Abstract
Atherosclerosis occurs in the subendothelial space (intima) of medium-sized arteries at regions of disturbed blood flow and is triggered by an interplay between endothelial dysfunction and subendothelial lipoprotein retention. Over time, this process stimulates a nonresolving inflammatory response that can cause intimal destruction, arterial thrombosis, and end-organ ischemia. Recent advances highlight important cell biological atherogenic processes, including mechanotransduction and inflammatory processes in endothelial cells, origins and contributions of lesional macrophages, and origins and phenotypic switching of lesional smooth muscle cells. These advances illustrate how in-depth mechanistic knowledge of the cellular pathobiology of atherosclerosis can lead to new ideas for therapy.
Atherosclerotic vascular disease is the underlying cause of myocardial infarction (heart attack), stroke, unstable angina (ischemic heart pain), and sudden cardiac death (Lusis, 2000). Collectively, these diseases account for the leading cause of death in the world, and the incidence is continuing to rise as a result of the international epidemic of obesity and type 2 diabetes, which are potent risk factors for atherosclerosis (Braunwald, 1997; World Health Organization, 2014). The disease is initiated by the subendothelial retention of apolipoprotein B (apoB)–containing lipoproteins (LPs) in focal areas of arteries, particularly regions in which laminar flow is disturbed by bends or branch points in the arteries (Williams and Tabas, 1995). Various modifications of the retained LPs likely mimic pathogen- and/or damage-associated molecular patterns (DAMPs) and thereby trigger a low-grade inflammatory response. This response lead to activation of endothelial and vascular smooth muscle cells (SMCs); recruitment of monocytes; and accumulation of cellular, extracellular, and lipid material in the subendothelial space, or intima. The cells include monocyte-derived macrophages, other inflammatory cells, including T cells, B cells, dendritic cells, and mast cells, and SMCs that take on myofibroblast characteristics. Atherosclerotic lesions most often undergo a partial resolution process characterized by the formation of an overlying scar, or fibrous cap (Libby, 2008; Falk et al., 2013). This fibrous cap provides a “protective” barrier between platelets in the blood stream and prothrombotic material in the plaque. Moreover, outward remodeling of the arterial wall, resulting in preservation of lumenal blood flow, and collateral vessel formation help prevent end organ ischemia. Thus, most atherosclerotic lesions do not cause acute vascular disease (Virmani et al., 2002).
However, certain types of atherosclerotic lesions over time develop features that can lead to acute thrombotic vascular disease. The features of these so-called “vulnerable plaques” include a large area of necrosis in the intima, called the necrotic or lipid core, thinning of the fibrous cap, and a heightened inflammatory state. These features can lead to breakdown of the aforementioned fibrous cap barrier and thereby promote acute lumenal thrombosis. If the thrombosis is occlusive, end organ damage occurs. Plaque necrosis results from a combination of defective efferocytosis, or clearance of apoptotic cells, and primary necrosis of these cells (Moore and Tabas, 2011). Fibrous cap thinning is likely caused by both defective collagen synthesis by intimal SMCs and increased degradation by matrix metalloproteinases secreted by inflammatory cells. Activation of innate and adaptive immune pathways contribute to the inflammatory response (Hansson and Hermansson, 2011), and this is likely amplified in advanced lesions by the increased production of DAMPs from necrotic cells. Moreover, there are many features of defective inflammation resolution, which may be caused by defective production and/or action of proresolving mediators, which are lipid and protein factors that promote repair and healing after the initial inflammatory assault (Libby et al., 2014).
In this review, we will focus on how three cell types that participate in atherosclerosis—endothelial cells, macrophages, and intimal SMC—contribute to atherogenesis and vulnerable plaque formation. Rather than an all-inclusive review of how these three cell types contribute to atherosclerosis, we emphasize overall principles of cellular pathophysiology and new areas of investigation.
Endothelial cells
Endothelial cell function, dysfunction, and atherogenesis.
The endothelial lining of the vascular system comprises a dynamic interface with the blood and acts as an integrator and transducer of both humoral and mechanical stimuli. The vascular endothelium responds to these stimuli by synthesizing and metabolizing products that then act in an autocrine and paracrine manner to maintain vascular homeostasis. In this regard, alterations of the endothelial phenotype into a dysfunctional state constitute a pathogenic risk factor for several vascular diseases including atherosclerosis. Atherosclerosis is a spatially nonrandom and temporally nonlinear process that initially affects so-called lesion-prone areas of the arterial tree. These areas display a unique endothelial dysfunctional phenotype (proinflammatory, prothrombotic, impaired barrier function), which is triggered by the distinct type of biomechanical forces present in these regions. These prelesional but susceptible areas are also distinguished by their predisposition to the retention of apoB LPs, which then further exacerbates the endothelial dysfunctional phenotype, particularly after the LPs become modified by oxidation and perhaps other processes (Tabas et al., 2007). This amplifying combination of endothelial dysfunction and apoB LP retention stimulates monocyte entry and provokes their differentiation into macrophages, which become loaded with LP cholesterol (“foam cell” formation) beneath a physically intact but dysfunctional endothelial lining. Additional factors important for the activation of the arterial endothelium in a pro-atherogenic manner include cytokines, advanced glycosylation end products, and possibly pathogen-associated molecular patterns from bacteria or viruses. The concept that biomechanical forces generated by the flow of blood can act as local risk factors for atherosclerosis provides an interesting conceptual framework, which is the central topic of this section.
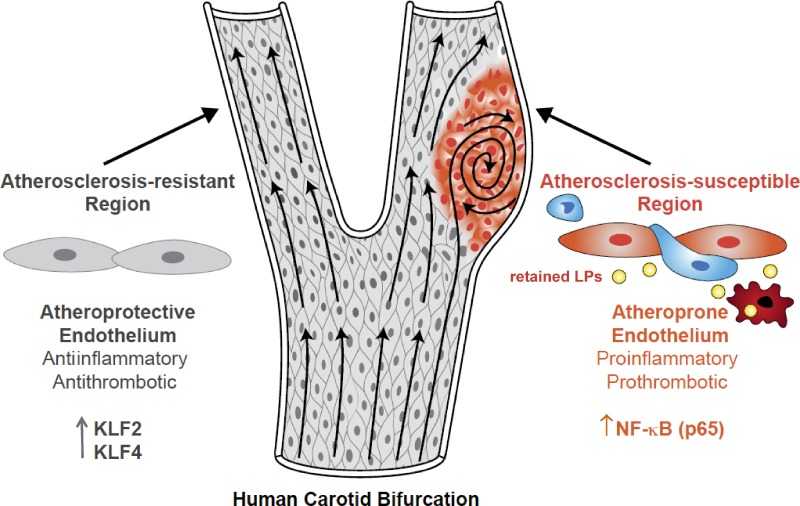
An intriguing aspect of the disease is that atherosclerotic lesions develop in a nonrandom fashion—typically around areas where blood vessels branch or curve. Physical and computational models have determined that these susceptible regions have low time-average shear stress, a high oscillatory shear index, and a steep temporal and spatial gradient in shear stress (atheroprone flow). In contrast, unbranched arteries that are exposed to uniform laminar shear stress (atheroprotective flow) largely do not develop lesions. It is now well documented that the endothelial cells overlying atherosclerosis-resistant versus -susceptible regions of the human carotid or mouse aorta have unique structural, molecular, and functional differences that help explain, at least in part, their atheroprotective versus atheroprone phenotypes (Fig. 1; Gimbrone and García-Cardeña, 2013).
Figure 1.
Vascular endothelial cells and the development of early atherosclerotic lesions. Early lesions of atherosclerosis in the human carotid artery develop in the area of a major curvature (carotid sinus) exposed to low time-average shear stress, a high oscillatory shear index, and steep temporal and spatial gradients. Endothelial cells at this site display an atheroprone phenotype, which promotes a proinflammatory milieu driven by the priming of the NF-κB signaling pathway, which is then perpetuated in response to subendothelial apoB LPs. NF-κB activation promotes the entry of blood-borne monocytes (blue cells) through the junctions of endothelial cells (orange cells) into the intima, and there, monocytes differentiate into macrophages (red cells). In contrast, arterial geometries that are exposed to uniform laminar flow evoke an atheroprotective endothelial cell phenotype driven by the transcriptional integrators KLF2 and KLF4. This atheroprotective endothelial phenotype, together with a decrease in LP retention, promotes an antiinflammatory and antithrombotic environment that affords relative protection from atherosclerotic lesion development.
