Abstract
Alzheimer´s disease (AD), a neurodegenerative illness involving synaptic dysfunction with extracellular accumulation of Aβ1-42 toxic peptide, glial activation, inflammatory response and oxidative stress, can lead to neuronal death. Endogenous cannabinoid system is implicated in physiological and physiopathological events in central nervous system (CNS), and changes in this system are related to many human diseases, including AD. However, studies on the effects of cannabinoids on astrocytes functions are scarce. In primary cultured astrocytes we studied cellular viability using MTT assay. Inflammatory and oxidative stress mediators were determined by ELISA and Western-blot techniques both in the presence and absence of Aβ1-42 peptide. Effects of WIN 55,212-2 (a synthetic cannabinoid) on cell viability, inflammatory mediators and oxidative stress were also determined. Aβ1-42 diminished astrocytes viability, increased TNF-α and IL-1β levels and p-65, COX-2 and iNOS protein expression while decreased PPAR-γ and antioxidant enzyme Cu/Zn SOD. WIN 55,212-2 pretreatment prevents all effects elicited by Aβ1-42. Furthermore, cannabinoid WIN 55,212-2 also increased cell viability and PPAR-γ expression in control astrocytes. In conclusion cannabinoid WIN 55,212-2 increases cell viability and anti-inflammatory response in cultured astrocytes. Moreover, WIN 55,212-2 increases expression of anti-oxidant Cu/Zn SOD and is able to prevent inflammation induced by Aβ1-42 in cultured astrocytes. Further studies would be needed to assess the possible beneficial effects of cannabinoids in Alzheimer's disease patients.
Introduction
Alzheimer’s disease (AD) is a common neurodegenerative disease implicated in the aging process, affecting nearly 50% of people over 75 [1,2]. It involves neurofibrillary degeneration, extracellular accumulation of beta-amyloid peptide (Aβ) and synaptic dysfunction, resulting in neural cell death in the hippocampus and cerebral cortex, and in activation of glial cells [3,4]. Aβ can interact with different cellular components producing Ca2+ deregulation, oxidative stress and inflammation [5,6].
Astrocytes are specialized neural cells serving as a structural and metabolic support and trophic help to the brain [7]. Astrocytes also release cytokines and chemokines involved both in protective and toxic roles in neuroinflammatory processes [8]. However, released cytokines in neuroinflammation may induce deleterious effects on the viability and functionality of astrocytes [9]. Furthermore, in pathological situations such as hypoxia, cytokines induce activation of vascular endothelial cells thereby modulating inflammatory responses [10]. In AD, astrocytes are found around senile plaques producing phagocytosis, and cleaning up toxic compounds such as Aβ [11]. Moreover, when stimulated with compounds such as genistein or estradiol, astrocytes block the release of pro-inflammatory mediators and induce the synthesis of anti-inflammatory proteins [12].
Endocannabinoids have been implicated in various physiopathological events in different organs, including the peripheral and central nervous system (CNS) [13], and changes in the endocannabinoid system have been related to many human diseases, such as metabolic syndrome [14], neurodegeneration [15], inflammatory diseases [16], psychiatric disorders [17] and cancer [18]. The endocannabinoid signaling system is composed of anandamide and 2-arachidonoyl glycerol interacting with CB1 and CB2 cannabinoid receptors. Receptor signaling may involve mechanisms such as adenylyl cyclase blockade or activation of mitogen-activated protein kinases or ceramide signaling [13].
Different authors have proposed cannabinoids as preventive treatment in AD [19] due to their anti-inflammatory and neuroprotective properties [16]. In this sense, cannabinoids prevented microglial activation and cognitive impairment in Aβ-treated rats [19]. In mice exposed to Aβ, cannabinoids also suppress neuroinflammation by inhibiting inducible nitric oxide synthase (iNOS) expression and interleukin-1β generation [20]. However, the effects of cannabinoids on astrocytes functions have been poorly investigated. Therefore, we investigated the role of WIN 55,212–2 (WIN) as a neuroprotective agent against lesions induced by Aβ1–42 on cultured astrocytes.
Material and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco (Gibco Invitrogen Corporation, Barcelona, Spain). The oligomers Aβ (40–1 and 1–42), were prepared following manufacture instructions (Sigma-Aldrich biotechnology). Briefly, the peptides were dissolved in H2O, and, for assembly the oligomers, preparations were heated for 24 h at 37ºC. WIN and 3-(4,5-dimethyl-2-thiazolyl)-2,5-dipheniyl-2H-tetrazolium bromide (MTT) were purchased from Sigma Chemical Co. (St Louis, MO). Enzyme-linked immunosorbent assay (ELISA) kits for interleukin 1β (IL-1β) and tumor necrosis factor (TNF-α) from Pierce Biotechnology, Inc. (Rockford, USA). Western Blot Chemiluminescent Detection System (ECL) was from Amersham (Amersham Biosciences, Barcelona, Spain). Monoclonal anti-peroxisome proliferator-activated receptor antibody (PPAR-γ) (1:250) and polyclonal anti-cyclooxigenase-2 antibody (COX-2) (1:250) from Sigma Aldrich (Madrid, Spain). Monoclonal p65 antibody (p65) (1:250) and monoclonal anti-Mn superoxide dismutase antibody (Mn-SOD) (1:250) from Santa Cruz Biotechnology (Madrid, Spain). Polyclonal anti-Cu/Zn superoxide dismutase antibody (Cu/Zn SOD) (1:250) from Assay Designs (Madrid, Spain). Monoclonal inducible nitric oxide synthetize (iNOS) (1:250) and anti-tubuline (1:1000) antibodies from Cell Signaling (Beverly, MA, USA). All other reagents are analytical or culture grade purity.
Primary culture of cortical astrocytes
All animals were handled according to the recommendations of the Bioethics Committee of the School of Medicine of the University of Valencia, Spain. Ethics committee specifically approved this study. Cortical astrocytes were isolated from rat fetuses of 21 days gestation. Fetuses were obtained by cesarean section and decapitated. Cerebral cortices were removed and triturated 10–15 times through a Pasteur pipette with 10 ml DMEM. The cell suspension was filtered through nylon mesh with a pore size of 90 μm and re-suspended in DMEM containing 20% fetal bovine serum (FBS), supplemented with L-glutamine (1%), HEPES (10 mM), fungizone (1%), and antibiotics (1%). Cells were plated on T75 culture flask and maintained in a humidified atmosphere of 5% CO2/95% air at 37°C during 15 days. After 4 days of culture, the FBS was maintained at 20% and after 1 week of culture, the FBS content was reduced to 10%, and the medium was changed twice a week. The purity of astrocytes was assessed by immunofluorescence using anti-glial fibrillary acidic protein (astrocyte marker; Sigma-Aldrich), anti-CD-68 (microglial marker; Serotec), anti-myelin basic protein (olygodendroglial marker; Sigma-Aldrich) and anti-microtubule-associated protein 2 (neuronal marker; Sigma-Aldrich). The astrocyte cultures were found to be at least 99% glial fibrillary acidic protein positive. No cells were found to express CD-68, myelin basic protein, or microtubule-associated protein-2.
Cell treatments
Ten days after seeding, WIN (10 μM) was added to culture flasks. Twenty-four hours later, 10 μM Aβ1–42 (toxic peptide) or Aβ40–1 (control peptide) (Sigma-Aldrich) were added to the flasks. Aβ1–42 concentration used in our study is in the range of toxic concentrations of the peptide [21,22]. Before incubation, the peptides were diluted in 100 μM of phosphate-buffered saline (PBS) and incubated for 24 h at 37º C. Assays were performed 24 h after peptide addition.
