Abstract
Objective
To determine if intraprostatic injection of gadofosveset trisodium mixed with human serum albumin (HSA) can identify sentinel lymph nodes draining the prostate on magnetic resonance imaging (MRI) in a canine model.
Material-Methods
Three male canines weighing between 25.7 and 41.3 kg were anesthetized, placed in a 3T MRI and a needle was placed transrectally into one side of the prostate using a commercially available intrarectal needle guide. Gadofosveset trisodium premixed with 10% HSA was then administered at doses ranging from 0.1 to 2.5ml. T1W MRI was performed immediately after injection and two readers evaluated images for visualization of LNs draining the prostate.
Results
Intraprostatic injection of 0.2 ml gadofosveset trisodium premixed with HSA identified the draining periprostatic lymph nodes in all cases. Delayed images demonstrated upper echelon nodes in the pelvis and abdomen. Higher volume injections resulted in excessive periprostatic extravasation whereas lower volume injections resulted in suboptimal visualization of LNs.
Conclusion
We demonstrate that gadofosveset trisodium (premixed with 10% HSA) injected intraprostatically at 0.2ml visualized lymph nodes draining the prostate. This approach can be readily adapted for clinical applications such as sentinel lymph node imaging in prostate cancer patients prior to surgery.
Keywords: gadofosveset trisodium, lymph node, MRI, human serum albumin
Introduction
Identification of lymph node metastasis is important for the accurate staging of prostate cancer (PCa). Current anatomic and functional imaging techniques have limited accuracy in the detection of metastatic lymph nodes (1, 2). Therefore, surgical pelvic lymph node dissection (PLND) remains the gold standard for nodal staging (3). The most common surgical approach to PLND includes resection of nodes in the obturator fossa; however, this technique fails to detect almost 30% of metastatic lymph nodes (4). For this reason, extended PLND (EPLND), including additional pelvic wall nodes and iliac lymph node chains, is currently the preferred method in higher risk patients (5). While decreasing the false negative rate for PCa, EPLND results in more complications such as lymphoceles, thromboembolic events, ureteral injury, and neurovascular injury due to the invasiveness of the surgery (6) and therefore a more targeted approach to lymph node resection is desirable.
A variety of imaging approaches have been used to identify malignant nodes in the pelvis. Computed tomography (CT) and magnetic resonance imaging (MRI) use size criteria to judge whether a node is malignant. Unfortunately, both methods suffer from a low sensitivity and specificity around 0.39-0.42 and 0.82, respectively (7). Recently, functional MRI, specifically diffusion weighted MRI (DW MRI), has been used to detect normal-sized metastatic nodes in patients with prostate and bladder cancer diagnosed as N0 with conventional cross-sectional imaging techniques (8). However, these results require further validation as the specificity may not be sufficient for clinical purposes. MRI performed 24 hours after intravenous injection of an ultrasmall superparamagnetic iron oxide agent (USPIO) has been reported to identify metastatic nodes by the absence of uptake of iron. However, this method is not yet widely available as the ideal agent, ferumoxtran, is not currently commercially available (9). Positron emission tomography (PET) with Fluorodeoxyglucose (FDG) has a low sensitivity in prostate cancer but good specificity for nodal metastases (10).
