Abstract
Virus-induced gene silencing (VIGS) offers a powerful approach for functional analysis of individual genes by knocking down their expression. We have adopted this approach to dissect gene functions in cotton resistant to Verticillium wilt, one of the most devastating diseases worldwide. We showed here that highly efficient VIGS was obtained in a cotton breeding line (CA4002) with partial resistance to Verticillium wilt, and GhMKK2 and GhVe1 are required for its resistance to Verticillium wilt. Arabidopsis AtBAK1/SERK3, a central regulator in plant disease resistance, belongs to a subfamily of somatic embryogenesis receptor kinases (SERKs) with five members, AtSERK1 to AtSERK5. Two BAK1 orthologs and one SERK1 ortholog were identified in the cotton genome. Importantly, GhBAK1 is required for CA4002 resistance to Verticillium wilt. Surprisingly, silencing of GhBAK1 is sufficient to trigger cell death accompanied with production of reactive oxygen species in cotton. This result is distinct from Arabidopsis in which AtBAK1 and AtSERK4 play redundant functions in cell death control. Apparently, cotton has only evolved SERK1 and BAK1 whereas AtSERK4/5 are newly evolved genes in Arabidopsis. Our studies indicate the functional importance of BAK1 in Verticillium wilt resistance and suggest the dynamic evolution of SERK family members in different plant species.
Keywords: Cell death, Gossypium hirsutum, Verticillium dahliae, virus-induced gene silence
Cotton (Gossypium spp.) is one of the most important crops around the world because of the significant economic value of its textile fiber, feed, foodstuff, oil, and biofuel products. There are 50 species with 45 diploid and five allotetraploid species in the Gossypium genus, among which Gossypium hirsutum, a tetraploid species, produces more than 95% of the annual cotton yield worldwide (Chen et al. 2007). Diploid Gossypium species can be classified into eight subgenome types designated as A to G and K. The D genome of G. raimondii represents the smallest genome size among Gossypium species (880 Mb for the haploid), and possesses high levels of synteny or collinearity with other Gossypium genomes (Wendel 2000). The recent release of the draft G. raimondii D genome sequence provides a reference for the assembly of the G. hirsutum genome and a foundation for the functional genomic analysis of cotton genes in the post-genomic era (Wang et al. 2012).
With the availability of the cotton genome sequence, largescale molecular and genetic approaches are needed to understand cotton gene functions at the genome-wide level. We have developed an Agrobacterium-mediated virus-induced gene silencing (VIGS) assay in cotton to study individual gene functions by knocking down the expression of endogenous genes (Gao et al. 2011a, 2011b). VIGS is a type of RNA-mediated post-transcriptional gene silencing and functions as an antivirus defense mechanism in plants (Hamilton and Baulcombe 1999). Through Agrobacterium infiltration, the T-DNA containing the partial viral genome and gene of interest is delivered into host cells. The production of double-stranded RNAs between the endogenous gene and DNA fragment from the T-DNA vector results in the silencing of endogenous genes both locally and systemically throughout the plant tissues (Burch-Smith et al. 2004; Becker and Lange 2010). This rapid and efficient loss-of-function approach bypasses plant stable transformation and overcomes functional redundancy (Burch-Smith et al. 2004; Becker and Lange 2010). The Agrobacterium carrying the gene of interest is inoculated in cotton cotyledons at the 2-w-old seedling stage, and the silencing will be observed within 2 weeks after inoculation (Gao and Shan 2013). We further used the VIGS approach to study the genetic and molecular mechanisms of cotton resistance to Verticillium wilt, one of the most devastating cotton diseases worldwide (Gao et al. 2011b). Cotton Verticillium wilt is caused by the soil-borne pathogen Verticillium dahliae. This pathogen is particularly difficult to control by fungicides because the fungi reside in the woody vascular tissues and can be transmitted systemically in cotton plants (Fradin and Thomma 2006). By using a VIGS approach, we have shown that silencing cotton GhNDR1 (Nonrace-specific Disease Resistance 1), GhMKK2 (MAPK kinase 2), or GhVe1 compromised its resistance to V. dahliae infection (Gao et al. 2011b). Recently, the tobacco rattle virus (TRV)-based VIGS assay has been extended to study cotton gene function in cotton fiber development (Qu et al. 2012). Thus, the TRV-VIGS system provides a powerful tool for rapid functional analysis of cotton genes involved in biotic and abiotic stresses and the development of seedlings to reproductive organs.
Being sessile, plants have evolved a large number of membrane-resident receptor-like kinases (RLKs) to cope with potential microbial invasions and maintain active growth and development (Shiu and Bleecker 2001, 2003; Shan et al. 2007; Boller and Felix 2009). Arabidopsis BAK1, a leucine-rich-repeat RLK (LRR-RLK) originally identified as a BRI1-associated receptor kinase mediating the signaling of plant growth hormone brassinosteroid (BR) signaling (Li et al. 2002; Nam and Li 2002), has emerged as an important player in plant disease resistance by association with bacterial flagellin sensor FLS2 and other immune sensors (Chinchilla et al. 2007; Heese et al. 2007; Schulze et al. 2010; Roux et al. 2011). Arabidopsis BAK1 is also known as somatic embryogenesis receptor kinase 3 (SERK3), belonging to a subfamily of RLKs with five members, SERK1 to SERK5 (Chinchilla et al. 2009). SERKs were named because overexpression of SERK1 enhanced the somatic embryogenesis ability of suspension cells (Schmidt et al. 1997; Hecht et al. 2001). Further phenotypic analyses of serk1serk2 double mutants suggest that SERK1 and SERK2 play crucial and redundant functions in anther development and male gametophyte maturation (Colcombet et al. 2005). In addition, SERK1, BAK1/SERK3, and SERK4 (also known as BKK1) play a redundant role in BR signaling by association with BRI1 (Karlova et al. 2006; He et al. 2007; Gou et al. 2012). Remarkably, BAK1 and SERK4 also exhibit redundant functions in plant disease resistance via association with multiple immune sensors (Roux et al. 2011). In addition to their roles in BR signaling and plant disease resistance, BAK1 and SERK4 negatively regulate plant cell death because the bak1serk4 double mutant exhibits seedling lethality accompanied with constitutive defense gene activation and spontaneous cell death (He et al. 2007). How BAK1 and SERK4 are involved in cell death control is still not clear.
