Introduction
Microparticles (MPs) are sub-micron sized vesicles released by activated or apoptotic cells. They are generally defined as 0.1–1 µm membrane particles that expose the anionic phospholipid phosphatidylserine (PS) and membrane antigens representative of their cellular origin [1]. It is now well recognized that MPs behave as vectors of bioactive molecules, playing a role in blood coagulation, inflammation, cell activation and cancer metastasis. In clinical practice, circulating MPs originating from blood and vascular cells are elevated in a variety of prothrombotic and inflammatory disorders, cardiovascular diseases, autoimmune conditions, infectious diseases and cancer [1–3]. As potential disease biomarkers, MP counts may provide useful diagnostic or prognostic information or may be used to monitor response to treatment; however, currently the assessment of their clinical relevance is hampered by methodological concerns. Further standardization of protocols for microparticle determination are needed to allow multicentre, prospective trials to be conducted, which will be critical in establishing the clinical value of these emerging biomarkers and ultimately in incorporating MP measurements into routine diagnostic laboratories.
Accordingly, the International Society on Thrombosis and Haemostasis (ISTH) Vascular Biology Standardisation Subcommittee (VB SSC) previously organized a first collaborative working party aimed at standardizing platelet-derived MP (PMP) measurement by flow cytometry [4]. This study demonstrated the utility of fluorescent calibrated beads to improve the reproducibility of MP counts; despite significant progress in the analytical phase, a wide range of pre-analytical variables (blood handling and centrifugation conditions, use of frozen vs. fresh samples, etc.) critically impact on MP measurements and remain a major source of variability and potential artifacts in MP analysis, as shown by several studies [5–11]. Because there is as yet no consensus on which are the major pre-analytical variables and which preparation protocol to recommend, the multicenter evaluation of a common pre-analytical protocol was identified as a priority by the VB SSC. Therefore, the main objective of this new workshop was to determine whether it is possible to reduce the inter-laboratory variability in MP count and procoagulant activity using a common pre-analytical protocol [9] compared with a non-standardized approach.
To this end, 14 participating laboratories prepared platelet-free plasma (PFP) samples from well-defined healthy donors (n = 15 per laboratory [range 6–17]) using predetermined common pre-analytical conditions (protocol A) on one hand and their own pre-analytical conditions (protocol B) on the other hand. In order to reduce the inter-individual variability related to gender and potentially age, all healthy volunteers were men aged from 20 to 40 years of age. Also, stringent conditions were imposed regarding biological data and absence of unusual effort on the day before blood collection and/or medication. Detailed information regarding protocols was carefully collected in the ‘checking files’ (specimen form provided in supporting information).
Protocol A was defined as follows. (i) Blood was collected from fasting subjects in the morning (08.00 to 11.00). (ii) Venipuncture of the antecubital vein was performed with a ≥21-gauge needle following the application of a light tourniquet. The first 2–3 mL of blood was discarded. (iii) Citrated plastic tubes (0.109 mol L−1 or 3.2%) with a minimum volume of 3.5 mL were used as collection tubes. All the following tubes were not used: citrate-theophylline-adenosine-dipyridamole (CTAD), ethylenediamine tetraaceticacid (EDTA), heparin, acid-citrate-dextrose (ACD) tubes and glass tubes. Anticoagulant was mixed with blood by gentle inversion. (iv) When possible, samples were drawn directly in the laboratory. If tube transportation was needed, this was carried out carefully to avoid unnecessary agitation. For this purpose, a box maintaining blood tubes in a steady vertical position was used. Samples were kept at room temperature (20–24 °C) and the delay before the first centrifugation was < 2 h. (v) Samples were centrifuged at 2500 ×g for 15 min at room temperature avoiding application of the centrifuge brake. Plasma was collected in a plastic tube with a 1000-µL pipette, leaving 1 cm of plasma above the buffy layer and taking care not to disturb it. The plasma was then centrifuged a second time at 2500 ×g for 15 min at room temperature. PFP was collected into a new plastic tube using a 200-µL pipette, and leaving approximately 100 µL at the bottom of the plastic tube. (vi) Homogenized PFP was snap-frozen in liquid nitrogen and stored at −80 °C. Collected PFP from both protocols was sent in adequate cardboard cryoboxes to the core laboratory in dry ice. This was then analyzed using flow cytometry (Gallios; Beckman-Coulter, Villepinte, France) to enumerate MP and MP-dependent functional assays to measure their procoagulant activity: thrombin generation was measured using the Calibrated Automated Thrombogram® (CAT) and phospholipid-dependent coagulation time was measured using the STA®-Procoag-PPL assay, both from Diagnostica Stago (Asnières, France). Details regarding materials and methods can be found in the Supporting Information.
