption. Additionally, in subgroups based on the chemotherapy method (chemotherapy and chemoradiotherapy), this association remained unchanged after adjusting for known prognostic factors. Thus, our results strongly support that cigarette smoking would impact the treatment outcome in resected ESCC who received chemotherapy.
Although the exact mechanism for the association between cigarette smoking and chemotherapy is not known, one probable explanation would involve nicotine's interaction with the PI3K/Akt/mTOR signaling pathway [29, 30], which has been proven to participate in the regulation of chemosensitivity by its downstream effects, specifically, stimulation of angiogenesis [31] and overexpression of DNA repair enzymes [32]. Recently, several studies in vitro have demonstrated that nicotine decreased the chemosensitivity of esophageal cancer cell lines [20, 21]. Besides, presence of nicotine and carbon monoxide in the blood may also work in this process, because the smoking impact was more pronounced in current smoker in our study. Additionally, the fact that the smoking effect is remarkable in long term smokers possibly has implications for an alternate, more aggressive molecular phenotype, which may has intrinsically more chemoresistance effect, induction of detoxifying pathways, reduced oxygenation, or altered immune function.
As expected, in the subgroup analysis for SC group, we found that the increased risks associated with cigarette smoking were restricted to patients with advanced stage. A meta-analysis of ESCC observed a significantly higher OS rate among patients with stage III–IV tumors, but not in those with stage I–II tumors, with surgery plus adjuvant chemotherapy compared with surgery alone [33], which is accordance with previous studies on adjuvant chemoradiotherapy [34, 35]. Although series of studies have proven the positive prognostic impact of neoadjuvant chemoradiotherapy for esophageal cancer [2, 36], it has still failed to help those with stage I–II esophageal cancer [37]. As indicated by our results, cigarette smoking may achieve its prognostic impact by interacting with chemotherapy, therefore the mediocre effect of chemotherapy/chemoradiotherapy in patients with early stage tumors may suppress the impact of cigarette smoking. Second, cigarette smoking was not associated with survival in patients with a tumor diameter of ≤3cm. Although we cannot clearly explain the reason, this may be partially associated to the fact that 83.3% (55/66) of these patients were diagnosed as early stage (0–II).
The current study observed an adverse association between cigarette smoking and long-term survival in patients with ESCC. Therefore, control of cigarette usage should be emphasized to reduce mortality of patients of ESCC. Furthermore, our results indicated an interaction between cigarette smoking and chemotherapy for patients with ESCC. Thus, further studies should include cigarette smoking as a potential indicator for chemosensitivity and focus on the underlying mechanism.
A major limitation of our study is that we relied on self-reported smoking status and possibly mistook the exact cumulative amount of cigarette smoking. However, previous studies have demonstrated the reliability of such information [4, 16, 18, 28]. Another limitation is that we could not collect information on the age at which patients began smoking and the age at which patients stopped smoking, as these parameters might alter the effect of cigarette smoking. The third concern is of competing risks from smoking-related mortality, however, in this study, most patients died from esophageal cancer (95.2%, 610/641). We assume that if the difference in OS in the SC group were mainly due to this confounding factor, then a similar result would be seen in the S group, which has a bigger sample size.
Conclusion
This study observed an adverse effect of cigarette smoking on the long-term survival in patients with resected ESCC who received chemotherapy. Further studies are needed to explore the biological mechanism and the effect of genetic or behavioral differences in ESCC patients regarding the presence of cigarette smoking.
Supporting Information
(A) The median survival time of non-smoker, ex-smoker, and current smoker was 44.9, 34.7, and 28.2 months, respectively (P = 0.002). (B) The median survival time of patients with cumulative smoking of 0, >0&≤20, and >20 pack-year were 44.9, 40.5, and 25.5 months, respectively (P = 0.001).
(TIF)
Acknowledgments
This study was supported by Chinese Ministry of Health Key Program (grant number 179) and National Natural Science Foundation of China General Program(grant number 81272635).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by Chinese Ministry of Health Key Program (grant number 179) and National Natural Science Foundation of China General Program (grant number 81272635).
