Abstract
Introduction
We hypothesize that surgical decompression for Chiari malformation type 1 (CM-1) is associated with statistically significant decrease in tonsillar pulsatility, and that the degree of pulsatility can be reliably assessed regardless of the experience level of the reader.
Methods
IRB-approved HIPAA-compliant retrospective study was performed on 22 children with CM-1 (8 males; mean age 11.4 years) who had cardiac-gated trueFISP sequence and phase contrast CSF flow imaging as parts of routine MR imaging before and after surgical decompression. The surgical technique (decompression with or without duraplasty) was recorded for each patient. Three independent radiologists with different experience levels assessed tonsillar pulsatility qualitatively and quantitatively, and assessed peri-tonsillar CSF flow qualitatively. Results were analyzed. To evaluate reliability, Fleiss Kappa for multiple raters on categorical variables, and intra-class correlation for agreement in pulsatility ratings were calculated.
Results
After surgical decompression, degree of tonsillar pulsatility appreciably decreased, confirmed by T-test, both qualitatively (P values 0.0004, 0.0004, and 0.0451 for 3 readers), and quantitatively (amount of decrease /P values for 3 readers: 0.7 mm/0.0002, 0.7 mm/0.0002, and 0.5 mm/0.0220). There was better agreement among the readers in quantitative assessment of tonsillar pulsatility (Kappa 0.753 – 0.834), compared to qualitative assessment of pulsatility (Kappa 0.472 – 0.496), and qualitative assessment of flow (Kappa 0.056 to 0.203). Posterior fossa decompression with duraplasty led to larger decrease in tonsillar pulsatility, compared to posterior fossa decompression alone.
Conclusion
Tonsillar pulsatility in CM-1 is significantly reduced after surgical decompression. Quantitative assessment of tonsillar pulsatility was more reliable across readers than qualitative assessments of tonsillar pulsatility or CSF flow.
Keywords: Chiari malformation, cerebellar tonsil, true-FISP, flow imaging, posterior fossa decompression
Introduction
Chiari Malformation Type 1 (CM-1) is associated with impaired flow of cerebrospinal fluid (CSF) at the craniovertebral junction [1]. This abnormal CSF flow is believed to contribute to disease manifestations such as development of syringomyelia [2,3]. However, the exact pathophysiology is poorly understood [4].
Phase-contrast magnetic resonance imaging (MRI) has been extensively used for qualitative and quantitative assessment of CSF flow at the craniovertebral junction. Experts have used parameters such as flow velocity, flow pulsation magnitude, and temporal variations during the cardiac cycle for assessment of CSF flow dynamics [2,5-8]. Several studies demonstrated that phase contrast MRI findings correlate to syrinx size and disease progression [2,5,9], and can help predict symptomatic outcome after surgical decompression [6,7].
Despite these important advances, quantitative phase-contrast MRI is not routinely used in many clinical departments, in part due to its time- and computation-intensive nature, and in part due to the measurement errors related to low CSF velocities and lack of signal.
Cardiac gated balanced steady state technologies have been adopted from cardiac imaging in order to study brain motion [10-12]. This technique enables direct measurement of cerebellar tonsillar pulsation on T2-weighted images [13]. Recent work suggested that tonsillar motion is increased in patients with CM-1 compared to healthy controls, and that tonsillar pulsatility might be further increased in patients with symptomatic disease [14]. However, such findings have not yet been published in peer-reviewed journals. In our institution, the MRI protocol for children with CM-1 includes cardiac-gated cine TRUE Fast Imaging with Steady state Precession (trueFISP) sequence since December 2009. TrueFISP sequence allows transverse magnetization equilibrium and hence the desired T1/T2 weighting to be achieved even in the presence of motion [15]. Therefore, it provides high CSF-soft tissue contrast. We have observed in several cases that the degree of pulsatility of the cerebellar tonsil after surgery is less than the pulsatility before surgery. However, to the best of our knowledge, no study has been published to evaluate the pulsatility change associated with posterior decompression surgery in CM-1.
Our first hypothesis was that the degree of tonsillar pulsatility in CM-1 decreases after neurosurgical decompression. Our second hypothesis was that readers of different experience level could reliably measure the degree of tonsillar pulsatility using cardiac-gated cine trueFISP sequence.
