Abstract
Objectives
Children in families of low socioeconomic status (SES) have been found to have poor sleep, yet the reasons for this finding are unclear. Two possible mediators, presleep worries and home environment conditions, were investigated as indirect pathways between SES and children’s sleep.
Participants/Methods
The participants consisted of 271 children (M (age) = 11.33 years; standard deviation (SD) = 7.74 months) from families varying in SES as indexed by the income-to-needs ratio. Sleep was assessed with actigraphy (sleep minutes, night waking duration, and variability in sleep schedule) and child self-reported sleep/wake problems (e.g., oversleeping and trouble falling asleep) and sleepiness (e.g., sleeping in class and falling asleep while doing homework). Presleep worries and home environment conditions were assessed with questionnaires.
Results
Lower SES was associated with more subjective sleep/wake problems and daytime sleepiness, and increased exposure to disruptive sleep conditions and greater presleep worries were mediators of these associations. In addition, environmental conditions served as an intervening variable linking SES to variability in an actigraphy-derived sleep schedule, and, similarly, presleep worry was an intervening variable linking SES to actigraphy-based night waking duration. Across sleep parameters, the model explained 5–29% of variance.
Conclusions
Sleep environment and psychological factors are associated with socioeconomic disparities, which affect children’s sleep.
Keywords: Children, Sleep, Environment, Poverty, Worry
1. Introduction
Sleep is increasingly being linked to multiple aspects of health and well-being in youth [1]. Experimental and observational studies have shown that sleep is critical to maintaining metabolic, endocrine, and immune functioning [2], and it is related to health-related behaviors such as decreased physical activity, increased risk of substance use, and suicidal ideation [3]. Other research suggests that daytime sleepiness is related to behavior problems and mood [4], to lower levels of self-reported general health [5], and to an increased risk of unintentional injury [6].
Given the important role of sleep for overall health, it has been proposed that differences in various sleep parameters along socioeconomic lines may explain, at least in part, health disparities for a number of diseases [7,8]. This proposed link is underscored by research documenting socioeconomic disparities in sleep beginning in childhood. Studies on associations between family-level economic disadvantage and sleep have shown that lower socioeconomic status (SES) is associated with shorter sleep duration when assessed objectively using actigraphy [9–11] and through self- and parent-report methods [12,13]. In addition to sleep duration, self-reported subjective sleep problems (e.g., trouble falling asleep, maintaining sleep, and oversleeping) may be associated with family SES. Findings from the National Sleep Foundation’s 2006 [14] survey showed that adolescents with family income in the lowest bracket (<$50,000) were more likely to report difficulty falling asleep and staying asleep as compared to those in the highest bracket (>$100,000) [14]. Related research has found that, during late childhood, a lower income-to-needs ratio was associated with greater self-reported sleep/wake problems [9].
1.1. Possible mediators linking SES and sleep
The reasons why youths from lower socioeconomic backgrounds are at a greater risk of shorter and worse-quality sleep have not been well studied, and there is a need to identify mechanisms linking lower SES to poorer sleep [15]. The physical sleep environment is considered an important domain of “sleep hygiene” [16]. The National Sleep Foundation’s sleep hygiene recommendations include keeping the bedroom “comfortable, free from light and noise,” and these recommendations are supported by research showing links between the sleep environment and sleep problems. A study of families in China, for example, found noisy home conditions to double the risk of children experiencing more than three symptoms of insomnia [17]. Fewer economic resources may make it more challenging for families to maintain children’s sleep environments that are quiet, dark, and kept at a comfortable temperature, however. Smaller domiciles, for example, make it more likely that young children will share a bedroom with siblings and tighter living conditions are associated with greater difficulty falling asleep [18]. Research has also found that children from lower-income homes are three to four times more likely than those from the middle- and upper-income brackets to have a television in their bedroom [19]. In a review of the literature, Cain and Gradisar [20] found consistent evidence that the presence of a television in the bedroom is related to shorter total sleep and higher levels of sleep disturbance. Therefore, it is possible that the sleep environment may be an important consideration when examining links between SES and sleep. Indeed, limited research suggests that the sleep environment may at least partially explain differences in sleep along socioeconomic lines. In a sample of adults, sleep environment factors were found to partially mediate links between SES and poor self-reported sleep quality in a diverse sample of adults [21].
Another possible mechanism that could link economic disadvantage and sleep is that lower-SES children’s sleep may be compromised due to worries they have that prevent them from easily falling asleep. Nicassio, Mendlowitz, Fussell,&Petras [22] created the Pre-sleep Arousal Scale that separated arousal into somatic arousal (e.g., heart racing or stomach upset) and cognitive arousal (e.g., being distracted by sounds or worry about falling asleep). Associations between cognitive arousal at bedtime, including worry, and sleep disturbance have been demonstrated in children and adolescents. Alfano, Pina, Zerr, and Villalta [23] reported that greater cognitive (but not somatic) presleep arousal was associated with shorter sleep duration and more sleep problems reported by parents in an ethnically diverse sample of 7–14-year-olds. It is possible that youths who are economically disadvantaged experience greater levels of cognitive arousal as a result of greater exposure to daytime stressors. Economic disadvantage is associated with high levels of family stress and numerous specific stressors, including exposure to events that are unpredictable and uncontrollable, harsh discipline, and violence at home, school, or neighborhood [24]. However, no research has examined whether the higher rates of sleep disturbances in lower-SES children may be related to greater presleep worries in that population.