MTT assay
Cell viability was determined by MTT assay. The MTT assay is a well-established, widely used and easily reproducible method for the assessment of cell viability and cytotoxicity [23,24]. Astrocytes were plated in 96-well culture plate and incubated with WIN during 24h. Subsequently, Aβ40–1 (control) and Aβ1–42 peptides were added to wells for another 24h. After cell treatments, the medium was removed and cells were incubated with red free medium and MTT solution [0.5 mg/ml, prepared in phosphate buffer saline (PBS) solution] for 4 h at 37ºC. Finally, the medium was removed and formazan particles were dissolved in dimethyl sulfoxide. Cell viability, defined as the relative amount of MTT reduction, was determined by spectrophotometry at 570 nm.
Cytokine determination, IL-1 and TNFα
Astrocytes were seeded as previously published [12]. At the time of assay, the red phenol medium was removed and replaced by PBS containing 1 mg/ml bovine serum albumin, either in the presence or absence of Aβ1–42 (10 μM). IL-1β and TNF-α concentration (pg/ml) were ascertained using ELISA kits (Pierce Biotechnology, Inc.).
Western blot analysis
Cultured cells were treated with lysis buffer and mechanically degraded to release the proteins. Protein concentration was determined using modified Lowry method [25]. Loading buffer (0.125 M Tris-HCl, pH 6.8, 2% SDS, 0.5% (v/v) 2-mercaptoethanol, 1% bromophenolblue and 19% glycerol) was added to protein sample and heated for 5 min at 95ºC. Proteins were separated on SDS-PAGE gels and transferred to nitrocellulose membranes in a humid environment using a transfer buffer (25mM Tris, 190mM glycine, 20% methanol). Membranes were blocked with 5% milk in TBS-T (0.05% Tween-20) and incubated with primary antibodies overnight at 4ºC. Membranes were washed 3 times with wash buffer TBS-T and incubated with a secondary anti-rabbit IgG or anti-mouse IgG (Cell Signalling Technologies Danvers, MA) antibody conjugated to the enzyme horseradish peroxidase (HRP) for 1 h. Membranes were washed three times and proteins were detected using the ECL method as specified by the manufacturer. Autoradiography signals were assessed using digital image system ImageQuant LAS 4000 (GE Healthcare).
Statistical analyses
Values are expressed as mean±S.D. Differences between groups were assessed by one-way analysis of variance (ANOVA). Statistical significance was accepted at P ≤ 0.05. Data sets in which F was significant were examined by a modified t-test.
Results
Protective role of WIN on cell viability
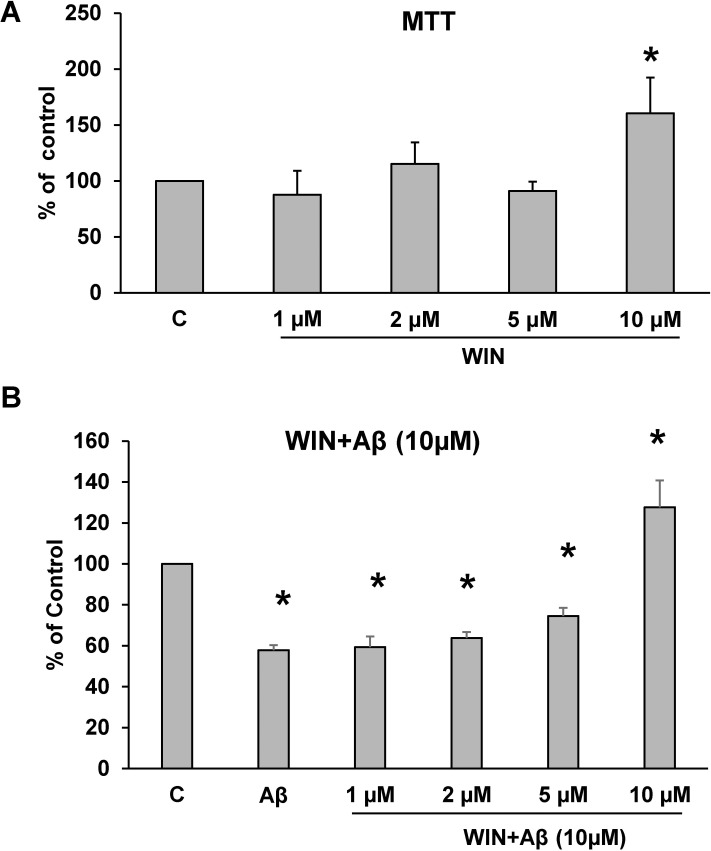
The role of WIN on cell viability was studied using MTT conversion assay. Fig 1A shows that incubation with WIN at different concentrations induced a significant increase in cell viability at 10 μM. Consequently, that concentration was used in future experiments. Astrocytes previously incubated with 10 μM Aβ1–42 for 24 h significantly decreased cell viability compared to control cells (Fig 1B). Furthermore, pretreating astrocytes during 48 h with WIN (10 μM) prevented the decrease in cell viability induced by Aβ1–42 (WIN + Aβ), conversely WIN (1, 2, 5μM) did not have any effect (Fig 1B).
Fig 1. Astrocytes viability.
(A) Astrocytes viability induced by WIN. Concentration-dependent viability of WIN (1, 2, 5, 10 μM) was determined by MTT assay for 24 h. Data are means ± SD for 4 independent experiments. *p<0.04 comparing WIN vs control cells. (B) Astrocytes viability in cells treated during 24 h with 10 μM Aβ40–1 (control peptide, C), 10 μM Aβ1–42 (Aβ) and WIN (1, 2, 5, 10 μM) + 10 μM Aβ1–42 (WIN + Aβ). Data are means ± SD of 3 independent experiments. *p<0.05 vs control cells.
WIN prevents IL-1β and TNF-α increase elicited by Aβ1–42
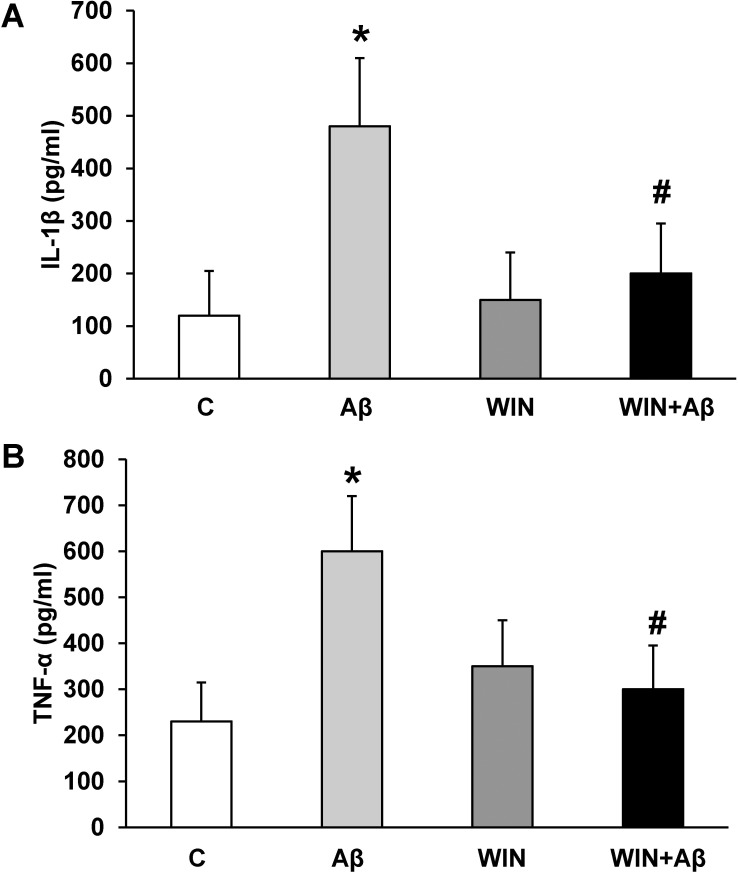
Cultured astrocytes were incubated with 10 μM Aβ1–42 and proinflammatory mediators TNF-α and IL-1β were detected by ELISA. Aβ1–42 increased 4.5-fold IL-1β release (480.4±150.3 pg/ml) compared with control (103.9±82.9 pg/ml) (Fig 2A) and 2.4 fold TNF-α release (605.3±103.4 pg/ml vs 210.5±85.3 pg/ml in control group) (Fig 2B). Furthermore, WIN pre-treatment (10 μM) prevented the increase in pro-inflammatory mediators induced by Aβ1–42 (Fig 2A and 2B).