Materials and methods
Our Institutional Review Board (IRB) approved all procedures of this retrospective study (IRB # 201102012) and issued a waiver of informed consent. The study was in complete compliance with Health Insurance Portability and Accountability Act (HIPAA).
Patients
The inclusion criteria for this retrospective study were patients with CM-1 who underwent neurosurgical decompression at ***** Children's Hospital between December 2009 and May 2013, and had both preoperative and postoperative CSF flow study as well as cardiac-gated cine trueFISP sequence. Twenty-four patients met these inclusion criteria. The exclusion criterion was presence of MRI artifact on trueFISP or phase-contrast images of the craniovertebral junction perceived by at least one of the 3 readers to be compromising evaluation of tonsillar pulsatility or CSF flow. Two cases were excluded from this study based on the exclusion criterion.
MRI acquisition
MR imaging was performed on 1.5 Tesla scanners (Siemens, Erlangen, Germany). The cardiac gated trueFISP sequence consisted of 20-25 5-mm-thick T2-weighted images in mid sagittal plane, and could be viewed in a cine loop or individually to assess tonsillar pulsatility. Scan parameters used were TR of 120-131 ms, TE of 3.9-4.1 ms, FOV of 240 mm, and matrix 320 × 320.
Scan parameters for sagittal phase contrast (flow) imaging included TR 34-44 ms, TE 9-12 ms, FOV 220 mm, matrix 192 × 256, phases 18-24, NEX 2, and Venc 6.
MRI data analysis
A board-certified, CAQ-certified neuroradiology attending with more than 15 years of experience (AS), a board-certified neuroradiology fellow (RC), and a fourth-year radiology resident who had matched for neuroradiology fellowship (AR) reviewed all pre- and post-operative images independently using institutional commercially available image viewer application, Clinical Desktop, and blinded to the clinical history and surgical outcome. Each reader first reviewed all preoperative images. Following a minimum interval of 2 weeks to eliminate recall bias, readers reviewed all postoperative images. In regards to each examination, the readers first qualitatively assessed the tonsillar motion and then measured it quantitatively. Therefore, the assessment was at least partially subjective. All readers were blinded to the surgical technique used (posterior fossa decompression with versus without duraplasty. Please see Surgical Technique section for details).
Qualitative assessment of tonsillar motion
Each reader reviewed the trueFISP images of each examination in a cine mode, and visually graded the degree of tonsillar pulsatility on a three-point scale, as 0 (no appreciable tonsillar motion), 1 (minimal tonsillar motion), or 2 (marked tonsillar motion). A similar method for qualitative assessment of tonsillar pulsatility was previously used in other articles [14].
Quantitative assessment of tonsillar motion
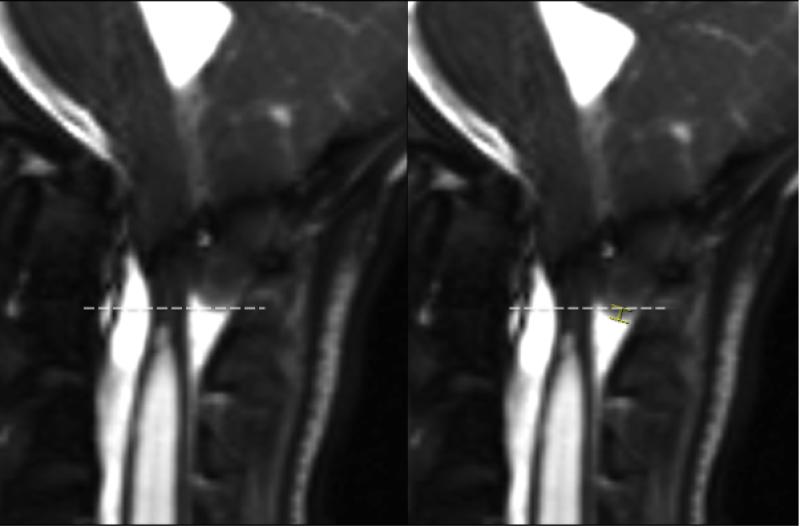
Each reader scrolled through the trueFISP images of each examination, and measured (in millimeters) the degree of translation of the inferior edge of the cerebellar tonsils during cardiac cycle using inbuilt measurement tool in our institutional image viewing application (Figure 1). Considering the submillimeter nature of pulsatility in children with CM-1, readers were advised to zoom in the cerebellar tonsil area approximately 10 times before doing measurements, and also to use the sagittal conventional T1-weighted and/or T2-weighted sequences to ensure that flow artifact is not perceived as tonsillar tip on trueFISP images.