Atheroprotective and atheroprone endothelium.
Endothelial cells from atherosclerosis-resistant regions display an ellipsoidal cell and nuclear morphology and coaxial alignment in the primary flow direction, in contrast to the atherosclerosis-susceptible regions where this orderly pattern is not present and where the cells display a cuboidal morphology. Moreover, a thick glycocalyx layer forms on the endothelium in atherosclerosis-resistant regions, and long-term exposure of cultured human endothelial cells to atheroprotective flow promotes the cell surface expression of key molecular components of the endothelial glycocalyx (Koo et al., 2013). Although the functional significance of the glycocalyx layer in the context of atherosclerosis is not known, some studies suggest that an intact glycocalyx layer decreases extravasation of low-density LP particles into the subendothelial space (van den Berg et al., 2009). Endothelial cells in atherosclerosis-susceptible regions also display impaired endothelial barrier function (McGill et al., 1957) and higher rates of cell turnover and cellular senescence compared with cells present in atherosclerosis-resistant regions (Gerrity et al., 1977; Hansson et al., 1985). They also express markers of chronic ER stress (Civelek et al., 2009), which may promote atherosclerosis by causing endothelial apoptosis (Zeng et al., 2009).
At the molecular level, atheroprotective and atheroprone endothelial phenotypes are associated with distinct patterns of gene expression and mechanoactivated signaling pathways. Among the most important distinctions are the activation of the transcriptional integrators KLF2 (Kruppel-like factor 2) and KLF4 (Kruppel-like factor 4) in the atheroprotective endothelium and activation of the NF-κB pathway in the atheroprone endothelium. Interestingly, these two pathways appear to play antagonistic roles in endothelial cells (Atkins and Simon, 2013). The expression of KLF2 and KLF4 is activated by the MEK5/ERK5/MEF2 signaling cascade (Parmar et al., 2006; Ohnesorge et al., 2010; Villarreal et al., 2010). This central signaling pathway can be modulated by AMP kinase, SIRT1, protein kinase C-ζ, SUMO-specific protease 2, histone deacetylase 5, and micro-RNAs (Abe and Berk, 2014). The importance of KLF2 for atheroprotection in vivo was documented by showing that Klf2+/−Apoe−/− mice exhibit a 31–37% increase in atherosclerotic lesion area compared with littermate control Apoe−/− mice (Atkins et al., 2008). Nevertheless, the contribution of endothelial KLF2 expression to this phenotype remains to be defined. Of interest, KLF2 in other atherosclerosis-relevant cell types, including monocytes (Das et al., 2006), T cells (Bu et al., 2010; Takada et al., 2011; Pabbisetty et al., 2014), and dendritic cells (Fang et al., 2013) maintains an antiinflammatory state and, in the case of myeloid cells, is atheroprotective (Lingrel et al., 2012). The role of endothelial KLF4 in atherosclerosis is clearer: recent work using endothelial-specific loss-of-function and gain-of-function approaches in Apoe−/− mice suggests that endothelial KLF4 is atheroprotective (Zhou et al., 2012).
A critical role for the endothelial NF-κB signaling pathway in early atherogenesis was demonstrated by a seminal study showing its activation in prelesional atherosclerosis-susceptible regions of the mouse aorta (Hajra et al., 2000). Atheroprone flow activates the NF-κB pathway in endothelial cells (Dai et al., 2004; Won et al., 2007), leading to expression of proatherogenic cell surface receptors, including VCAM-1 and the Toll-like receptor 2 (Mullick et al., 2008). In addition, the atheroprone endothelium produces several proinflammatory cytokines and chemokines, extracellular matrix proteins, growth factors, and micro-RNAs (Thomas et al., 2009; Feaver et al., 2010; Marin et al., 2013; Zhou et al., 2014a; Kumar et al., 2014), which may act as autocrine or paracrine factors to foster a local proatherogenic environment.
Disturbed flow also modulates global DNA methylation patterns via alterations in DNA methyltransferase activity, in particular DNMT1 (Dunn et al., 2014; Zhou et al., 2014b). More specifically, disturbed flow increases methylation of the proximal promoter of KLF4, thus inhibiting KLF4 transcription in atherosclerosis-susceptible regions (Jiang et al., 2014). These observations suggest that, during the development of the vascular system, the initial exposure of endothelial cells to atheroprotective or atheroprone flow may “mark” their DNA, leading to unique flow-dependent endothelial epigenetic landscapes. Atheroprone flow may also affect mRNA splicing. For example, disturbed flow suppresses a “protective” switch in the splicing of the EIIIA and EIIIB exons of the fibronectin gene (Murphy and Hynes, 2014).
Endothelial mechanotransduction.
The capacity displayed by endothelial cells to sense and discriminate distinct flow patterns raises the fundamental cell biological question of how these cells sense mechanical forces. Although the true nature of the mechanosensing and mechanotransduction systems in endothelial cells remains poorly characterized, several plausible and promising hypotheses supported by experimental data have been put forward in recent years (Conway and Schwartz, 2013). Here, we will highlight some recent developments in this area with a focus on cell surface–proximal sensors.
Previous work had shown a mechanotransducing role for PECAM-1, in complex with VE-cadherin and VEGFR2, at endothelial cell junctions, leading to downstream changes in NF-κB activation and cell alignment. Recent studies have led to a more in-depth understanding of the molecular mechanism. In particular, a flow-dependent GTP exchange factor called TIAM1 links PECAM-1 mechanotransduction to focal activation of the small GTPase Rac1 at the flow-downstream region of the cell (Liu et al., 2013). Rac1 activation then triggers the NF-kB pathway as well as production of reactive oxygen species.
A role for the G protein–coupled S1P1 (S1P receptor-1) in endothelial mechanotransduction has been documented (Jung et al., 2012). The expression of S1P1 was demonstrated to be important for flow-mediated directional alignment in cultured endothelial cells and for the characteristic alignment of the endothelium of the descending mouse aorta. Using endothelial cell–specific inducible S1p1−/− mice, this study also demonstrated that an activating phosphorylation site of endothelial nitric oxide synthase is decreased in the retinal vasculature of these mice when compared with wild-type littermate controls.
Another mechanosensor of interest is Piezo1, a mediator of shear stress–evoked ionic current and calcium influx in endothelial cells (Li et al., 2014). Piezo1-mediated calcium influx was shown to be important for an increase in calpain activity and subsequent rearrangement of focal adhesions. As such, endothelial cells isolated from Piezo1−/− mice failed to align in the direction of flow when exposed to atheroprotective flow. Moreover, endothelial cells in intact cerebral arteries of Piezo1−/− mice showed the same alignment defect. A second study also documented a role for Piezo1 in endothelial cell alignment using a siRNA approach in human endothelial cells exposed to atheroprotective flow (Ranade et al., 2014).
Finally, syndecan 4, which is a transmembrane heparan sulfate proteoglycan, was recently shown to be also required for endothelial cell alignment in cultured endothelial cells exposed to laminar shear stress and in the mouse thoracic aorta (Baeyens et al., 2014). When mice deficient for syndecan 4 were placed on a genetic background of elevated cholesterol and fed a high-fat diet, i.e., to promote atherosclerosis, they displayed an increase in atherosclerotic lesion formation when compared with similar mice with normal expression of syndecan 4.
Future challenges in this area include understanding how the several documented mechanosensors are integrated at the cellular and molecular level and how they affect specific endothelial cell processes. These efforts should ultimately lead to a better understanding of the function of endothelial mechanoactivated pathways in physiology, their dysregulation in atherogenesis, and their potential as therapeutic targets for the prevention and treatment of atherosclerotic vascular disease.
Macrophages
Origins of lesional macrophages.
Chemokine-induced influx of bone marrow–derived monocytes is triggered by endothelial activation in nascent lesions, i.e., as initiated by LP retention and endothelial cell alterations. In certain settings, the monocytes first seed the spleen, where they undergo additional rounds of proliferation and activation before reentering the blood stream and homing to atherosclerotic lesions (Dutta et al., 2012). The monocytes that most readily enter developing and progressing atherosclerotic lesions in mice are Ly6hi monocytes, which is the subset that participates in the inflammatory response. However, atherosclerosis is maximally inhibited only when the entry of both Ly6hi and Ly6lo monocytes is blocked (Tacke et al., 2007), suggesting a more complex picture. Moreover, the nature and functions of monocyte subpopulations in humans differ from those in mice, and the roles of human monocyte subpopulations in atherosclerosis is not known.