In this study, we first established the Agrobacterium-mediated VIGS assay in a newly developed and released cotton germplasm line CA4002 that is partially resistant to Verticillium wilt (Dever et al. 2013). Consistent with our previous report that VIGS is not variety-specific, the VIGS efficiency reaches 100% in this line, and GhMKK2 and GhVe1 are required for its resistance to Verticillium wilt. An examination of the G. raimondii D genome and G. hirsutum unigene database identified two BAK1 orthologs and one SERK1 ortholog in cotton. Interestingly, silencing of GhBAK1 by VIGS caused the compromised resistance to Verticillium wilt, pronounced cell death, and the production of reactive oxygen species (ROS) in cotton. Our results suggest that cotton has only evolved SERK1 and BAK1, and lacks SERK4/5 orthologs, whereas AtSERK4 and AtSERK5 are newly evolved genes in Arabidopsis, which play redundant roles with AtBAK1 in plant disease resistance and cell death control.
Results
Involvement of GhMKK2 and GhVe1 in cotton CA4002 resistance to Verticillium wilt
We have shown that VIGS of cotton Cla1 (GhCla1), a gene involved in chloroplast development, results in an albino phenotype on the newly emerging leaves (Gao et al. 2011b). This phenotype is readily observed. Thus, we employed silencing of GhCla1 as a visual marker to monitor the VIGS efficiency in cotton germplasm CA4002. The newly emerging true leaves from the infiltrated plants with Agrobacterium carrying GhCla1 exhibited an albino phenotype (Figure 1). Consistent with our previous report, the albino phenotype was observed on the leaves of the GhCla1-silenced plants approximately 10 d post-infiltration, and this phenotype was further developed and uniformly distributed over the entire leaves along with plant growth (Figure 1). Thus, we have established an efficient Agrobacterium-mediated VIGS assay in the cotton germplasm line CA4002 for transient silencing of interested genes. This result also substantiated our previous finding that VIGS is independent of cotton cultivars.
Figure 1. Establishment of Agrobacterium-mediated virus-induced gene silencing (VIGS) assay in the cotton CA4002 line.

The cotyledons of 10-d-old CA4002 seedlings were hand-infiltrated with Agrobacterium carrying either pYL156-GhCla1 or VIGS-vector control (pYL156-GFP). The albino phenotypes were observed from the newly emerging true leaves and pictures were taken at approximately 2 w after infiltration.
We have recently shown that silencing of GhMKK2, GhNDR1, or GhVe1 in a commercial cotton cultivar (Fibermax 9160B2F) compromised its resistance to V. dahliae infection (Gao et al. 2011b). The newly released breeding line CA4002 confers partial resistance to Verticillium wilt (Dever et al. 2013). Yet, the molecular basis of this resistance remains unknown. Here, we tested whether a similar genetic requirement exists in CA4002 resistance to Verticillium wilt and examined the functional importance of GhVe1 and GhMKK2 genes for resistance to the V. dahliae King isolate, a “defoliating” type and aggressive cotton pathogen isolated in TX.
The Agrobacterium cultures carrying the recombinant pTRV vectors with either vector control, GhMKK2 or GhVe1, were hand-infiltrated into the cotyledons of cotton CA4002 seedlings, and then V. dahliae was inoculated into the stems of cotton seedlings. The progressive wilting of the true leaves was scored. Consistent with an earlier report, CA4002 exhibited lower incidence of Verticillium wilt and less defoliation compared to most other cultivars and breeding lines (Dever et al. 2013). Silencing of GhVe1 or GhMKK2 enhanced plant susceptibility to V. dahliae infection, and plants exhibited a more severe wilting phenotype than the vector control-inoculated plants (Figure 2A, B). In particular, at a later infection stage, almost 100% of GhMKK2 or GhVe1-VIGSed plants were severely infected by V. dahliae and most of the leaves showed the wilting phenotype compared to approximately 60% of control vector-inoculated plants (Figure 2C). Together, our loss-of-function assays indicated that GhMKK2 and GhVe1 partially contribute to cotton CA4002 resistance to V. dahliae infection.
Figure 2. GhMKK2 and GhVe1 are required for resistance to Verticillium dahliae in the cotton CA4002 line.