Figure 1.
Two individual trueFISP images of a 14 year-old girl with Chiari malformation type-I before surgical decompression. The image on the viewer's left demonstrates the cerebellar tonsillar tip in its highest position, and the image on the viewer's right demonstrates the cerebellar tonsillar tip in its lowest position. The tonsillar translation (marked by a caliper on the right side image) measured 2 mm in this case. TrueFISP: True Fast Imaging with Steady state Precession
Qualitative assessment of peri-tonsillar CSF flow
Each reader reviewed the phase contrast MR images, and graded the anterior flow (anterior to the cervicomedullary junction), and the posterior flow (posterior to the cervicomedullary junction) on a three-point scale, as 0 (no flow), 1 (reduced flow), or 2 (normal/robust flow). In our institution, quantitative assessment of CSF flow based on phase contrast imaging is not routinely performed.
Assessment of cerebellar tonsillar position
For each preoperative examination, the perpendicular distance from the tip of the cerebellar tonsil to McRae's line (basion-opisthion line) was measured on sagittal T1 weighted images, and recorded as a measure of tonsillar position/ectopia.
Surgical technique
For all patients, a midline incision was made from the inion to the upper cervical spine, followed by subperiosteal dissection, a suboccipital craniectomy, extending from the foramen magnum to the inferior nuchal line, and C1 laminectomy. For patients who underwent posterior fossa decompression with duraplasty (PFDD), the dura was opened in a Y-shaped fashion. Arachnoid adhesions were released with sharp dissection and tonsillar coagulation or tonsillar resection, if indicated. Dural closure was performed using a triangular patch of Durepair Dura Regeneration Matrix (TEI Biosciences, Medtronic Neurosurgery, Waltham, MA) or a pericranial graft. For patients who underwent less invasive extradural decompression (PFD), the dura was not opened, and intraoperative ultrasound was used to ensure adequate decompression of the craniovertebral junction by visualizing the space around the cerebellar tonsils before, and again after sectioning of the atlanto-occipital membrane. Typically after sectioning of the extradural membrane, the surgeon would see expansion of the subarachnoid space posterior to the tonsils, which would persist throughout the cardiac cycle. If the dura did not contact the tonsils throughout the cardiac cycle, then extradural decompression was considered adequate. In rare cases when inadequate expansion was noted, the dura would be expanded by multiple vertically oriented incisions in the outer leaf, taking care not to open the arachnoid membrane. Following hemostasis, the wound was closed using interrupted suture.
Surgical outcome
The Chicago Chiari Outcome Scale (CCOS) is a standardized and recently validated tool to assess the postoperative outcome in CM-1 [16,17]. The CCOS uses 4 postoperative outcome categories (pain, nonpain symptoms, functionality, and complications) graded 1 to 4 for a total possible score of 16.
Statistical analysis
We used SAS 9.3 (SAS Institute, Cary, NC) to perform Fisher exact tests, analysis of variance, two-sided parametric t-tests, logistic regressions, and linear regressions. To evaluate reliability, we used R 3.0.2 irr library (The R Foundation for Statistical Computing) to calculate Fleiss Kappa for multiple raters on categorical variables, and intra-class correlation for agreement in pulsatility ratings [18].
Results
Patients
Out of 24 patients who met the inclusion criteria, two were excluded because MRI artifact at the craniovertebral junction on their trueFISP images compromised evaluation of tonsillar pulsatility. Twenty-two patients were studied, consisting of 8 males and 14 females. The age of the patients at the time of surgery ranged from 2 years to 18 years, with a mean age of 11.4 years, and standard deviation of 4.9 years. The mean time interval between preoperative MRI and decompression surgery was 5.6 weeks (standard deviation of 6.6 weeks). The mean time interval between decompression surgery and postoperative MRI was 17 weeks (standard deviation of 4.6 weeks).