Until recently, it was generally assumed that each lesional macrophage originated from one monocyte despite hints in the literature that macrophages in human and animal atherosclerotic lesions undergo proliferation. Recent work has provided more convincing evidence that macrophage proliferation may be a quantitatively important process in macrophage accumulation in advanced lesions, at least in murine models of atherosclerosis (Robbins et al., 2013). Previous in vitro work had shown that activation of type A scavenger receptors on macrophages can promote macrophage proliferation, perhaps by activating a phosphatidylinositol-3-kinase pathway (Sakai et al., 2000), but the relevance of this mechanism in vivo remains to be shown.
Roles of macrophages in early atherosclerosis.
Monocyte-derived macrophages are key drivers of the atherogenic process (Fig. 2). Processes that promote the proliferation of bone marrow–derived hematopoietic stem cells, including cholesterol accumulation caused by defective cholesterol efflux, increase circulating monocytes and promote atherogenesis (Murphy et al., 2014). Indeed, there is a significant and independent correlation between blood monocyte count and atherosclerotic vascular disease in humans. Lesional macrophages encounter and internalize subendothelially retained LPs, which can be native or modified by oxidation, aggregation, and other processes. In vitro studies suggest that the LPs can be internalized by a combination of phagocytosis of aggregated LPs, scavenger receptor-mediated uptake of modified LPs, and fluid-phase pinocytosis of native LPs. In the traditional pathway, internalized LPs are delivered to late endosomes and lysosomes, where various LP lipids and proteins are degraded by lysosomal hydrolases. However, recent studies examining the interaction of cultured macrophages with matrix-bound aggregated LPs, which may be particularly relevant to atherosclerosis, raise the possibility that LP hydrolysis can also occur in sealed-off, acidic extracellular compartments that receive hydrolases through lysosomal exocytosis (Haka et al., 2009).
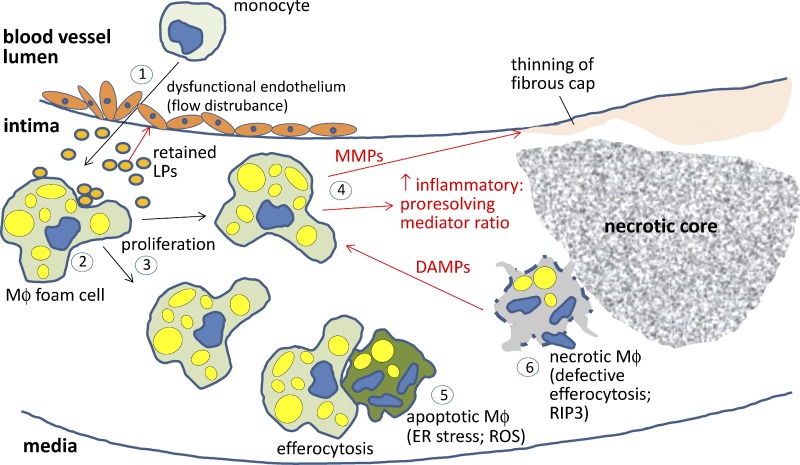
Figure 2.
Proatherogenic roles of lesional macrophages. (1) The two-way interplay between activated, dysfunctional endothelium, i.e., as a result of flow disturbances, and apoB LP retention triggers the entry of inflammatory monocytes into the subendothelial intima (red arrows depict endothelial dysfunction triggered by retained LPs in the intima). (2) The macrophages (MΦ) ingest the retained LPs through various pathways and become lipid-loaded foam cells. (3) Lesional macrophages can proliferate, particularly in advanced lesions. (4) Macrophages promote plaque progression by propagating a maladaptive, nonresolving inflammatory response characterized by an imbalance of inflammatory-to-proresolving mediators. Moreover, matrix metalloproteinases (MMPs) secreted by inflammatory macrophages can lead to thinning of the fibrous cap and plaque rupture. (5) Environmental factors in advanced lesions promote macrophage apoptosis, e.g., as a result of prolonged ER stress and/or oxidative stress. Apoptotic cell death may not be problematic if cleared efficiently by lesional phagocytes (efferocytosis). (6) However, in advanced atherosclerosis, this process goes awry, leading to postapoptotic necrosis. Necrotic cells, which can also develop through RIP3 activation (primary necrosis), release DAMPs, which amplify inflammation. These cells can also coalesce into areas, called necrotic cores, that promote plaque breakdown and thrombosis. ROS, reactive oxygen species.
The activation of inflammatory pathways in lesional macrophages is a critical proatherogenic process. In particular, certain subpopulations of lesional macrophages take on an inflammatory, M1-like phenotype, which further activates the endothelium and leads to additional rounds of monocyte recruitment (Peled and Fisher, 2014). The exact mechanisms of macrophage activation in lesions remain to be fully explored, but modified LPs and other lesional molecules can activate receptors involved in inflammatory signaling, such as toll-like receptors and nucleotide-binding oligomerization domain–like receptors. Furthermore, LP cholesterol can accumulate in the plasma membrane, where it enhances inflammatory receptor signaling by altering membrane properties (Fessler and Parks, 2011; Westerterp et al., 2014). Oxidative stress induced by modified LPs, oxysterols, and other lesional factors can also activate inflammatory pathways (Glass and Witztum, 2001). For example, mitochondrial oxidative stress occurs in both human and animal atherosclerosis and can be induced in cultured macrophages by oxidized LPs and sterols. Mitochondrial oxidative stess enhances NF-κB activation, which leads to induction of the monocyte chemokine MCP-1 and further recruitment of monocytes (Wang et al., 2014).
In addition, there is ample evidence of inflammasome activation in atherosclerotic lesions, and IL-1β likely plays an important role in early atherogenesis (Lu and Kakkar, 2014). Based on in vitro studies and in vivo observations, a leading hypothesis posits that cholesterol microcrystals derived from the cellular ingestion of retained LPs activate the inflammasome pathway (Duewell et al., 2010). However, it is not clear whether cholesterol crystallization would be robust enough at body temperature to activate the inflammasome pathway, and so other mechanisms of are being explored. For example, activation of CD36 by modified LPs promotes the conversion of cytoplasmic soluble molecules, such as β-amyloid, into inflammasome-activating stimuli (Sheedy et al., 2013). In addition, oxidized mitochondrial DNA molecules resulting from mitochondrial oxidative stress can activate the inflammasome (Zhou et al., 2011).
The net proatherogenic effect of lesional macrophages is best conceived as a tipping of a delicate balance between inflammatory and proresolving responses (Tabas, 2010). For example, the proinflammatory consequence of excess plasma membrane cholesterol is counterbalanced by cholesterol efflux mediated in large part by the ABCA1 and ABCG1 transporters. Moreover, when macrophages internalize atherogenic LPs, there is an accumulation of the cholesterol intermediate desmosterol, which triggers a liver X receptor–mediated antiinflammatory response (Spann et al., 2012). Another example is the balance between the synthesis of proinflammatory (and proatherogenic) leukotriene B4 and proresolving lipoxin A4 in macrophages, which is regulated by the subcellular localization of the enzyme 5-lipoxygenase and by mediators of inflammation resolution (Fredman et al., 2014). In addition, inflammatory processes can induce compensatory proresolving signaling pathways. For example, when the NF-κB pathway is genetically blocked in macrophages in fat-fed Ldlr−/− mice, early lesion development is actually accelerated, which may be tied to inhibition of a compensatory IL-10 response (Kanters et al., 2003). The implication of this balancing concept is that it may be very difficult to prevent atherosclerosis by simply blocking a specific inflammatory pathway (Tabas and Glass, 2013).
Roles of macrophages in vulnerable plaque formation.
The subtype of atherosclerotic lesions that cause acute atherothrombotic vascular events are characterized by large areas of necrosis, nonresolving inflammation, and thinning of the subendothelial fibrous cap. Plaque necrosis results in large part by the combination of lesional macrophage apoptosis and defective efferocytosis, which leads to postapoptotic necrosis, loss of efferocytosis-mediated antiinflammatory signaling, and generation of proinflammatory DAMPs. RIP3-mediated primary necrosis may also contribute to plaque necrosis (Lin et al., 2013). Macrophages likely contribute to fibrous cap thinning by the secretion of matrix metalloproteinases (Libby, 2013), although this has been difficult to prove in vivo because mouse models of atherosclerosis do not mimic the type of plaque rupture that occurs in humans (Fig. 2).