1.2. Current study
The aim of the current study was to explore novel possible mediators in relations between SES (as indexed by family income-to-needs ratio) and sleep in a community sample of children with a wide range of SES. Given the importance of examining multiple parameters via objective and subjective methods [25], sleep duration and quality (continuity/fragmentation and schedule)were assessed using actigraphy, and subjective sleep/wake problems and daytime sleepiness were assessed with a self-report questionnaire. Presleep worry and the sleep environment were examined with children’s self-reports. We predicted that lower SES would be directly related to sleep and that both sleep environment conditions and greater presleep worries would function as mediating variables in the link between lower SES and more subjective and objective sleep problems.
2. Method
2.1. Participants
The initial pool of participants consisted of 278 children and their parents who enlisted in a larger study examining biopsychosocial influences on health (Auburn University Sleep Study).
The study was approved by the Auburn University Institutional Review Board. The current investigation is based on data collected during the third study wave in 2011–2012. To recruit families, letters inviting participation were distributed to children at semirural public schools in the southeastern United States. Interested families were asked to call our research laboratory. The exclusion criteria were based on mothers’ reports, and the criteria included the child having been diagnosed with a sleep disorder or learning disability.
The sample used in the current analyses excluded children without any data on primary study variables and included 271 children (47% girls, 53% boys; M (age) = 11.33 years, standard deviation (SD) = 7.74 months, range = 10.00–12.67 years). Representative of the community, 62% of children were European American and 38% were African American. Regarding the education level of mothers and fathers, 3% and 4% had less than a high school education, 24% and 41% had a high school education, 35% and 31% had some college education, 27% and 17% had a college education, and 11% and 7% had a graduate degree, respectively. Based on mothers’ reports of pubertal status (1 = prepubertal, 2 = early pubertal, 3 = midpubertal, 4 = late pubertal, and 5 = postpubertal; Petersen et al. [26]), boys were prepubertal (M = 1.80, SD = 0.53) and girls were early pubertal on average (M= 2.35, SD = 0.61). Further, 79% of children were from two parent homes (e.g., both biological parents, or one biological parent and a spouse or partner) and 21% were from single-parent families (mostly single mothers).
2.2. Procedures
Sleep data were collected during the regular school year, excluding holidays. Actigraphs were delivered to the child’s home and parents were instructed to place them on the child’s nondominant wrist prior to bedtime for seven consecutive nights. To cross-validate the actigraphy data, parents completed child sleep diary logs [27]. Nights during which medication was used were excluded from analyses. Shortly after the actigraph assessment, children and their parents visited the on-campus laboratory to complete questionnaires; 77% of children visited the laboratory on the day following the last night of actigraphy and the rest shortly after. Children’s height and weight were measured using a Tanita digital weight scale and wall-mounted stadiometer (Arlington Heights, IL, USA) to compute the body mass index (BMI) (scores ranged from 1 (underweight) to 4 (obese)).
2.3. Measures
2.3.1. Income-to-needs ratio
Mothers reported on annual family income using the following categories: (a) $10,000–$20,000; (b) $20,000–$35,000; (c) $35,000– $50,000; (d) $50,000–$75,000; and (e) > $75,000. The mean of family income range and family/household size were used in the calculation of the income-to-needs ratio, specifically by dividing family income by the federal poverty threshold for that household/ family size (U.S. Department of Commerce; http://www.commerce.gov). Families who received an income-to-needs ratio of <1 were considered to be living in poverty (39% of families in the current sample), 1–2 living near the poverty line (28% of families), >2–3 lower middle class (24% of families), and ≥3 middle class standing (9% of families).
2.3.2. Actigraphy-measured sleep
Actigraphy was used to objectively measure children’s sleep. Participants wore Octagonal Basic Motionloggers (Ambulatory Monitoring, Inc, Ardsley, NY, USA). The actigraphs measured motion in 1-min epochs using the zero-crossing mode. The Octagonal Motionlogger Interface with Actme software and the analysis software package (Action W2, 2000 Ambulatory Monitoring Inc.) were used for analyses. Children were considered awake or asleep using the Sadeh scoring algorithm [28,29]. Actigraphy has good reliability, especially when used for multiple consecutive nights [30]. The actigraph and analysis software package have established validity for the assessment of children’s sleep when compared with polysomnography [28,29].
The inclusion of multiple sleep parameters is recommended to tap a wide range of sleep-related problems. We assessed the following parameters: (a) sleep minutes: number of minutes scored as sleep during the sleep period (actigraphy-based sleep-onset time to wake time) and (b) night waking duration: average length (in minutes) of each night waking. These parameters are commonly assessed in the child sleep literature [31]. To capture the variability in the sleep schedule over the week of actigraphic assessment, variability in sleep onset and variability in wake time were computed, using the mean-centered coefficient of variance statistic [32], and they were then mean-composited to create (c) variability in sleep schedule.
In total, 38% of children had actigraphy data for all seven nights, 22% had six nights, 20% had five nights, 8% had four nights, 6% had three nights, and 7% had fewer than three nights or no actigraphy data. These rates of valid actigraphy data are considered very good [30]. The reasons for missing data included mechanical problems (n = 4 children), forgetting to wear the actigraph (n = 5), having actigraphy-measured sleep times that were inconsistent with diary-based bed and wake times (n = 7), and using medicine known to influence sleep for at least one night (e.g., antiallergenics, cough syrup; n = 19) and exclusion of these nights from analyses. Researchers have recommended that actigraphy assessments include at least five nights [25,30]. We conducted secondary analyses excluding cases that had fewer than five nights of actigraphy data, and the findings were similar in nature to the study results. Consequently, all cases, regardless of the number of available nights, were retained to enhance power. Further, in support of using all available actigraphy nights, the well-established full information maximum likelihood estimation was used to handle missing data [33]. Intraclass correlations indicated good night-to-night stability for sleep minutes (α = 0.79), night waking duration (α = 0.83), variability in sleep onset (α = 0.88) and variability in wake time (α = 0.77) over the week.