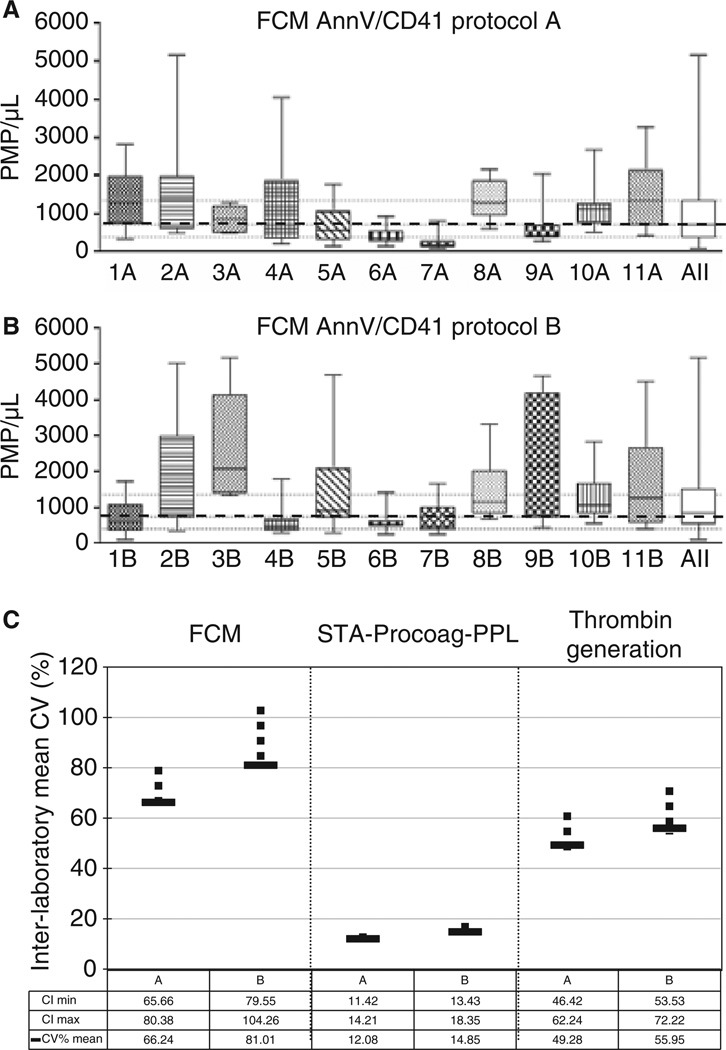
Among 14 participating laboratories, 11 complied with protocol A’s instructions and were included in the analysis. As shown in Fig. 1(A), median PMP counts determined in PFP prepared from healthy individuals varied from 180 (7A) to 1400 (2A) PMP per µL, with an inter-individual variability ranging from 37% (7A) to 130% (4A). As a whole, PMP counts were higher with the non-standardized protocols (Protocol B, Fig. 1B), 420 (4B) to 2100 (3B) PMP per µL, and showed higher inter-individual variability (62% [6B] to 170% [9B]). Interestingly, as seen in Fig. 1, in most laboratories (9/11) this variability was reduced by using a standardized protocol rather than the in-house method. Thus, MP counts determined in healthy donors were lower and less variable using a common pre-analytical protocol (A). This tendency was confirmed by the thrombin generation velocity (Fig. S1) and the phospholipid-dependent coagulation time (Fig. S2), with (respectively) a higher thrombin generation and a shorter clotting time for protocol B. It varied from a median velocity range of 2 (1A) to 10 (2A) nmol L−1min−1 and a median clotting time range of 70 (2A) to 95 (8A) seconds for protocol A, as compared with median velocity range of 1 (1B) to 12 (5B) nmol L−1 min−1 and median clotting time range of 65 (3B) to 88 (11B) seconds for protocol B. However, it cannot be concluded from higher MP counts and thrombin generation and shorter clotting time for protocol B, that protocol A is better.