Advanced lesional macrophage apoptosis is likely induced by a variety of factors. Examples include oxidized LPs, oxidized phospholipids, and excess accumulation of LP-derived cholesterol in the ER. Moreover, in view of the importance of obesity and type 2 diabetes as a major driver of coronary artery disease, systemic risk factors associated with insulin resistance as well as direct effects of defective insulin signaling on macrophages can promote macrophage cell death (Bornfeldt and Tabas, 2011). A common process associated with a variety of death-inducing factors in advanced lesions is activation of a prolonged unfolded protein response, which can trigger several apoptotic pathways (Tabas and Ron, 2011). In vivo studies in mice have indicated a direct, causative role for the ER effector C/EB-homologous protein (CHOP) in lesional apoptosis (Thorp et al., 2009), and there is a very strong correlation among CHOP expression, apoptosis, and the degree of plaque vulnerability in human coronary and carotid arteries (Myoishi et al., 2007; Dorweiler et al., 2014). CHOP induces a variety of apoptotic pathways, but one that may be particularly relevant to advanced lesional macrophages involves activation of the inositol-3-phosphate receptor ER calcium release channel (Li et al., 2009). The resulting increase in cytosolic calcium activates CaMKII (calcium/calmodulin-dependent proteinase II), which, via downstream signaling, engages both death receptor and mitochondrial pathways of apoptosis (Timmins et al., 2009). CHOP has also been shown to decrease the expression of the cell survival protein Bcl-2, and Bcl-2 deficiency promotes advanced lesional macrophage death and plaque necrosis.
Macrophage efferocytosis becomes defective in advanced lesions of both humans and animals (Schrijvers et al., 2005; Tabas, 2005). Efferocytosis is carried out by the interaction of apoptotic cell recognition motifs, macrophage receptors, and molecules that bridge these two components (Hochreiter-Hufford and Ravichandran, 2013). Several of these molecules have been shown to mediate efferocytosis in atherosclerotic lesions, and thus, defective efferocytosis and subsequent plaque necrosis could develop as a consequence of compromised expression or function of these molecules (Thorp et al., 2011a). As one possible example, a macrophage receptor called MerTK, which is functionally important in lesional efferocytosis, undergoes an ADAM17 protease-mediated cleavage reaction (Sather et al., 2007; Thorp et al., 2011b). MerTK cleavage both destroys the receptor and creates a long-lived extracellular portion, called soluble Mer, that acts as a competitor inhibitor of apoptotic cell uptake by sequestering efferocytosis bridging molecules. MerTK cleavage is triggered by inflammation, which makes it a plausible contributor to defective efferocytosis in advanced atherosclerosis. Indeed, there is evidence of MerTK cleavage in advanced human plaques, particularly in plaque necrosis (Garbin et al., 2013). This hypothesis and alternative ones related to other efferocytosis molecules awaits validation in vivo.
Much of the maladaptive behavior of macrophages in advanced atherosclerosis, including their persistent inflammatory state, continued influx of monocytes, and defective efferocytosis, can be explained on the basis of defective inflammation resolution. In physiological host defense and response to injury, the inflammatory phase directly triggers a resolving phase that promotes repair of collateral tissue damage and return to homeostasis (Nathan and Ding, 2010). Resolution is mediated by proteins, such as IL-10, TGF-β, and annexin A1, and by small lipids derived from arachidonic acid and omega-3 fatty acids, such as lipoxins, resolvins, protectins, and maresins (Buckley et al., 2014). Although the persistence and amplification of the major inflammatory stimulus in atherosclerosis—retained subendothelial LPs—goes a long way in explaining defective resolution, it is also possible that defective expression of proresolving mediators and/or their receptors also occurs. Moreover, this paradigm provides a potentially unique therapeutic opportunity to inhibit advanced plaque progression, because proresolving mediators, unlike direct inhibitors of inflammatory cytokines or chemokines, are less likely to compromise host defense. Two recent studies demonstrated the potential promise of proresolving therapy for atherosclerosis using mouse models of atherosclerosis (Drechsler et al., 2015; Fredman et al., 2015).
SMCs
Origins and fates of vascular SMCs in atherosclerosis.
LP accumulation, endothelial activation, and inflammatory responses in developing atherosclerotic lesions result in “activation” or “phenotypic switching” of SMCs. During this process, quiescent, fully contractile SMCs down-regulate expression of differentiation marker genes, such as those encoding smooth muscle α-actin (Acta2) and smooth muscle myosin heavy chain (Myh11). As a consequence, the SMCs undergo cell proliferation and migration, and they increase their production of extracellular matrix, proteoglycans, and other proteins believed to be beneficial in outward vessel remodeling and plaque stabilization (Alexander and Owens, 2012). Indeed, the current dogma is that lesions that are more vulnerable to plaque rupture, and associated acute thrombotic events have a reduced fraction of SMC relative to inflammatory lipid-loaded macrophages, particularly in the vicinity of the fibrous plaque. However, there is considerable ambiguity as to which cells in atherosclerotic lesions are SMC-derived versus macrophage-derived, largely because of lack of rigorous, definitive lineage tracing studies.
Beginning efforts in this critical area can be illustrated by a few studies. For example, SMC lineage tracing in atheroprone Apoe−/− mice (Wamhoff et al., 2004; Gomez et al., 2013) revealed that in advanced lesions, intimal SMCs lacked detectable expression of smooth muscle α-actin (Acta2), smooth muscle myosin heavy chain (Myh11), and SM22α/transgelin (Tagln), which are the markers traditionally used to identify lesional SMCs. Moreover, cholesterol loading of cultured SMCs was reported to down-regulate SMC marker genes and induce macrophage markers, including CD68 and Mac2 (Rong et al., 2003). This phenomenon also appears to occur in atherosclerotic lesions: studies using SM22α ERT2 Cre-LacZ lineage tracing mice on the Apoe−/− background showed that SMC-derived cells in advanced lesions expressed Mac2 and CD68, although the very low labeling efficiency in these studies (11%) precludes assessing the overall contributions of these cells within lesions (Feil et al., 2014). In addition, it is unclear what function these cells have in overall lesion pathogenesis or if they are present within human atherosclerotic lesions. Importantly, the converse is also true: macrophages, or at least hematopoietic-derived cells, can express SMC markers, including smooth muscle α-actin and SM22α. For example, treatment of cultured macrophages with TGF-β or thrombin results in the expression of SMC markers on these cells, and lineage tracing studies have shown that hematopoietic-derived cells express early but not late stage markers of SMCs in Apoe−/− lesions (Stewart et al., 2009; Martin et al., 2009; Iwata et al., 2010). The latter studies are partially consistent with lineage tracing studies reporting that virtually all SMC marker–positive cells in lesions of Apoe−/− mice are of local SMC origin (Bentzon et al., 2006). Finally, Y chromosome lineage tracing studies in humans who have undergone cross-gender bone marrow transplantation have shown that ≥10% of smooth muscle α-actin–positive cells in advanced coronary artery lesions are of hematopoietic and not SMC origin (Caplice et al., 2003).
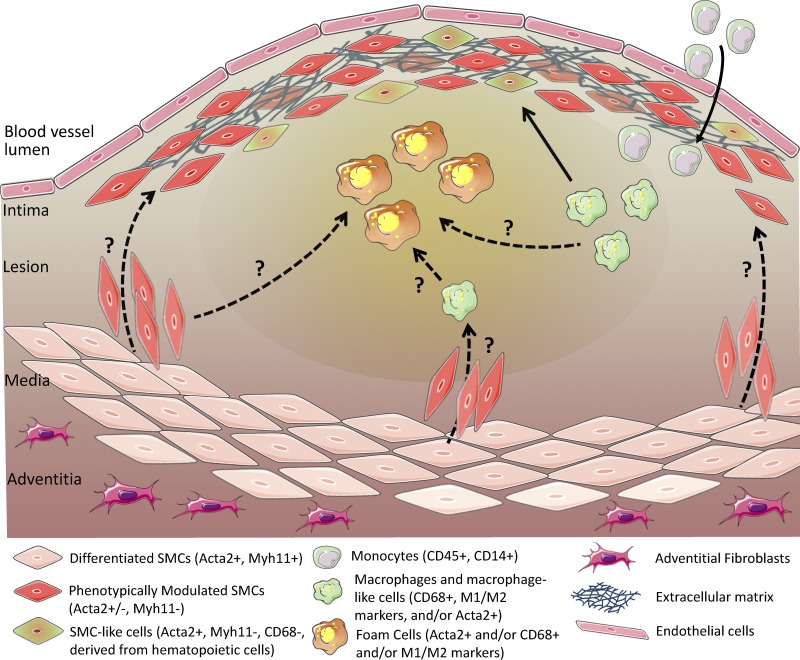
Collectively, the results from these various studies support the following conclusions: (a) it is highly likely, indeed certain, that SMCs and macrophages in atherosclerotic lesions have been misidentified in most previous studies in the field; (b) SMC marker–positive cells in lesions can be derived from multiple cell types other than SMCs; (c) the majority of SMC-derived cells in lesions have not been identified in previous studies as a result of loss of expression of SMC markers; and (d) macrophage marker–positive cells in lesions may not be macrophages or even of hematopoietic origin (Fig. 3).