MRI data
Agreement among readers
The Kappa values for preoperative and postoperative pulsatility assessment were 0.496 and 0.472, respectively (p-value < 0.001 for both). Therefore, there was moderate agreement among the 3 readers in qualitative assessment of tonsillar pulsatility [18].
The interclass correlation for quantitative assessment of preoperative tonsillar pulsatility among the readers was 0.834 (p-value<0.001, 95% confidence interval 0.690-0.924). The interclass correlation for quantitative assessment of postoperative tonsillar pulsatility among the readers was 0.753 (p-value < 0.001, 95% confidence interval 0.551-0.886). Therefore, there was substantial to almost perfect agreement among the 3 readers in quantitative assessment of tonsillar pulsatility [18].
The Kappa values (p-value) for qualitative assessment of peri-tonsillar CSF flow for preoperative images were 0.163 (0.089) and 0.203 (0.036) for anterior and posterior flow, respectively. The Kappa values (p-value) for qualitative assessment of peri-tonsillar CSF flow for postoperative images were 0.056 (0.581) and 0.183 (0.038) for anterior and posterior flow, respectively. Therefore, there was slight agreement among the 3 readers in qualitative assessment of peri-tonsillar flow (Kappa 0.056 to 0.203) [18].
Qualitative assessment of tonsillar motion
In order to analyze qualitative data, 0, 1, and 2 were assigned to no, minimal, and marked pulsatility, respectively. Delta pulsatility was defined as preoperative tonsillar pulsatility minus postoperative tonsillar pulsatility. The parametric t-test with the null hypothesis being “delta pulsatility is zero” revealed p-values of <0.001, <0.001, and 0.0451 for the 3 readers, respectively. Therefore, the change in pulsatility following neurosurgical decompression as perceived visually by the 3 readers was statistically significant.
Quantitative assessment of tonsillar motion
The mean (standard deviation) of preoperative and postoperative pulsatility respectively measured 1.2 mm (0.6 mm) and 0.6 mm (0.4 mm) by the first reader, 1.1 mm (0.6 mm) and 0.5 mm (0.4 mm) by the second reader, and 1.0 mm (0.6 mm) and 0.6 mm (0.3 mm) by the third reader.
More prominent tonsillar ectopia (lower position of the cerebellar tonsil) was associated with larger tonsillar pulsatility on preoperative images (p-values of 0.033, 0.007, and 0.013 for the 3 readers). Preoperative tonsillar pulsatility showed no meaningful correlation to the age of the subjects (p-value of 0.87).
Delta pulsatility for each patient was defined as the preoperative pulsatility measure minus the postoperative pulsatility measure. The delta pulsatility mean was 0.64 mm (p-value: <0.001, 95% confidence interval 0.35 – 0.93) for the first reader; 0.66 mm (p-value <0.001, 95% CI: 0.37 – 0.95) for the second reader; and 0.43 mm (p-value 0.022, 95% CI: 0.07 – 0.79) for the third reader. Therefore, based on the quantitative measurements reported by all 3 readers, postoperative tonsillar pulsatility was significantly less than preoperative tonsillar pulsatility in all cases.
Qualitative assessment of peri-tonsillar CSF flow
In order to evaluate difference in peritonsillar flow before and after the surgery, each anterior or posterior flow assessment result was designated 0 for “no flow”, 1 for “reduced flow”, and 2 for “normal/robust” flow. Delta flow was defined as postoperative flow minus preoperative flow. The t-test was performed for the anterior and posterior flow separately with the null hypothesis being “delta pulsatility is zero”. According to all 3 readers, the anterior flows were not significantly different before and after the surgery (p-values > 0.1 for all 3 readers). The posterior flows were significantly different after the surgery (p-values 0.011, 0.003, <0.001 for the 3 readers).
Surgical technique
Seven out of 22 cases underwent PFDD, while others underwent less invasive PFD. Delta pulsatility, defined as preoperative pulsatility minus postoperative pulsatility, was calculated for each case. Delta pulsatility mean (standard deviation) for PFDD and PFD methods respectively measured 1.0 mm (0.6 mm) and 0.5 mm (0.5 mm) for the first reader, 1.1 mm (0.8 mm) and 0.5 mm (0.5 mm) for the second reader, and 1.1 mm (0.9 mm) and 0.3 mm 0.5 mm) for the third reader. The two-sided parametric t-test was run for each reader's results separately to see if the mean delta pulsatility for the PFDD method is significantly different from the mean delta pulsatility for the PFD method. Based on the results of the three readers, PFDD was associated with more prominent decrease in tonsillar pulsatility in comparison to PFD. Such difference was statistically significant based on the results from 2 of the 3 readers (p-values of 0.166, 0.043 and 0.019 for the 3 readers).