2.3.3. Subjective reports of sleep
Children reported on their sleep over the past 2 weeks using the School Sleep Habits Survey (SHS; [34]). The SHS has good reliability and validity [35]. The 10-item Sleep/Wake Problems Scale (α = 0.70) and the nine-item Sleepiness Scale (α = 0.59) were used. The Sleep/Wake Problems Scale assessed the frequency of problems related to several sleep and wake parameters including oversleeping, trouble falling asleep, staying up all night, extreme tiredness at night, and difficulty waking up. The Sleepiness Scale measured the frequency of struggling to stay awake or falling asleep during nine daily activities including while in class, doing homework, attending a performance, watching television, and during a test; one item on driving was excluded. For each scale, higher scores reflect greater sleep-related problems.
2.3.4. Environmental conditions
Children completed a brief instrument similar to the Sleep Environment Inventory [21] to assess factors commonly reported as reasons for disrupted sleep. Seven items assessing physical environmental conditions (see Table 1 for items) were presented and children were asked to rate each item that “keeps them from sleeping well” during the last week. The response choices included 0 = no/ not applicable and 1 = yes. The scores on the seven items were summed to create an overall score (M = 1.67, SD = 1.48; range = 0– 6); Cronbach’s α = 0.52, which is acceptable given the small number of items and the diversity in circumstances that could interrupt sleep.
Table 1.
Correlations between environmental conditions and presleep worries Items and income-to-needs ratio and the sleep parameters.
| Percent endorsement |
r with income-to-needs ratio |
r with sleep minutesa |
r with night waking durationa |
r with variability in sleep schedulea |
r with sleep/wake problems b |
r with sleepinessb |
|
|---|---|---|---|---|---|---|---|
| Environmental conditions | |||||||
| Noise outside | 27.2% | −0.08 | −0.12t | 0.16* | 0.05 | 0.25** | 0.17** |
| Noise inside | 32.5% | −0.07 | 0.01 | 0.02 | 0.07 | 0.17** | 0.20** |
| Someone snoring | 19.6% | −0.11t | −0.01 | −0.05 | 0.02 | 0.24** | 0.18** |
| Uncomfortable bed | 18.9% | −0.01 | −0.05 | 0.03 | 0.05 | 0.27** | 0.25** |
| Temperature of room | 36.9% | −0.04 | −0.09 | 0.04 | 0.15* | 0.22** | 0.13* |
| TV, radio, or computer | 24.9% | −0.10 | 0.01 | 0.10 | 0.02 | 0.23** | 0.19** |
| Too much light | 9.2% | −0.04 | −0.03 | 0.03 | −0.06 | 0.05 | 0.14* |
| Presleep worries | |||||||
| About schoolwork | 19.7% | −0.01 | −0.08 | 0.07 | 0.14* | 0.19** | 0.17** |
| About family | 29.7% | −0.18** | −0.16* | 0.20** | −0.04 | 0.37** | 0.36** |
| About friends | 21.3% | −0.10 | −0.13* | 0.19** | −0.03 | 0.24** | 0.24** |
| About other kids | 12.8% | −0.10 | −0.12t | 0.18** | −0.06 | 0.27** | 0.27** |
| About other things | 30.4% | −0.03 | −0.04 | 0.01 | −0.05 | 0.40** | 0.32** |
This measure is actigraphy derived.
This is a child-reported sleep measure.
p < 0.10;
p < 0.05;
p < 0.01;
p < 0.001.
2.3.5. Presleep worries
Five items assessing presleep worries (“…worried about my family,” “…worried about my friends,” “…worried about school work”, “…worried about other kids,” and “…worried about other things”) were used. Children were asked to endorse items that “keep me from sleeping well” in the last week (see Table 1 for items). Similar to modifications used in other published research [23], items were adapted for youths from the Pre-Sleep Arousal Scale originally developed by Nicassio et al. [22]. The response choices included 0 = no/not applicable and 1 = yes. The items were summed to create a total score (M = 1.13, SD = 1.50; range = 0–5; Cronbach’s α = 0.78).
3. Results
3.1. Plan of analysis
We examined environmental conditions and presleep worries as mediators in the association between the income-to-needs ratio and children’s sleep. Five sleep parameters were examined: Actigraphy-derived sleep minutes, night waking duration, and variability in sleep schedule as well as self-reported sleep–wake problems and daytime sleepiness. Actigraphy-derived sleep efficiency (percentage of minutes scored as sleep between sleep onset and morning wake time) and long wake episodes (number of wake episodes ≥5 min) were assessed initially but neither yielded significant effects. For power considerations, these sleep parameters were excluded fromthe final model. Environmental conditions and presleep worries were included simultaneously in the same model (rather than fitting separate models for each). This approach is conservative and it allows for the assessment of the unique effects of one mediator (e.g., environmental conditions) while controlling for the other (e.g., presleep worries) and vice versa. Similarly, all five sleep variables were examined simultaneously to decipher “unique” effects.