Figure 3.
Ambiguity regarding the identity and origins of SMC, macrophages, and putative derivatives of these cells within advanced human atherosclerotic lesions. Lesional cells display remarkable heterogeneity as a result of effects of microenvironmental factors, including cytokines, inflammatory lipids, growth factors, dead cell debris, oxygen tension variations, and oxidative stress. For purposes of this figure, we have only considered data in intact human tissue specimens rather than studies in cultured cells or animal models. The solid arrows illustrate known pathways that give rise to lesion cells, whereas the dotted arrows indicate putative pathways not yet directly validated in humans. For example, cross-gender bone marrow transplant Y-chromosome lineage tracing studies provide clear evidence that myeloid cells, presumably monocytes, give rise to CD68+ macrophages but also Acta2+ SMC-like cells within advanced human coronary artery lesions. In contrast, there is no direct evidence that SMCs are the primary source of fibrous cap cells that produce extracellular matrix that stabilizes lesions because Acta2+ cells may be derived from SMCs, macrophages, or other cell types. Similarly, there is evidence that approximately half of the foam cells within advanced human coronary artery atherosclerotic lesions are Acta2+ and CD68+ (Allahverdian et al., 2014), but the origin of these cells is not clear.
Functional significance and mechanisms of SMC phenotypic switching.
In the final analysis, the most critical issue is how knowledge of the origin and phenotypic features of lesional cells helps us to understand the pathogenesis of lesion progression and formulate new ideas for cell-based therapies. As an example, we can consider a study showing that 50% of foam cells in advanced human coronary artery lesions express smooth muscle α-actin (Allahverdian et al., 2014). However, the majority of these cells also expressed the macrophage marker CD68, and thus, their origin is uncertain, particularly when one considers that cells of myeloid origin can be induced to express smooth muscle α-actin. Most importantly, expression of the cholesterol exporter ABCA1 (ATP-binding cassette transporter A1) was reduced in smooth muscle α-actin+ foam cells compared with smooth muscle α-actin−CD68+ cells, suggesting that the former subpopulation of foam cells might exhibit impaired reverse cholesterol transport and thereby contribute to plaque cholesterol burden and associated inflammation. Consistent with this idea, a recent study demonstrated that although cholesterol-loaded cultured SMCs show diminished expression of SMC markers and express some markers of macrophages, their overall transcriptome indicates they are likely to show impaired macrophage functions including phagocytosis and efferocytosis (Vengrenyuk et al., 2015).
The origin of vascular cells can also be important in understanding cell-specific consequences of common signaling pathways. For example, as reviewed in the Endothelial cells section, conditional knockout studies suggest that KLF4 is atheroprotective in endothelial cells and macrophages (Sharma et al., 2012). In SMCs, however, the results of vascular injury experiments (Yoshida et al., 2008) predict that KLF4 may have detrimental effects by lowering SMC content and hence decreasing plaque stability. SMC-specific deletion of KLF4 in the setting of atherosclerosis will be needed to test this prediction. In this regard, it is interesting to note that KLF4 is required for phenotypic switching of cultured SMCs in response to treatment with PDGF-BB or oxidized phospholipids (Gomez and Owens, 2012). As another example, whereas IL-1β signaling in macrophage-like cells is almost certainly proatherogenic, in vascular SMCs, it may promote plaque stability (Alexander et al., 2012).
In terms of identifying new cell-based therapeutic targets, these examples of how different cell types respond differently to environmental cues present within lesions highlight the importance of defining the cellular origins of lesional cells and understanding what controls their phenotypic transitions. In the case of SMCs, this knowledge will be critical if we wish to promote plaque stabilization. With regard to the regulation of phenotypic switching, cholesterol loading of cultured SMCs induces phenotypic switching to a macrophage-like state, but whether this is a key factor linking hypercholesterolemia and LP retention to the progression of atherosclerosis remains to be seen. There is also extensive evidence showing that various cytokines and growth factors can induce phenotypic switching in cultured SMCs, including PDGF-BB/DD, IL-1β, TNF, oxidized phospholipids, basic FGF, and SDF-1α. However, these observations need to be validated in vivo through studies combining rigorous SMC lineage tracing with SMC-specific conditional deletions of the candidate regulatory pathway of interest.
Conclusion
In diseases with well-defined initiating events, the most successful strategy is to prevent these events from occurring or to curtail them as quickly and efficiently as possible after they occurs. For atherosclerotic disease, this principle translates into the goals of reversing endothelial dysfunction in atherosusceptible sites and lowering apoB LPs as earlier and robustly as is safe. The rationale for this dual approach is that the interplay between apoB LP retention and endothelial dysfunction initiates and then sustains the maladaptive, nonresolving inflammatory response that ultimately leads to atherothrombotic clinical disease. In theory, this interplay could be broken by early, robust, and safe apoB LP lowering. However, there is some variability in the response to apoB-lowering drugs in terms of threshold for efficacy and safety, and this is likely to be the case with newer drugs as well. Moreover, the population at risk is extremely large, and it continues to grow in response to the epidemic of obesity and metabolic disease. Therefore, the most successful therapies will combine apoB LP-lowering therapy with complementary approaches that target endothelial dysfunction and other pathogenic cellular responses to these LPs.
In-depth knowledge of endothelial biology will be the key to solving the mystery of how apoB LPs traverse the endothelium and how this process, as well as the physical process of LP retention after entry, can be prevented. The focal nature of atherosclerosis, which is likely caused by focal blood flow disturbances, speaks to the necessity of fully understanding endothelial cell mechanotransduction biology to achieve this goal. In terms of the cellular responses after LP retention, the key issue is to elucidate how the LPs trigger a nonresolving inflammatory response in the various lesional cell types. To achieve this goal, it will be important to fully understand the interplay between events occurring in the subendothelial space per se and those being “communicated” to this space from the circulation through endothelial cell mechanotransduction. Ultimately, it is the inflammatory cells in the lesions that trigger the type of plaque changes that lead to cardiovascular disease. In that regard, the older notion that monocyte-derived macrophages are the culprit and that media-derived SMCs are protective needs to be reevaluated in view of lineage tracing studies that raise questions regarding the identify of these cells within lesions (Fig. 3). Integrating this new insight with more traditional mechanisms of innate and adaptive immunity will be key to achieving the ultimate goal of suppressing plaque inflammation in a manner that does not compromise host defense. For these newer efforts, further understanding the cellular biology of atherosclerosis will be essential.
Acknowledgments
This work was supported by National Institutes of Health grants HL107497 and HL075662 (I. Tabas), AG032443 and HL118826 (G. García-Cardeña), and HL057353, HL121008, and HL087867 (G.K. Owens).
