Abstract
Saliva is a complex body fluid that comprises secretions from the major and minor salivary glands, nourished by body’s vasculature. While many circulatory molecules (DNA, RNA and proteins) could also be present in saliva, saliva harbors unique molecular constituents that can be discriminatory for oral and systemic disease screening and detection. Many studies have reported that salivary constituents can discriminate oral diseases (oral cancer and Sjögren’s Syndrome) and also systemic diseases (lung cancer, breast cancer, pancreatic cancer and ovarian cancer). Non-coding RNAs (ncRNAs) are emerging new regulators of diverse biological functions, playing important roles in oncogenesis and tumor progression. Indeed, the short size of these molecules makes them very stable in different body fluids such as urine, blood and saliva, being not as susceptible as mRNAs to degradation by RNases. Here, we reviewed the current status and clinical implications of the ncRNAs present in human saliva for translational applications and basic biological research. The development of non-invasive salivary test (based on ncRNAs profiles) for disease detection, could have impactful applications into the clinical context with a translational significance as emerging molecular biomarkers for non-invasively disease detection, not only by reducing the cost to the healthcare system, but also benefitting patients.
Keywords: saliva, body fluid, cancer, diagnostics, non-invasiveness, biomarkers, non-coding RNA, small ncRNAs, long ncRNAs
1. Introduction
Saliva has critical roles in maintaining the oral health and function of the upper gastrointestinal tract. It is mostly water but also contains protein molecules that lubricate our tongue, inhibit the growth of bacteria, prevent excessive swings in pH and begin the process of digestion [1,2]. Unfortunately, the importance of saliva is often appreciated only when it’s gone, as commonly happens in patients who get radiation treatments or have oral cancer [3]. Saliva comes primarily from three paired major salivary glands (parotid, submandibular and sublingual) where specialized cells take up water, salts and macromolecules from the blood that sum to their individual gland secretions. Hence, most compounds found in blood are also present in saliva, giving rise to the notion that saliva “mirror of the body” [4,5].
Saliva is a highly desirable body fluid for biomarker development for clinical applications as it provides a non-invasive, simple and low-cost method for disease screening and detection [6–8]. Much efforts have been implied in elucidating the molecular profiles in healthy saliva, both at protein and mRNA levels [9], by using several techniques such as 2-D gel electrophoresis, mass spectrometry and western blot for protein profiling [10–14], and by using qPCR, microarray analysis and sequencing techniques for mRNA profiling [15–21]. Furthermore, efforts have been made on establishing procedures for saliva collection, storage and analysis [22,23], as well as methods for increasing stability of proteins and mRNA [24–27] present in saliva. These studies have generated a vast amount of saliva-omics data, which had lead to develop the Salivaomics Knowledge Base (SKB) [28] (Figure 1), a data management system and Web resource that support salivary diagnostics research [29,30].
Figure 1.
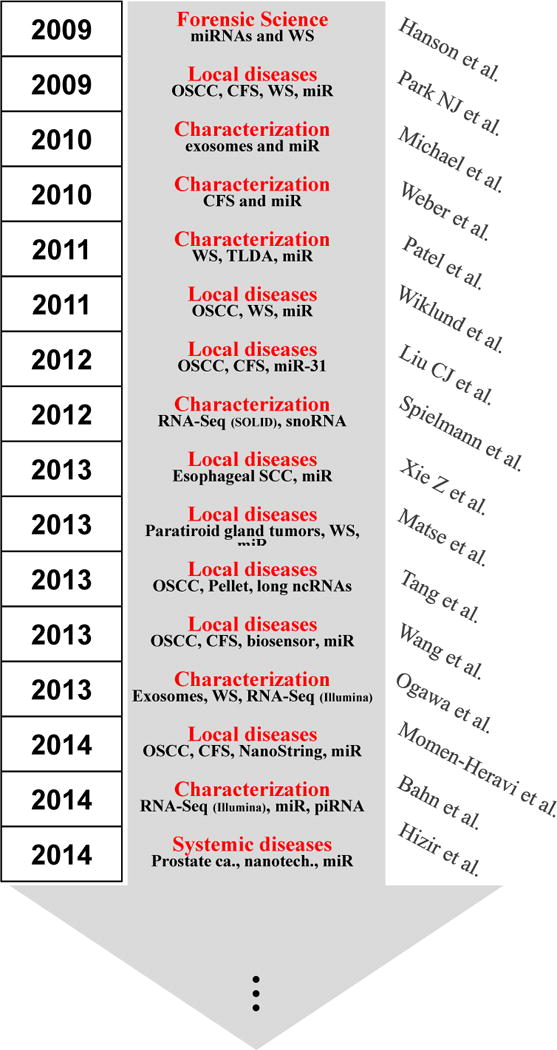
The rise of non-coding RNA in saliva
In the last decade, the potential use of saliva has not only been demonstrated useful for detecting various local diseases including Sjögren’s syndrome [31–33], and oral and head and neck cancers [34–38], through proteomic and transcriptomic discovery phases and preclinical validation phases; but also for systemic diseases detection such as type-2 diabetes [39], lung [40–42], pancreatic [43,44], breast [45,46] and ovarian cancers [47]. It should be noted that while salivary diagnostics is recognized for oral diseases, its clinical utility and scientific credibility for systemic diseases are still largely unsubstantiated. The clinical and scientific credentialing of saliva for systemic disease detection will present a groundbreaking technology that is impactful, sustainable, and will transform molecular screening for diseases globally. Studies are in progress to address this gap and unmet need. We hypothesize that disease-specific molecular targets are shuttled from the primary organ of pathology to the vasculature and then salivary gland and appear altered in cancer saliva compared to control saliva [40] in tumor-bearing mice models of melanoma and lung cancer. Each tumor-type is associated a different salivary transcriptome profile. At the basic mechanistic levels, we have shown that by using a rodent pancreatic cancer model, exosomes-like vesicles carry, drive and deliver tumor-specific biomarkers into the saliva [44].
Altogether, we can clearly note that after a decade of scientific advancements, the maturation of these basic and translational research is leading to eventual clinical utilities that can benefit patients. Nonetheless, the majority of all these efforts have been made on revealing the underpinning of the presence of mRNA and proteins as disease discriminatory biomarkers, little is known about the emerging new class of biomarkers in body fluids: the non-coding RNAs (ncRNAs). Here, we review the current status, power, advantages and future applications of ncRNAs in saliva as a source of biological information, disease status and biomarker performance.
