Abstract
A low-power wearable ECG monitoring system has been developed entirely from discrete electronic components and a custom PCB. This device removes all loose wires from the system and minimizes the footprint on the user. The monitor consists of five electrodes, which allow a cardiologist to choose from a variety of possible projections. Clinical tests to compare our wearable monitor with a commercial clinical ECG recorder are conducted on ten healthy adults under different ambulatory conditions, with nine of the datasets used for analysis. Data from both monitors were synchronized and annotated with PhysioNet's waveform viewer WAVE (physionet.org) [1]. All gold standard annotations are compared to the results of the WQRS detection algorithm [2] provided by PhysioNet. QRS sensitivity and QRS positive predictability are extracted from both monitors to validate the wearable monitor.
Keywords: Ambulatory monitoring, ECG, low power, QRS, wearable devices, WQRS
I. Introduction
Cardiovascular disease is the leading cause of death in the United States, accounting for nearly half of all major causes of death in the U.S. in 2008 [3]. In 2010, the U.S. spent $316.4 billion on health care services, medications, and lost productivity due to heart disease [4]. Almost half of all sudden cardiac deaths occur outside of the hospital, which suggests that many people may not be familiar with early warning signs [5].
Typically, patients who are having heart problems or discomfort are fitted with a Holter monitor for 24–48 h [6]. The Holter monitor samples data continuously, with resolution of 8–12 bits at 128–256 Hz. Although these devices produce manageable datasets, they have several shortcomings. First, the form factor requires that a device be clipped onto a belt or be worn in a pocket. The monitor attaches to electrodes on the patient's chest via long wires. These wires can be a significant noise source and can be inadvertently detached if pulled [7]. Although new Holter monitors can take data for up to 1 week [8], patients can have arrhythmias that occur once every ten days to one month that are not recorded by the Holter.
To capture these occasional episodes, patients can be fitted with event monitors, which can be worn for a month or longer [9]. However, event monitors typically only save ECG data around the irregular cardiac episode, which limits the degree of analysis that can be performed. Continuous long-term data allow the clinician to detect overall heart health trends and possibly see initial markers before the cardiac irregularity occurs [10].
To improve the existing Holter monitor use model, several groups have worked on wearable monitors to improve patient acceptance [11]–[13]. These monitors often adhere directly to the chest to avoid long wires. However, these monitors either protrude far from the chest, which can limit patient acceptance [11], [13], have bulky base-stations [13], or do not save all of the raw data which makes them ineffective for analyses such as morphological variability [10], [12]. They all rely on transmitting data to a base station, which also limits their effectiveness when a cell phone or computer is not nearby, for example, during periods of exercise.
To address these issues, we have developed a wearable monitor that can record one of several ECG projections of clinical quality without wires, can be used reliably during exercise, is less than 1 cm thick and contains 1 Gb of flash memory to save all of the raw data for long-term monitoring without a base station.
II. System Design
A. Mechanical Design
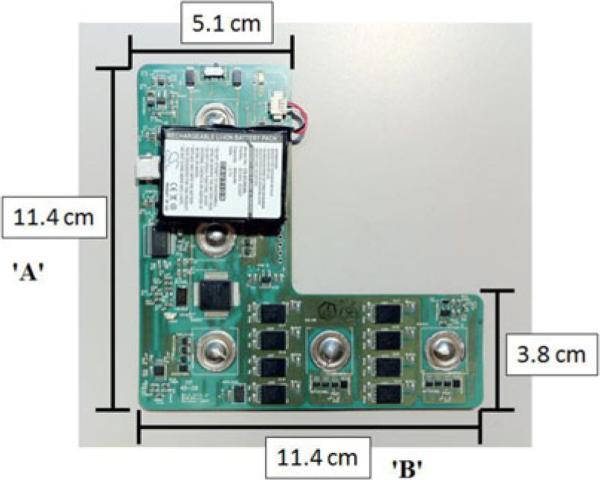
The cardiac monitor is designed using a flexible PCB fabricated by Fit4Flex (Milipitas, CA). Fig. 1 shows the “L”-shaped board, which is 11.4 cm tall and 5.1 cm wide in the vertical section, and 11.4 cm long and 3.8 cm wide in the horizontal section. The 2-layer PCB is fabricated in Dupont FR Material and is 0.1625 mm thick. An extra 0.25 mm of stiffener, also composed of Dupont FR Material, is attached to the bottom layer to increase rigidity. The board is designed in an “L” shape to allow the clinician to choose one of several possible ECG vectors to record, depending on a patient's specific needs. All corners are rounded to remove sharp edges, to minimize the chance of prodding the user. After fabrication, the board is coated in parylene-C by Paratronix (Attleboro, MA) for biocompatibility and water proofing.