References
- 1. Rice TW, Rusch VW, Apperson-Hansen C, Allen MS, Chen LQ, Hunter JG et al. (2009) Worldwide esophageal cancer collaboration. Dis Esophagus 22:1–8. 10.1111/j.1442-2050.2008.00901.x [DOI] [PubMed] [Google Scholar]
- 2. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE (2009) Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 27:5062–5067. 10.1200/JCO.2009.22.2083 [DOI] [PubMed] [Google Scholar]
- 3. Liu J, Hu Y, Xie X, Fu J (2012) Subcarinal node metastasis in thoracic esophageal squamous cell carcinoma. Ann Thorac Surg 93:423–427. 10.1016/j.athoracsur.2011.10.011 [DOI] [PubMed] [Google Scholar]
- 4. Castellsague X, Munoz N, De Stefani E, Victora CG, Castelletto R, Rolon PA et al. (1999) Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer 82:657–664. [DOI] [PubMed] [Google Scholar]
- 5. Oze I, Matsuo K, Ito H, Wakai K, Nagata C, Mizoue T et al. (2012) Cigarette smoking and esophageal cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol 42:63–73. 10.1093/jjco/hyr170 [DOI] [PubMed] [Google Scholar]
- 6. Wakai K, Inoue M, Mizoue T, Tanaka K, Tsuji I, Nagata C et al. (2006) Tobacco smoking and lung cancer risk: an evaluation based on a systematic review of epidemiological evidence among the Japanese population. Jpn J Clin Oncol 36:309–324. [DOI] [PubMed] [Google Scholar]
- 7. Giovannucci E (2001) An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 10:725–731. [PubMed] [Google Scholar]
- 8. Walter V, Jansen L, Hoffmeister M, Brenner H (2014) Smoking and survival of colorectal cancer patients: systematic review and meta-analysis. Ann Oncol 25:1517–1525. 10.1093/annonc/mdu040 [DOI] [PubMed] [Google Scholar]
- 9. Sharp L, McDevitt J, Carsin AE, Brown C, Comber H (2014) Smoking at Diagnosis Is an Independent Prognostic Factor for Cancer-Specific Survival in Head and Neck Cancer: Findings from a Large, Population-Based Study. Cancer Epidemiol Biomarkers Prev. [DOI] [PubMed] [Google Scholar]
- 10. Seibold P, Vrieling A, Heinz J, Obi N, Sinn HP, Flesch-Janys D et al. (2014) Pre-diagnostic smoking behaviour and poorer prognosis in a German breast cancer patient cohort—Differential effects by tumour subtype, NAT2 status, BMI and alcohol intake. Cancer Epidemiol 38:419–426. 10.1016/j.canep.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 11. Han MA, Kim YW, Choi IJ, Oh MG, Kim CG, Lee JY et al. (2013) Association of smoking history with cancer recurrence and survival in stage III-IV male gastric cancer patients. Cancer Epidemiol Biomarkers Prev 22:1805–1812. 10.1158/1055-9965.EPI-13-0385 [DOI] [PubMed] [Google Scholar]
- 12. Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A et al. (2010) Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol 5:620–630. 10.1097/JTO.0b013e3181d2dcd9 [DOI] [PubMed] [Google Scholar]
- 13. Jiang JM, Zeng XJ, Chen JS, Li JY, Zhang KL, Wu YP et al. (2006) Smoking and mortality from esophageal cancer in China: a large case-control study of 19,734 male esophageal cancer deaths and 104,846 living spouse controls. Int J Cancer 119:1427–1432. [DOI] [PubMed] [Google Scholar]
- 14. Yaegashi Y, Onoda T, Morioka S, Hashimoto T, Takeshita T, Sakata K et al. (2014) Joint effects of smoking and alcohol drinking on esophageal cancer mortality in Japanese men: findings from the Japan collaborative cohort study. Asian Pac J Cancer Prev 15:1023–1029. [DOI] [PubMed] [Google Scholar]
- 15. Zhang F, Han H, Wang C, Wang J, Zhang G, Cao F et al. (2013) A retrospective study: the prognostic value of anemia, smoking and drinking in esophageal squamous cell carcinoma with primary radiotherapy. World J Surg Oncol 11:249 10.1186/1477-7819-11-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsao AS, Liu D, Lee JJ, Spitz M, Hong WK (2006) Smoking affects treatment outcome in patients with advanced nonsmall cell lung cancer. Cancer 106:2428–2436. [DOI] [PubMed] [Google Scholar]
- 17. Fountzilas G, Kosmidis P, Beer M, Sridhar KS, Banis K, Vritsios A et al. (1992) Factors influencing complete response and survival in patients with head and neck cancer treated with platinum-based induction chemotherapy. A Hellenic Co-operative Oncology Group Study. Ann Oncol 3:553–558. [DOI] [PubMed] [Google Scholar]
- 18. Aksoy S, Harputluoglu H, Guler N, Altundag K, Hayran M, Tekuzman G et al. (2007) Influence of smoking history on breast cancer prognosis: retrospective study of 240 operable breast cancer patients who received adjuvant cyclophosphamide, doxorubicin, and 5-fluorouracil chemotherapy regimen. Breast J 13:431–432. [DOI] [PubMed] [Google Scholar]
- 19. Johnston-Early A, Cohen MH, Minna JD, Paxton LM, Fossieck BE Jr., Ihde DC et al. (1980) Smoking abstinence and small cell lung cancer survival. An association. JAMA 244:2175–2179. [PubMed] [Google Scholar]