Interest has therefore turned to the sentinel lymph node (SLN) approach whereby, the draining nodes or sentinel nodes are identified and removed during surgery. Since these nodes are most likely to harbor metastases, a negative SLN sampling implies that upper echelon nodes will also be negative, sparing the patient extensive lymph node dissection. This approach has been most extensively evaluated in breast cancer where SLN imaging involves the injection of the radioactive tracers 99mTc nano-colloid or sulfur-colloid followed by scintigraphic imaging and intraoperative gamma probes to localize the draining node during surgery (11, 12). While, highly successful in breast cancer, it has been less successfully applied in prostate cancer. Criticisms of this approach include exposure of the patient and surgeon to radiation, poor spatial resolution and possible false positives related to improper orientation of the gamma probe during surgery, which may result in detection of bladder or periprostatic activity (13, 14). The spatial resolution of lymphoscintigraphy is quite limited (~7-8mm) making identification of smaller nodes, typical of pelvic nodes, difficult. Optical dyes such as blue dye and indocyanine green (ICG) have also been proposed for intraoperative SLN imaging but cannot be used pre-operatively because the optical signal cannot be detected outside the body. Combinations of ICG and 99mTc nano-colloid have also been proposed. For prostate SLN imaging magnetic resonance imaging has several advantages including, the ability to detect the tumor within the prostate so as to better localize the injection site, no radiation exposure and high spatial resolution, however, no suitable contrast agent has been available since lymph node mapping requires a macromolecular contrast agent that is retained by the lymphatics and all of the approved agents are small molecules. Recently however, gadofosveset trisodium (Ablavar® Lantheus Medical, North Billerica, MA) an albumin-binding small molecule gadolinium chelate was approved by the FDA for intravenous use. Gadofosveset trisodium demonstrates a prolonged intravascular half-life, secondary to its reversible binding to albumin, temporarily making it a macromolecule and therefore, suitable for lymphatic imaging after interstitial injection. This agent has been reported to be useful in identifying the deep lymphatics and nodes in rodent, primate and porcine models after interstitial injection in the paw indicating some utility in identifying the lymphatics (15-17). Unlike other macromolecules that are often retained in the liver, gadofosveset is rapidly excreted through the kidney after it dissociates from albumin due to weak, non-covalent binding. However, because gadofosveset is itself a small molecule and canine albumin characteristics are different than human albumin, we reasoned that premixing gadofosveset with human albumin would be advantageous for an MR lymphographic agent, Thus, in this study, we investigated the utility of gadofosveset trisodium (premixed with 10% human serum albumin [HSA]) as an SLN mapping agent in a canine model.
Materials and Methods
Contrast Agent
All studies were approved by the institutional Animal Care and Use Committee (ACUC) and all applicable institutional and/or national guidelines for care and use of animals were followed. Gadofosveset trisodium (Ablavar® Lantheus Medical Imaging, Billerica, MA, USA) was purchased commercially; it has a Gd concentration of 250mM. Gadofosveset trisodium is designed to reversibly bind HSA. Because the lymphatics preferentially take up macromolecules compared to small molecules, the gadofosveset trisodium was mixed with 10% HSA (Grifols Therapeutics Inc, Research Triangle Park, NC, USA) prior to injection so that it would be albumin-bound upon injection. Prior preliminary data suggested the injection of gadofosveset trisodium injection without HSA would be suboptimal for SLN imaging.
Study Design
Three dogs (two year old mixed-breed hounds varying in weight between 25.7-41.3 kg) were studied although each dog underwent several imaging sessions. All studies were conducted using a 3T MRI (Achieva 3.0T TX, Philips Healthcare, Best, The Netherlands). All procedures were performed in accordance with NIH guidelines on the use of animals in research with approval by the local ACUC and were conducted under the auspices of a veterinarian and trained staff. Intraprostatic injections of 50mM gadofosveset trisodium premixed with 10% human serum albumin (HSA) were performed at volumes per prostatic lobe of 2.5ml, 2ml, 0.5ml, 0.2ml and 0.1ml.
In vivo Imaging
After an overnight fast with access to water ad libitum, the animal was pre-anesthetized with a combination of midazolam (0.5mg/kg) and butorphanol (0.3mg/kg) administered intramuscularly followed by intravenous propofol (2.5-3.0mg/kg). The animal was intubated and was transferred to the MRI scanner where it was placed in the prone position. General anesthesia was maintained with inhaled 1-2% isoflurane delivered through a vaporizer and circle absorption breathing system using an MRI compatible anesthetic machine and ventilator (Penlon, Nuffield Anesthesia Ventilator Series 200, Oxfordshire, UK or Surgivet, Smiths Medical PM, Inc., Waukesha, WI, USA). During the MR imaging, the animal‘s vital signs were monitored with an MRI compatible monitoring system (In vivo systems, Orlando, FL, USA). A commercially available MR-compatible needle guide (DynaTRIM®, Invivo corp., Gainesville FL, USA) was used for directing intraprostatic injections. This system includes an endorectal needle guide whose insertion angle into the rectum, cranial-caudal location and left-right angle can be adjusted manually to target a particular region in the prostate (18). After initial calibration of the MR biopsy device, the provided software was then used to determine the coordinates for the placement of the needle guide to a defined region within each lobe of the prostate. After correct alignment, the needle guide was fixed in position for intraprostatic injection of gadofosveset trisodium human serum albumin (Gd-HSA) mixture using a 200mm 22-gauge needle (MReye Disposable Chiba needle, Cook Medical,Bloomington, IN, USA).