Fig. 1.
“L”-shaped wearable cardiac monitor. Clearly visible are the battery, MSP430 and eight spansion FLASH chips as well as the five electrode buttons used to connect the monitor to wet electrodes.
The board can be mounted on the user's chest in two preferred configurations. The first configuration (type 1) consists of the “A”-axis aligned with the sternum and the “B”-axis underneath the left pectoralis muscle. The monitor can also be rotated 90° clockwise, so the “A”-axis rests horizontally above the left pectoralis and the “B”-axis is aligned along the sternum (type 2). In both configurations, projections can be obtained that mimic standard leads of electrocardiography. The board is connected to conventional 3M 2560 red dot wet ECG electrodes via five metal buttons on the back of the PCB.
B. Electrical Design
The cardiac monitor has three subsystems: the analog front end (AFE) which consists of the ECG buffers and amplifiers; the digital back end, which includes a Texas Instruments MSP430 and eight Spansion flash memory chips; and power management circuits. The MSP430 configures the electrodes via USB input from a computer and the on-chip 12 bit ADC is used to digitize the ECG signal. The MSP is also used to write the data to flash memory.
The device can be programmed for low- or high-resolution modes. Low-resolution mode samples the ECG with 8 bit resolution at 250 Hz and consumes 0.65 mA of current. High resolution mode has 12 bits of resolution at 250 Hz and the device consumes 0.9 mA. Power is supplied by a 3.7 V, 600 mA-h Liion battery (Cameron-Sino CS-EC003 SL, Hong Kong, China). Table I summarizes the battery and memory usage during both power modes. The device achieves low-power operation through system level optimizations such as low quiescent current components, saving data locally instead of wireless transmission, and efficient use of clocking and low-power modes of the flash and MSP430.
TABLE I.
Memory and Power Usage During Both Power Modes
| Specification | Low Resolution | High Resolution |
|---|---|---|
| Current Consumption | 0.65 mA | 0.9 mA |
| Memory Capacity | 5.9 days | 3.9 days |
| Battery Life | 38.5 days | 27.8 days |
| ECG Resolution | 8 bits | 12 bits |
| ECG Sample Rate | 250 Hz | 250 Hz |
Each electrode can be configured as an ECG input (either positive or negative), a ground drive output or be tristated (see Fig. 2). When configured as an input, the biosignal is immediately buffered to convert the high impedance biosignal node to a low-impedance output node. Actively buffering the signal at the electrode site can reduce power line interference by up to 40 dB [14]. When configured as an output, the electrode is used as the right leg drive interface to the body (see Fig. 2).
Fig. 2.
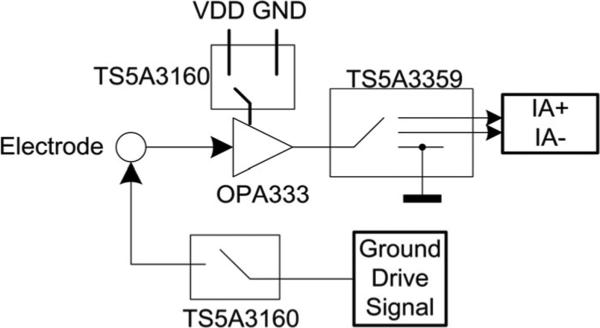
Electrode and surrounding circuitry with switching network. This switching network allows for configurability of the electrodes without sacrificing power consumption.