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- apoB
- apolipoprotein B
- CHOP
- C/EB-homologous protein
- DAMP
- damage-associated molecular pattern
- LP
- lipoprotein
- SMC
- smooth muscle cell
References
- Abe J., and Berk B.C.. 2014. Novel mechanisms of endothelial mechanotransduction. Arterioscler. Thromb. Vasc. Biol. 34:2378–2386 10.1161/ATVBAHA.114.303428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M.R., and Owens G.K.. 2012. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu. Rev. Physiol. 74:13–40 10.1146/annurev-physiol-012110-142315 [DOI] [PubMed] [Google Scholar]
- Alexander M.R., Moehle C.W., Johnson J.L., Yang Z., Lee J.K., Jackson C.L., and Owens G.K.. 2012. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J. Clin. Invest. 122:70–79 10.1172/JCI43713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdian S., Chehroudi A.C., McManus B.M., Abraham T., and Francis G.A.. 2014. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 129:1551–1559 10.1161/CIRCULATIONAHA.113.005015 [DOI] [PubMed] [Google Scholar]
- Atkins G.B., and Simon D.I.. 2013. Interplay between NF-κB and Kruppel-like factors in vascular inflammation and atherosclerosis: location, location, location. J Am Heart Assoc. 2:e000290 10.1161/JAHA.113.000290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins G.B., Wang Y., Mahabeleshwar G.H., Shi H., Gao H., Kawanami D., Natesan V., Lin Z., Simon D.I., and Jain M.K.. 2008. Hemizygous deficiency of Krüppel-like factor 2 augments experimental atherosclerosis. Circ. Res. 103:690–693 10.1161/CIRCRESAHA.108.184663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens N., Mulligan-Kehoe M.J., Corti F., Simon D.D., Ross T.D., Rhodes J.M., Wang T.Z., Mejean C.O., Simons M., Humphrey J., and Schwartz M.A.. 2014. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc. Natl. Acad. Sci. USA. 111:17308–17313 10.1073/pnas.1413725111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzon J.F., Weile C., Sondergaard C.S., Hindkjaer J., Kassem M., and Falk E.. 2006. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler. Thromb. Vasc. Biol. 26:2696–2702 10.1161/01.ATV.0000247243.48542.9d [DOI] [PubMed] [Google Scholar]
- Bornfeldt K.E., and Tabas I.. 2011. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 14:575–585 10.1016/j.cmet.2011.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunwald E.1997. Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N. Engl. J. Med. 337:1360–1369 10.1056/NEJM199711063371906 [DOI] [PubMed] [Google Scholar]
- Bu D.X., Tarrio M., Grabie N., Zhang Y., Yamazaki H., Stavrakis G., Maganto-Garcia E., Pepper-Cunningham Z., Jarolim P., Aikawa M., et al. . 2010. Statin-induced Krüppel-like factor 2 expression in human and mouse T cells reduces inflammatory and pathogenic responses. J. Clin. Invest. 120:1961–1970 10.1172/JCI41384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley C.D., Gilroy D.W., and Serhan C.N.. 2014. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 40:315–327 10.1016/j.immuni.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplice N.M., Bunch T.J., Stalboerger P.G., Wang S., Simper D., Miller D.V., Russell S.J., Litzow M.R., and Edwards W.D.. 2003. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc. Natl. Acad. Sci. USA. 100:4754–4759 10.1073/pnas.0730743100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelek M., Manduchi E., Riley R.J., Stoeckert C.J. Jr, and Davies P.F.. 2009. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ. Res. 105:453–461 10.1161/CIRCRESAHA.109.203711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway D.E., and Schwartz M.A.. 2013. Flow-dependent cellular mechanotransduction in atherosclerosis. J. Cell Sci. 126:5101–5109 10.1242/jcs.138313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai G., Kaazempur-Mofrad M.R., Natarajan S., Zhang Y., Vaughn S., Blackman B.R., Kamm R.D., García-Cardeña G., and Gimbrone M.A. Jr. 2004. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc. Natl. Acad. Sci. USA. 101:14871–14876 10.1073/pnas.0406073101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das H., Kumar A., Lin Z., Patino W.D., Hwang P.M., Feinberg M.W., Majumder P.K., and Jain M.K.. 2006. Kruppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc. Natl. Acad. Sci. USA. 103:6653–6658 10.1073/pnas.0508235103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorweiler B., Grechowa I., Wallrath A., Vahl C.F., and Horke S.. 2014. Activation of the proapoptotic unfolded protein response in plaques of the human carotid artery. Eur. J. Vasc. Endovasc. Surg. 48:248–257 10.1016/j.ejvs.2014.06.038 [DOI] [PubMed] [Google Scholar]
- Drechsler M., de Jong R., Rossaint J., Viola J.R., Leoni G., Wang J.M., Grommes J., Hinkel R., Kupatt C., Weber C., et al. . 2015. Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ. Res. 116:827–835 10.1161/CIRCRESAHA.116.305825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duewell P., Kono H., Rayner K.J., Sirois C.M., Vladimer G., Bauernfeind F.G., Abela G.S., Franchi L., Nuñez G., Schnurr M., et al. . 2010. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 464:1357–1361 10.1038/nature08938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J., Qiu H., Kim S., Jjingo D., Hoffman R., Kim C.W., Jang I., Son D.J., Kim D., Pan C., et al. . 2014. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J. Clin. Invest. 124:3187–3199 10.1172/JCI74792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta P., Courties G., Wei Y., Leuschner F., Gorbatov R., Robbins C.S., Iwamoto Y., Thompson B., Carlson A.L., Heidt T., et al. . 2012. Myocardial infarction accelerates atherosclerosis. Nature. 487:325–329 10.1038/nature11260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E., Nakano M., Bentzon J.F., Finn A.V., and Virmani R.. 2013. Update on acute coronary syndromes: the pathologists’ view. Eur. Heart J. 34:719–728 10.1093/eurheartj/ehs411 [DOI] [PubMed] [Google Scholar]
- Fang H., Lin J., Wang L., Xie P., Wang X., Fu J., Ai W., Chen S., Chen F., Zhang F., et al. . 2013. Kruppel-like factor 2 regulates dendritic cell activation in patients with acute coronary syndrome. Cell. Physiol. Biochem. 32:931–941 10.1159/000354496 [DOI] [PubMed] [Google Scholar]
- Feaver R.E., Gelfand B.D., Wang C., Schwartz M.A., and Blackman B.R.. 2010. Atheroprone hemodynamics regulate fibronectin deposition to create positive feedback that sustains endothelial inflammation. Circ. Res. 106:1703–1711 10.1161/CIRCRESAHA.109.216283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil S., Fehrenbacher B., Lukowski R., Essmann F., Schulze-Osthoff K., Schaller M., and Feil R.. 2014. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ. Res. 115:662–667 10.1161/CIRCRESAHA.115.304634 [DOI] [PubMed] [Google Scholar]
- Fessler M.B., and Parks J.S.. 2011. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J. Immunol. 187:1529–1535 10.4049/jimmunol.1100253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman G., Ozcan L., Spolitu S., Hellmann J., Spite M., Backs J., and Tabas I.. 2014. Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calcium-activated kinase pathway. Proc. Natl. Acad. Sci. USA. 111:14530–14535 10.1073/pnas.1410851111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman G., Kamaly N., Spolitu S., Milton J., Ghorpade D., Chiasson R., Kuriakose G., Perretti M., Farokzhad O., and Tabas I.. 2015. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl. Med. 7:275ra20 10.1126/scitranslmed.aaa1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbin U., Baggio E., Stranieri C., Pasini A., Manfro S., Mozzini C., Vallerio P., Lipari G., Merigo F., Guidi G., et al. . 2013. Expansion of necrotic core and shedding of Mertk receptor in human carotid plaques: a role for oxidized polyunsaturated fatty acids? Cardiovasc. Res. 97:125–133 10.1093/cvr/cvs301 [DOI] [PubMed] [Google Scholar]
- Gerrity R.G., Richardson M., Somer J.B., Bell F.P., and Schwartz C.J.. 1977. Endothelial cell morphology in areas of in vivo Evans blue uptake in the aorta of young pigs. II. Ultrastructure of the intima in areas of differing permeability to proteins. Am. J. Pathol. 89:313–334. [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M.A. Jr, and García-Cardeña G.. 2013. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc. Pathol. 22:9–15 10.1016/j.carpath.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C.K., and Witztum J.L.. 2001. Atherosclerosis. the road ahead. Cell. 104:503–516 10.1016/S0092-8674(01)00238-0 [DOI] [PubMed] [Google Scholar]