2. Salivary non-coding RNAs associated with (patho) physiological states
About 98% of all transcriptional output in humans is non-coding RNA. RNA-mediated gene regulation is widespread in higher eukaryotes and complex genetic phenomena like RNA interference, co-suppression, transgene silencing, imprinting, methylation, and possibly position-effect variegation and transvection, all involve intersecting pathways based on or connected to RNA signaling [48,49]. Although proteins are the fundamental effectors of cellular function, the basis of eukaryotic complexity and phenotypic variation may lie primarily in a control architecture composed of a highly parallel system of trans-acting RNAs: the noncoding RNAs [48]. NcRNAs are short RNAs that have been widely described to be stable in many body fluids [50] and are emerging new regulators of diverse biological functions, playing an important role in oncogenesis and tumor progression [51–53]. NcRNAs are grouped into two major classes based on their transcript sizes: small ncRNAs (<200 bp) and long ncRNAs (lncRNAs) (≥200 bp) [48,54,55].
As the complex compositional profiles of salivary extracellular RNA molecules are emerging, encompassing mRNAs, microRNAs (miRNAs), small nucleolar RNAs (snoRNAs), and other non-coding RNAs [21,56,57]. However, the entire spectrum of extracellular RNA (exRNA) from saliva has not been extensively described, thus warranting the need of further comprehensive deciphering and analyses. Using high-throughput RNA sequencing (RNA-Seq), we have recently described a landscape of miRNA, piwi-interacting RNA (piRNA) and circular RNA (circRNA) in human saliva as a source of new molecular biomarkers [58]. In addition, there is also increasing interest in understanding the functional aspects of salivary exRNA in oral and systemic biology [40,44]. Such studies will be facilitated by a detailed delineation of the landscapes of salivary exRNAs.
2.1. Characterization of salivary non-coding RNAs
Compared with other biofluids, saliva can be collected easily and noninvasively. However, low RNA abundance, small sample volumes, highly fragmented mRNA and high abundance of bacterial contents create challenges for downstream RNA sequencing assays [21]. Thus, ncRNAs rise to the first position of ideal and suitable salivary biomarkers because of its short size body fluid stability, and also due to their main location inside exosomes [44,50,57,59].
Small ncRNAs are the most exploited and widely described ncRNAs in saliva. In particular, miRNAs, which are small, 19- to 23-nucleotide- long, single-stranded RNA molecules, that play an important role in regulating various biological processes through their interaction with cellular messenger RNAs (mRNA) [60], have been widely characterized in saliva body fluid. Weber et al. [61], with the goal of assessing the distribution of miRNAs and demonstrating the potential use of miRNAs as biomarkers, they examined the presence of miRNAs in 12 human body fluids including saliva, conducting a global survey of the miRNA distribution in these fluids, finding that miRNAs were present in all fluids tested and showing distinct compositions in different fluid types. Actually, they found that several of the highly abundant miRNAs in the body fluids tested were common among multiple fluid types, and some of the miRNAs were enriched in specific fluids. Interestingly, saliva, breast milk, and seminal fluid had a higher number of detectable miRNA species, whereas urine, cerebrospinal fluid, and pleural fluid had far fewer. The miRNA spectrum in plasma was different from that of most of the other body fluids, indicating an extensive “filtering” process separating the plasma from other body fluid types, biases caused by the differential uptake or release of miRNAs from different circulating cell types that come in contact with the blood, or other processes not yet understood.
Two other groups have studied the miRNAs composition of the whole saliva (WS), including the cell content, cell debris and abundant bacteria present in oral cavity. On one hand, Patel et al. [62] used commercial kits (Oragen and miRVana) for collection and isolation of total RNA from WS, and performed miRNA analysis aiming to profile salivary miRNAs. They have described the five most abundantly expressed miRNAs (miR-223, miR-191, miR-16, miR-203, and miR-24) that were similarly described in other published reports [59,63,64]. Additionally, many previously undetected miRNAs were also identified, what was justified by the improved RNA isolation method they describe, and the high quality of miRNAs isolated from WS. On the other hand, Spielmann et al. [21] aimed the comparison between the whole saliva (WS) and the cell-free saliva (CFS) miRNA content by using massively parallel sequencing. They found that more than 90% of the uniquely mapped genes were coding (i.e., mRNAs), and the remaining small percentage was noncoding. Rank ordering the genes by RPKM showed, however, that 95 of the top 100 highest-expressing genes encoded ncRNAs, being mostly and 224 small nucleolar RNAs (snoRNAs) present in saliva. Nonetheless, they found measurable differences between CFS and WS; with the greatest difference being the percentage of reads aligning to microbial genomes, with higher fraction of microbial RNA in WS compared to CFS, which markedly decreased the sensitivity of human RNA in WS analysis. Therefore, they suggest that a low-speed centrifugation step cannot reduce the presence of microbial RNA, and adding subsequent steps might remove more of the microbial cells and cell debris.
Consistently with the microbial issue and cellular contamination, some groups start to investigate the miRNA content in exosomes isolated from saliva. This methodology is technically more complicated to implement in the clinics once you get the specific-disease biomarkers, but it is a better method to characterize the exRNA present in saliva. In 2010, Michael et al. [59], although they could isolate exosomes from both glandular and whole saliva, the viscosity and cellular contamination of whole saliva made it less than ideal for exosomes isolation. Therefore, they focused the study on glandular saliva only by using miRNA microarray as a proof of concept to profile miRNA in salivary exosomes.
Despite several studies have been focused on characterizing salivary exosomes at nanostructural, transcriptomic [65,66] and proteomic [67] levels, very little is known about ncRNA content in salivary exosomes. Gallo et al. [68] wanted to aim whether miRNAs, that are easily accessible in many body fluids, are circulating freely or are encapsulated in microvesicles (particularly exosomes). They extracted the RNA from the exosomes in the pellet and from the exosomes-depleted supernatant from both serum and saliva samples, and the miRNA concentration was lower in salivary exosomes than serum exosomes, but still predominantly present in the exosomes fraction compared to exosomes-depleted salivary supernatant. Furthermore, Ogawa et al. [57] examined small RNA transcriptomes by using next generation sequencing technology to elucidate a full transcriptome set of small RNAs expressed in two types of salivary exosomes and in whole saliva (WS). Many types of small RNA, such as miRNA, piRNA, snoRNAs and other small RNAs are contained in salivary exosomes. Specifically, both salivary exosomes and WS commonly expressed a total of 143 miRNAs, and 147 miRNAs were detected between both exosomes fractions but not in WS. Importantly, piRNA and snoRNAs have been described for the first time in saliva samples: 129 piRNAs were mostly expressed in exosomes, while WS contained only 90. On the other hand, the number of snoRNAs detected in one exosomes fraction was less than 50% than in the other exosomes fraction and WS. Thus, again specific ncRNAs appear differentially expressed in depleted or non-depleted exosomes fraction, and further studies need to be addressed to define the function of small ncRNAs in salivary exosomes.