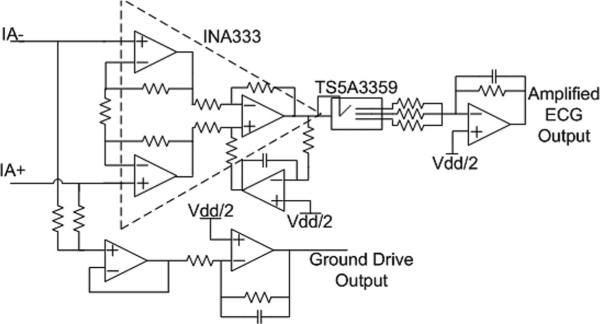
The amplification section of the AFE includes an INA333 instrumentation amplifier with a gain of 19, followed by a band-limited inverting amplifier with programmable gain of 21, 37, or 50. The total signal gain is therefore 400, 700, or 950. The band-limited inverting amplifier has a low pass cutoff frequency of approximately 125 Hz. Along with noise bandwidth reduction this amplifier serves as the antialiasing filter for the system (see Fig. 3).
Fig. 3.
Instrumentation and configurable gain stage including ground drive circuity. The overall system gain is configured during user setup to be between 400 and 950. The output of amplified ECG signal passes through a simple low-pass filter before digitization by the built-in ADC of the MSP430.
III. Usage Model
The use model of the cardiac monitor is designed to maximize patient and clinician acceptance. The use model leverages the positives of both the Holter use model (reliable, low-power local data storage) and the wearable device use model (discreet, no long wires) while avoiding their negatives (reliance on a base-station and long, annoying, and noise-introducing wires) to maximize acceptance. The device is comfortable to wear and weighs just over 30 g (1 oz). To ensure that the device is not seen, it is designed to be less than 1 cm thick so as not to protrude from the chest. Finally, it is designed to be “stick and go.” Once the device is set up by the clinician, the user does not have to download any data, or recharge the battery in order to wear the device every day.
The ECG is sampled at 250 Hz with a bandwidth of 0.5–125 Hz at 8 or 12 bits and all of the raw data are saved for clinician viewing. As previously mentioned, the device has three different gain settings, from 400 to 950, to allow for intersubject variability. Additionally, the device is easy to setup on the patient, which reduces the training required for a clinician to use the monitor. Table II compares the wearable cardiac monitor with [11]–[13].
TABLE II.
Comparison of This Work to Other Monitors
The cardiac monitor is designed for experimental purposes, specifically for long-term monitoring to develop new heart health algorithms using heart rate variability and other ECG risk-based metrics [10]. Compared to the existing Holter monitor usage model, the cardiac monitor is an improvement for ECG data acquisition in ambulatory settings, specifically for healthy and/or active users.
IV. Clinical Test
The cardiac monitor was tested at the Clinical Research Center (CRC) at MIT. The study protocol was approved by MIT's Committee on the Use of Humans as Experimental Subjects, and written informed consent was obtained prior to testing. We tested ten healthy male subjects aged 19 to 56 with an average age of 30 years. Data from nine subjects were deemed acceptable for analysis. Test subjects wore the cardiac monitor in the type-2 configuration to mimic a lead I ECG. A Criticare 504-US ECG recorder with electrodes placed adjacent to the cardiac monitor also recorded a lead I ECG [15]. Subjects also wore a respiration monitor (Inductrotrace), pulse oximeter (Criticare 504-US), and continuous blood pressure monitor (Portapres) to acquire other important physiological variables during the test. The test is split into three parts: rest, moderate movement, and heavy movement. Between most interventions, a minute of stationary rest is used for the calibration of the continuous blood pressure monitor to ensure accuracy.