A baseline 3D T1 weighted (T1W) sequence with fat suppression was acquired with a 32-channel cardiac coil (SENSE; Philips Healthcare, Best, the Netherlands) (Table 1). The anatomic coverage included the diaphragm to the anal verge. Then, 50mM Gd-HSA was rapidly administered intraprostatically into one lobe of the prostate and four post-injection T1W MRI scans were acquired spanning approximately 20 minutes. If an additional injection was performed in the contralateral lobe, it was performed at least 30 minutes after the initial injection. Upon completion of image acquisition, all animals were recovered and returned to their cages for observation. No complications were encountered.
Table 1. Pulse sequence parameters used for SLN MRI after gadofosveset trisodium injection.
| TR/TE (msec) |
Matrix | Field of view (mm) |
Slice thickness (mm) |
Flip Angle |
Voxel size (mmxmmxmm) |
Fat suppression |
Acquisition time (sec) |
|---|---|---|---|---|---|---|---|
| 6.1/2.9 | 508×200 | 306×200×180 | 1.2 | 25 | 0.6×0.6×0.6 | SPIR | 218 |
Image Analysis
All MR image analysis was performed by two readers (BT and PLC with >7 and >20 years of experience on body MRI) on a commercially available work station (Extended MR WorkSpace [EWS], Philips, The Best, Netherlands). For each imaging session, baseline MR images were subtracted from the post-injection MR images at each phase and the degree of contrast enhancement within the visualized lymph nodes (LN) was evaluated on both subtracted and raw MR images. A single composite score derived from joint evaluation of both subtracted and raw MR images was assigned to each LN chain evaluatedusing a 2 point scoring system (0=no enhancement within the LN, 1=positive enhancement of the LN [hyperintense signal change within the LN] compared to baseline MR images). Additionally, wash out of the contrast from LNs was evaluated qualitatively over time. The readers assessed internal, external, common iliac and retroperitoneal lymph node chains.
Results
Intraprostatic Injection of 2.0-2.5ml Gadofosveset trisodium premixed with 10% Human Serum Albumin
The first two dogs (weight=41.3kg and 28kg) underwent injection of 2.5ml and 2.0ml of 50mM Gd-HSA (Gd dose=3μmol Gd/kg) into a lobe of the prostate. In both cases there was a large amount of extravasation around the prostate interfering with the identification of periprostatic nodes, however, the right common iliac, and higher retroperitoneal lymph nodes were visible (Figure 1).
Figure 1.



Oblique coronal T1W MRI demonstrates the needle placed in the right prostate lobe (arrow) prior to 2ml Gadofosveset trisodium premixed with 10% Human Serum Albumin injection in a 28 kg dog (dog number 2) (A). Maximum intensity projection T1W MRI obtained 7 minutes after injection of 2ml Gadofosveset trisodium-HSA shows significant contrast extravasation around the prostate (white arrow), into the bladder through the urethra (asterix), additionally the lymphatic channels are enhanced (black arrow) (B). Axial T1W MRI obtained 7 minutes after injection of 2ml Gadofosveset trisodium-HSA shows enhancing retroperitoneal lymph nodes (white arrows) and the lymphatic channels (black arrows) (C).
Intraprostatic Injection of 0.5ml Gadofosveset trisodium premixed with 10% Human Serum Albumin
The next dog (weight=25.7kg) underwent injection of 0.5ml of 50mM Gd-HSA (Gd dose=0.97μmol Gd/kg) into the left and right lobe of the prostate sequentially. The respective internal iliac and bilateral common iliac lymph nodes were the only visible lymph node chains (score=1) after these injections. The enhancement within the lymph nodes started in the first phase after injection and the contrast began to wash out of the lymph nodes by the fourth phase. Periprostatic contrast leakage after these injections was seen but it was much less than with the higher injection volumes.