Similarly, the peri-tonsillar flow values were assigned 0, 1, and 2 for no flow, reduced flow, and normal/robust flow respectively. Delta flow was defined as postoperative flow minus preoperative flow. These steps were done separately for the anterior and posterior flow and for each reader's results. Subsequently, The parametric t-test was performed to explore any significant difference in mean delta flow between PFDD and PFD techniques. The analysis for the results obtained by all three readers revealed no statistically significant difference in the anterior or the posterior flow between PFD and PFDD techniques (p-values for the anterior and posterior flow respectively were 0.500 and 0.086 for the first reader, 0.130 and 0.133 for the second reader, and 0.351 and 0.842 for the third reader).
Surgical outcome
The Chicago Chiari Outcome Scale was in the range of 9-16 (median of 14) for all patients. The outcome scale did not show a statistically significant correlation with the amount of change in tonsillar pulsatility after surgery (p-values 0.53, 0.32, and 0.10 for the 3 readers).
Discussion
Based on our findings, posterior fossa decompression surgery in children with CM-1 is associated with statistically significant decrease in cerebellar tonsillar pulsatility. Such decrease is appreciable both qualitatively (visually) and quantitatively.
These findings are concordant with available literature. The anatomical features of the posterior fossa have been shown to normalize after decompression surgery where tonsillar ectopia diminishes by 51% [1]. Researchers have found that tonsillar pulsatility correlates positively with the degree of tonsillar ectopia, while tonsillar ectopia correlates with clinical symptoms [14]. Pujol et al. showed that the amplitude of tonsillar pulsation was associated with the symptom of cough-strain headache [8].
The readers in our study detected a mean preoperative tonsillar pulsatility of 1.0-1.2 mm (SD 0.6 mm). Cousins et al. studied 11 CM-1 patients of 29-50 years of age, and reported a tonsillar pulsatility of 0.57 mm (SD 0.04 mm) [10]. Different factors may contribute to the noticeable difference in the reported numbers between the two studies. Unlike the age range studied by Cousins et al. all our subjects were 18 years old or younger. We only included the subjects that had both pre- and post-operative imaging, and such cases might conceivably have been more symptomatic or possibly have had more severe craniovertebral junction abnormality necessitating postoperative follow up imaging. In addition, our subjective method of assessing and measuring tonsillar motion might have affected the accuracy of our reported values, while Cousins et al. used a software program based on pixel shifting for measuring tonsillar motion [10]. Hofmann et al. reviewed 18 patients of 3-79 years of age with CM-1 and reported a faster cord displacement in patients relative to healthy controls but did not report cerebellar tonsillar motion measurements [19].
A reliability study by Sharma, et al in 2012 showed good agreement between two attending neuroradiologists with more than a decade of experience each, in assessing the degree of tonsillar pulsatility both qualitatively and quantitatively. In this study, we showed that such agreement also exists among users of different experience levels, including a senior radiology resident, a neuroradiology fellow, and an attending neuroradiologist with several years of experience. Sharma et al. also showed good intra-observer agreement in determining and measuring tonsillar pulsatility [13].
Brain and spine MRI in patients with CM-1 commonly includes phase-contrast sequences to assess CSF flow dynamics at the level of the foramen magnum. Some studies have shown findings of phase-contrast imaging in CM-1 to correlate with symptoms and surgical outcome, while others have not shown such correlation. [4,6,20]. In centers, like ours, where quantitative assessment of the CSF flow is not available, images are reviewed and assessed qualitatively and visually by a neuroradiologist. Our study showed that readers of different experience levels had only slight agreement in their qualitative assessment of peri-tonsillar flow robustness. Therefore, we suggest assessment of tonsillar pulsatility as a more reliable complementary or alternative approach to visual assessment of peri-tonsillar flow, wherever quantitative CSF flow study is not available. It should be noted that 4D phase-contrast flow imaging with its three-dimensional velocity encoding and volumetric coverage has been shown to provide a great deal of information about the complex flow of CSF, and although not tested in this study, may prove equally or more reliable compared to tonsillar pulsatility measurement [21].