During the rest phase, the test subject starts in a supine position, then sits up and finally stands up. Each of these three positions is held for 5 min, with 1 min of calibration after each positional transition.
The moderate movement phase begins with light hopping, followed by an arm movement and finishes with a Valsalva maneuver. The arm movement requires the test subject to move his left arm across his chest in order to induce motion artifact and muscle noise into the recording. The hopping and arm movement are performed for 1 min each. The Valsalva maneuver is 10 s long. There is 1 min of calibration between each positional transition.
The heavy movement portion of the test begins with 5 min of walking on a treadmill, followed by 5 min of light jogging/running on a treadmill, followed by 1 min of stair stepping. There are 2 min of calibration in between running and stepping to allow the patient time to rest.
The entire protocol is conducted twice in a row for each test subject. Fig. 4 shows a timeline for the protocol.
Fig. 4.
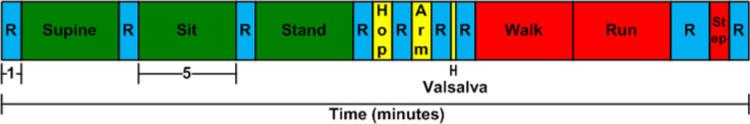
Timeline of the clinical protocol. Boxes labeled C signify calibration of the continuous blood pressure monitor. All boxes labeled C are 1 min long except for calibration after the running portion, which was 2 min to allow the patient extra rest. Lying down, sitting, standing, walking and running all lasted 5 min. The green blocks indicate light movement, yellow blocks indicate moderate movement, red indicate heavy movement and blue indicate rest.
V. Experimental Results
To characterize the wearable monitor, the experimental data are converted into PhysioNet's WaveForm DataBase (WFDB) format [1] and a set of gold-standard beat annotations are developed through the visual inspection of both ECG signals. The WQRS algorithm [2] is run on both datasets and then compared to the gold standard annotations. The first comparison is QRS sensitivity, defined as the percentage of positive QRS matches the WQRS algorithm finds compared to the gold standard. A positive QRS match is defined as an algorithm-determined QRS annotation within 150 ms [16], [17] of a gold-standard QRS annotation. The second comparison is QRS positive predictability, defined as the number of correct QRS detections over the total number of QRS detections the WQRS algorithm detected.
Table III summarizes the ECG experimental results of the clinical test. Under supine, sitting, standing, hopping, Valsalva, walking, running, and stepping, the Wearable Cardiac Monitor had a sensitivity over 99% of correctly detecting beats. Only the arm movement has sensitivity below 99%. The Criticare monitor has sensitivity over 99% for every intervention. Overall, the QRS sensitivity is 99.67% (std dev—0.35%) for the Wearable Monitor and 99.77% (std dev—0.27%) for the Criticare monitor.
TABLE III.