We examined both environmental conditions and presleep worries as mediators of relations. If no mediation effects emerged, we considered environmental conditions and presleep worries as intervening variables in the link between the income-to-needs ratio and sleep. In both a mediation model and an intervening variable model, the independent variable shares a significant relation with the mediator or intervening variable, which in turn is significantly related with the outcome variable [36]. Thus, the mediating and intervening variables bridge the association between the independent and dependent variable, and they play a role in explaining why two variables are related. In a mediation model, the relation between the independent and dependent variable is significant prior to the inclusion of the mediating variable [37], whereas in an intervening model, no such significant relation exists. An examination of the intervening processes is common in the literature and a significant effect suggests that the relation between two variables might be contingent on an intervening variable [36,38]. Further, the study utilized a cross-sectional design and, thus, reference to a prediction of change in the mediator or dependent variable is used in the statistical sense rather than the prediction of change that might be inferred with a multi-wave design.
Monte Carlo simulation was used to assess the indirect effects [39]. This method produces confidence intervals (CIs) of the hypothesized indirect effects by generating a large number of estimates (20,000 in this study) of an indirect effect by resampling from the distributions of each direct effect. The CIs for testing indirect effects were generated using Selig and Preacher’s [40] interactive tool; an indirect effect was demonstrated when the CI did not contain zero. To minimize the outlier effects among primary study variables, the data points that surpassed three SDs were recoded as the highest observed value below three SDs [41]. Five values were recoded for sleep minutes, four values for night waking duration, three values for variability in sleep schedule, one value for sleep/wake problems, and five values for daytime sleepiness.
Child gender, ethnicity, medication use (e.g., acetaminophen), and BMI were included as covariates given their known associations with several primary study variables and their significant impact on model fit. Pubertal status, asthma (12% of children had asthma based on parents’ reports), and chronic illness (6% of children had a chronic illness; e.g., sickle cell, eczema, and ulcers) were initially considered as covariates, but none yielded a significant influence on model fit and thus were excluded. The control variables were allowed to correlate with each other as well as with the income-to-needs ratio, and these variables were allowed to predict environmental conditions, presleep worries, and the sleep parameters.
Analyses were conducted using Amos 21 [42]. Full information maximum likelihood estimation was used to handle the missing data [33]. The residual variances among environmental conditions and presleep worries were allowed to correlate as were the residual variances among each of the sleep parameters. Nonsignificant covariances among exogenous variables were omitted to increase the degrees of freedom (vs. fully saturating the model). Acceptable model fit was based on satisfying at least two of the three following criteria: χ2/df < 3, comparative fit index (CFI) > 90, and root mean square error of approximation (RMSEA) ≤ 0.08 [43]. In initial analyses, we examined the exacerbation of risk by assessing whether child gender and ethnicity served as moderators in the mediation model (moderated mediation); no significant interaction effects were detected and thus these variables were not considered further.
3.2. Preliminary analyses
Descriptive statistics are presented in Table 2. Based on actigraphy, children slept on average 7 h 16 min per night and the average night waking duration was nearly 6 min. Actigraphy-based information indicated that the average sleep-onset time was 10:06 pm (SD = 47 min) and the average morning wake time was 6:16 am (SD = 42 min). A relatively wide range of variability in sleep schedules and other primary study variables was observed. Bivariate correlations among primary study variables are also presented in Table 2.
Table 2.
Descriptive statistics and correlations among study variables.
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | M | SD | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Income-to-needs ratio | – | 1.49 | 0.99 | |||||||
| 2. Environmental conditions | −0.14* | – | 1.69 | 1.50 | ||||||
| 3. Presleep worries | −0.14* | 0.50*** | – | 1.14 | 1.50 | |||||
| 4. Sleep minutesa | 0.16** | −0.07 | −0.14t | – | 436 min | 55 min | ||||
| 5. Night waking durationa | −0.08 | 0.09 | 0.16** | 0.63*** | – | 5min and 51 s | 3 min and 25 s | |||
| 6. Variability in sleep schedulea | 0.10 | 0.11t | −0.01 | −0.03 | 0.07 | – | 0.08 | 0.05 | ||
| 7. Sleep/wake problemsb | −0.22*** | 0.40*** | 0.41*** | −0.21*** | 0.19** | 0.05 | – | 17.39 | 5.38 | |
| 8. Sleepinessb | −0.14* | 0.36*** | 0.38*** | −0.04 | 0.08 | −0.06 | 0.39*** | – | 12.29 | 3.22 |
Note. In sleep minutes, 436 min translates into 7 h and 16 min.
This measure is actigraphy derived.
This is a child-reported sleep measure.
p < 0.10;
p < 0.05;
p < 0.01;
p < 0.001.
3.3. Examination of direct effects between income-to-needs ratio and children’s sleep
Prior to mediation analyses, a model was first fit to examine the direct associations between the income-to-needs ratio and sleep (the direct effects model is not depicted in Fig. 1). After controlling for the covariates, a lower income-to-needs ratio was moderately related to fewer sleep minutes (B = 6.35, β = 0.12, p = 0.08) as well as greater sleep/wake problems (B = −0.84, β = −0.16, p = 0.01) and increased sleepiness (B = −0.42, β = −0.15, p = 0.01).