The tonsillar pulsatility before surgery is significantly greater than the pulsatility after neurosurgical decompression, and the difference was appreciable both qualitatively and quantitatively by all 3 users. These findings pave the way for future studies of larger number of cases to investigate the correlation between delta pulsatility and surgical outcome as hypothesized based on our findings and available literature [14,22]. We could not prove such correlation using the Chicago Scale as the measure of outcome. If such correlation is confirmed in future studies and using larger number of cases or alternative outcome measures, tonsillar pulsatility can be used as a prognostic biomarker in postoperative care of CM-1.
Other researchers have shown that from engineering perspective, CSF biomechanics including fluid velocity, pressure, and motion of the cord are significantly different between patients with CM-1 and healthy subjects [23] [20]. Larger decrease in tonsillar pulsatility following PFDD compared to PFD is probably related to the role of duraplasty as an extra step during PFDD to correct the impaired biomechanics at the level of the foramen magnum. This finding is concordant with previous works showing that PFDD is associated with lower risk of reoperation compared to PFD [22]. The small number of cases in our series that underwent PFDD (7 cases) should be considered as a limitation for this conclusion. In our institution, the choice between the two techniques is made primarily based on individual surgeon's practice patterns/preferences. However, for patients with severe symptoms and/or large syrinx, surgeons were more likely to choose PFDD over PFD.
Beside the small sample size, the primary limitation of this study is the fact that the visual assessment and then manual measurement of tonsillar pulsatility is inherently subjective. An objective method of assessment such as using pixel threshold technique or quantitative assessment of motion based on phase-contrast MRI, as used by Yiallourou et al. could address this limitation [24].
In our institution, postoperative MRI including CSF flow study is usually performed to monitor syrinx resolution, or if there is concern for persistent or progressive symptoms. We included the patients who had both pre- and postoperative imaging; therefore, there is potential for a selection bias in our series. Such bias might have led to inclusion of patients who had less favorable postoperative outcome necessitating postoperative imaging. Therefore, the degree of difference in tonsillar pulsatility that we noticed before and after surgery could be on the conservative side, and, if patients with more favorable postoperative outcome are included in such analysis, the difference will likely be more significant than we found. This assumption is based on prior studies that showed positive correlations between tonsillar pulsatility, tonsillar ectopia, and clinical symptoms [14].
Another limitation of this study is the fact that the 3 readers could not be blinded to the pre- versus post-operative state of the images they reviewed, because postsurgical changes of sub occipital decompression are radiologically evident. A minimum interval of 2 weeks was established for each reader between reviewing the preoperative images and post-operative images in an attempt to eliminate recall bias.
Lack of CSF flow quantification is a limitation of this study. Future studies to evaluate correlations between tonsillar pulsatility and quantitative phase contrast imaging, and between tonsillar pulsatility and surgical outcome will be of interest.
With the current state of technology, susceptibility of trueFISP images to artifact (especially after suboccipital decompression surgery) remains a limiting factor in accurately assessing tonsillar pulsatility.
Conclusion
Tonsillar pulsatility was reliably measured using trueFISP sequence, and was found to have reduced after decompressive surgery in children with Chiari malformation type 1.
Supplementary Material
Acknowledgments
This study was supported by the Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and TL1 TR000449 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This study was also supported in part through philanthropic funding provided by the Park-Reeves Syringomyelia Research Consortium, the O'Keefe family and Mateo Dalla Fontana. The authors would like to thank Michael Wallendorf from the Department of Biostatistics at Washington University in St Louis for his contributions to this manuscript.
Footnotes
Ethical Standards and Patient Consent
We declare that all human and animal studies have been approved by the Institutional Review Board and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that IRB # 201102012 waived informed consent for this retrospective study.
Conflict of interest
We declare that we have no conflict of interest.