Fig. 1.
Impact of the pre-analytical protocol on the inter-individual and inter-laboratory variability of MP measurements (A & B). Impact of pre-analytics on inter-individual variability of PMP counts by FCM. PMP counts by FCM in normal plasma samples prepared by different laboratories using a common pre-analytical protocol (protocol A, upper graph) or their in house pre-analytical approach (protocol B, lower graph). Median (dashed line) and 25–75% interquartile range (dotted lines) of PMP using protocol A. For example, 1A means laboratory number 1, protocol A. ‘All’ accounts for the combination of data from all the laboratories. (C). Impact of pre-analytics standardization on interlaboratory variability of PMP counts and procoagulant activities. To determine if the inter-laboratory variability was different between protocols for each analyzed parameter, the coefficient of variation was calculated for each laboratory. Then a mean coefficient of variation was derived by averaging all coefficients related on a parameter. Differences between protocols were evaluated used the Bootstrap method and expressed by determination of the confidence intervals. The normal approximations method has been used for computing 95% confidence intervals. CV, coefficient of variation; CI, confidence interval determined by the Boopstrap method (vertical bars).
The inter-laboratory reproducibility for MP counts and procoagulant assays was compared between protocols. As illustrated in Fig. 1(C), the mean coefficient of variation of the PMP counts decreased in protocol A as compared with protocol B (66% [range 64.8–80.3] vs. 80% [79.6–104.3] respectively), clearly demonstrating the improved reproducibility using protocol A. However, the study failed to demonstrate the superiority of protocol A over protocol B based on MP-dependent procoagulant assays.
These data illustrate that, despite a significant reduction of the inter-laboratory variability of FCM-based PMP counts when using a common sampling protocol, this is still unacceptably high for clinical use. Along this line, we noticed critical differences between the requested specifications of the common protocol (protocol A) and what was actually applied in three laboratories, leading to significant differences in MP counts and procoagulant activities and, thus, rejection of these samples from protocol A analysis. Interestingly, and as illustrated in Fig. S3, careful re-analysis of these specific cases provided useful information on major pre-analytical parameters impacting MP analysis, such as the volume of the collection tube (1.8 or 2.4 mL vs. 3.5 mL) and the amount of plasma left above the buffy layer (5 mm vs. 10 mm). As a comparison, incidental thawing of PFP samples during transportation to the core laboratory showed a lower influence than the volume of the collection tube.
Furthermore, as could be seen from the checking files, nine laboratories had a different centrifugation protocol in protocol B as compared with A. As illustrated in Fig. S4, centrifugation force inversely correlates with PMP counts. This result confirms data in the literature that identify centrifugation as the main factor affecting MP analysis [8,12–14]. Other variables have been shown to have a significant impact on MP pre-analytics, such as the time before the first centrifugation and the agitation of the tube transportation [8]; however, both these variables have been well controlled during this study. Other parameters have not been taken into account, such as the type of the collection and storage plastic tubes [15] or the operator experience, and may partly explain the remaining variability.