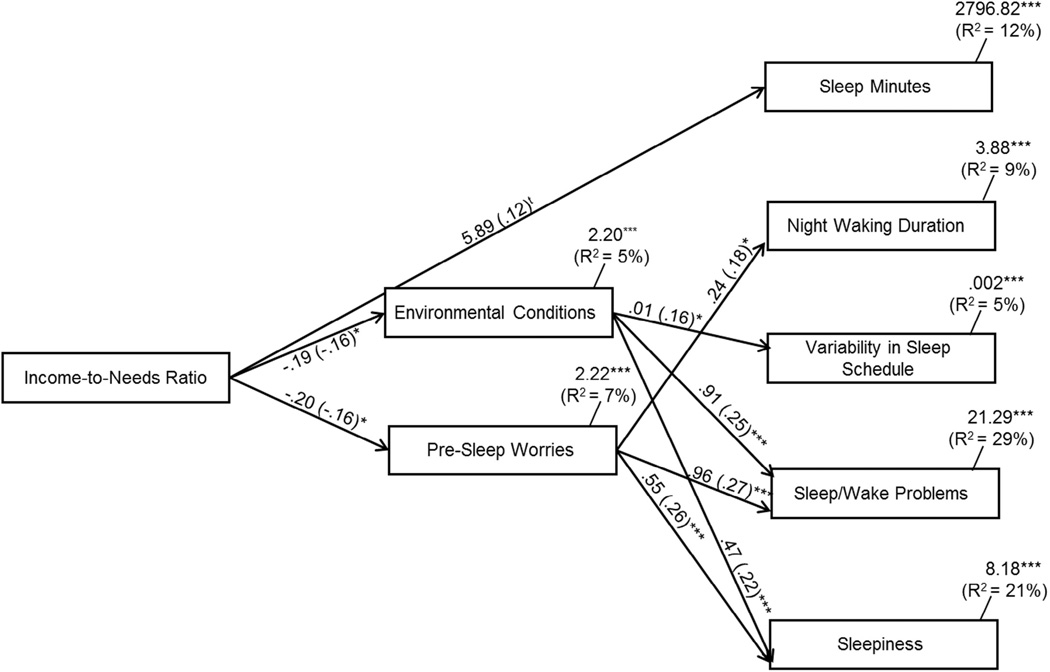
Fig. 1.
Path model of environmental conditions and presleep worries as mediators of relations between the income-to-needs ratio and sleep. Model fit: χ2(4) = 4.85 ns; χ2/df = 0.82; CFI = 0.99; RMSEA = 0.03 ns, 95% CI (0.00–0.10). Residual variances among environmental conditions and presleep worries were allowed to correlate as were the residual variances among the sleep parameters. Statistically significant lines are solid and nonsignificant lines are dotted. The covariates were child gender, ethnicity, medication use, and body mass index. tp < 0.10; *p < 0.05; **p < 0.01; ***p < 0.001.
3.4. Income-to-needs ratio and children’s sleep: environmental conditions and presleep worries as mechanisms of risk
Next, environmental conditions and presleep worries were added to the model for their assessment as mediators and intervening processes among relations between the income-to-needs ratio and children’s sleep (Fig. 1). The model fit the data well: χ2(4) = 4.85 ns, χ2/df = 0.82, CFI = 0.99, RMSEA = 0.03 ns, and 95% CI (0.00–0.10). Overall, the model explained 12% of the variance in sleep minutes, 9% in night waking duration, 5% in variability in sleep schedule, 29% in sleep/wake problems, and 21% in sleepiness.
Many control variables were related to the primary study variables (not shown in Fig. 1 for clarity). The male gender was related to fewer sleep minutes (B = −14.41, β = −0.13, p = 0.03) and less sleepiness to a moderate extent (B = −0.70, β = −0.11, p = 0.06). African American ethnicity was related to more presleep worries (B = 0.55, β = 0.18, p = 0.004), fewer sleep minutes (B = −21.35, β = −0.19, p = 0.002), longer night waking duration (B = 0.53, β = 0.13, p = 0.04), and greater sleep/wake problems (B = −0.69, β = −0.11, p = 0.06). Medication use was related to more sleep/wake problems (B = 2.00, β = 0.13, p = 0.02). Higher BMI was related to fewer sleep minutes (B = −2.32, β = −0.24, p < 0.001) as well as to longer night waking duration (B = 0.06, β = 0.17, p = 0.006) and increased sleep/wake problems (B = 0.12, β = 0.12, p = 0.03).
As shown in Fig. 1, and to a moderate extent, a lower income-to-needs ratio was directly related to fewer sleep minutes (B = 5.89, β = 0.12, p = 0.06) as well as to greater disruptive environmental conditions (B = −0.19, β = −0.16, p = 0.04) and presleep worries (B = −0.20, β = −0.16, p = 0.04; Fig. 1). Greater environmental conditions that disrupt sleep were associated with more variability in sleep schedule (B = 0.01, β = 0.16, p = 0.03) and greater sleep/wake problems (B = 0.91, β = 0.25, p < 0.001) and sleepiness (B = 0.47, β = 0.22, p < 0.001). Further, a heightened level of presleep worries was related to longer night waking duration (B = 0.24, β = 0.18, p = 0.03), greater sleep/wake problems (B = 0.96, β = 0.27, p = 0.001), and more sleepiness (B = 0.55, β = 0.26, p < 0.001).