References
- 1.Heiss JD, Suffredini G, Bakhtian KD, Sarntinoranont M, Oldfield EH. Normalization of hindbrain morphology after decompression of Chiari malformation Type I. Journal of neurosurgery. 2012;117(5):942–946. doi: 10.3171/2012.8.JNS111476. doi:10.3171/2012.8.jns111476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhadelia RA, Bogdan AR, Wolpert SM, Lev S, Appignani BA, Heilman CB. Cerebrospinal fluid flow waveforms: analysis in patients with Chiari I malformation by means of gated phase-contrast MR imaging velocity measurements. Radiology. 1995;196(1):195–202. doi: 10.1148/radiology.196.1.7784567. doi:10.1148/radiology.196.1.7784567. [DOI] [PubMed] [Google Scholar]
- 3.Wetjen NM, Heiss JD, Oldfield EH. Time course of syringomyelia resolution following decompression of Chiari malformation Type I. Journal of neurosurgery Pediatrics. 2008;1(2):118–123. doi: 10.3171/PED/2008/1/2/118. doi:10.3171/ped/2008/1/2/118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hekman KE, Aliaga L, Straus D, Luther A, Chen J, Sampat A, Frim D. Positive and negative predictors for good outcome after decompressive surgery for Chiari malformation type 1 as scored on the Chicago Chiari Outcome Scale. Neurological research. 2012;34(7):694–700. doi: 10.1179/1743132812Y.0000000066. doi:10.1179/1743132812y.0000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heiss JD, Patronas N, DeVroom HL, Shawker T, Ennis R, Kammerer W, Eidsath A, Talbot T, Morris J, Eskioglu E, Oldfield EH. Elucidating the pathophysiology of syringomyelia. Journal of neurosurgery. 1999;91(4):553–562. doi: 10.3171/jns.1999.91.4.0553. doi:10.3171/jns.1999.91.4.0553. [DOI] [PubMed] [Google Scholar]
- 6.McGirt MJ, Nimjee SM, Floyd J, Bulsara KR, George TM. Correlation of cerebrospinal fluid flow dynamics and headache in Chiari I malformation. Neurosurgery. 2005;56(4):716–721. doi: 10.1227/01.neu.0000156203.20659.14. discussion 716-721. [DOI] [PubMed] [Google Scholar]
- 7.McGirt MJ, Nimjee SM, Fuchs HE, George TM. Relationship of cine phase-contrast magnetic resonance imaging with outcome after decompression for Chiari I malformations. Neurosurgery. 2006;59(1):140–146. doi: 10.1227/01.NEU.0000219841.73999.B3. discussion 140-146. doi:10.1227/01.neu.0000219841.73999.b3. [DOI] [PubMed] [Google Scholar]
- 8.Pujol J, Roig C, Capdevila A, Pou A, Marti-Vilalta JL, Kulisevsky J, Escartin A, Zannoli G. Motion of the cerebellar tonsils in Chiari type I malformation studied by cine phase-contrast MRI. Neurology. 1995;45(9):1746–1753. doi: 10.1212/wnl.45.9.1746. [DOI] [PubMed] [Google Scholar]
- 9.Oldfield EH, Muraszko K, Shawker TH, Patronas NJ. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. Journal of neurosurgery. 1994;80(1):3–15. doi: 10.3171/jns.1994.80.1.0003. doi:10.3171/jns.1994.80.1.0003. [DOI] [PubMed] [Google Scholar]
- 10.Cousins J, Haughton V. Motion of the cerebellar tonsils in the foramen magnum during the cardiac cycle. AJNR American journal of neuroradiology. 2009;30(8):1587–1588. doi: 10.3174/ajnr.A1507. doi:10.3174/ajnr.A1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hentschel S, Mardal KA, Lovgren AE, Linge S, Haughton V. Characterization of cyclic CSF flow in the foramen magnum and upper cervical spinal canal with MR flow imaging and computational fluid dynamics. AJNR American journal of neuroradiology. 2010;31(6):997–1002. doi: 10.3174/ajnr.A1995. doi:10.3174/ajnr.A1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reubelt D, Small LC, Hoffmann MH, Kapapa T, Schmitz BL. MR imaging and quantification of the movement of the lamina terminalis depending on the CSF dynamics. AJNR American journal of neuroradiology. 2009;30(1):199–202. doi: 10.3174/ajnr.A1306. doi:10.3174/ajnr.A1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma A, Parsons MS, Pilgram TK. Balanced steady-state free- precession MR imaging for measuring pulsatile motion of cerebellar tonsils during the cardiac cycle: a reliability study. Neuroradiology. 2012;54(2):133–138. doi: 10.1007/s00234-011-0861-3. doi:10.1007/s00234-011-0861-3. [DOI] [PubMed] [Google Scholar]