- 20. Hamano R, Miyata H, Yamasaki M, Kurokawa Y, Hara J, Moon JH et al. (2011) Overexpression of miR-200c induces chemoresistance in esophageal cancers mediated through activation of the Akt signaling pathway. Clin Cancer Res 17:3029–3038. 10.1158/1078-0432.CCR-10-2532 [DOI] [PubMed] [Google Scholar]
- 21. Yoshioka A, Miyata H, Doki Y, Yasuda T, Yamasaki M, Motoori M et al. (2008) The activation of Akt during preoperative chemotherapy for esophageal cancer correlates with poor prognosis. Oncol Rep 19:1099–1107. [PubMed] [Google Scholar]
- 22. Dasgupta P, Kinkade R, Joshi B, Decook C, Haura E, Chellappan S (2006) Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc Natl Acad Sci U S A 103:6332–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsurutani J, Castillo SS, Brognard J, Granville CA, Zhang C, Gills JJ et al. (2005) Tobacco components stimulate Akt-dependent proliferation and NFkappaB-dependent survival in lung cancer cells. Carcinogenesis 26:1182–1195. [DOI] [PubMed] [Google Scholar]
- 24. Chen RJ, Ho YS, Guo HR, Wang YJ (2010) Long-term nicotine exposure-induced chemoresistance is mediated by activation of Stat3 and downregulation of ERK1/2 via nAChR and beta-adrenoceptors in human bladder cancer cells. Toxicol Sci 115:118–130. 10.1093/toxsci/kfq028 [DOI] [PubMed] [Google Scholar]
- 25. Liu Y, Liu BA (2011) Enhanced proliferation, invasion, and epithelial-mesenchymal transition of nicotine-promoted gastric cancer by periostin. World J Gastroenterol 17:2674–2680. 10.3748/wjg.v17.i21.2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edge SB BD, Compton CC, Fritz AG, Greene FL, Trotti A. (2010) American joint committee on cacer (AJCC) cancer staging manual, 7th edn. Chicago: Springer:67–72. [Google Scholar]
- 27. Ji X, Zhang W, Xie C, Wang B, Zhang G, Zhou F (2011) Nasopharyngeal carcinoma risk by histologic type in central China: impact of smoking, alcohol and family history. Int J Cancer 129:724–732. 10.1002/ijc.25696 [DOI] [PubMed] [Google Scholar]
- 28. Shitara K, Matsuo K, Hatooka S, Ura T, Takahari D, Yokota T et al. (2010) Heavy smoking history interacts with chemoradiotherapy for esophageal cancer prognosis: a retrospective study. Cancer Sci 101:1001–1006. 10.1111/j.1349-7006.2009.01466.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang S, Takayama K, Tanaka K, Takeshita M, Nakagaki N, Ijichi K et al. (2013) Nicotine induces resistance to epidermal growth factor receptor tyrosine kinase inhibitor by alpha1 nicotinic acetylcholine receptor-mediated activation in PC9 cells. J Thorac Oncol 8:719–725. 10.1097/JTO.0b013e31828b51d4 [DOI] [PubMed] [Google Scholar]
- 30. West KA, Castillo SS, Dennis PA (2002) Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat 5:234–248. [DOI] [PubMed] [Google Scholar]
- 31. Cooke JP, Bitterman H (2004) Nicotine and angiogenesis: a new paradigm for tobacco-related diseases. Ann Med 36:33–40. [DOI] [PubMed] [Google Scholar]
- 32. Luo B, Soesanto Y, McClain DA (2008) Protein modification by O-linked GlcNAc reduces angiogenesis by inhibiting Akt activity in endothelial cells. Arterioscler Thromb Vasc Biol 28:651–657. 10.1161/ATVBAHA.107.159533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang SS, Yang H, Xie X, Luo KJ, Wen J, Bella AE et al. (2013) Adjuvant chemotherapy versus surgery alone for esophageal squamous cell carcinoma: a meta-analysis of randomized controlled trials and nonrandomized studies. Dis Esophagus. [DOI] [PubMed] [Google Scholar]
- 34. Wang ZW, Luan ZP, Zhang W, Dong W, Fu CR, Wang YN et al. (2014) Postoperative chemoradiotherapy improves survival in esophageal squamous cell cancer with extracapsular lymph node extension. Neoplasma 61:732–738. 10.4149/neo_2014_089 [DOI] [PubMed] [Google Scholar]
- 35. Kofoed SC, Muhic A, Baeksgaard L, Jendresen M, Gustafsen J, Holm J et al. (2012) Survival after adjuvant chemoradiotherapy or surgery alone in resectable adenocarcinoma at the gastro-esophageal junction. Scand J Surg 101:26–31. [DOI] [PubMed] [Google Scholar]
- 36. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP et al. (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366:2074–2084. 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 37. Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B et al. (2014) Surgery Alone Versus Chemoradiotherapy Followed by Surgery for Stage I and II Esophageal Cancer: Final Analysis of Randomized Controlled Phase III Trial FFCD 9901. J Clin Oncol. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The median survival time of non-smoker, ex-smoker, and current smoker was 44.9, 34.7, and 28.2 months, respectively (P = 0.002). (B) The median survival time of patients with cumulative smoking of 0, >0&≤20, and >20 pack-year were 44.9, 40.5, and 25.5 months, respectively (P = 0.001).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.