3.4.1. Environmental conditions
The mediating role of environmental conditions in relations between the income-to-needs ratio and sleep was examined. As previously reported, a lower income-to-needs ratio was related to greater disruptive environmental conditions, which in turn were associated with more variability in sleep schedule (Fig. 1). Because the income-to-needs ratio was not directly related to variability in sleep schedule, environmental conditions served as an intervening process in these relations; the indirect effect was significant (95% CI (−0.01 to −0.001)). Further, because the income-to-needs ratio was directly related to sleep/wake problems and sleepiness as well as environmental conditions, and environmental conditions were related to sleep/wake problems and sleepiness, the mediating role of environmental conditions was assessed. While examining one potential mediator, we temporarily constrained the pathway between the other potential mediator and the sleep outcome variable of interest. Along this line, the pathways from presleep worries to sleep/ wake problems and sleepiness were constrained. Analyses indicated that the level of significance between the income-to-needs ratio and sleep/wake problems was reduced (p-value changed from 0.01 to 0.04), thus suggesting that disruptive environmental conditions partially mediated relations between the income-to-needs ratio and sleep/wake problems; the indirect effect was significant (95% CI (−0.58 to −0.04)). Similarly, the p-value of the direct effect between the income-to-needs ratio and sleepiness became nonsignificant (the p-value changed from 0.01 to 0.14), indicating that environmental conditions fully mediated relations between the income-to-needs ratio and sleepiness (95% CI (−0.36 to −0.03)).
3.4.2. Presleep worries
The mediating role of presleep worries in relations between the income-to-needs ratio and children’s sleep was assessed. As previously reported, a lower income-to-needs ratio was related to more presleep worries, which in turn was related to greater night waking duration (Fig. 1). Because the income-to-needs ratio was not directly related to night waking duration, presleep worries served as an intervening variable in these relations (95% CI (−0.15 to −0.002)). Further, we assessed whether presleep worries mediated relations between the income-to-needs ratio and sleep/wake problems and sleepiness. The paths from environmental conditions to sleep/ wake problems and sleepiness were first constrained to remove the effect of environmental conditions. The level of significance between the income-to-needs ratio and sleep/wake problems was reduced (the p-value changed from 0.01 to 0.05), thereby indicating that presleep worries partially mediated relations between the income to-needs ratio and sleep/wake problems (95% CI (−0.54 to −0.08)). Lastly, the p-value of the direct effect between the income-to-needs ratio and sleepiness became nonsignificant (the p-value changed from 0.01 to 0.17), indicating that presleep worries fully mediated relations between the income-to-needs ratio and sleepiness (95% CI (−0.31 to −0.04)).
4. Discussion
This study adds to a scant literature by exploring novel pathways linking lower SES to actigraphically derived and self-reported sleep problems during late childhood. The results showed a few direct associations between lower SES and sleep problems. Further, central to study hypotheses, models testing indirect effects indicated that increased exposure to disruptive sleep conditions and greater presleep worries serve as either mediators or intervening variables explaining some of the associations between SES and sleep. Across sleep parameters, the model explained 5–29% of variance, suggesting that both sleep environment and psychological factors contribute to socioeconomic disparities in sleep for children in important ways.
A growing number of studies have demonstrated that lower-SES living conditions are not conducive to children’s sleep [9,10,13]; however, critical questions remain regarding why such relations exist [15]. Toward addressing this gap, our findings pertaining to environmental conditions indicate that they serve as mediators of direct significant associations between the income-to-needs ratio and children’s sleep/wake problems and daytime sleepiness. Specifically, disruptive environmental living conditions partially mediated the relations between the income-to-needs ratio and subjective sleep/ wake problems and fully mediated the relations between the income-to-needs ratio and daytime sleepiness. In addition to mediation effects, an intervening process was observed in which environmental conditions linked SES and variability in the sleep schedule. Intervening processes are of importance and they help identify variables that could potentially bridge the relations between SES and sleep.
Several mediation and intervening effects involving presleep worries were observed. Specifically, presleep worries partially mediated the relations between the income-to-needs ratio and subjective sleep/wake problems and fully mediated the relations between this index of SES and daytime sleepiness. Further, presleep worries served as an intervening variable linking SES with night waking duration. The simultaneous inclusion of both environmental conditions and presleep worries in the same model helps more closely ascertain the unique effect of each mediating/intervening variable and lends confidence in the findings. Overall, our study advances the child literature in important ways, and it is one of the first to demonstrate that environmental concerns and presleep worries explain, at least partially, why lower SES relates to children’s sleep.
Inconsistent with expectations, lower SES was not directly related to actigraphy-measured night waking duration and variability in sleep schedule. It is unclear why such relations were not detected. The conservative nature of assessments including controlling for influential covariates and reducing mono-reporter bias (e.g., parent reports of SES and actigraphy-measured sleep) might have contributed to the null effects. However, evidence of intervening processes was found, such that lower SES was related to environmental conditions, which in turn was related to greater variability in sleep schedule. In addition, lower SES was related to longer night waking duration through their shared relations with presleep worries. The intervening rather than mediation effect suggests that the inclusion of environmental conditions and presleep worries are required to observe the indirect effect between lower SES and either night waking duration or variability in sleep schedule ([36]; MacKinnon, 2000). Further, evidence of a direct effect between lower SES and sleep is not a required criterion for our conceptual model, which proposes that lower SES ultimately undermines children’s sleep by creating living conditions that are not conductive to sleep and setting in motion greater presleep worries.