Fig 2. IL-1β and TNF-α secretion.
WIN prevents the increase of IL-1β and TNF-α secretion caused by Aβ1–42 in astrocytes. Cells were incubated with 10 μM Aβ40–1 (control peptide, C), 10 μM Aβ1–42 (Aβ), 10 μM WIN + 10 μM control peptide (WIN) and 10 μM WIN + 10 μM Aβ1–42 (WIN + Aβ). Cell culture supernatants were harvested, and IL-1β (panel A) and TNF-α (panel B) secretion were determined by ELISA. Values are means ± SD of replicate experiments from 4 independent astrocytes cultures. *p<0.05 vs control astrocytes. #p<0.05 vs Aβ1–42 treated cells.
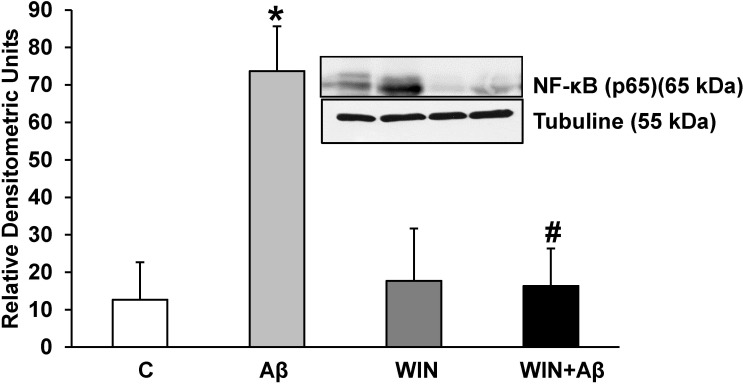
Effect of Aβ1–42 and WIN on p65 protein expression
Nuclear factor κB (NF-κB), the pro-inflammatory transcription factor is formed by different subunits. We measured p65 protein expression by western-blot. Incubation with Aβ1–42 increased p65 protein expression compared with control astrocytes (Fig 3), which was prevented by WIN pretreatment. (p<0.05 compared with Aβ1–42 treated astrocytes).
Fig 3. p65 protein expression.
WIN 55, 212–2 prevents p65 expression induced by Aβ1–42 in astrocytes in primary culture. p65 and α-tubulin expressions were determined by Western-blot in astrocytes treated for 24 h with 10 μM Aβ40–1 (control peptide, C), 10 μM Aβ1–42 (Aβ), 10 μM WIN + 10 µM control peptide (WIN) and 10 μM WIN + 10 μM Aβ1–42 (WIN + Aβ). A representative immunoblot of each protein is shown and tubulin was used as control amount of protein. Data are means ± SD of 5 independent experiments. *p<0.05 vs control cells. #p<0.05 vs Aβ1–42.
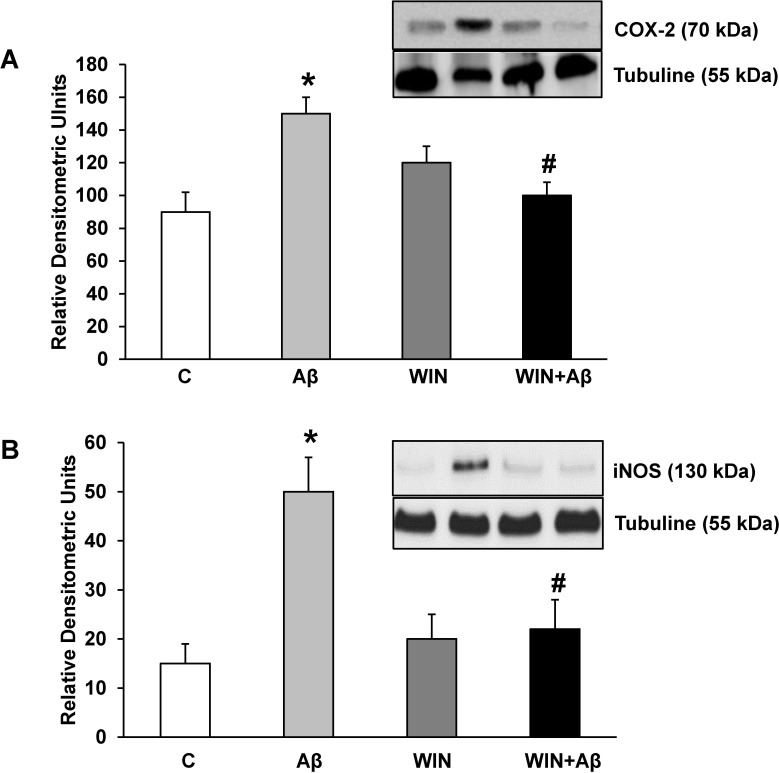
WIN prevents COX-2 and iNOS protein increase induced by Aβ1–42 peptide
Incubation with Aβ1–42 significantly increased inflammatory proteins COX-2 (Fig 4A) and iNOS (Fig 4B) expressions compared to control. Furthermore, pretreating astrocytes with WIN prevented the effects produced by Aβ1–42.
Fig 4. COX-2 and iNOS protein expression.
WIN prevents COX-2 and iNOS expression induced by Aβ1–42. COX-2 (panel A), iNOS (panel B) and α-tubulin expressions were determined by Western-blot in astrocytes treated for 24 h with 10 μM Aβ40–1 (control peptide, C), 10 μM Aβ1–42 (Aβ), 10 μM WIN + 10 μM control peptide (WIN) and 10 μM WIN + 10 μM Aβ1–42 (WIN + Aβ). A representative immunoblot of each protein is shown and tubulin was used as control amount of protein. Data are means ± SD of 6 independent experiments. *p<0.05 vs control cells. #p<0.05 vs Aβ1–42.