- Gomez D., and Owens G.K.. 2012. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc. Res. 95:156–164 10.1093/cvr/cvs115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez D., Shankman L.S., Nguyen A.T., and Owens G.K.. 2013. Detection of histone modifications at specific gene loci in single cells in histological sections. Nat. Methods. 10:171–177 10.1038/nmeth.2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra L., Evans A.I., Chen M., Hyduk S.J., Collins T., and Cybulsky M.I.. 2000. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc. Natl. Acad. Sci. USA. 97:9052–9057 10.1073/pnas.97.16.9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haka A.S., Grosheva I., Chiang E., Buxbaum A.R., Baird B.A., Pierini L.M., and Maxfield F.R.. 2009. Macrophages create an acidic extracellular hydrolytic compartment to digest aggregated lipoproteins. Mol. Biol. Cell. 20:4932–4940 10.1091/mbc.E09-07-0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G.K., and Hermansson A.. 2011. The immune system in atherosclerosis. Nat. Immunol. 12:204–212 10.1038/ni.2001 [DOI] [PubMed] [Google Scholar]
- Hansson G.K., Chao S., Schwartz S.M., and Reidy M.A.. 1985. Aortic endothelial cell death and replication in normal and lipopolysaccharide-treated rats. Am. J. Pathol. 121:123–127. [PMC free article] [PubMed] [Google Scholar]
- Hochreiter-Hufford A., and Ravichandran K.S.. 2013. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb. Perspect. Biol. 5:a008748 10.1101/cshperspect.a008748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata H., Manabe I., Fujiu K., Yamamoto T., Takeda N., Eguchi K., Furuya A., Kuro-o M., Sata M., and Nagai R.. 2010. Bone marrow-derived cells contribute to vascular inflammation but do not differentiate into smooth muscle cell lineages. Circulation. 122:2048–2057 10.1161/CIRCULATIONAHA.110.965202 [DOI] [PubMed] [Google Scholar]
- Jiang Y.Z., Jiménez J.M., Ou K., McCormick M.E., Zhang L.D., and Davies P.F.. 2014. Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-Like Factor 4 promoter in vitro and in vivo. Circ. Res. 115:32–43 10.1161/CIRCRESAHA.115.303883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung B., Obinata H., Galvani S., Mendelson K., Ding B.S., Skoura A., Kinzel B., Brinkmann V., Rafii S., Evans T., and Hla T.. 2012. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell. 23:600–610 10.1016/j.devcel.2012.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanters E., Pasparakis M., Gijbels M.J., Vergouwe M.N., Partouns-Hendriks I., Fijneman R.J., Clausen B.E., Förster I., Kockx M.M., Rajewsky K., et al. . 2003. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 112:1176–1185 10.1172/JCI200318580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo A., Dewey C.F. Jr, and García-Cardeña G.. 2013. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. Am. J. Physiol. Cell Physiol. 304:C137–C146 10.1152/ajpcell.00187.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Kim C.W., Simmons R.D., and Jo H.. 2014. Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: mechanosensitive athero-miRs. Arterioscler. Thromb. Vasc. Biol. 34:2206–2216 10.1161/ATVBAHA.114.303425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Mongillo M., Chin K.T., Harding H., Ron D., Marks A.R., and Tabas I.. 2009. Role of ERO1-α–mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress–induced apoptosis. J. Cell Biol. 186:783–792 10.1083/jcb.200904060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Hou B., Tumova S., Muraki K., Bruns A., Ludlow M.J., Sedo A., Hyman A.J., McKeown L., Young R.S., et al. . 2014. Piezo1 integration of vascular architecture with physiological force. Nature. 515:279–282 10.1038/nature13701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P.2008. The molecular mechanisms of the thrombotic complications of atherosclerosis. J. Intern. Med. 263:517–527 10.1111/j.1365-2796.2008.01965.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P.2013. Collagenases and cracks in the plaque. J. Clin. Invest. 123:3201–3203 10.1172/JCI67526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Tabas I., Fredman G., and Fisher E.A.. 2014. Inflammation and its resolution as determinants of acute coronary syndromes. Circ. Res. 114:1867–1879 10.1161/CIRCRESAHA.114.302699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Li H., Yang M., Ren J., Huang Z., Han F., Huang J., Ma J., Zhang D., Zhang Z., et al. . 2013. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Reports. 3:200–210 10.1016/j.celrep.2012.12.012 [DOI] [PubMed] [Google Scholar]
- Lingrel J.B., Pilcher-Roberts R., Basford J.E., Manoharan P., Neumann J., Konaniah E.S., Srinivasan R., Bogdanov V.Y., and Hui D.Y.. 2012. Myeloid-specific Krüppel-like factor 2 inactivation increases macrophage and neutrophil adhesion and promotes atherosclerosis. Circ. Res. 110:1294–1302 10.1161/CIRCRESAHA.112.267310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Collins C., Kiosses W.B., Murray A.M., Joshi M., Shepherd T.R., Fuentes E.J., and Tzima E.. 2013. A novel pathway spatiotemporally activates Rac1 and redox signaling in response to fluid shear stress. J. Cell Biol. 201:863–873 10.1083/jcb.201207115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., and Kakkar V.. 2014. Inflammasome and atherogenesis. Curr. Pharm. Des. 20:108–124 10.2174/13816128113199990586 [DOI] [PubMed] [Google Scholar]
- Lusis A.J.2000. Atherosclerosis. Nature. 407:233–241 10.1038/35025203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin T., Gongol B., Chen Z., Woo B., Subramaniam S., Chien S., and Shyy J.Y.. 2013. Mechanosensitive microRNAs-role in endothelial responses to shear stress and redox state. Free Radic. Biol. Med. 64:61–68 10.1016/j.freeradbiomed.2013.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K., Weiss S., Metharom P., Schmeckpeper J., Hynes B., O’Sullivan J., and Caplice N.. 2009. Thrombin stimulates smooth muscle cell differentiation from peripheral blood mononuclear cells via protease-activated receptor-1, RhoA, and myocardin. Circ. Res. 105:214–218 10.1161/CIRCRESAHA.109.199984 [DOI] [PubMed] [Google Scholar]
- McGill H.C. Jr, Geer J.C., and Holman R.L.. 1957. Sites of vascular vulnerability in dogs demonstrated by Evans blue. AMA Arch. Pathol. 64:303–311. [PubMed] [Google Scholar]
- Moore K.J., and Tabas I.. 2011. Macrophages in the pathogenesis of atherosclerosis. Cell. 145:341–355 10.1016/j.cell.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick A.E., Soldau K., Kiosses W.B., Bell T.A. III, Tobias P.S., and Curtiss L.K.. 2008. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J. Exp. Med. 205:373–383 10.1084/jem.20071096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A.J., Dragoljevic D., and Tall A.R.. 2014. Cholesterol efflux pathways regulate myelopoiesis: a potential link to altered macrophage function in atherosclerosis. Front. Immunol. 5:490 10.3389/fimmu.2014.00490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P.A., and Hynes R.O.. 2014. Alternative splicing of endothelial fibronectin is induced by disturbed hemodynamics and protects against hemorrhage of the vessel wall. Arterioscler. Thromb. Vasc. Biol. 34:2042–2050 10.1161/ATVBAHA.114.303879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myoishi M., Hao H., Minamino T., Watanabe K., Nishihira K., Hatakeyama K., Asada Y., Okada K., Ishibashi-Ueda H., Gabbiani G., et al. . 2007. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 116:1226–1233 10.1161/CIRCULATIONAHA.106.682054 [DOI] [PubMed] [Google Scholar]
- Nathan C., and Ding A.. 2010. Nonresolving inflammation. Cell. 140:871–882 10.1016/j.cell.2010.02.029 [DOI] [PubMed] [Google Scholar]
- Ohnesorge N., Viemann D., Schmidt N., Czymai T., Spiering D., Schmolke M., Ludwig S., Roth J., Goebeler M., and Schmidt M.. 2010. Erk5 activation elicits a vasoprotective endothelial phenotype via induction of Kruppel-like factor 4 (KLF4). J. Biol. Chem. 285:26199–26210 10.1074/jbc.M110.103127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabbisetty S.K., Rabacal W., Maseda D., Cendron D., Collins P.L., Hoek K.L., Parekh V.V., Aune T.M., and Sebzda E.. 2014. KLF2 is a rate-limiting transcription factor that can be targeted to enhance regulatory T-cell production. Proc. Natl. Acad. Sci. USA. 111:9579–9584 10.1073/pnas.1323493111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar K.M., Larman H.B., Dai G., Zhang Y., Wang E.T., Moorthy S.N., Kratz J.R., Lin Z., Jain M.K., Gimbrone M.A. Jr, and García-Cardeña G.. 2006. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Invest. 116:49–58 10.1172/JCI24787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled M., and Fisher E.A.. 2014. Dynamic aspects of macrophage polarization during atherosclerosis progression and regression. Front. Immunol. 5:579 10.3389/fimmu.2014.00579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade S.S., Qiu Z., Woo S.H., Hur S.S., Murthy S.E., Cahalan S.M., Xu J., Mathur J., Bandell M., Coste B., et al. . 2014. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. USA. 111:10347–10352 10.1073/pnas.1409233111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins C.S., Hilgendorf I., Weber G.F., Theurl I., Iwamoto Y., Figueiredo J.L., Gorbatov R., Sukhova G.K., Gerhardt L.M., Smyth D., et al. . 2013. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19:1166–1172 10.1038/nm.3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong J.X., Shapiro M., Trogan E., and Fisher E.A.. 2003. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc. Natl. Acad. Sci. USA. 100:13531–13536 10.1073/pnas.1735526100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai M., Kobori S., Miyazaki A., and Horiuchi S.. 2000. Macrophage proliferation in atherosclerosis. Curr. Opin. Lipidol. 11:503–509 10.1097/00041433-200010000-00008 [DOI] [PubMed] [Google Scholar]