It is notable that, among all of the individual items on both presleep and environmental condition measures, only one item (worry about family) was more likely to be endorsed by lower-SES children. Yet, when considered as summed scale scores, both greater sleep environment concerns and presleep worry were associated with lower SES. This suggests a complex challenge for prevention and intervention efforts in that there is likely no single target for eliminating disparities in sleep. Still, improvements to the physical sleep environment may help children attain better sleep, and relatively inexpensive devices (e.g., fans, dehumidifiers, and white noise generators) should be empirically evaluated for efficacy in ameliorating some sleep problems. Helping children deal with worries that disrupt their sleep has already been incorporated into some sleep intervention studies [44] and may be a particularly potent intervention for children from lower-SES backgrounds.
The diverse sample, which included a wide range of family SES, is scarce in the literature, and multi-method sleep assessments were important strengths of the present study. However, there are some limitations worth noting that highlight the need for further research aimed at understanding socioeconomic disparities in sleep. We chose to focus on the income-to-needs ratio as it may best reflect the material resources of a family, yet other aspects of SES including parental education may relate to children’s sleep through similar or different pathways. Although our measures of environmental concerns and presleep worries were based on prior scales, it is possible that there are important additional concerns or worries that were not included in these measures. Further, because our scales were sum scores, they reflect the number of stressors endorsed by children, but not the severity of those stressors. There may be important qualitative and quantitative differences in the concerns or worries that children have based on SES. For example, the “noise outside” that affects sleep may also be threatening and presleep worries may vary greatly in terms of their intensity or potential consequences. Further, our assessment of pubertal status and the exclusion of clinical sleep disorders relied on maternal report and could have been improved through child reports or clinical assessment. Moreover, our study has not examined coping mechanisms that children employ to deal with these bedtime stressors or catastrophizing cognitions, which may represent an additional vulnerability factor for children from low-SES homes.
The importance of research exploring the processes that underlie links between sleep behaviors and economic disadvantage in youth is becoming more evident as a growing body of literature shows that sleep during childhood and adolescence is not only important concurrently [1] but also predictive of health outcomes into adulthood, above and beyond the effects of adult sleep (Landhuis, Poulton, Welch, & Hancox, 2008). The findings of this study represent an early step in explaining sleep disparities in youth and serve as a jumping-off point for future studies in this important area. It is likely that, through a more fine-grained, nuanced understanding of the social, psychological, and physical context within which sleep occurs, effective prevention programs can be developed.
Acknowledgments
The project described was supported by Grant Number R01HL093246 from the National Heart, Lung, and Blood Institute awarded to Mona El-Sheikh. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: http://dx.doi.org/10.1016/j.sleep.2014.10.008.
References
- 1.Shochat T, Cohen-Zion M, Tzischinsky O. Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep Med Rev. 2014;18(1):75–87. doi: 10.1016/j.smrv.2013.03.005. http://dx.doi.org/10.1016/j.smrv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 2.AlDabal L, BaHammam AS. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J. 2011;5:31–43. doi: 10.2174/1874306401105010031. PMCID:PMC3132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKnight-Eily LR, Eaton DK, Lowry R, Croft JB, Presley-Cantrell L, Perry GS. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev Med. 2011;53(4–5):271–273. doi: 10.1016/j.ypmed.2011.06.020. http://dx.doi.org/10.1016/j.ypmed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Fallone G, Owens JA, Deane J. Sleepiness in children and adolescents: clinical implications. Sleep Med Rev. 2002;6(4):287–306. doi: 10.1053/smrv.2001.0192. http://dx.doi.org/10.1053/smrv.2001.0192. [DOI] [PubMed] [Google Scholar]
- 5.Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Ancoli-Israel S, et al. Relationships among sleepiness, sleep time, and psychological functioning in adolescents. J Pediatr Psychol. 2009;34(10):1175–1183. doi: 10.1093/jpepsy/jsp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahl RE. Biological, developmental, and neurobehavioral factors relevant to adolescent driving risks [Review] Am J Prev Med. 2008;35(3S):S278–S284. doi: 10.1016/j.amepre.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Hale L, Peppard PE, Young T. Does the demography of sleep contribute to health disparities? In: Leger D, Pandi-Perumal SR, editors. Sleep disorders: their impact on public health. Oxon, UK: Informa Healthcare; 2007. pp. 1–17. [Google Scholar]
- 8.Moore PJ, Adler NE, Williams DR, Jackson JS. Socioeconomic status and health: the role of sleep. Psychosom Med. 2002;64(2):337–344. doi: 10.1097/00006842-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 9.El-Sheikh M, Bagley EJ, Keiley M, Elmore-Staton L, Chen E, Buckhalt JA. Economic adversity and children’s sleep problems: multiple indicators and moderation of effects. Health Psychol. 2013;32(8):849–859. doi: 10.1037/a0030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marco CA, Wolfson AR, Sparling M, Azuaje A. Family socioeconomic status and sleep patterns of young adolescents. Behav Sleep Med. 2012;10(1):70–80. doi: 10.1080/15402002.2012.636298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Redline S. Correlates of adolescent sleep time and variability in sleep time: the role of individual and health related characteristics. Sleep Med. 2011;12(3):239–245. doi: 10.1016/j.sleep.2010.07.020. http://dx.doi.org/10.1016/j.sleep.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adam EK, Snell EK, Pendry P. Sleep timing and quantity in ecological and family context: a nationally representative time-diary study. J Fam Psychol. 2007;21(1):4–19. doi: 10.1037/0893-3200.21.1.4. [DOI] [PubMed] [Google Scholar]