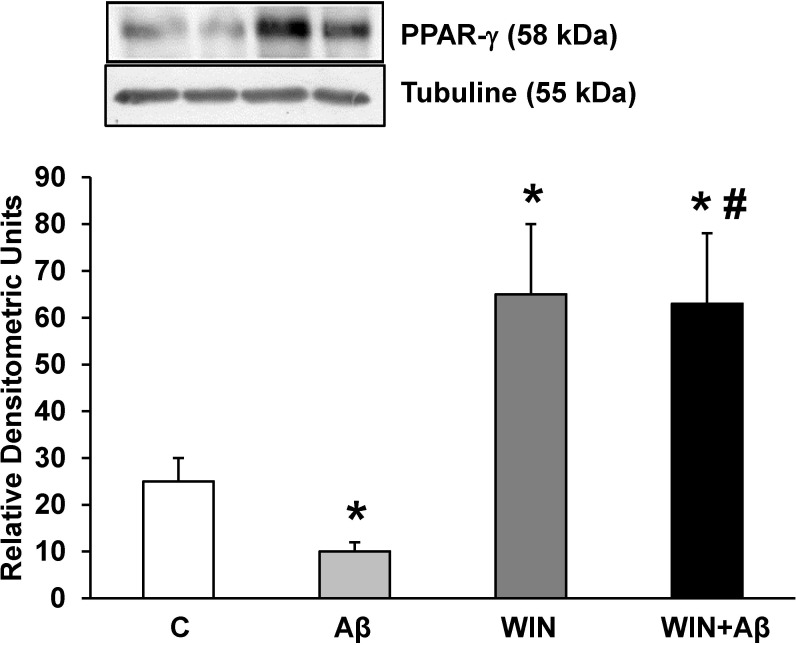
Effect of Aβ1–42 and WIN on PPAR-γ protein expression
Pro-inflammatory gene expression is downregulated by PPARs family [26]. We found that pretreatment with WIN (10 μM) increased PPAR-γ expression compared to control cells (Fig 5). Incubation with Aβ1–42 significantly decreased PPAR-γ expression that was prevented by WIN pretreatment.
Fig 5. PPAR-γ protein expression.
WIN induces PPAR-γ expression in astrocytes in primary culture treated with Aβ1–42. PPAR-γ and α-tubulin expressions were determined by Western-blot in astrocytes treated for 24 h with 10 μM Aβ40–1 (control peptide, C), 10 μM Aβ1–42 (Aβ), 10 μM WIN + 10 μM control peptide (WIN) and 10 μM WIN + 10 μM Aβ1–42 (WIN + Aβ). A representative immunoblot of each protein is shown and tubulin was used as control amount of protein. Data are means ± SD of 4 independent experiments. *p<0.05 vs control cells. #p<0.05 vs Aβ1–42.
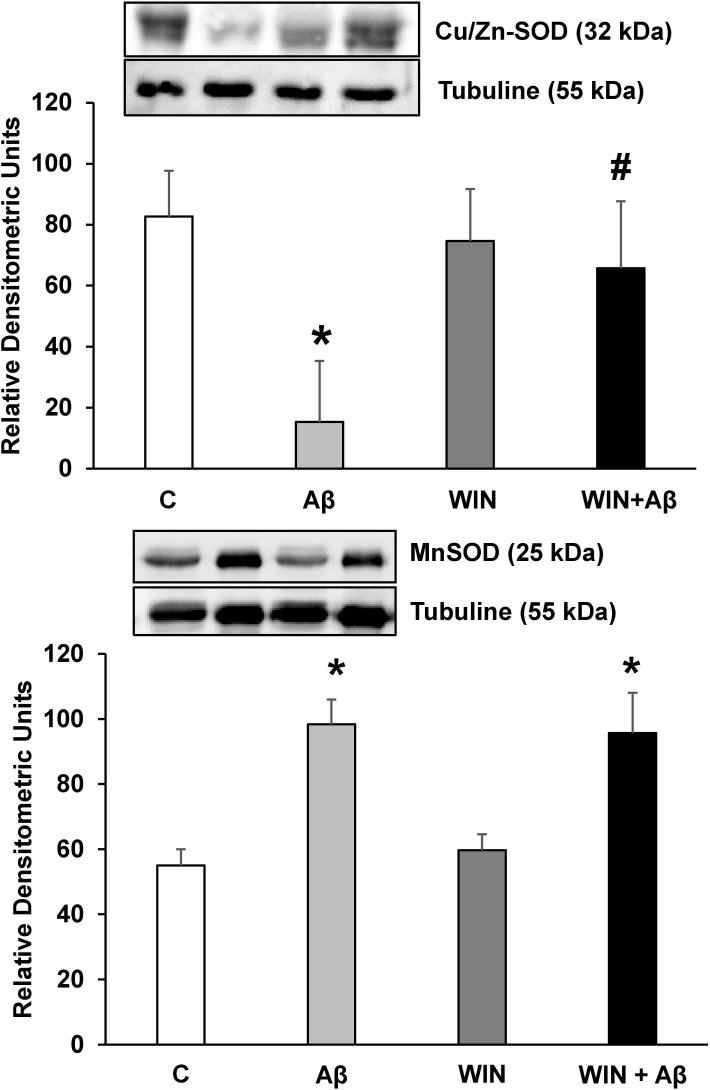
Effect of Aβ1–42 and WIN on Cu/Zn SOD and Mn SOD protein expression
Superoxide dismutase is a key antioxidant enzyme. In our study, incubation with Aβ1–42 decreased Cu/Zn SOD expression in astrocytes in primary culture which was prevented by WIN pretreatment, evidencing that WIN could play a neuro-protective role against oxidative stress induced by Aβ1–42 peptide (Fig 6A). On the other hand, our results indicated that Mn SOD protein expression is increased in presence of Aβ1–42. Pretreatment with WIN did not prevent Mn SOD increase induced by Aβ1–42 (Fig 6B).
Fig 6. Cu/Zn-SOD and Mn-SOD protein expressions.
WIN prevents Cu/Zn-SOD expression decrease in astrocytes in primary culture treated with Aβ1–42. Cu/Zn-SOD, Mn-SOD and α-tubulin expressions were determined by Western-blot in astrocytes treated for 24 h with 10 μM Aβ40–1 (control peptide, C), 10 μM Aβ1–42 (Aβ), 10 μM WIN + 10 μM control peptide (WIN) and 10 μM WIN + 10 μM Aβ1–42 (WIN + Aβ). A representative immunoblot of each protein is shown and tubulin was used as control amount of protein. Data are means ± SD of 4 independent experiments. *p<0.05 vs control cells. #p<0.05 vs Aβ1–42.
Discussion
Oxidative stress and inflammation are the main mechanisms in the progression of various neurodegenerative diseases, including AD [27–30]. In our study, we determined different markers involved in inflammation and oxidative stress induced by the Aβ1–42 peptide in primary cultures of astrocytes, with the aim to assess the antioxidant and anti-inflammatory effects of cannabinoid WIN. We found that WIN significantly increased astrocytes viability compared to control cells. Furthermore, WIN prevented the decrease in astrocytes viability induced by Aβ1–42.
It has been shown that cannabinoids preserve neurons from Aβ exposure by activating MAP kinase cascade [31] and by anti-oxidative and anti-apoptotic effects [32]. Moreover, some studies demonstrated that cannabinoids protect glial cells from death [33,34]. Nevertheless, in cancer, where cells are highly proliferative and undifferentiated, treatment with cannabinoids can block cell proliferation in a dose dependent manner [35–38], demonstrating that the effects of cannabinoids on cell viability are probably dependent on cell type [39] and developmental stage [40].
Expression of CB1 [41] and CB2 [42] receptors in rat culture astrocytes have been published and also dual activation of both cannabinoid receptors by WIN (the mixed non-selective CB1/CB2 agonist) in rat cortical astrocytes have been detected [41] On the other hand, WIN confers its protective and anti-inflammatory effects against Aβ injury through both CB1 and CB2 receptors [43]. Given that our results there is expression of both types of cannabinoid receptors, CB1 and CB2, it is likely that the effect of WIN observed in our study is due to the interaction with both types of receptors, consistent with published results by Fakhfouri and cols [44].