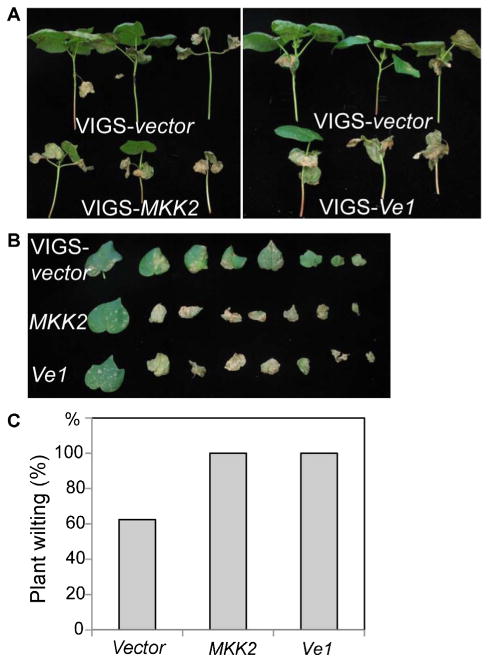
Ten-d-old CA4002 seedlings were hand-infiltrated with Agrobacterium carrying individual genes in the virus-induced gene silencing (VIGS) vector. When GhCla1-silenced plants showed albino phenotype (∼2 w after infiltration), the seedlings that silenced MKK2 or Ve1 were stem-inoculated with V. dahliae (isolate King) suspension spores at a concentration of 1 × 106/mL
(A) Whole plants and (B) detached leaves are shown at 34 d after V. dahliae infection.
(C) Percentage of plants showing Verticillium wilt phenotype at 34 d after infection. The disease ratio was scored with at least 15 plants per treatment and the assays were repeated for three times with similar results.
Cotton SERK gene family
By using a VIGS approach, tomato BAK1 was shown to be required for Verticillium wilt resistance in tomato (Fradin et al. 2009). It remains unknown how many SERK family members exist in cotton and what their biological functions are. Here, we systematically characterized the SERK gene subfamily in cotton. We used the full-length AtBAK1 amino acid sequence as a query and Blasted against the G. raimondii D genome database at http://cgp.genomics.org.cn/page/species/blast.jsp. Three genes (10025422 in chromosome (Chr.) 12; 10029586 in Chr. 5; 10039700 in Chr. 10) with an e value of 0.0 were retrieved. These three genes encode proteins with more than 70% identity to AtBAK1 (Figure 3A). The gene 10025423, next to 10025422 in Chr. 12, encodes a protein with 68% identity with AtBAK1. The 10025423 and 10025422 may be a result of local gene duplication. However, the protein encoded by 10025423 lacks the intact transmembrane domain and juxtamembrane domain (Figure S1). Thus, this gene is unlikely to be functional. The gene next to these three genes (10005636) encodes a protein with only 49% identity to AtBAK1 full length and 39% identity to the AtBAK1 LRR domain (Figure S2), suggesting that 10005636 does not belong to the SERK subfamily anymore. When any of the AtSERK1 to AtSERK5 SERKs were used as a query for Blast search, similar sequences with three genes (10025422, 10029586, and 10039700) were retrieved as top hits compared to those using AtBAK1 as a query. Sequence analysis indicates that 10025422 and 10029586 share 90% identity and belong to the same clade as AtBAK1 based on the phylogenetic tree (Figure 3). Thus, we named 10025422 as GrBAK1.1 and 10029586 as GrBAK1.2. The gene 10039700 belongs to the same clade as AtSERK1 and AtSERK2, and was named GrSERK1. Similarly, when searching the G. hirsutum unigene database at http://www.cottondb.org/blast/blast.html, we also retrieved three SERK family members, GhSERK1 (ADR00582.1), GhSERK2 (AEA76434.1), and GhBAK1 (AEG25668.1), in G. hirsutum (Figure S2). Sequence and phylogenetic analysis indicates that GhSERK2 (AEA76434.1) is almost identical to GhBAK1, and they all belong to the same clade as AtBAK1 (Figure S2). Therefore, we renamed GhBAK1 (AEG25668.1) as GhBAK1.1 and GhSERK2 (AEA76434.1) as GhBAK1.2, which correspond to GrBAK1.1 and GrBAK1.2 in G. raimondii. Thus, there are two BAK1 orthologs and one SERK1/SERK2 ortholog in the cotton genome, and we did not find a cotton SERK4/SERK5 ortholog.
Figure 3. (A) Sequence alignment and (B) phylogenetic analysis of Arabidopsis SERK (AtSERK) and cotton Gossypium raimondii SERK (GrSERK) family members.
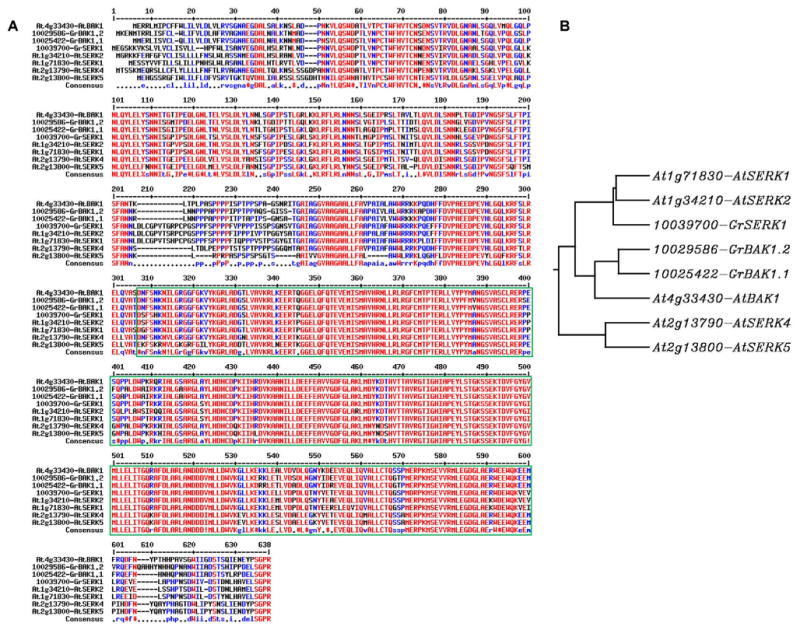
The amino acid sequences were used for the alignment. The sequences squared in green are the kinase domain conserved among AtSERKs and GrSERKs. The alignment was performed using the Multalin website (http://multalin.toulouse.inra.fr/multalin/) with a hierarchical clustering approach, and the phylogenetic tree was constructed using CLUSTALW and rooted phylogenetic tree (http://www.genome.jp/tools/clustalw/).