- 13.Fredriksen K, Rhodes J, Reddy R,Way N. Sleepless in Chicago: tracking the effects of adolescent sleep loss during the middle school years. Child Dev. 2004;75(1):84–95. doi: 10.1111/j.1467-8624.2004.00655.x. [DOI] [PubMed] [Google Scholar]
- 14.National Sleep Foundation. 2006 Sleep in America poll. 2006 Retrieved from http://sleepfoundation.org/sleep-polls-data/sleep-in-america-poll/2006-teens-and-sleep. [Google Scholar]
- 15.Knutson KL. Sociodemographic and cultural determinants of sleep deficiency: implications for cardiometabolic disease risk. Soc Sci Med. 2013;79(0):7–15. doi: 10.1016/j.socscimed.2012.05.002. http://dx.doi.org/10.1016/j.socscimed.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeBourgeois MK, Giannotti F, Cortesi F, Wolfson AR, Harsh J. The relationship between reported sleep quality and sleep hygiene in Italian and American adolescents. Pediatrics. 2005;115(10):257–265. doi: 10.1542/peds.2004-0815H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Li AM, Kong APS, Lai KYC, Tang NLS, Wing YK. A community-based study of insomnia in Hong Kong Chinese children: prevalence, risk factors, and familial aggression. Sleep Med. 2009;10(9):1040–1046. doi: 10.1016/j.sleep.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Milan S, Snow S, Belay S. The context of preschool children’s sleep: racial/ethnic differences in sleep locations, routines, and concerns. J Fam Psychol. 2007;21(1):20–28. doi: 10.1037/0893-3200.21.1.20. [DOI] [PubMed] [Google Scholar]
- 19.Tandon PS, Zhou C, Sallis JF, Cain KL, Frank LD, Saelens BE. Home environment relationships with children’s physical activity, sedentary time, and screen time by socioeconomic status. Int J Behav Nutr Phys Act. 2012;9(88) doi: 10.1186/1479-5868-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cain M, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: a review. Sleep Med. 2010;11:735–742. doi: 10.1016/j.sleep.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Mezick EJ, Matthews KA, Hall M, Strollo PJJ, Buysse DJ, Kamarck TW, et al. Influence of race and socioeconomic status on sleep: Pittsburgh Sleep SCORE Project. Psychosom Med. 2008;70(4):410–416. doi: 10.1097/PSY.0b013e31816fdf21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther. 1985;23(3):263–271. doi: 10.1016/0005-7967(85)90004-x. http://dx.doi.org/10.1016/0005-7967(85)90004-X. [DOI] [PubMed] [Google Scholar]
- 23.Alfano C, Pina A, Zerr A, Villalta I. Pre-sleep arousal and sleep problems of anxiety-disordered youth. Child Psychiatry Hum Dev. 2010;41(2):156–167. doi: 10.1007/s10578-009-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller GE, Chen E. The biological residue of childhood poverty. Child Dev Perspect. 2013;7(2):67–73. doi: 10.1111/cdep.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadeh A. Sleep assessment methods. In: El-Sheikh M, editor. Sleep and development: familial and socio-cultural considerations. New York: Oxford University Press; 2011. pp. 355–371. [Google Scholar]
- 26.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 27.Acebo C, Carskadon M. Scoring actigraph data using ACTION-W2. Providence, RI: Bradley Sleep Center, Brown University; 2001. [Google Scholar]
- 28.Sadeh A, Acebo C, Seifer R, Aytur S, Carskadon MA. Activity-based assessment of sleep-wake patterns during the 1st year of life. Infant Behav Dev. 1995;18(3):329–337. [Google Scholar]
- 29.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 30.Acebo C, Sadeh A, Seifer R, Tzischinsky O, Wolfson A, Hafer A, et al. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22:95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- 31.Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 2012;16(5):463–475. doi: 10.1016/j.smrv.2011.10.002. http://dx.doi.org/10.1016/j.smrv.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snedecor GW, Cochran WG. Statistical methods. 6th ed. Ames, IA: Iowa State University Press; 1967. [Google Scholar]
- 33.Acock AC. Working with missing values. J Marriage Fam. 2005;67(4):1012–1028. [Google Scholar]
- 34.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69(4):875–887. [PubMed] [Google Scholar]
- 35.Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak SE, et al. Evidence for the validity of a sleep habits survey of adolescents. Sleep. 2003;26(2):213–216. doi: 10.1093/sleep/26.2.213. [DOI] [PubMed] [Google Scholar]
- 36.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research. Conceptual, strategic, and statistical consideration. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 38.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- 39.Preacher KJ, Selig JP. Advantages of Monte Carlo confidence intervals for indirect effects. Commun Methods Meas. 2012;6(2):77–98. [Google Scholar]
- 40.Selig JP, Preacher KJ. Monte Carlo method for assessing mediation: an interactive tool for creating confidence intervals for indirect effects [Computer software] 2008 Retrieved from http://www.quantpsy.org. [Google Scholar]
- 41.Cousineau D, Chartier S. Outliers detection and treatment: a review. Int J Psychol Res. 2010;3(1):58–67. [Google Scholar]
- 42.Arbuckle JL. Amos 21 reference guide. Chicago: SmallWaters Corporation; 2012. [Google Scholar]
- 43.Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–262. [Google Scholar]
- 44.Britton WB, Bootzin RR, Cousins JC, Hasler BP, Peck T, Shapiro SL. The contribution of mindfulness practice to a multicomponent behavioral sleep intervention following substance abuse treatment in adolescents: a treatment-development study. Subst Abus. 2010;31(2):86–97. doi: 10.1080/08897071003641297. [DOI] [PubMed] [Google Scholar]