We found that WIN prevented the increase of inflammatory mediators IL-1β, TNF-α, NF-κB, iNOS and COX-2, as well as the decrease of the anti-inflammatory mediator PPAR-γ induced by Aβ1–42 in astrocytes in primary culture. The inflammatory process is a characteristic mechanism in the development of AD, and pro-inflammatory agents are involved in the progression of cell damage [45,46,47,12]. Moreover, it is known that astrocytes participate in the inflammatory process induced by Aβ1–42 [27,28,48]. Initially, inflammation is beneficial since it produces pro-inflammatory substances involved in tissue protection, limiting the survival and proliferation of cells exposed to toxic agents, such as Aβ1–42 [49,50]. However, sustained inflammatory response could lead to neurotoxic damage or cell death [12,51,8]. NF-κB proteins are up-regulated in inflammation conditions such as astroglial activation induced by Aβ1–42 oligomers [52]. In this regard, we found an increase in NF-kB/p-65 expression in astrocytes after addition of Aβ1–42 that was prevented by WIN pretreatment. Valles and collaborators [53] found that the cytokine-receptor complex is able to bind to cytokines and other proteins of the extracellular matrix, producing inflammatory signals which could be important in pathologies such as Alzheimer's disease [53,54]. In agreement with our results, different authors have reported that cannabinoids mitigate neural cell activation in the neuroinflammatory response induced by Aβ1–42, reducing the levels of pro-inflammatory molecules such as IL-1β, TNF-α, COX-2 and iNOS [55,56,57]. Likewise, the activation of cannabinoid receptors diminishes the release of IL-1β, IL-6 and TNF-α in microglial cells [58,59,60] as well as COX-2 and iNOS [61]. Studies conducted in rats pretreated with the Aβ peptide found that WIN prevented cognitive impairment, glial activation and neuronal loss [19,62,63], and also reduced COX-2, iNOS and TNF-α levels [63,64].
Kainu et al. [65] demonstrated for the first time the presence of mRNA and protein PPAR-γ in CNS cells. Subsequent studies have detected PPAR-γ expression in microglial and astrocytic cells [66,12]. PPAR-γ agonists protect against Aβ-induced inflammatory and neuronal damage [67,68], thus making neurons and astrocytes potential therapeutic targets for PPAR-γ ligands [69,12]. Astrocytes also express the largest levels of PPAR-γ in the neural tissues [70,71]. As other authors [72,73,74], we found a decreased expression of PPAR-γ in astrocytes treated with Aβ1–42. Esposito et al. [56] showed in neurons that cannabinoids may act as neuro-protective agents by PPAR-γ activation. In this study, we demonstrate an increase in this protein expression in astrocytic cells previously incubated with WIN. Furthermore, we found for the first time that WIN prevents PPAR-γ expression decrease induced by Aβ1–42 peptide in astrocytes in primary culture. There is strong evidence to suggest that some cannabinoids can act on PPARs through either direct or indirect pathways. In order to directly act on nuclear transcriptional factors PPARs, exogenous cannabinoids need to pass through plasma membrane and be transported into nucleus which may involve certain membrane and intracellular transporters. However, we still cannot rule out that cannabinoids effects could be indirect through the binding of other cellular targets which in turn induces PPARs activation [75]. In fact, WIN attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-γ pathway [44].
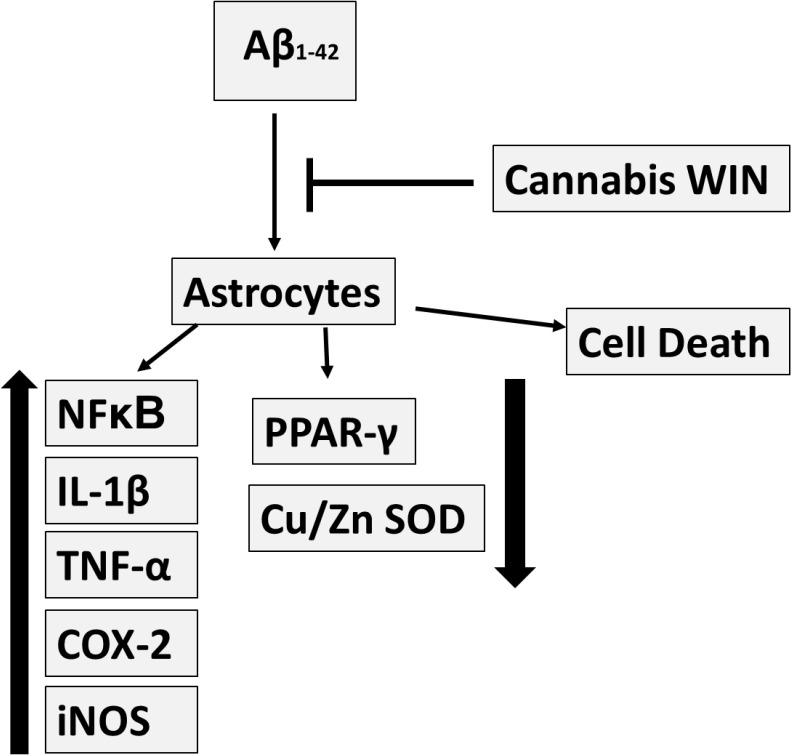
Different authors have demonstrated the role of oxidative stress in AD [76–79]. The cumulative damage caused by free radicals induces alterations in the activity or expression of antioxidant enzymes like catalase or SOD. These enzymes were found to be decreased in both CNS and peripheral tissues of AD patients [80,81]. In this sense, we demonstrate that Cu/Zn SOD is decreased in astrocytes treated with Aβ1–42. Our results are consistent with those reported by other authors, highlighting the role of oxidative stress in the development of AD [82]. New substances are under research to reduce damage caused by oxidative stress in this disease. Widely distributed in the body, cannabinoids receptors were discovered few decades ago and are still under research [57]. Few studies address the effect of cannabinoids on oxidative stress. For instance, cannabinoids were found to prevent or antagonize oxidative stress toxicity in cortical neurons in cultures [83,84], and in lymphoblastic cells [85]. Studies with PC12 cells exposed to Aβ1–42 peptide demonstrated that cannabinoids reduced reactive oxygen species production and membrane lipid oxidation [86,32]. Our results provide evidence that Aβ1–42 decreases Cu/Zn SOD expression in astrocytes in primary culture, and pretreatment with WIN increases Cu/Zn SOD expression, preventing the decrease caused by Aβ1–42. These findings indicate that cannabinoids could act as a protective agent against oxidative stress caused by Aβ1–42. In Fig 7 the set of results are summarized. However our results indicate that Aβ1–42 elevated Mn SOD protein expression, increasing mitochondrial biogenesis mechanism, such as we previously published [74]. Pretreatment with WIN did not prevent Mn SOD overexpression induced by Aβ1–42. Mn SOD plays a role in the adaptive response which protects brain cells from damage, as in the case of AD. In fact, Mn SOD preserves neurons against oxidative stress [87] and protects developing neurons from β-amyloid toxicity [88]. This enzyme catalyzes the conversion of superoxide radicals to molecular oxygen and H2O2, whereas glutathione peroxidase, peroxiredoxin reductase and catalase neutralize H2O2. Overexpression of Mn SOD induces cognitive recovery and reduces Aβ levels in AD animal models [89]. Furthermore, Mn SOD deficiency increases β-amyloid levels and amyloid plaque burden, promoting the development of behavioural disturbances [90].