Recently, Bahn et al. [58] by using high-throughput RNA sequencing (RNA-Seq) conducted an in-depth bioinformatic analysis of ncRNAs in human CFS from healthy individuals, with a focus on miRNAs, piRNAs, and circular RNAs (circRNAs). Their data demonstrated robust reproducibility of miRNA and piRNA profiles across individuals. Furthermore, individual variability of these salivary exRNA species was highly similar to those in other body fluids or cellular samples, despite the direct exposure of saliva to environmental impacts. By comparative analysis of >90 RNA-Seq datasets of different origins, they observed that piRNAs were surprisingly abundant in CFS compared with other body fluid or intracellular samples, with expression levels in CFS comparable to those found in embryonic stem cells and skin cells. Summarizing, the most abundant types of small ncRNAs in their data included human miRNAs (6.0% of reads on average), piRNAs (7.5% of reads), and snoRNAs (0.02% of reads). In addition, 58.8% of reads corresponded to microbial RNA sequences, reflecting the enriched presence of microorganisms in saliva [21]. Furthermore, using a customized bioinformatics method, they identified >400 circRNAs in CFS. These data represent the first global characterization and experimental validation of circRNAs in any type of extracellular body fluid. These results suggest that the small ncRNA sequencing experiment can capture a wide spectrum of noncoding exRNAs in human saliva [58].
The identification of biological markers of disease is a major impetus in current research. Ideal biomarkers have the capacity to identify a disease, with a strong degree of accuracy, before it can be diagnosed clinically. Thus, the search for a minimally invasive, easily accessible body medium such as saliva, housing biological information reflective of disease status is clinically very relevant. Whilst several groups have characterized the use of isolated small ncRNAs from saliva to further use this information as diagnostic biomarkers, there is no information on long ncRNAs quality and yield and also not consistent and standardized protocols for isolation, characterization and analyses have been established; hence emphasizing the discrepancies of the published findings. However, it is clear the emerging interest of this field and the recent publications revealing new ncRNAs present in saliva including miRNAs, piRNAs, circRNAs will generate future interests for biomarker development studies.
2.2. Salivary non-coding RNAs: biomarkers for local/systemic diseases
Human saliva has been used increasingly for biomarker development to enable noninvasive detection of diseases. The term “salivaomics” was coined to highlight the omics constituents in saliva that can be used for biomarker development and personalized medicine [30]. Salivary extracellular (exRNA) [21] was discovered 10 years ago; since then, the nature, origin, and characterization of salivary RNA have been actively pursued [17,21,31,35,43]. These studies have demonstrated the potential for the use of salivary RNA to detect local diseases such as oral cancer [34,35], and Sjögren’s syndrome [31], but also systemic diseases including resectable pancreatic cancer [43], lung cancer [40,42], ovarian cancer [47], and breast cancer [46].
Several studies have been implied the efforts on deciphering miRNA profiles for oral cancer detection by using saliva body fluid. In 2009, Park et al. demonstrate that several miRNAs present in CFS and WS of 12 healthy donors can be validated on 50 oral cancer patient-cohort and found that miR-125a and miR-200a where differentially expressed in both CFS and WS from patients with oral cancer than patients without [63]. In 2011, pursuing the same disease, Wiklund et al. [69] demonstrated that a panel of miRNAs and DNA methylation patterns found in oral squamous cell carcinoma (OSCC) tissues could be validated in oral rinse and saliva from OSCC patients and healthy controls, with aberrant miR-375 and miR-200a expression and miR-200c-141 methylation which could be detected in and distinguish OSCC patient oral rinse and saliva from healthy volunteers, suggesting a potential clinical application for OSCC specific miRNA signatures in oral fluids. In 2012, Li et al. exploited miR-31 as a clinical biomarker of OSCC [70] in oral lesions, plasma and saliva, founding that miR-31 was significantly increased in saliva from patients with oral carcinoma at all clinical stages, including very small tumors. However, preliminary analysis showed no increase of salivary miR-31 in patients with oral verrucous leukoplakia relative to controls. The miR-31 was more abundant in saliva than in plasma, suggesting salivary miR-31 was a more sensitive marker for oral malignancy. Furthermore, they found that after excision of oral carcinoma, salivary miR-31 was remarkably reduced, indicating that most of the up-regulated salivary miR-31 came from tumor tissues.
More recently, Wang et al. [71], developed a electrochemical biosensor method for the ultra sensitive and specific detection of attomolar level oral cancer-related miRNA. In order to evaluate the applicability of the novel RNA biosensor, the saliva samples were spiked with different concentrations of target miRNA (miR-200a, miR-142-3p, miR-93 and miR-125a). The results shown clear indications that the magnetic-controllable electro-chemical biosensor had a strong resistance to the complex matrix of saliva, and can be used to detect ultra-trace target miRNA in real saliva samples with a recovery of 93–108%. In 2013, Yang et al. [72] reported firstly the use of microRNA microarray to profile low-grade dysplasia (LGD) oral premalignant lesions (OPLs) from progressing and non-progressing LGD OPLs, in order to explore the possible microRNAs, which later could lead the progression into high-grade dysplasia (HGD) or OSCC. They identified 25 miRNAs differentially expressed between progressive and non-progressive LGD leukoplakias. Compared to non-progressive LGD leukoplakias, 13 miRNAs were down-regulated and 12 miRNAs were up-regulated in progressive LGD leukoplakias. Finally, the latest report on OSCC and salivary miRNA was done in 2014 by Momen-Heravi et al. [73]. Of more than 700 miRNAs tested by the newly technology NanoString nCounter [74], 13 were identified as being significantly deregulated in saliva of OSCC patients compared to HCs: 11 miRNAs were down-regulated (miRNA-136, miRNA-147, miRNA-1250, miRNA-148a, miRNA-632, miRNA-646, miRNA668, miRNA-877, miRNA-503, miRNA-220a, miRNA-323-5p), and 2 miRNAs were overexpressed (miRNA-24, miRNA-27b).