Conserved function of BAK1 in plant resistance to Verticillium wilt
We further tested whether GhBAK1 was required for cotton resistance to Verticillium wilt. We designed the primers to amplify the most identical region of GhBAK1.1 and GhBAK1.2 into TRV2 vector pYL156, which will likely silence both genes at once (Figure S2 A). Notably, this region shows the limited similarity with GhSERK1 at the nucleotide level. The wilting phenotype in CA4002 plants silenced with GhBAK1 by VIGS was observed and the percentage of wilting plants was scored after V. dahliae infection. Clearly, plants silenced with GhBAK1 showed a more severe wilting phenotype than plants infiltrated with the vector control (Figure 4A). The percentage of wilting plants silenced with GhBAK1 was also higher than those with control infiltration (Figure 4B). Thus, GhBAK1 is an essential component for cotton resistance to V. dahliae infection.
Figure 4. Silencing of GhBAK1 compromises cotton resistance to Verticillium dahliae infection.
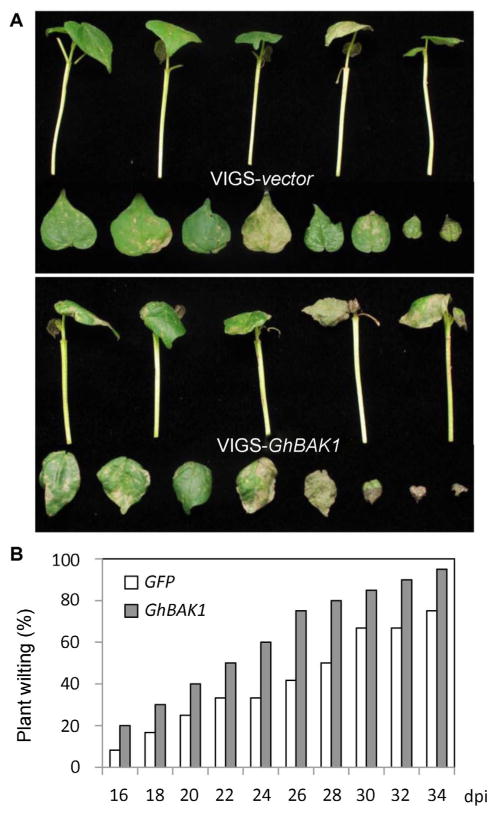
Ten-d-old CA4002 plants were first infiltrated with Agrobacterium carrying pYL156-GhBAK1 or vector control (pYL156-GFP), and then inoculated with V. dahliae (isolate King) suspension spores at a concentration of 1 × 106/mL.
(A) Whole plants (top panel) and detached leaves (bottom) are shown at 14 d after V. dahliae infection.
(B) Percentage of plants showing Verticillium wilt phenotype at the indicated days post-infection. The disease ratio was scored using 21 plants per treatment and the assays were repeated three times with similar results.
The V. dahliae strain (King isolate) that we used to infect cotton is also able to infect Arabidopsis (Figure 5). Using a rootdipping inoculation assay, the newly emerging leaves of infected Arabidopsis plants showed a glassy and water-soaking phenotype 20 d post-inoculation and plant growth became retarded (Figure 5A). The water-soaking phenotype further progresses into the older leaves, which leads to the similar leaf wilting phenotype as observed in cotton at the later time point of infection. Importantly, two bak1 null mutants, bak1-3 and bak1-4, showed a more sensitive and severe wilting phenotype to V. dahliae infection than wild-type (WT) Col-0 plants (Figure 5B). The mutation in SERK2 did not affect the Arabidopsis resistance to V. dahliae, indicating the involvement of specific SERK family members in Verticillium wilt resistance (Figure 5B). BAK1 has been shown to be required for multiple pathogen recognition receptor (PRR)-mediated responses, basal defense, and restriction of pathogen growth in Arabidopsis, tomato, and Nicotiana benthamiana. Cotton possesses highly conserved orthologs of BAK1 genes which are required for Verticillium resistance (Figures 4, 5), implying the potential conserved signaling mechanisms mediating fungal Verticillium resistance.
Figure 5. Arabidopsis bak1 mutants are more sensitive to Verticillium dahliae infection.

(A) Four week old Arabidopsis wild-type (WT) Col-0 plants were inoculated with V. dahliae using the root-dipping method. Photos were taken 20 days post-inoculation. The left panel shows intact plants and the right panel shows detached leave.
(B) The whole plant disease phenotype of WT, bak1-3, bak1-4, and serk2 mutant plants infected with V. dahliae. Photos were taken 20 days post-inoculation.