- 14.Sharma A, Moran K, Smyth M, Limbrick D, Pilgram T. Assessment of Tonsillar Pulsatility in Patients with and without Tonsillar Ectopia Using Cardiac-Gated Balanced Steady-State Precession MR Imaging.. Paper presented at the Annual meeting of the American Society of Neuroradiology; Seattle, WA. 2011.2011. [Google Scholar]
- 15.Haacke EM, Wielopolski PA, Tkach JA, Modic MT. Steady-state free precession imaging in the presence of motion: application for improved visualization of the cerebrospinal fluid. Radiology. 1990;175(2):545–552. doi: 10.1148/radiology.175.2.2326480. doi:10.1148/radiology.175.2.2326480. [DOI] [PubMed] [Google Scholar]
- 16.Aliaga L, Hekman KE, Yassari R, Straus D, Luther G, Chen J, Sampat A, Frim D. A novel scoring system for assessing Chiari malformation type I treatment outcomes. Neurosurgery. 2012;70(3):656–664. doi: 10.1227/NEU.0b013e31823200a6. discussion 664-655. doi:10.1227/NEU.0b013e31823200a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yarbrough CK, Greenberg JK, Smyth MD, Leonard JR, Park TS, Limbrick DD., Jr External validation of the Chicago Chiari Outcome Scale. Journal of neurosurgery Pediatrics. 2014;13(6):679–684. doi: 10.3171/2014.3.PEDS13503. doi:10.3171/2014.3.peds13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003;228(2):303–308. doi: 10.1148/radiol.2282011860. doi:10.1148/radiol.2282011860. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann E, Warmuth-Metz M, Bendszus M, Solymosi L. Phase-contrast MR imaging of the cervical CSF and spinal cord: volumetric motion analysis in patients with Chiari I malformation. AJNR American journal of neuroradiology. 2000;21(1):151–158. [PMC free article] [PubMed] [Google Scholar]
- 20.Alperin N, Loftus JR, Oliu CJ, Bagci A, Lee SH, Ertl-Wagner B, Green B, Sekula R. MRI Measures of Posterior Cranial Fossa Morphology and CSF Physiology in Chiari Malformation Type I. Neurosurgery. 2014 doi: 10.1227/NEU.0000000000000507. doi:10.1227/neu.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bunck AC, Kroeger JR, Juettner A, Brentrup A, Fiedler B, Crelier GR, Martin BA, Heindel W, Maintz D, Schwindt W, Niederstadt T. Magnetic resonance 4D flow analysis of cerebrospinal fluid dynamics in Chiari I malformation with and without syringomyelia. European radiology. 2012;22(9):1860–1870. doi: 10.1007/s00330-012-2457-7. doi:10.1007/s00330-012-2457-7. [DOI] [PubMed] [Google Scholar]
- 22.Durham SR, Fjeld-Olenec K. Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation Type I in pediatric patients: a meta-analysis. Journal of neurosurgery Pediatrics. 2008;2(1):42–49. doi: 10.3171/PED/2008/2/7/042. doi:10.3171/ped/2008/2/7/042. [DOI] [PubMed] [Google Scholar]
- 23.Shaffer N, Martin B, Loth F. Cerebrospinal fluid hydrodynamics in type I Chiari malformation. Neurological research. 2011;33(3):247–260. doi: 10.1179/016164111X12962202723805. doi:10.1179/016164111x12962202723805. [DOI] [PubMed] [Google Scholar]
- 24.Yiallourou TI, Kroger JR, Stergiopulos N, Maintz D, Martin BA, Bunck AC. Comparison of 4D phase-contrast MRI flow measurements to computational fluid dynamics simulations of cerebrospinal fluid motion in the cervical spine. PloS one. 2012;7(12):e52284. doi: 10.1371/journal.pone.0052284. doi:10.1371/journal.pone.0052284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.