In 2013, a meta-analysis study was published addressing esophageal squamous cell carcinoma (ESCC) investigations [75], including 6 data sets from plasma/serum circulating miRNAs and 2 data sets from saliva miRNAs profile for ESCC, which had been downloaded from two previous studies published on the field [76,77] on 2013. Seventeen studies from eight articles, including 995 ESCC patients and 733 healthy controls, were included in this meta- analysis. The pooled AUC was 0.91 (95 % CI 0.88–0.93), but subgroup analyses indicated that blood-based miRNA assay displays better diagnostic accuracy than saliva-based miRNA assay. However, the individual study reported by Xie et al. [76] showed that after validation by RT-qPCR, miR-10b*, miR-144, and miR-451 in whole saliva and miR-10b*, miR-144, miR-21, and miR-451 in saliva supernatant were significantly upregulated in patients, with sensitivities of 89.7, 92.3, 84.6, 79.5, 43.6, 89.7, and 51.3% and specificities of 57.9, 47.4, 57.9%, 57.9, 89.5, 47.4, and 84.2%, respectively, showing that miRNAs possess discriminatory power for detection of esophageal cancer by using saliva body fluid.
Most of the published studies described small ncRNAs, in particular miRNA, in saliva. However, there is only one study that describes long ncRNAs in saliva for oral cancer detection aberrantly expressed in oral cancer and metastasis tissues [78]. Moreover, whole saliva contained a detectable amount of some long ncRNAs, which appeared to be potential salivary biomarker candidates. Among several long ncRNAs investigated, MALAT-1 was present in all the participants (n=9), but HOTAIR was only detected in 5/9 patients with higher expression in patients with lymph node metastasis. Taken together, these data indicate that whole saliva contains detectable amount of certain long ncRNAs that may be potential markers for OSCC diagnosis. Since there are much evidence that long ncRNAs can serve as circulating diagnostic biomarkers for several diseases such as B-cell neoplasms and prostate cancer [79–81], and have the sufficient power to discriminate between cancer and healthy status [78,82], we truly believe that it is just a matter of time that long ncRNAs will appear as the new spectrum of diagnostic biomarkers in saliva, specific for either local and systemic diseases. Last but not least, a study focused on salivary and circulating small ncRNAs as miRNA signatures for prostate cancer detection [83], supporting the translational utility of the salivary ncRNA for systemic diseases detection.
4. Conclusions and Future perspectives
Non-coding RNA expression profiles in human cancers have highlighted the potential value of this class of RNAs as tumor markers in patient diagnosis and prognosis. The rapidly expanding and continuously catalog of salivary ncRNAs holds promises that in the near future ncRNAs will become ever more important in cancer patient management. The potential relevance to oral diseases were proposed (84). An analogy can be made with the impact of salivary mRNA profiling in many types of cancer, which has provided different experimental lines of evidence that deregulation of mRNAs not only results as consequence of cancer progression but also directly affects gene networks that promote tumor initiation and progression in a cause-effect manner. As the catalog of salivary ncRNAs grows, it will become important to elucidate the genetic networks and pathways regulated by the abnormally expressing ncRNAs in saliva from cancer patients as a means to understanding the role and biomarker performance of these ncRNAs in the induction of malignant transformation as well as their ability to create significant profiles for salivary diagnostics.
Table 1.
Salivary non-codingRNAs as disease-related molecular markers
| Characterization | |||||
|---|---|---|---|---|---|
| Study # | Saliva fraction | Disease | Study cohort | Technique | Molecular profile |
| Weber et al., Clin. Chem. 2010 | CFS | characterization | 5 healthy donors | Human miScript Assay panel (Qiagen) – 714 miRNA | miR-182*, miR-450b-5p, miR-622, miR-141, miR-26a, miR-145*, miR-135b*, miR-381, miR-96*, miR-1228, miR-431* |
| Michael et al., Oral Dis. 2010 | exosomes | characterization | 2 healthy donors | miRCURY LNA microRNA Array, v.10.0, (Exiqon, Denmark) | let-7b, let-7c*, miR-128, miR-150*, miR-17, miR-1908, miR-212, miR-27b*, miR-29b, miR-29c, (Top-10) |
| Patel et al., Arch Oral Biol. 2011 | WS | characterization | 20 healthy donors | TaqMan1 Low Density Array Card (TLDA) Human miRNA Panel v2.0 (Applied Biosystems). | miR-223, miR-191, miR-16, miR-203, and miR-24 |
| Spielmann et al., Clin. Chem. 2012 | CFS and WS | characterization | 8 healthy donors | SOLiDTM Total RNA-Seq Kit and Barcoding Kit (modules 1–16) (Applied Biosystems) | 224 snoRNAs |
| Gallo et al., PLoS One 2012 | exosomes | characterization | Healthy donors (# N/A) | TaqMan MicroRNA Assay, PN 4427975, Applied Biosystems | miR-22, miR202, miR-203, miR-1273d |
| Ogawa et al., Biol. Pharm. Bull. 2013 | exosomes and WS | characterization | 1 healthy donor (7 saliva collection replicates) | Illumina Genome Analyzer Iix by Hokkaido System Sciences Co., Ltd. (Japan) | miR-378a, miR-143, let-7c, miR-146b, miR-21, let-7f-1, let-7f-2, miR-30a, miR-9-1, miR-9-2, miR-9-3, let-7a-1, let-7a-2, miR-20a, miR-30d, miR-30e; piR-39980, piR-48209, piR-52207, piR-38581, piR-36095, piR-59293, piR-61648, piR-55361; U78, U44, U21, U31, U104, U15A, snR39B. |
| Bahn et al., Clin. Chem. 2014 | CFS | characterization | 8 healthy donors | Illumina HiSeq 50SE | 127– 418 miRNAs (Top-2: miR-223–3p and miR-148a-3p) |
| Local & Systemic diseases | |||||
| Study | Saliva fraction | Disease | Study cohort | Technique | Molecular profile |
| Park NJ et al., Clin. Cancer Res. 2009 | CFS and WS | oral squamous cell carcinoma | 50 OSCC patients and 50 healthy matched control subjects. | RT-preamp-qPCR | miR-125a and miR-200a |