Function of GhBAK1 in cell death control
Surprisingly, we also observed certain developmental defects in plants silenced with GhBAK1. The GhBAK1 VIGSed plants showed wrinkled true leaves that hardly fully expanded (Figure 6A). Trypan blue staining indicated that silencing of GhBAK1 triggered pronounced cell death in the newly emerging true leaves compared with the vector control inoculated leaves (Figure 6B). 3,3′-Diaminobenzidinetetrachloride (DAB) staining further revealed elevated ROS production as detected by the massive brown precipitates in GhBAK1-silenced leaves (Figure 6C). This result is distinct from Arabidopsis in which AtBAK1 and AtSERK4 play redundant functions in the control of cell death (He et al. 2007). However, this result is consistent with our genomic analysis of the cotton SERK family, in which cotton lacks SERK4 and SERK5 orthologs. Thus, cotton has only evolved orthologs of SERK1 and BAK1 (Figure 3) whereas AtSERK4 and AtSERK5 are newly evolved genes in Arabidopsis.
Figure 6. Silencing of GhBAK1 triggers cell death and reactive oxygen species (ROS) production.
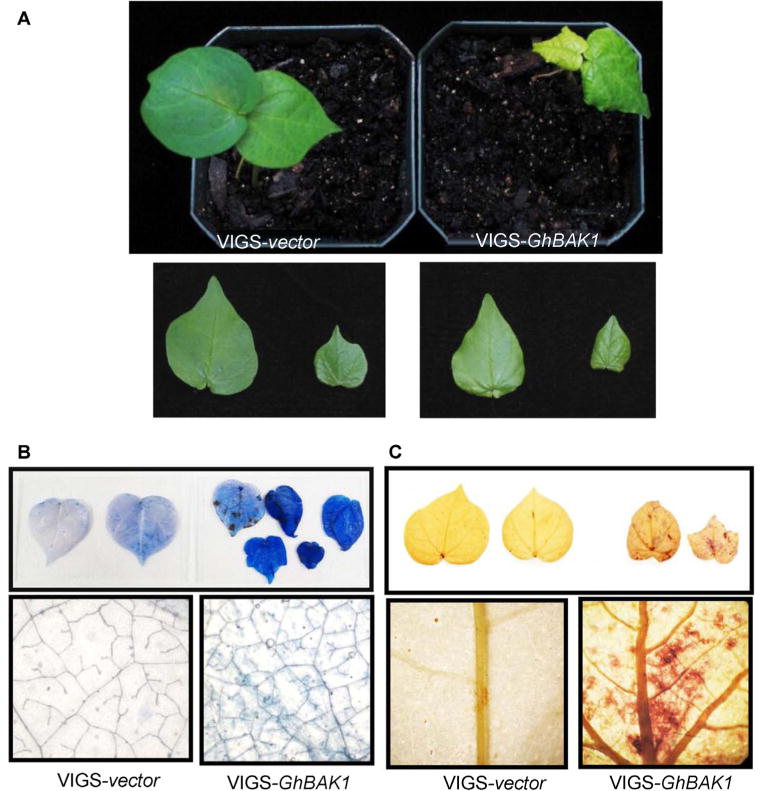
(A) The GhBAK1-silenced plants display abnormal growth phenotype (top panel) and the true leaves do not fully expand (bottom panel). The pictures were taken at 28 d post-virus-induced gene silencing (VIGS) infiltration.
(B) Silencing of GhBAK1 triggers cell death in true leaves as shown by blue-stained cells by Trypan blue staining. The leaves were detached and stained at 28 d post-VIGS infiltration. The bottom panels showed the close-up view of one of the leaves in the corresponding top panels.
(C) Silencing of GhBAK1 induces ROS accumulation as shown by 3,3′-diaminobenzidine-tetrachloride (DAB) staining. The leaves were detached and stained at 28 d post-VIGS infiltration.
Discussion
The development of Agrobacterium-mediated VIGS assay and the availability of the whole cotton genome sequence provide a cornerstone for systematic characterization of cotton gene functions at a genome-wide level and an understanding of the conserved and unique functions of individual cotton genes. An examination of the G. raimondii and G. hirsutum database identified two BAK1 orthologs and one SERK1 ortholog in the cotton genome. Silencing of GhBAK1 compromised the cotton CA4002 line's resistance to Verticillium wilt. Consistent with our previous report, silencing of GhMKK2 or GhVe1 also compromised Verticillium wilt resistance in the cotton CA4002 line. The variety Fibermax 9160B2F (Bayer CropSciences, Lubbock, TX, USA) and breeding line CA4002 were developed from different parents and so the effects of these genes may be sufficiently broad as to be used in a marker selection program. This study provides a mechanism to partially explain the resistance phenotype in this newly developed breeding line (Dever et al. 2013). Interestingly, AtBAK1 is also required in Arabidopsis resistance to Verticillium wilt, suggesting the conserved Verticillium resistance mechanisms in different plant species. Surprisingly, we did not find any SERK4/5 orthologs in the cotton genome, suggesting that Arabidopsis SERK4 and SERK5 are newly evolved genes. However, SERK1/2 and BAK1 have evolved before the divergence of cotton and Arabidopsis. Notably, cotton has evolved two copies of BAK1. It is also possible that GhBAK1.1 and GhBAK1.2 are homologous genes in the G. hirsutum genome. Genome-wide characterization of SERK family members in different plant species will provide information about the dynamic evolution of this important RLK subfamily. Our study also suggests that cotton may be a better model to study the biological functions of SERK family members due to less functional redundancy. Consistent with this hypothesis, silencing GhBAK1 is sufficient to induce cell death and ROS production in cotton.