- Sather S., Kenyon K.D., Lefkowitz J.B., Liang X., Varnum B.C., Henson P.M., and Graham D.K.. 2007. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 109:1026–1033 10.1182/blood-2006-05-021634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers D.M., De Meyer G.R., Kockx M.M., Herman A.G., and Martinet W.. 2005. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 25:1256–1261 10.1161/01.ATV.0000166517.18801.a7 [DOI] [PubMed] [Google Scholar]
- Sharma N., Lu Y., Zhou G., Liao X., Kapil P., Anand P., Mahabeleshwar G.H., Stamler J.S., and Jain M.K.. 2012. Myeloid Krüppel-like factor 4 deficiency augments atherogenesis in ApoE-/- mice—brief report. Arterioscler. Thromb. Vasc. Biol. 32:2836–2838 10.1161/ATVBAHA.112.300471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy F.J., Grebe A., Rayner K.J., Kalantari P., Ramkhelawon B., Carpenter S.B., Becker C.E., Ediriweera H.N., Mullick A.E., Golenbock D.T., et al. . 2013. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 14:812–820 10.1038/ni.2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann N.J., Garmire L.X., McDonald J.G., Myers D.S., Milne S.B., Shibata N., Reichart D., Fox J.N., Shaked I., Heudobler D., et al. . 2012. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 151:138–152 10.1016/j.cell.2012.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart H.J., Guildford A.L., Lawrence-Watt D.J., and Santin M.. 2009. Substrate-induced phenotypical change of monocytes/macrophages into myofibroblast-like cells: a new insight into the mechanism of in-stent restenosis. J. Biomed. Mater. Res. A. 90A:465–471 10.1002/jbm.a.32100 [DOI] [PubMed] [Google Scholar]
- Tabas I.2005. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol. 25:2255–2264 10.1161/01.ATV.0000184783.04864.9f [DOI] [PubMed] [Google Scholar]
- Tabas I.2010. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10:36–46 10.1038/nri2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., and Glass C.K.. 2013. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 339:166–172 10.1126/science.1230720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., and Ron D.. 2011. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 13:184–190 10.1038/ncb0311-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Williams K.J., and Borén J.. 2007. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 116:1832–1844 10.1161/CIRCULATIONAHA.106.676890 [DOI] [PubMed] [Google Scholar]
- Tacke F., Alvarez D., Kaplan T.J., Jakubzick C., Spanbroek R., Llodra J., Garin A., Liu J., Mack M., van Rooijen N., et al. . 2007. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Wang X., Hart G.T., Odumade O.A., Weinreich M.A., Hogquist K.A., and Jameson S.C.. 2011. Kruppel-like factor 2 is required for trafficking but not quiescence in postactivated T cells. J. Immunol. 186:775–783 10.4049/jimmunol.1000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J.A., Deaton R.A., Hastings N.E., Shang Y., Moehle C.W., Eriksson U., Topouzis S., Wamhoff B.R., Blackman B.R., and Owens G.K.. 2009. PDGF-DD, a novel mediator of smooth muscle cell phenotypic modulation, is upregulated in endothelial cells exposed to atherosclerosis-prone flow patterns. Am. J. Physiol. Heart Circ. Physiol. 296:H442–H452 10.1152/ajpheart.00165.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp E., Li G., Seimon T.A., Kuriakose G., Ron D., and Tabas I.. 2009. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe-/- and Ldlr-/- mice lacking CHOP. Cell Metab. 9:474–481 10.1016/j.cmet.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp E., Subramanian M., and Tabas I.. 2011a. The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. Eur. J. Immunol. 41:2515–2518 10.1002/eji.201141719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp E., Vaisar T., Subramanian M., Mautner L., Blobel C., and Tabas I.. 2011b. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cδ, and p38 mitogen-activated protein kinase (MAPK). J. Biol. Chem. 286:33335–33344 10.1074/jbc.M111.263020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmins J.M., Ozcan L., Seimon T.A., Li G., Malagelada C., Backs J., Backs T., Bassel-Duby R., Olson E.N., Anderson M.E., and Tabas I.. 2009. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J. Clin. Invest. 119:2925–2941 10.1172/JCI38857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg B.M., Spaan J.A., and Vink H.. 2009. Impaired glycocalyx barrier properties contribute to enhanced intimal low-density lipoprotein accumulation at the carotid artery bifurcation in mice. Pflugers Arch. 457:1199–1206 10.1007/s00424-008-0590-6 [DOI] [PubMed] [Google Scholar]
- Vengrenyuk Y., Nishi H., Long X., Ouimet M., Savji N., Martinez F.O., Cassella C.P., Moore K.J., Ramsey S.A., Miano J.M., and Fisher E.A.. 2015. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler. Thromb. Vasc. Biol. 35:535–546 10.1161/ATVBAHA.114.304029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal G. Jr, Zhang Y., Larman H.B., Gracia-Sancho J., Koo A., and García-Cardeña G.. 2010. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem. Biophys. Res. Commun. 391:984–989 10.1016/j.bbrc.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virmani R., Burke A.P., Kolodgie F.D., and Farb A.. 2002. Vulnerable plaque: the pathology of unstable coronary lesions. J. Interv. Cardiol. 15:439–446 10.1111/j.1540-8183.2002.tb01087.x [DOI] [PubMed] [Google Scholar]
- Wamhoff B.R., Hoofnagle M.H., Burns A., Sinha S., McDonald O.G., and Owens G.K.. 2004. A G/C element mediates repression of the SM22α promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ. Res. 95:981–988 10.1161/01.RES.0000147961.09840.fb [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang G.Z., Rabinovitch P.S., and Tabas I.. 2014. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-κB-mediated inflammation in macrophages. Circ. Res. 114:421–433 10.1161/CIRCRESAHA.114.302153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerterp M., Bochem A.E., Yvan-Charvet L., Murphy A.J., Wang N., and Tall A.R.. 2014. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ. Res. 114:157–170 10.1161/CIRCRESAHA.114.300738 [DOI] [PubMed] [Google Scholar]
- Williams K.J., and Tabas I.. 1995. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 15:551–561 10.1161/01.ATV.15.5.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won D., Zhu S.N., Chen M., Teichert A.M., Fish J.E., Matouk C.C., Bonert M., Ojha M., Marsden P.A., and Cybulsky M.I.. 2007. Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow. Am. J. Pathol. 171:1691–1704 10.2353/ajpath.2007.060860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2014. The top 10 causes of death. Available at: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed March 26, 2015)
- Yoshida T., Kaestner K.H., and Owens G.K.. 2008. Conditional deletion of Krüppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ. Res. 102:1548–1557 10.1161/CIRCRESAHA.108.176974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Zampetaki A., Margariti A., Pepe A.E., Alam S., Martin D., Xiao Q., Wang W., Jin Z.G., Cockerill G., et al. . 2009. Sustained activation of XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed flow. Proc. Natl. Acad. Sci. USA. 106:8326–8331 10.1073/pnas.0903197106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Hamik A., Nayak L., Tian H., Shi H., Lu Y., Sharma N., Liao X., Hale A., Boerboom L., et al. . 2012. Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J. Clin. Invest. 122:4727–4731 10.1172/JCI66056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Li Y.S., and Chien S.. 2014a. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler. Thromb. Vasc. Biol. 34:2191–2198 10.1161/ATVBAHA.114.303422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Li Y.S., Wang K.C., and Chien S.. 2014b. Epigenetic mechanism in regulation of endothelial function by disturbed flow: Induction of DNA hypermethylation by DNMT1. Cell Mol Bioeng. 7:218–224 10.1007/s12195-014-0325-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Yazdi A.S., Menu P., and Tschopp J.. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature. 469:221–225 10.1038/nature09663 [DOI] [PubMed] [Google Scholar]