In conclusion, this workshop showed the ability of a common pre-analytical protocol to reduce the inter-laboratory variability of flow cytometric enumeration of PMPs in healthy individuals. However, significant variability does remain in some cases even when a common protocol is correctly applied, suggesting that other, as yet unidentified, parameters could be important in the setting of multicenter studies. Moreover, besides PMPs, which are the most sensitive to pre-analytical variables, the benefit of a common standardized pre-analytical protocol has yet to be evaluated in other MP sub-populations, such as erythrocyte-, leukocyte- and endothelial-derived MPs.
Supplementary Material
Acknowledgements
We want to thank P. Poncelet and T. Bouriche (BioCytex) for providing CD41-PE and its matched isotype control IgG1-PE conjugates, as well as Megamix-Plus beads, X. Teuma and A. Berthier (Diagnostica Stago Company) for providing CAT® and STA®s-ProcoagPPL reagents and Beckman-Coulter for providing AnnexinV/7AAD kits. We thank R. Bradford for technical assistance.
Appendix 1
ISTH SSC Workshop
Ambrozic A.1, Bailly N.2, Buffat C.3, Buzas E.4, Charpentier A.5, Chatelain B.2, Dogne J.M.D.2, Falanga A.6, Garcia Arias-Salgado E.7, Gyorgy B.4, Harrison M.8, Kwaan H.9, Lakota K.1, Latger-Cannard V.10,11, Massin F.10, Mobarrez F.12, Mojca F.1, Mullier F.2, Phelan JP.8, Snoj N.16, Sodin-Semrl S.1, Stezpień E.13, Susen S.14, Terrisse A-D.15, Tintillier V.14, Vignoli A.6
1 University Medical Center, Department of Rheumatology, Ljubljana, Slovenia;2 CHU UCL Mont-Godinne, Laboratoire d’Hématologie & Namur Thrombosis and Hemostasis Center, Yvoir, Belgium;3 Hôpital de la Conception, Laboratoire de Biochimie, Marseille, France;4 Semmelweis University, Department of Genetics, Cell and Immunobiology, Budapest, Hungary;5 Laboratoire de l’Hôpital St Philibert, Groupe Hospitalier de l’Institut Catholique de Lille, Lomme, France;6 Ospedali Riuniti di Bergamo, Division of Immunohematology and Transfusion Medicine, Bergamo, Italy;7 Hospital Universitario La Paz-idiPaz, Paseo de la Castellana, Madrid, Spain;8 School of Health Sciences, Waterford Institute of Technology, Waterford, Ireland;9 Northwestern University Feinberg School of Medicine, Chicago, USA;10 Plateforme Nancytomique, CHU Nancy, Vandouvre-les-Nancy, France;11 Service d’Hématologie Biologique, CHU Nancy, Vandouvre-les-Nancy, France;12 Karolinska Institute, Department of Clinical Sciences, Danderyd Hospital, Clinical Research Center, Stockholm, Sweden;13 Laboratory for Molecular Biology and Research, John Paul II Hospital, Krakow, Poland;14 CHU de Lille, Institut d’Hématologie et Transfusion, Centre de Biologie Pathologie et Génétique, EA 2693, Université Lille Nord de France, Lille, France;15 CHU Rangueil, Laboratoire d’Hématologie, Toulouse, France;16 University Medical Center, Clinical Institute of Clinical Chemistry and Biochemistry, Ljubljana, Slovenia.
Footnotes
See Appendix 1.
Disclosure of Conflict of Interests
C. Judicone is a full-time employee of the BioCytex company.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1. Material and methods
Data S2. Checking file
Figure S1. Impact of the pre-analytical protocol on the inter-individual and inter-laboratory variability of the thrombin generation (TG).
Figure S2. Impact of the pre-analytical protocol on the inter-individual and inter-laboratory variability of the phospholipids-dependent coagulation time (CT).
Figure S3. Impact of critical changes in the common protocol’s instructions.