BAK1 functions in plant disease resistance through association with FLS2 and other PRRs (Chinchilla et al. 2007; Heese et al. 2007; Schulze et al. 2010; Roux et al. 2011). In addition, BAK1 is involved in plant hormone BR signaling via hetero-dimerization with receptor BRI1 (Li et al. 2002; Nam and Li 2002). With a relatively short extracellular LRR domain, BAK1 does not directly bind to ligands but instead functions as a regulatory partner to positively modulate FLS2 and BRI1 signaling via trans-phosphorylation. Rather than being involved in direct ligand binding (Kinoshita et al. 2005; Chinchilla et al. 2007), BAK1 more likely functions as a signaling partner for the regulation of receptors FLS2 and BRI1 (Chinchilla et al. 2009). Interestingly, GhVe1 encodes a receptor-like protein, which might function in the receptor complex to perceive an unknown elicitor from V. dahliae. The requirement of both GhVe1 and GhBAK1 in Verticillium wilt resistance suggests that GhBAK1 and GhVe1 may associate with each other in mediating resistance to V. dahliae infection. Future examination of GhBAK1 and GhVe1 interaction in vivo and in vitro will shed light on the mechanisms underlying plant resistance to this devastating disease.
AtBAK1 and AtSERK4 redundantly control Arabidopsis cell death (He et al. 2007). The Arabidopsis bak1 serk4 double mutant exhibited spontaneous cell death with constitutive defense gene expression and ROS accumulation (He et al. 2007), which is similar to the silencing of GhBAK1 in cotton. Despite the unclear mechanism of BAK1/SERK4 in the control of cell death, BAK1 interacts with another RLK BIR1 (BAK1-interacting receptor-like kinase) constraining cell death and defense activation. Interestingly, a suppressor of BIR1-mediated cell death SOBIR also encodes an RLK, suggesting a BAK1-mediated receptor complex in the control of cell death (Gao et al. 2009). It will be interesting to test whether cotton GhBIR1 also interacts with GhBAK1 in the control of cell death, and which one functions as a bona fide receptor to perceive the death signal. Nevertheless, the multiple functions of BAK1 and other SERKs in plant growth, development, and disease resistance raise the question of how this important RLK subfamily dictates the bifurcate cellular outputs in response to distinct extrinsic and intrinsic signals.
Materials and Methods
Plant materials and growth
Cotton (Gossypium hirsutum) CA4002 line (Reg. No. GP-956, PI 665226) was grown in pots containing Metro Mix 900 (SunGR, Beavile, WA, USA) in a growth room at 23 °C, 60% relative humidity and 75 μE/m2 s1 light with a 12 h photoperiod. Arabidopsis WT Col-0, and mutants bak1-3, bak1-4, and serk2 (all in Col-0 background), were obtained from the Arabidopsis Biological Resource Center (ABRC) as previously reported (Shan et al. 2008) and grown in soil Metro Mix 366 (SunGR, Beavile, WA, USA) in a growth room at 23 °C, 60% relative humidity, and 75 μE/m2 s1 light with a 12 h photoperiod.
Construction of VIGS vectors and Agrobacterium-mediated VIGS
GhCLA1 was cloned into the pYL156 (pTRV-RNA2) vector as previously described (Gao et al. 2011b). GhBAK1 was amplified by polymerase chain reaction from a cDNA library of G. hirsutum leaf tissues with primers GhBAK1-F, 5′-CGGAATTCGCA-CACTCGGAGCTGCAAGG-3′, GhBAK1-R, 5′- GGGGTACC-GAGTGCACAACAGAGCC-3′, and inserted into the pYL156 vector with restriction enzymes EcoRI and KpnI digestion. VIGS constructs of pYL156-GhMKK2 and pLY156-GhVe1 were conducted as previously described (Gao et al. 2011b).
The plasmids containing binary TRV vectors pTRV-RNA1 and pTRV-RNA2 (pYL156) vector, pYL156-GhBAK1, pYL156-GhMKK2, or pLY156-GhVe1, were transformed into Agrobacterium tumefaciens strain GV3101, respectively. Agrobacterium cultures were harvested and infiltrated into two fully expanded cotyledons of 2-w-old plants as previously described (Gao and Shan 2013). VIGS experiments were repeated at least three times with more than 15 plants for each construct per repeat.
Verticillium infection and disease scoring
Verticillium dahliae (isolate King) was maintained and cultured, and suspension spores were prepared as described previously (Gao et al. 2011b). Spores of V. dahliae were collected from mycelium growing on potato dextrose agar in a plate by scratching the surface using sterile H2O and filtering through two layers of cheese cloth. For stem inoculation of cotton, spore suspension at a concentration of 1 × 106/mL containing 0.001% Tween-20 was injected slowly into the stem site approximately 1 cm below the cotyledons with a syringe needle (20 G) until the suspension dripped from the injection site. The plants were covered with a transparent plastic dome to maintain a high humidity at room temperature overnight. The disease phenotype was observed and wilting was scored at designated time points. The experiments were repeated three times with similar results.
For root inoculation of Arabidopsis, 2-w-old Arabidopsis seedlings were uprooted from the soil and extra soil was removed by washing with water and gentle tapping without damaging the roots. The roots were slightly blotted with a paper towel to remove excessive water, and subsequently inoculated by immersing the roots in the conidial suspension for 5 min. The seedlings were immediately transplanted into fresh soil, and covered with the dome at room temperature overnight. At least 10 seedlings were inoculated per treatment. The disease ratio was calculated as the percentage of wilting plants to the total infected plants.