Fig 7. Preventive function of cannabinoid WIN on Aβ1-42-induced toxic effects in astrocytes in primary culture.
Cannabinoid WIN 55,212–2 increases cell viability and anti-inflammatory response in cultured astrocytes and prevents inflammatory effects induced by Aβ1–42.
Preclinical data suggest a beneficial role of some cannabinoids for treatment of different diseases. Dronabidol, an oil-based solution of Δ9-THC, is used as anti-emetic and appetite stimulant [91]. Δ9-THC also decreases agitation present in the advanced stage of AD [92]. In 2003, the FDA granted the patent for cannabinoids as antioxidants and neuro-protectants (U.S. Department of Health and Human Services). Despite these promising preliminary results, the clinical utility of cannabinoids in AD is still to be determined [93].
Conclusions
Taken together, our findings show that cannabinoid WIN increases cell viability and anti-inflammatory response in astrocytes in primary culture and prevents cell death induced by Aβ1–42. Furthermore, WIN increases expression of anti-oxidant Cu/Zn SOD and is able to prevent inflammation induced by Aβ1–42 in astrocytes. In this sense, clinical studies are needed to evaluate the neuro-protective effects of cannabinoids in Alzheimer´s disease.
Data Availability
All relevant data are within the paper.
Funding Statement
Generalitat Valenciana AP-073/09. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Selkoe DJ. Alzheimer's disease: genotypes, phenotypes, and treatments. Science. 1977;275: 630–631. [DOI] [PubMed] [Google Scholar]
- 2. Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Alzheimer's Disease International. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366: 2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heneka MT, O'Banion MK. Inflammatory processes in Alzheimer's disease. J Neuroimmunol. 2007;184: 69–91. [DOI] [PubMed] [Google Scholar]
- 4. Pratico D. Evidence of oxidative stress in Alzheimer's disease brain and antioxidant therapy: lights and shadows. Ann N Y Acad Sci. 2008;1147: 70–78. 10.1196/annals.1427.010 [DOI] [PubMed] [Google Scholar]
- 5. Inestrosa NC, Reyes AE, Chacón MA, Cerpa W, Villalón A, Montiel J, et al. Human-like rodent amyloid-beta-peptide determines Alzheimer pathology in aged wild-type Octodon degu. Neurobiol Aging. 2005;26: 1023–1028. [DOI] [PubMed] [Google Scholar]
- 6. Vallés SL, Borrás C, Gambini J, Furriol J, Ortega A, Sastre J, et al. Oestradiol or genistein rescues neurons from amyloid beta-induced cell death by inhibiting activation of p38. Aging Cell. 2008;7: 112–118. [DOI] [PubMed] [Google Scholar]
- 7. Sofroniew MV and Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119: 7–35. 10.1007/s00401-009-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi SS, Lee HJ, Lim I, Satoh J, Kim SU. Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One. 2014;1: 9(4):e92325 10.1371/journal.pone.0092325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Kralingen C, Kho DT, Costa J, Angel CE, Graham ES. Exposure to inflammatory cytokines IL-1β and TNFα induces compromise and death of astrocytes; implications for chronic neuroinflammation. PLoS One. 2013;19;8(12): e84269 10.1371/journal.pone.0084269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang W, Smith C, Howlett C, Stanimirovic D. Inflammatory activation of human brain endothelial cells by hypoxic astrocytes in vitro is mediated by IL-1beta. J Cereb Blood Flow Metab. 2000;20: 967–978. [DOI] [PubMed] [Google Scholar]
- 11. Li C, Zhao R, Gao K, Wei Z, Yin MY, Lau LT et al. Astrocytes: implications for neuroinflammatory pathogenesis of Alzheimer's disease. Curr Alzheimer Res. 2011; 8: 67–80. [DOI] [PubMed] [Google Scholar]
- 12. Valles SL, Dolz-Gaiton P, Gambini J, Borras C, Lloret A, Pallardo FV. Estradiol or genistein prevent Alzheimer's disease-associated inflammation correlating with an increase PPAR gamma expression in cultured astrocytes. Brain Res. 2010;1312: 138–144. 10.1016/j.brainres.2009.11.044 [DOI] [PubMed] [Google Scholar]
- 13. Pacher P, Kunos G. Modulating the endocannabinoid system in human health and disease-successes and failures. FEBS J. 2013;280: 1918–1943. 10.1111/febs.12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kunos G and Tam J. The case for peripheral CB₁ receptor blockade in the treatment of visceral obesity and its cardiometabolic complications. Br J Pharmacol. 2011;163: 1423–1431. 10.1111/j.1476-5381.2011.01352.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centonze D, Finazzi-Agrò A, Bernardi G, Maccarrone M. The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends Pharmacol Sci. 2007;28: 180–187. [DOI] [PubMed] [Google Scholar]
- 16. Klein WK. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5: 400–411. [DOI] [PubMed] [Google Scholar]
- 17. Hillard CJ, Weinlander KM, Stuhr KL. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience. 2012;1: 207–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guindon J, Hohmann AG. The endocannabinoid system and cancer: therapeutic implication. Br J Pharmacol. 2011;163: 1447–1463. 10.1111/j.1476-5381.2011.01327.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramírez BG, Blázquez C, Gómez del Pulgar T, Guzmán M, de Ceballos ML. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25: 1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Esposito G, Scuderi C, Savani C, Steardo L Jr, De Filippis D, Cottone P, et al. Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br J Pharmacol. 2007;151: 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blanchard BJ, Chen A, Rozeboom LM, Stafford KA, Weigele P, Ingram VM. Efficient reversal of Alzheimer's disease fibril formation and elimination of neurotoxicity by a small molecule. Proc Natl Acad Sci. 2004;101: 14326–14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 2013;126: 479–497. 10.1007/s00401-013-1177-7 [DOI] [PubMed] [Google Scholar]
- 23. Rönicke R, Klemm A, Meinhardt J, Schröder UH, Fändrich M, Reymann KG. Abeta mediated diminution of MTT reduction—an artefact of single cell culture?. PLoS One. 2008;3: e3236 10.1371/journal.pone.0003236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palanca JM, Aguirre-Rueda D, Granell MV, Aldasoro M, Garcia A, Iradi A. Sugammadex, a neuromuscular blockade reversal agent, causes neuronal apoptosis in primary cultures. Int J Med Sci. 2013;10: 1278–1285. 10.7150/ijms.6254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83: 346–356. [DOI] [PubMed] [Google Scholar]
- 26. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391: 79–82. [DOI] [PubMed] [Google Scholar]
- 27. Eikelenboom P, van Gool WA. Neuroinflammatory perspectives on the two faces of Alzheimer's disease. J Neural Transm. 2004;111: 281–294. [DOI] [PubMed] [Google Scholar]
- 28. Zhu X, Smith MA, Perry G, Aliev G. Mitochondrial failures in Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2004;19: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiménez-Jiménez FJ, Alonso-Navarro H, Ayuso-Peralta L, Jabbour-Wadih T. Oxidative stress and Alzheimer's disease. Rev Neurol. 2006;42: 419–427. [PubMed] [Google Scholar]