Figure S4. Impact of the centrifugation protocol on microparticle enumeration by flow cytometry.
References
- 1.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Lynch SF, Ludlam CA. Plasma microparticles and vascular disorders. Br J Haematol. 2007;137:36–48. doi: 10.1111/j.1365-2141.2007.06514.x. [DOI] [PubMed] [Google Scholar]
- 3.Sabatier F, Camoin-Jau L, Anfosso F, Sampol J, Dignat-George F. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J Cell Mol Med. 2009;13:454–471. doi: 10.1111/j.1582-4934.2008.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacroix R, Robert S, Poncelet P, Kasthuri RS, Key NS, Dignat-George F. Standardization of platelet-derived microparticle enumeration by flow cytometry using calibrated beads: results of ISTH SSC collaborative workshop. J Thromb Haemost. 2010;8:2571–2574. doi: 10.1111/j.1538-7836.2010.04047.x. [DOI] [PubMed] [Google Scholar]
- 5.Ayers L, Kohler M, Harrison P, Sargent I, Dragovic R, Schaap M, Nieuwland R, Brooks SA, Ferry B. Measurement of circulating cell-derived microparticles by flow cytometry: sources of variability within the assay. Thromb Res. 2011;127:370–377. doi: 10.1016/j.thromres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Dey-Hazra E, Hertel B, Kirsch T, Woywodt A, Lovric S, Haller H, Haubitz M, Erdbruegger U. Detection of circulating microparticles by flow cytometry: influence of centrifugation, filtration of buffer, and freezing. Vasc Health Risk Manag. 2010;6:1125–1133. doi: 10.2147/VHRM.S13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shet AS. Characterizing blood microparticles: technical aspects and challenges. Vasc Health Risk Manag. 2008;4:769–774. doi: 10.2147/vhrm.s955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacroix R, Judicone C, Poncelet P, Robert S, Arnaud L, Sampol J, Dignat-George F. Impact of pre-analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J Thromb Haemost. 2012;10:437–446. doi: 10.1111/j.1538-7836.2011.04610.x. [DOI] [PubMed] [Google Scholar]
- 9.Jy W, Horstman LL, Jimenez JJ, Ahn YS. Measuring circulating cell-derived microparticles. J Thromb Haemost. 2004;2:1842–1851. doi: 10.1111/j.1538-7836.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- 10.Enjeti AK, Lincz LF, Seldon M. Detection and measurement of microparticles: an evolving research tool for vascular biology. Semin Thromb Hemost. 2007;33:771–779. doi: 10.1055/s-2007-1000369. [DOI] [PubMed] [Google Scholar]
- 11.Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb Haemost. 2010;105:396–408. doi: 10.1160/TH10-09-0595. [DOI] [PubMed] [Google Scholar]
- 12.Jayachandran M, Miller VM, Heit JA, Owen WG. Methodology for isolation, identification and characterization of microvesicles in peripheral blood. J Immunol Methods. 2011;375:207–214. doi: 10.1016/j.jim.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stagnara J, Garnache Ottou F, Angelot F, Mourey G, Seilles E, Biichle S, Saas P, Racadot E. Correlation between platelet-derived microparticle enumeration by flow cytometry and phospholipid-dependent procoagulant activity in microparticles: the centrifugation step matters! Thromb Haemost. 2012;107:1185–1187. doi: 10.1160/TH11-07-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Artoni A, Merati G, Padovan L, Scalambrino E, Chantarangkul V, Tripodi A. Residual platelets are the main determinants of microparticles count in frozen-thawed plasma. Thromb Res. 2012;130:561–562. doi: 10.1016/j.thromres.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Loeffen R, Kleinegris MC, Loubele ST, Pluijmen PH, Fens D, van Oerle R, ten Cate H, Spronk HM. Pre-analytical variables of thrombin generation: towards a standard procedure and validation of the method. J Thromb Haemost. 2012;10:2544–2554. doi: 10.1111/jth.12012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.