| Wiklund et al., PLoS One 2011 | WS | oral squamous cell carcinoma | 15 OSCC patients and 7 healthy control donors | TaqManH qRT-PCR assays (Applied Biosystems) | miR-375 and miR-200a expression and miR-200c-141 methylation |
| Liu CJ et al., Head Neck. 2012 | CFS | oral squamous cell carcinoma | 45 oral carcinoma, 10 oral verrucous leukoplakia, and 24 healthy controls | TaqMan miRNA assay system (Applied Biosystems, Foster City, CA) | miR-31 |
| Matse et al., Clin. Cancer Res. 2013 | WS | paratiroid gland tumors | 38 malignant tumors and 29 benign parotid gland tumors | TaqMan Human MicroRNA Cards (Applied Biosystems) and RTqPCR | hsa-miR-132, hsa-miR-15b, mmu-miR-140, and hsa-miR-22 |
| Tang et al., Mol. Med. Rep. 2013 | SP | oral squamous cell carcinoma | 4 OSCC saliva samples and 12 healthy donors | RT-qPCR of six lncRNAs found in OSCC tissue | MALALT-1, HOTAIR |
| Wang et al., Biosens and Bioelectr, 2013 | CFS | oral squamous cell carcinoma | 5 artificial saliva samples (spiked) | Novel home-made electrochemical biosensor magnetic-controllable gold electrode | miR- 200a, miR-142-3p, miR-93 and miR-125a |
| Xie Z et al., PLoS One 2013 | CFS and WS | esophageal squamous cell carcinoma | NA | Agilent miRNA microarray | miR-144, miR-10b*, miR-21 and miR-451 |
| Yang et al., BMC Cancer 2013 | SP | oral squamous cell carcinoma | 7 non-progressing LGD, 8 progressing LGD into OSCC and 7 healthy control donors | The TaqManW low density array (TLDA) qRT-PCR system (Applied Biosystems, Foster City CA) | miR-10b, miR-660, miR-708, miR- 30e, miR-145, miR-99b, miR-181c and miR-197 |
| Salazar et al., Cell Oncol. 2014 | WS | head and neck cancer | 61 HNSCC patients and 61 healthy controls | miScriptTM miRNA microarray, RTqPCR, TCGA | miR-9, miR-134 and miR-191 |
| Wang et al., Tumor Biol. 2014 | 2 saliva data sets, 6 plasma/serum data sets (meta-analysis) | esophageal squamous cell carcinoma | 995 ESCC patients and 733 healthy controls | Bioinformatic and Statistics tools | miR-144, miR-10, miR-451 |
| Momen-Heravi et al., J Dent Res, 2014 | CFS | oral squamous cell carcinoma | 9 OSCC patients before treatment, 8 patients with OSCC in remission, and 9 HCs. | NanoString nCounter miRNA expression assay (NanoString Technologies, Seattle, WA, USA) | miRNA-136, miRNA-147, miRNA-1250, miRNA-148a, miRNA- 632, miRNA-646, miRNA668, miRNA- 877, miRNA-503, miRNA-220a, miRNA-323-5p, miRNA-24, miRNA- 27b |
| Hizir et al., ACS appl. Mater. Interf. 2014 | CFS | prostate cancer | NA | nanographene oxide system | miR-21, miR141 |
| Forensic Science | |||||
| Study # | Saliva fraction | Disease | Study cohort | Technique | Molecular profile |
| Hanson et al., Anal. Biochem. 2009 | WS | body fluid identification | Healthy donors (# NA) | RTqPCR | miR-658, miR-205 |
| Zubakov et al., Int J Legal Med. 2010 | WS | body fluid identification | Healthy donors (# NA) | Microarray LNATM-modified oligo- nucleotides (Exiqon, Vedbæk, Denmark), and RTqPCR | miR-583, miR-518c*, miR-208b |
| Courts et al., J Forensic Sci. 2011 | WS | body fluid identification | Healthy donors (# NA) | Microarray Geniom Biochips (Heidelberg, Germany) and RTqPCR | miR-200c, miR-203, miR-205 |
| Wang et al., Forensic Sci. Int: Gen, 2012 | WS | body fluid identification | 10 healthy donors | RTqPCR | miR-658, miR-205 |
| Omelia et al., Analyt Biochem, 2013 | body fluid identification | ||||
| Park JL et al., Electrophoresis 2014 | WS | body fluid identification | 60 healthy donors | Affymetrix Gene Chip miRNA 3.0 array and RTqPCR | miR-203, miR-205 |
| Silva et al., Forensic Sci. Int: Gen, 2015 | – | body fluid identification | – | – | (Review) |
LEGEND
WS: whole saliva
CFS: cell free saliva
SP: saliva pellet
NA: not avaliable
Acknowledgments
Supported by research grants UH2 TR000923, U01DE17593, R01 CA139596, R56 DE23241, LC110207, 20PT-0032, 21RT-0112, Barnes Family Fund, O’Keeffe Foundation and George Richmond Foundation.
Footnotes
Conflicts of Interest
David Wong is co-founder of RNAmeTRIX Inc., a molecular diagnostic company. He holds equity in RNAmeTRIX, and serves as a company Director and Scientific Advisor. The University of California also holds equity in RNAmeTRIX. Intellectual property that David Wong invented and which was patented by the University of California has been licensed to RNAmeTRIX. Additionally, he is a consultant to PeriRx.
References and Notes
- 1.Mandel ID. The role of saliva in maintaining oral homeostasis. J Am Dent Assoc. 1989;119:298–304. doi: 10.14219/jada.archive.1989.0211. [DOI] [PubMed] [Google Scholar]
- 2.Amerongen AVN, Veerman ECI. Saliva–the defender of the oral cavity. Oral Dis. 2002;8:12–22. doi: 10.1034/j.1601-0825.2002.1o816.x. [DOI] [PubMed] [Google Scholar]
- 3.Teshima K, Murakami R, Tomitaka E, Nomura T, Toya R, Hiraki A, Nakayama H, Hirai T, Shinohara M, Oya N, Yamashita Y. Radiation-induced parotid gland changes in oral cancer patients: correlation between parotid volume and saliva production. Jpn J Clin Oncol. 2010;40:42–6. doi: 10.1093/jjco/hyp113. [DOI] [PubMed] [Google Scholar]
- 4.Loo JA, Yan W, Ramachandran P, Wong DT. Comparative human salivary and plasma proteomes. J Dent Res. 2010;89:1016–23. doi: 10.1177/0022034510380414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan W, Apweiler R, Balgley BM, Boontheung P, Bundy JL, Cargile BJ, Cole S, Fang X, Gonzalez-Begne M, Griffin TJ, Hagen F, Hu S, Wolinsky LE, Lee CS, Malamud D, Melvin JE, Menon R, Mueller M, Qiao R, Rhodus NL, Sevinsky JR, States D, Stephenson JL, Than S, Yates JR, Yu W, Xie H, Xie Y, Omenn GS, Loo JA, Wong DT. Systematic comparison of the human saliva and plasma proteomes. Proteomics Clin Appl. 2009;3:116–134. doi: 10.1002/prca.200800140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee Y-H, Wong DT. Saliva: an emerging biofluid for early detection of diseases. Am J Dent. 2009;22:241–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Baum BJ, Yates JR, Srivastava S, Wong DTW, Melvin JE. Scientific frontiers: emerging technologies for salivary diagnostics. Adv Dent Res. 2011;23:360–8. doi: 10.1177/0022034511420433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segal A, Wong DT. Salivary diagnostics: enhancing disease detection and making medicine better. Eur J Dent Educ. 2008;12(Suppl 1):22–9. doi: 10.1111/j.1600-0579.2007.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu S, Li Y, Wang J, Xie Y, Tjon K, Wolinsky L, Loo RRO, Loo JA, Wong DT. Human saliva proteome and transcriptome. J Dent Res. 2006;85:1129–33. doi: 10.1177/154405910608501212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramachandran P, Boontheung P, Xie Y, Sondej M, Wong DT, Loo JA. Identification of N-linked glycoproteins in human saliva by glycoprotein capture and mass spectrometry. J Proteome Res. 2006;5:1493–503. doi: 10.1021/pr050492k. [DOI] [PubMed] [Google Scholar]