Histological detection of ROS production and cell death in leaves
Histological ROS production in cotton plants was examined using a DAB staining method (ThordalChristensen et al. 1997) with modifications. Briefly, the leaves from plants approximately 2 w post-VIGS inoculation were excised and subsequently immersed in 1 mg/mL DAB (Sigma-Aldrich, St Louis, MO, USA; pH 3.8) solution with low vacuum pressure for 30 min, followed by overnight incubation at room temperature in the dark. The stained leaves were fixed and cleared in alcoholic lacto-phenol (95% ethanol: lactic acid: phenol = 2:1:1) at 65 °C, rinsed once with 50% ethanol, and twice with H2O. The destained leaves were stored in 50% glycerol or subjected to microscope observation.
To examine cell death, the leaves were collected and stained with Trypan blue in lactophenol (lactic acid : glycerol : liquid phenol : distilled water = 1:1:1:1) solution. The stained leaves were destained with 95% ethanol/lactophenol solution, washed with 50% ethanol, and mounted in 50% glycerol for microscope observation.
Bioinformatics analysis of the cotton SERK gene family
To retrieve the cotton SERK genes, the full length AtBAK1 and AtSERK1, 2, 4, or 5 amino acid sequences were used as a query to Blast against the G. raimondii D genome database (http://cgp.genomics.org.cn/page/species/blast.jsp) and the G. hirsutum unigene database (http://www.cottondb.org/blast/blast.html), respectively. Three genes (10025422 in Chr. 12; 10029586 in Chr.5; 10039700 in Chr.10) from the G.raimondii D genome with top hits with an e value of 0.0 were identified and analyzed. Three SERK genes from the G. hirsutum unigene database, GhSERK1 (ADR00582.1), GhSERK2 (AEA76434.1), and GhBAK1 (AEG25668.1), were retrieved. The amino acid sequence alignment between cotton and Arabidopsis SERKs was performed using the Multalin website (http://multalin.toulouse.inra.fr/multalin/) with a hierarchical clustering approach, and the phylogenetic tree was constructed using CLUSTALW and a rooted phylogenetic tree (http://www.genome.jp/tools/clustalw/).
Supplementary Material
Figure S1. Sequence alignment of Arabidopsis AtBAK1 with Gossypium raimondii SERK 10025423 and 10005636. The amino acid sequences were used for the alignment. The sequences squared in blue are the leucine-rich-repeat (LRR) domain and the sequences squared in red are the conserved transmembrane and juxtamembrane domains. The alignment was performed using the Multalin website (http://multalin.toulouse.inra.fr/multalin/) with a hierarchical clustering approach.
Figure S2. (A) Sequence alignment and (B) phylogenetic analysis of Arabidopsis SERK (AtSERK) and Gossypium hirsutum SERK (GhSERK) family members. The amino acid sequences were used for the alignment. The alignment was performed using the Multalin website (http://multalin.toulouse.inra.fr/multalin/) with a hierarchical clustering approach, and the phylogenetic tree was constructed using CLUSTALW and a rooted phylogenetic tree (http://www.genome.jp/tools/clustalw/). AEA76434.1 was originally named GhSERK2. The nucleotide sequence in the pTRV2-BAK1 vector corresponds to the residues that are underlined in yellow (A), which are almost identical between GhBAK1.1 and GhBAK1.2.
Acknowledgments
The work was supported by funds from the Texas AgriLife Research Cotton Improvement Program to J. D., T. W., P. H., and L. S., and the USDA National Institute of Food and Agriculture (Agriculture and Food Research Initiative Competitive Grants Program grant no. 2012-67013-19433) to P. H. and L. S. A. K. was supported by the NSF REU program. F. L. was supported by the China Scholarship Council.
Footnotes
Articles can be viewed online without a subscrption.
Supporting Information: Additional supporting information may be found in the online version of this article at the publisher's website:
References
- Becker A, Lange M. VIGS—genomics goes functional. Trends Plant Sci. 2010;15:1–4. doi: 10.1016/j.tplants.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Boller T, Felix G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- Burch-Smith TM, Anderson JC, Martin GB, Dinesh-Kumar SP. Applications and advantages of virus-induced gene silencing for gene function studies in plants. Plant J. 2004;39:734–746. doi: 10.1111/j.1365-313X.2004.02158.x. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Scheffler BE, Dennis E, Triplett BA, Zhang T, Guo W, Chen X, Stelly DM, Rabinowicz PD, Town CD, Arioli T, Brubaker C, Cantrell RG, Lacape JM, Ulloa M, Chee P, Gingle AR, Haigler CH, Percy R, Saha S, Wilkins T, Wright RJ, Van Deynze A, Zhu Y, Yu S, Abdurakhmonov I, Katageri I, Kumar PA, Mehboob UrR, Zafar Y, Yu JZ, Kohel RJ, Wendel JF, Paterson AH. Toward sequencing cotton (Gossypium) genomes. Plant Physiol. 2007;145:1303–1310. doi: 10.1104/pp.107.107672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Shan L, He P, de Vries S, Kemmerling B. One for all: The receptor-associated kinase BAK1. Trends Plant Sci. 2009;14:535–541. doi: 10.1016/j.tplants.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, Jones JD, Felix G, Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- Colcombet J, Boisson-Dernier A, Ros-Palau R, Vera CE, Schroeder JI. Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASES1 and 2 are essential for tapetum development and microspore maturation. Plant Cell. 2005;17:3350–3361. doi: 10.1105/tpc.105.036731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever J, Wheeler T, Kelly C. Registration of CA 4002 cotton germplasm line partially resistant to Verticillium wilt. J Plant Regist. 2013;7:1–7. [Google Scholar]
- Fradin EF, Thomma BP. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol Plant Pathol. 2006;7:71–86. doi: 10.1111/j.1364-3703.2006.00323.x. [DOI] [PubMed] [Google Scholar]