- 30. Quintanilla RA, Orellana JA, von Bernhardi R. Understanding risk factors for Alzheimer's disease: interplay of neuroinflammation, connexin-based communication and oxidative stress. Arch Med Res. 2012;43: 632–644. 10.1016/j.arcmed.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 31. Milton NG. Anandamide and noladin ether prevent neurotoxicity of the human amyloid-beta peptide. Neurosci Lett. 2002;332: 127–130. [DOI] [PubMed] [Google Scholar]
- 32. Iuvone T, Esposito G, Esposito R, Santamaria R, Di Rosa M, Izzo AA. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J Neurochem. 2004;89: 134–141. [DOI] [PubMed] [Google Scholar]
- 33. Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;1: 530–534. [DOI] [PubMed] [Google Scholar]
- 34. Gómez Del Pulgar T, De Ceballos ML, Guzmán M, Velasco G. Cannabinoids protect astrocytes from ceramide-induced apoptosis through the phosphatidylinositol 3-kinase/protein kinase B pathway. J. Biol. Chem. 2002;277: 36527–36533. [DOI] [PubMed] [Google Scholar]
- 35. Pourkhalili N, Ghahremani MH, Farsandaj N, Tavajohi S, Majdzadeh M, Parsa M, et al. Evaluation of anti-invasion effect of cannabinoids on human hepatocarcinoma cells. Toxicol Mech Methods. 2013;23: 120–126. 10.3109/15376516.2012.730559 [DOI] [PubMed] [Google Scholar]
- 36. De Petrocellis L, Ligresti A, Schiano Moriello A, Iappelli M, Verde R, Stott CG, et al. Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: pro-apoptotic effects and underlying mechanisms. Br J Pharmacol. 2013;168: 79–102. 10.1111/j.1476-5381.2012.02027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vara D, Salazar M, Olea-Herrero N, Guzmán M, Velasco G, Díaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011;18: 1099–1111. 10.1038/cdd.2011.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rocha FC, Dos Santos JG Júnior, Stefano SC, da Silveira DX. Systematic review of the literature on clinical and experimental trials on the antitumor effects of cannabinoids in gliomas. J Neurooncol. 2014:116: 11–24. 10.1007/s11060-013-1277-1 [DOI] [PubMed] [Google Scholar]
- 39. Sánchez C, Galve-Roperh I, Canova C, Brachet P, Guzmán M. Delta9-tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett. 1998;436: 6–10. [DOI] [PubMed] [Google Scholar]
- 40. Guzmán M, Sánchez C, Galve-Roperh I. Cannabinoids and cell fate. Pharmacol Ther. 2002;95: 175–184. [DOI] [PubMed] [Google Scholar]
- 41. Stella N. Cannabinoid signaling in glial cells. Glia. 2004;48: 267–277. [DOI] [PubMed] [Google Scholar]
- 42. Sheng WS, Hu S, min X, Cabral GA, Lokensgard JR, Peterson P. Synthetic cannabinoid WIN55,212–2 inhibits generation of inflammatory mediators by IL-1beta.stimulated human astrocytes. Glia. 2005;49: 211–219. [DOI] [PubMed] [Google Scholar]
- 43. Palmer SL, Thakur GA, Makriyannis A. Cannabinergic ligands. Chem Phys Lipids. 2002;121: 3–19. [DOI] [PubMed] [Google Scholar]
- 44. Fakhfouri G, Ahmadiani A, Rahimian R, Grolla AA, Moradi F, Haeri A. WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-γ pathway. Neuropharmacology. 2012;63: 653–666 10.1016/j.neuropharm.2012.05.013 [DOI] [PubMed] [Google Scholar]
- 45. Strohmeyer R, Rogers J. Molecular and cellular mediators of Alzheimer's disease inflammation. J Alzheimers Dis. 2001;3: 131–157. [DOI] [PubMed] [Google Scholar]
- 46. Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease-A doubleedged sword. Neuron. 2002;35: 419–432. [DOI] [PubMed] [Google Scholar]
- 47. Griffin WS, Mrak RE. Interleukin-1 in the genesis and progression of and risk for development of neuronal degeneration in Alzheimer's disease. J Leukoc Biol. 2002;72: 233–238. [PMC free article] [PubMed] [Google Scholar]
- 48. McGeer PL, Rogers J, McGeer EG. Inflammation, anti-inflammatory agents and Alzheimer disease: the last 12 years. J Alzheimers Dis. 2006;9: 271–276. [DOI] [PubMed] [Google Scholar]
- 49. Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2: 734–744. [DOI] [PubMed] [Google Scholar]
- 50. Allan SM, Rothwell NJ. Inflammation in central nervous system injury. Philos Trans R Soc Lond B Biol Sci. 2003;358: 1669–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Burkert K, Moodley K, Angel CE, Brooks A, Graham ES. Detailed analysis of inflammatory and neuromodulatory cytokine secretion from human NT2 astrocytes using multiplex bead array. Neurochem Int. 2012;60: 573–580. 10.1016/j.neuint.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 52. Carrero I, Gonzalo MR, Martin B, Sanz-Anquela JM, Arévalo-Serrano J, Gonzalo-Ruiz A. Oligomers of β-amyloid protein (Aβ1–42) induce the activation of cyclooxygenase-2 in astrocytes via an interaction with interleukin-1β, tumour necrosis factor-α, and a nuclear factor κ-B mechanism in the rat brain. Exp Neurol. 2012; 236: 215–227. 10.1016/j.expneurol.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 53. Vallés S, Tsoi C, Huang WY, Wyllie D, Carlotti F, Askari JA, et al. Recruitment of a heparan sulfate subunit to the interleukin-1 receptor complex. Regulation by fibronectin attachment. J Biol Chem. 1999;274: 20103–20109. [DOI] [PubMed] [Google Scholar]
- 54. Bondareff W. Age-related changes in brain extracellular space affect processing of amyloid-β peptides in Alzheimer's disease. J Alzheimers Dis. 2013;35: 1–6. 10.3233/JAD-122305 [DOI] [PubMed] [Google Scholar]
- 55. Sheng WS, Hu S, Min X, Cabral GA, Lokensgard JR, Peterson PK. Synthetic cannabinoid WIN55,212–2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia. 2005;49: 211–219. [DOI] [PubMed] [Google Scholar]
- 56. Esposito G, Scuderi C, Valenza M, Togna GI, Latina V, De Filippis D, et al. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One. 2011; 6:e28668 10.1371/journal.pone.0028668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scuderi C, Esposito G, Blasio A, Valenza M, Arietti P, Steardo L Jr, et al. Palmitoylethanolamide counteracts reactive astrogliosis induced by β-amyloid peptide. J Cell Mol Med. 2011;15: 2664–2674. 10.1111/j.1582-4934.2011.01267.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Puffenbarger RA, Boothe AC, Cabral GA. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia. 2000;29: 58–69. [PubMed] [Google Scholar]
- 59. Facchinetti F, Del Giudice E, Furegato S, Passarotto M, Leon A. Cannabinoids ablate release of TNFalpha in rat microglial cells stimulated with lypopolysaccharide. Glia. 2003;41: 161–168. [DOI] [PubMed] [Google Scholar]
- 60. Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, et al. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation. 2005;12: 2–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Castillo A, Tolón MR, Fernández-Ruiz J, Romero J, Martinez-Orgado J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB (2) and adenosine receptors. Neurobiol Dis. 2010;37: 434–440. 10.1016/j.nbd.2009.10.023 [DOI] [PubMed] [Google Scholar]