- 11.Whitelegge JP, Zabrouskov V, Halgand F, Souda P, Bassilian S, Yan W, Wolinsky L, Loo JA, Wong DTW, Faull KF. Protein-Sequence Polymorphisms and Post-translational Modifications in Proteins from Human Saliva using Top-Down Fourier-transform Ion Cyclotron Resonance Mass Spectrometry. Int J Mass Spectrom. 2007;268:190–197. doi: 10.1016/j.ijms.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sondej M, Denny PA, Xie Y, Ramachandran P, Si Y, Takashima J, Shi W, Wong DT, Loo JA, Denny PC. Glycoprofiling of the Human Salivary Proteome. Clin Proteomics. 2009;5:52–68. doi: 10.1007/s12014-008-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu S, Jiang J, Wong DT. Proteomic analysis of saliva: 2D gel electrophoresis, LC-MS/MS, and Western blotting. Methods Mol Biol. 2010;666:31–41. doi: 10.1007/978-1-60761-820-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halgand F, Zabrouskov V, Bassilian S, Souda P, Loo JA, Faull KF, Wong DT, Whitelegge JP. Defining intact protein primary structures from saliva: a step toward the human proteome project. Anal Chem. 2012;84:4383–95. doi: 10.1021/ac203337s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Zhou X, St John MAR, Wong DTW. RNA profiling of cell-free saliva using microarray technology. J Dent Res. 2004;83:199–203. doi: 10.1177/154405910408300303. [DOI] [PubMed] [Google Scholar]
- 16.Park NJ, Li Y, Yu T, Brinkman BMN, Wong DT. Characterization of RNA in saliva. Clin Chem. 2006;52:988–94. doi: 10.1373/clinchem.2005.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park NJ, Zhou X, Yu T, Brinkman BMN, Zimmermann BG, Palanisamy V, Wong DT. Characterization of salivary RNA by cDNA library analysis. Arch Oral Biol. 2007;52:30–35. doi: 10.1016/j.archoralbio.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Z, Zimmermann BG, Zhou H, Wang J, Henson BS, Yu W, Elashoff D, Krupp G, Wong DT. Exon-level expression profiling: a comprehensive transcriptome analysis of oral fluids. Clin Chem. 2008;54:824–32. doi: 10.1373/clinchem.2007.096164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei F, Wang J, Liao W, Zimmermann BG, Wong DT, Ho C-M. Electrochemical detection of low-copy number salivary RNA based on specific signal amplification with a hairpin probe. Nucleic Acids Res. 2008;36:e65. doi: 10.1093/nar/gkn299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palanisamy V, Wong DT. Transcriptomic analyses of saliva. Methods Mol Biol. 2010;666:43–51. doi: 10.1007/978-1-60761-820-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spielmann N, Ilsley D, Gu J, Lea K, Brockman J, Heater S, Setterquist R, Wong DTW. The human salivary RNA transcriptome revealed by massively parallel sequencing. Clin Chem. 2012;58:1314–21. doi: 10.1373/clinchem.2011.176941. [DOI] [PubMed] [Google Scholar]
- 22.Henson BS, Wong DT. Collection, storage, and processing of saliva samples for downstream molecular applications. Methods Mol Biol. 2010;666:21–30. doi: 10.1007/978-1-60761-820-1_2. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y-H, Zhou H, Reiss JK, Yan X, Zhang L, Chia D, Wong DTW. Direct saliva transcriptome analysis. Clin Chem. 2011;57:1295–302. doi: 10.1373/clinchem.2010.159210. [DOI] [PubMed] [Google Scholar]
- 24.Park NJ, Yu T, Nabili V, Brinkman BMN, Henry S, Wang J, Wong DT. RNAprotect saliva: An optimal room- temperature stabilization reagent for the salivary transcriptome. Clin Chem. 2006;52:2303–4. doi: 10.1373/clinchem.2006.075598. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J, Park NJ, Hu S, Wong DT. A universal pre-analytic solution for concurrent stabilization of salivary proteins, RNA and DNA at ambient temperature. Arch Oral Biol. 2009;54:268–73. doi: 10.1016/j.archoralbio.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JK, Zhou H, Nabili V, Wang MB, Abemayor E, Wong DTW. Utility of multiple sampling in reducing variation of salivary interleukin-8 and interleukin-1β mRNA levels in healthy adults. Head Neck. 2013;35:968–73. doi: 10.1002/hed.23063. [DOI] [PubMed] [Google Scholar]
- 27.Xiao H, Wong DTW. Method development for proteome stabilization in human saliva. Anal Chim Acta. 2012;722:63–9. doi: 10.1016/j.aca.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Salivaomics Knowledge Base. www.skb.ucla.edu (accessed Aug 19, 2012)
- 29.Ai J, Smith B, Wong DT. Saliva Ontology: an ontology-based framework for a Salivaomics Knowledge Base. BMC Bioinformatics. 2010;11:302. doi: 10.1186/1471-2105-11-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong DTW. Salivaomics. J Am Dent Assoc. 2012;143:19S–24S. doi: 10.14219/jada.archive.2012.0339. [DOI] [PubMed] [Google Scholar]