Summary of Clinical Test ECG Data
| Wearable | Criticare | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Total | Supine | Sitting | Standing | Hopping | Arm | Valsalva | Walk | Run | Step | Patient | Total | Supine | Sitting | Standing | Hopping | Arm | Valsalva | Walk | Run | Step | |
| Sensitivity | Avg | 99.68 | 99.98 | 99.70 | 99.98 | 99.18 | 95.61 | 100.00 | 99.88 | 99.39 | 99.95 | Avg | 99.77 | 100.00 | 99.89 | 99.98 | 99.80 | 99.33 | 100.00 | 100.00 | 99.82 | 99.95 |
| STD | 0.35 | 0.05 | 0.86 | 0.05 | 0.91 | 11.74 | 0.00 | 0.22 | 0.S8 | 0.15 | STD | 0.27 | 0.00 | 0.23 | 0.05 | 0.46 | 1.84 | 0.00 | 0.00 | 0.32 | 0.15 | |
| Total | Supine | Sitting | Standing | Hopping | Arm | Valsalva | Walk | Run | Step | Patient | Total | Supine | Sitting | Standing | Hopping | Arm | Valsalva | Walk | Run | Step | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictability | Avg | 89.21 | 91.77 | 95.54 | 99.62 | 83.05 | 72.93 | 88.91 | 92.54 | 80.76 | 94.66 | Avg | 85.56 | 86.62 | 88.13 | 86.18 | 89.09 | 75.04 | 95.12 | 84.02 | 87.04 | 88.77 |
| STD | 7.74 | 16.07 | 8.45 | 0.70 | 19.64 | 30.19 | 21.05 | 11.10 | 15.04 | 8.15 | STD | 18.05 | 20.42 | 21.72 | 21.26 | 14.05 | 26.40 | 14.65 | 23.99 | 13.13 | 19.97 |
QRS positive predictability is above 90% during supine, sitting, standing, walking, and stepping conditions for the wearable monitor, while it is above 90% during the Valsalva maneuver for the Criticare monitor. The arm intervention QRS positive predictability is below 80% for both monitors. Inter-subject variability and testing procedures may account for some of the large standard deviations for QRS predictability. There is also variability in the amount of muscle noise induced during arm movement. Overall the wearable monitor has a QRS positive predictability of 89.21% (std dev—7.74%) while the Criticare monitor has a positive predictability of 85.56% (std dev—18.05%).
To assess device comfort, each subject was asked to rate the device as either very comfortable, somewhat comfortable, neither particularly comfortable nor uncomfortable, somewhat uncomfortable, or very uncomfortable. Of the ten test subjects, eight filled out the survey, six rated the device as very comfortable, one rated the device as somewhat comfortable, and one rated the device as neither particularly comfortable nor uncomfortable.
VI. Summary and Future Work
A wearable cardiac monitor has been designed and tested in a clinical setting. The device was built on a flexible substrate and consumes between 2.4 and 3.33 mW depending on low- or high-resolution settings. The wearable cardiac monitor has an overall QRS sensitivity of 99.68%, which is within 0.1% of the Criticare 504-US clinical ECG recorder, and QRS positive predictability of 89.21%, which is 3.65% better than the Criticare recorder. These results compare favorably for the wearable cardiac monitor and prove that it is a viable alternative for everyday use compared to wired heart monitors.
Future design considerations for improved mechanical and electrical stability during high levels of activity, as well as increased memory size will allow the device to obtain the necessary data for long-term heart health algorithm analysis. A comparison study against current wireless solutions is also necessary to further validate the proposed device.
Acknowledgment
The authors would like to thank Dr. T. Heldt and Prof. G. Verghese for designing the clinical tests and writing the IRB. They would also like to thank B. Haslam, C. Ricciardi, A. Gordhandas for conducting the clinical tests, providing the data, and their help in annotating the ECG records. They would also like to thank Dr. C. Stultz MD, for discussions on cardiology and clinician acceptance of cardiac devices. Oversight for research subjects was provided by C. Ricciardi RN, BSN, and R. Thadhani MD, PhD.
This work was supported by Texas Instruments. The Clinical Research Center at MIT was supported under Grant UL1-RR025758 from the NIH through the National Center for Research Resources.
Contributor Information
Eric S. Winokur, Massachusetts Institute of Technology, Cambridge, MA 02139 USA..
Maggie K. Delano, Massachusetts Institute of Technology, Cambridge, MA 02139 USA..
Charles G. Sodini, Massachusetts Institute of Technology, Cambridge, MA 02139 USA..
REFERENCES
- 1.Moody G. [3/11/2012];Wave User’s Guide. (5th ed.). 2011 Mar 12; [Online]. Available: http://www.physionet.org/physiotools/wug/wug.pdf.