- 62. Martín-Moreno AM, Reigada D, Ramírez BG, Mechoulam R, Innamorato N, Cuadrado A, et al. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer’s disease. Mol Pharmacol. 2011;79: 964–973. 10.1124/mol.111.071290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Martín-Moreno AM, Brera B, Spuch C, Carro E, García-García L, Delgado M, et al. Prolonged oral cannabinoid administration prevents neuroinflammation, lowers β-amyloid levels and improves cognitive performance in Tg APP 2576 mice. J Neuroinflammation. 2012; Jan 16 9:8 10.1186/1742-2094-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Esposito G, De Filippis D, Carnuccio R, Izzo AA, Iuvone T. The marijuana component cannabidiol inhibits β-amyloid-induced tau protein hyperphosphorylation through Wnt/β-catenin pathway rescue PC12 cells. J Mol Med. 2006;84: 253–258. [DOI] [PubMed] [Google Scholar]
- 65. Kainu T, Wikström AC, Gustafsson JA, Pelto-Huikko M. Localization of the peroxisome proliferator-activated receptor in the brain. Neuroreport. 1994;5: 2481–2485. [DOI] [PubMed] [Google Scholar]
- 66. Luna-Medina R, Cortes-Canteli M, Alonso M, Santos A, Martínez A, Perez-Castillo A. Regulation of inflammatory response in neural cells in vitro by thiadiazolidinones derivatives through peroxisome proliferator-activated receptor gamma activation. J Biol Chem. 2005;280: 21453–21462. [DOI] [PubMed] [Google Scholar]
- 67.Bright JJ, Kanakasabai S, Chearwae W, Chakraborty S. PPAR Regulation of Inflammatory Signaling in CNS Diseases. PPAR Res. 2008;658520. 10.1155/2008/658520 [DOI] [PMC free article] [PubMed]
- 68. Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-γ agonists. Front Biosci. 2008;13: 1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Landreth G, Jiang Q, Mandrekar S, Heneka M. PPARgamma agonists as therapeutics for the treatment of Alzheimer's disease. Neurotherapeutics. 2008;5: 481–489. 10.1016/j.nurt.2008.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bodles AM, Barger SW. Secreted β-amyloid precursor protein activates microglia via JNK and p38-MAPK. Neurobiol Aging. 2005;26: 9–16. [DOI] [PubMed] [Google Scholar]
- 71. Ho GJ, Drego R, Hakimian E, Masliah E. Mechanisms of cell signaling and inflammation in Alzheimer's disease. Curr Drug Targets Inflamm Allergy. 2005;4: 247–256. [DOI] [PubMed] [Google Scholar]
- 72. Wang HM, Zhao YX, Zhang S, Liu GD, Kang WY, Tang HD, et al. PPARgamma agonist curcumin reduces the amyloid-beta-stimulated inflammatory responses in primary astrocytes. J Alzheimers Dis. 2010;20: 1189–1199. 10.3233/JAD-2010-091336 [DOI] [PubMed] [Google Scholar]
- 73. Chen YC, Wu JS, Tsai HD, Huang CY, Chen JJ, Sun GY, et al. Peroxisome proliferator-activated receptor gamma (PPAR-γ) and neurodegenerative disorders. Mol Neurobiol. 2012;46: 114–124. 10.1007/s12035-012-8259-8 [DOI] [PubMed] [Google Scholar]
- 74. Aguirre-Rueda D, Guerra-Ojeda S, Aldasoro M, Iradi A, Obrador E, Ortega A, et al. Astrocytes protect neurons from Aβ1–42 peptide-induced neurotoxicity increasing TFAM and PGC-1 and decreasing PPAR-γ and SIRT-1. Int J Med Sci. 2015;12: 48–56. 10.7150/ijms.10035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun Y, Bennett A Cannabinoids: a new group of agonists of PPARs. PPAR Res. 2007;23513. 10.1155/2007/23513 [DOI] [PMC free article] [PubMed]
- 76. Wallace DC. Mitochondrial defects in cardiomyopathy and neuromuscular disease. Am Heart J. 2000;139: S70–S85. [DOI] [PubMed] [Google Scholar]
- 77. Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia—reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33: 1065–1089. [DOI] [PubMed] [Google Scholar]
- 78. Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem. 2003;278: 36027–36031. [DOI] [PubMed] [Google Scholar]
- 79. Abeti R, Duchen MR. Activation of PARP by Oxidative Stress Induced by β-Amyloid: Implications for Alzheimer’s Disease. Neurochem Res. 2012;37: 2589–2596. 10.1007/s11064-012-0895-x [DOI] [PubMed] [Google Scholar]
- 80. Marcus DL, Thomas C, Rodriguez C, Simberkoff K, Tsai JS, Strafaci JA, et al. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer's disease. Exp Neurol. 1998;150: 40–44. [DOI] [PubMed] [Google Scholar]
- 81. Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neuroscience Letters. 2010;469: 6–10. 10.1016/j.neulet.2009.11.033 [DOI] [PubMed] [Google Scholar]
- 82. Pappolla MA, Chyan YJ, Omar RA, Hsiao K, Perry G, Smith MA, et al. Evidence of oxidative stress and in vivo neurotoxicity of beta-amyloid in a transgenic mouse model of Alzheimer's disease: a chronic oxidative paradigm for testing antioxidant therapies in vivo. Am J Pathol. 1998;152: 871–877. [PMC free article] [PubMed] [Google Scholar]
- 83. Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (-) Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci. 1998;95: 8268–8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hampson AJ, Grimaldi M. Cannabinoid receptor activation and elevated cyclic AMP reduce glutamate neurotoxicity. Eur J Neurosci. 2001;13: 1529–1536. [DOI] [PubMed] [Google Scholar]
- 85. Chen Y, Buck J. Cannabinoids protect cells from oxidative cell death: a receptor-independent mechanism. J. Pharmacol. Exp. Ther. 2000;293: 807–812. [PubMed] [Google Scholar]
- 86. Praticò D MY Lee V, Trojanowski JQ, Rokach J, Fitzgerald GA. Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998;12: 1777–1783. [DOI] [PubMed] [Google Scholar]
- 87. Keller JN, Kindy MS, Holtsberg FW, St Clair DK, Yen HC, Germeyer A, et al. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18: 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sompol P, Ittarat W, Tangpong J, Chen Y, Doubinskaia I, Batinic-Haberle I, et al. A neuronal model of Alzheimer's disease: an insight into the mechanisms of oxidative stress-mediated mitochondrial injury. Neuroscience. 2008;153: 120–130. 10.1016/j.neuroscience.2008.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dumont M, Wille E, Stack C, Calingasan NY, Beal MF, Lin MT. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer's disease. FASEB J. 2009;23: 2459–2466. 10.1096/fj.09-132928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Esposito L, Raber J, Kekonius L, Yan F, Yu GQ, Bien-Ly N, et al. Reduction in mitochondrial superoxide dismutase modulates Alzheimer's disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci. 2006;26: 5167–5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Volicer L, Stelly M, Morris J, McLaughlin J, Volicer BJ. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer's disease. Int J Geriatr Psychiatry. 1997;12: 913–919. [PubMed] [Google Scholar]
- 92. Walther S, Mahlberg R, Eichmann U, Kunz D. 9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology. 2006;185: 524–528. [DOI] [PubMed] [Google Scholar]
- 93. Krishnan S, Cairns R, Howard R. (2009) Cannabinoids for the treatment of dementia. Cochrane Database Syst Rev. 15;(2): CD007204 10.1002/14651858 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.