- 31.Hu S, Wang J, Meijer J, Ieong S, Xie Y, Yu T, Zhou H, Henry S, Vissink A, Pijpe J, Kallenberg C, Elashoff D, Loo JA, Wong DT. Salivary proteomic and genomic biomarkers for primary Sjögren’s syndrome. Arthritis Rheum. 2007;56:3588–600. doi: 10.1002/art.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu S, Gao K, Pollard R, Arellano-Garcia M, Zhou H, Zhang L, Elashoff D, Kallenberg CGM, Vissink A, Wong DT. Preclinical validation of salivary biomarkers for primary Sjögren’s syndrome. Arthritis Care Res (Hoboken) 2010;62:1633–8. doi: 10.1002/acr.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu S, Vissink A, Arellano M, Roozendaal C, Zhou H, Kallenberg CGM, Wong DT. Identification of autoantibody biomarkers for primary Sjögren’s syndrome using protein microarrays. Proteomics. 2011;11:1499–507. doi: 10.1002/pmic.201000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St John MAR, Li Y, Zhou X, Denny P, Ho C-M, Montemagno C, Shi W, Qi F, Wu B, Sinha U, Jordan R, Wolinsky L, Park N-H, Liu H, Abemayor E, Wong DTW. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:929–35. doi: 10.1001/archotol.130.8.929. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, St John MAR, Zhou X, Kim Y, Sinha U, Jordan RCK, Eisele D, Abemayor E, Elashoff D, Park N-H, Wong DT. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res. 2004;10:8442–50. doi: 10.1158/1078-0432.CCR-04-1167. [DOI] [PubMed] [Google Scholar]
- 36.Arellano-Garcia ME, Hu S, Wang J, Henson B, Zhou H, Chia D, Wong DT. Multiplexed immunobead-based assay for detection of oral cancer protein biomarkers in saliva. Oral Dis. 2008;14:705–12. doi: 10.1111/j.1601-0825.2008.01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu S, Arellano M, Boontheung P, Wang J, Zhou H, Jiang J, Elashoff D, Wei R, Loo JA, Wong DT. Salivary proteomics for oral cancer biomarker discovery. Clin Cancer Res. 2008;14:6246–52. doi: 10.1158/1078-0432.CCR-07-5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elashoff D, Zhou H, Reiss J, Wang J, Xiao H, Henson B, Hu S, Arellano M, Sinha U, Le A, Messadi D, Wang M, Nabili V, Lingen M, Morris D, Randolph T, Feng Z, Akin D, Kastratovic DA, Chia D, Abemayor E, Wong DTW. Prevalidation of salivary biomarkers for oral cancer detection. Cancer Epidemiol Biomarkers Prev. 2012;21:664–72. doi: 10.1158/1055-9965.EPI-11-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao PV, Reddy AP, Lu X, Dasari S, Krishnaprasad A, Biggs E, Roberts CT, Nagalla SR. Proteomic identification of salivary biomarkers of type-2 diabetes. J Proteome Res. 2009;8:239–45. doi: 10.1021/pr8003776. [DOI] [PubMed] [Google Scholar]
- 40.Gao K, Zhou H, Zhang L, Lee JW, Zhou Q, Hu S, Wolinsky LE, Farrell J, Eibl G, Wong DT. Systemic disease-induced salivary biomarker profiles in mouse models of melanoma and non-small cell lung cancer. PLoS One. 2009;4:e5875. doi: 10.1371/journal.pone.0005875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao H, Zhang L, Zhou H, Lee JM, Garon EB, Wong DTW. Proteomic analysis of human saliva from lung cancer patients using two-dimensional difference gel electrophoresis and mass spectrometry. Mol Cell Proteomics. 2012;11:M111.012112. doi: 10.1074/mcp.M111.012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Xiao H, Zhou H, Santiago S, Lee JM, Garon EB, Yang J, Brinkmann O, Yan X, Akin D, Chia D, Elashoff D, Park N-H, Wong DTW. Development of transcriptomic biomarker signature in human saliva to detect lung cancer. Cell Mol Life Sci. 2012;69:3341–50. doi: 10.1007/s00018-012-1027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Farrell JJ, Zhou H, Elashoff D, Akin D, Park N-H, Chia D, Wong DT. Salivary transcriptomic biomarkers for detection of resectable pancreatic cancer. Gastroenterology. 2010;138:949–57. doi: 10.1053/j.gastro.2009.11.010. e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lau C, Kim Y, Chia D, Spielmann N, Eibl G, Elashoff D, Wei F, Lin Y-L, Moro A, Grogan T, Chiang S, Feinstein E, Schafer C, Farrell J, Wong DTW. Role of pancreatic cancer-derived exosomes in salivary biomarker development. J Biol Chem. 2013;288:26888–97. doi: 10.1074/jbc.M113.452458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brooks MN, Wang J, Li Y, Zhang R, Elashoff D, Wong DT. Salivary protein factors are elevated in breast cancer patients. Mol Med Rep. 2008;1:375–8. [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L, Xiao H, Karlan S, Zhou H, Gross J, Elashoff D, Akin D, Yan X, Chia D, Karlan B, Wong DT. Discovery and preclinical validation of salivary transcriptomic and proteomic biomarkers for the non-invasive detection of breast cancer. PLoS One. 2010;5:e15573. doi: 10.1371/journal.pone.0015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee Y-H, Kim JH, Zhou H, Kim BW, Wong DT. Salivary transcriptomic biomarkers for detection of ovarian cancer: for serous papillary adenocarcinoma. J Mol Med (Berl) 2012;90:427–34. doi: 10.1007/s00109-011-0829-0. [DOI] [PubMed] [Google Scholar]
- 48.Mattick JS. Non-coding RNAs: the architects of eukaryotic complexity. EMBO Rep. 2001;2:986–91. doi: 10.1093/embo-reports/kve230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris KV, Mattick JS. The rise of regulatory RNA. Nat Rev Genet. 2014;15:423–437. doi: 10.1038/nrg3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orozco AF, Lewis DE. Flow cytometric analysis of circulating microparticles in plasma. Cytometry A. 2010;77:502–14. doi: 10.1002/cyto.a.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reis EM, Verjovski-Almeida S. Perspectives of Long Non-Coding RNAs in Cancer Diagnostics. Front Genet. 2012;3:32. doi: 10.3389/fgene.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 53.Dalmay T. MicroRNAs and cancer. J Intern Med. 2008;263:366–75. doi: 10.1111/j.1365-2796.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- 54.Brosnan CA, Voinnet O. The long and the short of noncoding RNAs. Curr Opin Cell Biol. 2009;21:416–25. doi: 10.1016/j.ceb.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–74. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 56.Tandon M, Gallo A, Jang S-I, Illei GG, Alevizos I. Deep sequencing of short RNAs reveals novel microRNAs in minor salivary glands of patients with Sjögren’s syndrome. Oral Dis. 2012;18:127–31. doi: 10.1111/j.1601-0825.2011.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogawa Y, Taketomi Y, Murakami M, Tsujimoto M, Yanoshita R. Small RNA transcriptomes of two types of exosomes in human whole saliva determined by next generation sequencing. Biol Pharm Bull. 2013;36:66–75. doi: 10.1248/bpb.b12-00607. [DOI] [PubMed] [Google Scholar]