- 2.Zong W, Moody GB, Jiang D. A robust open-source algorithm to detect onset and duration of QRS complexes. Proc. Comput. Cardiol., Sep. 2003;30:737–740. [Google Scholar]
- 3.Veronique RL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, M Makuc D, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation. 2011 Dec 15;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update. A report from the American Heath Association statistics committee and stroke statistics subcommittee. Circulation. 2010;121:e1–e170. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 5.State specific mortality from sudden cardiac death—United States, 1999. Morb. Mortal. Wkly. Rep. 2002 Feb 15;51(06):123–126. [PubMed] [Google Scholar]
- 6.Sugano H, Hara S, Tsujioka T, Nakajima S, Inoue T, Takeuchi K, Nakamura H. Continuous ECG data gathering by a wireless vital snsor—Evaluation of its sensing and transmission capabilities. IEEE Int. Symp. Spread Spectrum Appl., Taichung, Taiwan. 2010 Oct;:98–102. [Google Scholar]
- 7.Shin K, Hwang HT, Kim YH, Kim JP, Yeo H-S, Han W, Hwang J, Lee J-W, Park JC. WHAM: A novel, wearable heart activity monitor based on Laplacian potential mapping. IEEE Eng. Med. Biol. Soc. Conf., Shangha, China. 2005 Sep;:7361–7364. doi: 10.1109/IEMBS.2005.1616212. [DOI] [PubMed] [Google Scholar]
- 8.Goya-Esteban R, Mora-Jimenez I, Rojo-Alvarez JL, Bacquero-Perez O, Pastor-Perez FJ, Manzano-Fernandez S, Pascual-Figal DA, Garcia-Alberola A. Heart rate variability on 7-day Holter monitoring using a bootstrap rhythmometric procedure. IEEE Trans. Biomed. Eng. 2010 Feb 13;57(6):1366–1376. doi: 10.1109/TBME.2010.2040899. [DOI] [PubMed] [Google Scholar]
- 9.Hindricks G, Taborsky M, Wohlgemuth P, Rieger G, Beckers F, Albers B. Atrial fibrillation detection by a subcutaneous monitoring device. IEEE Comput. Cardiol. Conf., Bologna, Italy. 2008 Sep;:413–416. [Google Scholar]
- 10.Singh A, Lui J, Guttag JV. Discretization of continuous ECG based risk metrics using asymmetric and warped entropy measures. Comput. Cardiol. 2010 Sep;37:473–476. [Google Scholar]
- 11. [3/11/2012]; [Online]. Available: http://www.mindray.com/na/products/netguard.html?backUrl=pro_line1_pre.html.
- 12.Penders J, Gyselinckx B, Vullers R, De Nil M, Nimmala VSR, van de Molengraft J, Yazicioglu F, Torfs T, Leonov V, Merken P, Van Hoof C. Human++: From technology to emerging health monitoring concepts. IEEE 5th Int. Summer School Symp. Med. Dev. Biosens; Hong Kong. Jun. 2008.pp. 94–98. [Google Scholar]
- 13. [3/11/2012]; [Online]. Available: http://www.vpatchmedical.com/pages/vpms-components.php.
- 14.Searle A, Kirkup L. A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol. Meas. 2000 May;21:271–283. doi: 10.1088/0967-3334/21/2/307. [DOI] [PubMed] [Google Scholar]
- 15. [3/11/2012]; CSI Criticare 504-US product pages, [Online]. Available: http://www.dhbiomedical.com/product%20pages/CSI-504.pdf.
- 16.American National Standard for Ambulatory Electrocardiographs Association for the Advancement of Medical Instrumentation; ANSI/AAMI EC38-1998; Arlington, VA. 1998. [Google Scholar]
- 17.Testing and Reporting Performance Results of Cardiac.. American National Standard. ANSI/AAMI EC57; 1998/(R)2003. [Google Scholar]