- Fradin EF, Zhang Z, Juarez Ayala JC, Castroverde CD, Nazar RN, Robb J, Liu CM, Thomma BP. Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol. 2009;150:320–332. doi: 10.1104/pp.109.136762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Shan L. Functional genomic analysis of cotton genes with agrobacterium-mediated virus-induced gene silencing. Methods Mol Biol. 2013;975:157–165. doi: 10.1007/978-1-62703-278-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Wang X, Wang D, Xu F, Ding X, Zhang Z, Bi D, Cheng YT, Chen S, Li X, Zhang Y. Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe. 2009;6:34–44. doi: 10.1016/j.chom.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Gao X, Britt RC, Jr, Shan L, He P. Agrobacterium-mediated virus-induced gene silencing assay in cotton. J Vis Exp. 2011a;54:e2938. doi: 10.3791/2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Wheeler T, Li Z, Kenerley CM, He P, Shan L. Silencing GhNDR1 and GhMKK2 compromises cotton resistance to Verticillium wilt. Plant J. 2011b;66:293–305. doi: 10.1111/j.1365-313X.2011.04491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou X, Yin H, He K, Du J, Yi J, Xu S, Lin H, Clouse SD, Li J. Genetic evidence for an indispensable role of somatic embryogenesis receptor kinases in brassinosteroid signaling. PLoS Genet. 2012;8:e1002452. doi: 10.1371/journal.pgen.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286:950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J. BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol. 2007;17:1109–1115. doi: 10.1016/j.cub.2007.05.036. [DOI] [PubMed] [Google Scholar]
- Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt ED, Boutilier K, Grossniklaus U, de Vries SC. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol. 2001;127:803–816. [PMC free article] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA. 2007;104:12217–12222. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlova R, Boeren S, Russinova E, Aker J, Vervoort J, de Vries S. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell. 2006;18:626–638. doi: 10.1105/tpc.105.039412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Cano-Delgado A, Seto H, Hiranuma S, Fujioka S, Yoshida S, Chory J. Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature. 2005;433:167–171. doi: 10.1038/nature03227. [DOI] [PubMed] [Google Scholar]
- Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/s0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- Nam KH, Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/s0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- Qu J, Ye J, Geng YF, Sun YW, Gao SQ, Zhang BP, Chen W, Chua NH. Dissecting functions of KATANIN and WRINKLED1 in cotton fiber development by virus-induced gene silencing. Plant Physiol. 2012;160:738–748. doi: 10.1104/pp.112.198564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tor M, de Vries S, Zipfel C. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell. 2011;23:2440–2455. doi: 10.1105/tpc.111.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ED, Guzzo F, Toonen MA, de Vries SC. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development. 1997;124:2049–2062. doi: 10.1242/dev.124.10.2049. [DOI] [PubMed] [Google Scholar]
- Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, Boller T, Felix G, Chinchilla D. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem. 2010;285:9444–9451. doi: 10.1074/jbc.M109.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan LB, He P, Sheen J. Endless hide-and-seek: Dynamic co-evolution in plant-bacterium warfare. J Integr Plant Biol. 2007;49:105–111. [Google Scholar]
- Shan L, He P, Li J, Heese A, Peck SC, Nurnberger T, Martin GB, Sheen J. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe. 2008;4:17–27. doi: 10.1016/j.chom.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci USA. 2001;98:10763–10768. doi: 10.1073/pnas.181141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB. Expansion of the receptor-like kinase/pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 2003;132:530–543. doi: 10.1104/pp.103.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ThordalChristensen H, Zhang ZG, Wei YD, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- Wang K, Wang Z, Li F, Ye W, Wang J, Song G, Yue Z, Cong L, Shang H, Zhu S, Zou C, Li Q, Yuan Y, Lu C, Wei H, Gou C, Zheng Z, Yin Y, Zhang X, Liu K, Wang B, Song C, Shi N, Kohel RJ, Percy RG, Yu JZ, Zhu YX, Yu S. The draft genome of a diploid cotton Gossypium raimondii. Nat Genet. 2012;44:1098–1103. doi: 10.1038/ng.2371. [DOI] [PubMed] [Google Scholar]
- Wendel JF. Genome evolution in polyploids. Plant Mol Biol. 2000;42:225–249. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Sequence alignment of Arabidopsis AtBAK1 with Gossypium raimondii SERK 10025423 and 10005636. The amino acid sequences were used for the alignment. The sequences squared in blue are the leucine-rich-repeat (LRR) domain and the sequences squared in red are the conserved transmembrane and juxtamembrane domains. The alignment was performed using the Multalin website (http://multalin.toulouse.inra.fr/multalin/) with a hierarchical clustering approach.
Figure S2. (A) Sequence alignment and (B) phylogenetic analysis of Arabidopsis SERK (AtSERK) and Gossypium hirsutum SERK (GhSERK) family members. The amino acid sequences were used for the alignment. The alignment was performed using the Multalin website (http://multalin.toulouse.inra.fr/multalin/) with a hierarchical clustering approach, and the phylogenetic tree was constructed using CLUSTALW and a rooted phylogenetic tree (http://www.genome.jp/tools/clustalw/). AEA76434.1 was originally named GhSERK2. The nucleotide sequence in the pTRV2-BAK1 vector corresponds to the residues that are underlined in yellow (A), which are almost identical between GhBAK1.1 and GhBAK1.2.