- 58.Bahn JH, Zhang Q, Li F, Chan T-M, Lin X, Kim Y, Wong DTW, Xiao X. The Landscape of MicroRNA, Piwi-Interacting RNA, and Circular RNA in Human Saliva. Clin Chem. 2014 doi: 10.1373/clinchem.2014.230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michael A, Bajracharya SD, Yuen PST, Zhou H, Star Ra, Illei GG, Alevizos I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34–8. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson P, Kiriakidou M, Sharma A, Maniataki E, Mourelatos Z. The microRNA world: small is mighty. Trends Biochem Sci. 2003;28:534–40. doi: 10.1016/j.tibs.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–41. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel RS, Jakymiw A, Yao B, Pauley BA, Carcamo WC, Katz J, Cheng JQ, Chan EKL. High resolution of microRNA signatures in human whole saliva. Arch Oral Biol. 2011;56:1506–13. doi: 10.1016/j.archoralbio.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E, Wong DT. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res. 2009;15:5473–7. doi: 10.1158/1078-0432.CCR-09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanson EK, Lubenow H, Ballantyne J. Identification of forensically relevant body fluids using a panel of differentially expressed microRNAs. Anal Biochem. 2009;387:303–14. doi: 10.1016/j.ab.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 65.Palanisamy V, Sharma S, Deshpande A, Zhou H, Gimzewski J, Wong DT. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS One. 2010;5:e8577. doi: 10.1371/journal.pone.0008577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma S, Rasool HI, Palanisamy V, Mathisen C, Schmidt M, Wong DT, Gimzewski JK. Structural-mechanical characterization of nanoparticle exosomes in human saliva, using correlative AFM, FESEM, and force spectroscopy. ACS Nano. 2010;4:1921–6. doi: 10.1021/nn901824n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao H, Wong DTW. Proteomic analysis of microvesicles in human saliva by gel electrophoresis with liquid chromatography-mass spectrometry. Anal Chim Acta. 2012;723:61–7. doi: 10.1016/j.aca.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 68.Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7:e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiklund ED, Gao S, Hulf T, Sibbritt T, Nair S, Costea DE, Villadsen SB, Bakholdt V, Bramsen JB, Sørensen JA, Krogdahl A, Clark SJ, Kjems J. MicroRNA alterations and associated aberrant DNA methylation patterns across multiple sample types in oral squamous cell carcinoma. PLoS One. 2011;6:e27840. doi: 10.1371/journal.pone.0027840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu C-J, Lin S-C, Yang C-C, Cheng H-W, Chang K-W. Exploiting salivary miR-31 as a clinical biomarker of oral squamous cell carcinoma. Head Neck. 2012;34:219–24. doi: 10.1002/hed.21713. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, Zhang J, Guo Y, Wu X, Yang W, Xu L, Chen J, Fu F. A novel electrically magnetic-controllable electrochemical biosensor for the ultra sensitive and specific detection of attomolar level oral cancer-related microRNA. Biosens Bioelectron. 2013;45:108–13. doi: 10.1016/j.bios.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 72.Yang Y, Li Y, Yang X, Jiang L, Zhou Z, Zhu Y. Progress risk assessment of oral premalignant lesions with saliva miRNA analysis. BMC Cancer. 2013;13:129. doi: 10.1186/1471-2407-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Momen-Heravi F, Trachtenberg AJ, Kuo WP, Cheng YS. Genomewide Study of Salivary MicroRNAs for Detection of Oral Cancer. J Dent Res. 2014;93:86S–93S. doi: 10.1177/0022034514531018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.NanoString nCounter® miRNA Expression Assays. http://www.nanostring.com/products/miRNA.
- 75.Wang Y, Wang Q, Zhang N, Ma H, Gu Y, Tang H, Xu Z, Gao Y. Identification of microRNAs as novel biomarkers for detecting esophageal squamous cell carcinoma in Asians: a meta-analysis. Tumour Biol. 2014;35:11595–604. doi: 10.1007/s13277-014-2350-x. [DOI] [PubMed] [Google Scholar]
- 76.Xie Z, Chen G, Zhang X, Li D, Huang J, Yang C, Zhang P, Qin Y, Duan Y, Gong B, Li Z. Salivary microRNAs as promising biomarkers for detection of esophageal cancer. PLoS One. 2013;8:e57502. doi: 10.1371/journal.pone.0057502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu W, Hou W, Wu Z, Wang Y, Yi Y, Lin W. miRNA-144 in the saliva is a genetic marker for early diagnosis of esophageal cancer. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:1783–6. [PubMed] [Google Scholar]
- 78.Tang H, Wu Z, Zhang J, Su B. Salivary lncRNA as a potential marker for oral squamous cell carcinoma diagnosis. Mol Med Rep. 2013;7:761–6. doi: 10.3892/mmr.2012.1254. [DOI] [PubMed] [Google Scholar]
- 79.Isin M, Ozgur E, Cetin G, Erten N, Aktan M, Gezer U, Dalay N. Investigation of circulating lncRNAs in B-cell neoplasms. Clin Chim Acta. 2014;431:255–9. doi: 10.1016/j.cca.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 80.Ren S, Wang F, Shen J, Sun Y, Xu W, Lu J, Wei M, Xu C, Wu C, Zhang Z, Gao X, Liu Z, Hou J, Huang J, Sun Y. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. Eur J Cancer. 2013;49:2949–59. doi: 10.1016/j.ejca.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 81.Rönnau CGH, Verhaegh GW, Luna-Velez MV, Schalken JA. Noncoding RNAs as novel biomarkers in prostate cancer. Biomed Res Int. 2014;2014:591703. doi: 10.1155/2014/591703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kohls K, Schmidt D, Holdenrieder S, Müller SC, Ellinger J. Detection of cell-free lncRNA in serum of cancer patients. Urologe A. 2014 doi: 10.1007/s00120-014-3655-5. [DOI] [PubMed] [Google Scholar]
- 83.Hizir MS, Balcioglu M, Rana M, Robertson NM, Yigit MV. Simultaneous detection of circulating oncomiRs from body fluids for prostate cancer staging using nanographene oxide. ACS Appl Mater Interfaces. 2014;6:14772–8. doi: 10.1021/am504190a. [DOI] [PubMed] [Google Scholar]
- 84.Perez P, Jang SI, Alevizos I. Emerging landscape of non-coding RNAs in oral health and disease. Oral Dis. 2014;20:226–235. doi: 10.1111/odi.12142. (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]