Intraprostatic Injection of 0.2ml Gadofosveset trisodium premixed with 10% Human Serum Albumin
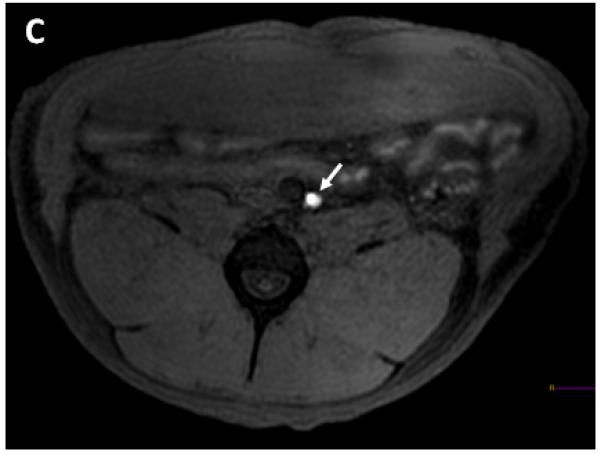
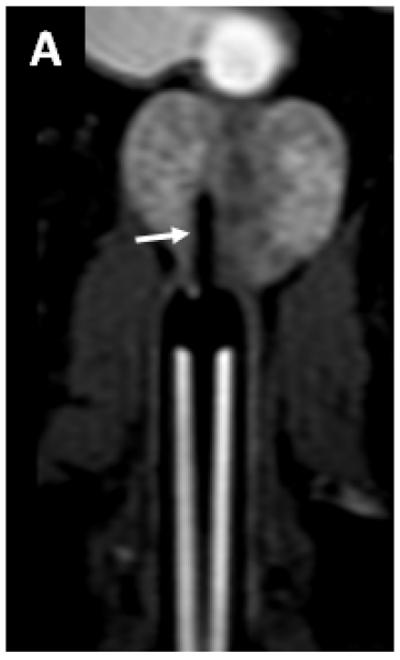
We further reduced the dose per lobe to 0.2ml in the next series of the experiments. In the next 3 injections (weight=38.6kg, 29.1 kg and 26.5kg) injections of 0.2ml of 50mM Gd-HSA (Gd dose=0.26μmol Gd/kg) were performed. The respective common iliac, retroperitoneal lymph nodes were visible after this injection. The enhancement within the lymph nodes started in the first phase after injection and the contrast began to wash out of the lymph nodes by the fourth phase after injection. There was reduced periprostatic extravasation (Figures 2, 3).
Figure 2.


Oblique coronal T1W MRI demonstrates the needle placed in the right prostate lobe (arrow) prior to 0.2ml Gadofosveset trisodium-HSA injection in a 29.1 kg dog (dog number 2) (A). Coronal T1W MRI obtained 7 minutes after injection of 0.2ml Gadofosveset trisodium-HSA shows enhancing right common iliac lymph node (long white arrow) and smaller amount of contrast extravasation around the prostate (short white arrow) (B). Axial T1W MRI obtained 7 minutes after injection of 0.2ml Gadofosveset trisodium-HSA shows enhancing left sided retroperitoneal lymph node (white arrow) (C).
Figure 3.


Oblique coronal T1W MRI demonstrates the needle placed in the right prostate lobe (arrow) prior to 0.2ml Gadofosveset trisodium-HSA injection in a 38.6 kg dog (dog number 1) (A). Coronal T1W MRI obtained 7 minutes after injection of 0.2ml Gadofosveset trisodium-HSA shows enhancing right common iliac lymph node (long white arrow) and smaller amount of contrast leaked around the prostate (short white arrow) (B). Axial T1W MRI obtained 7 minutes after injection of 0.2ml Gadofosveset trisodium-HSA shows enhancing right sided retroperitoneal lymph node (white arrow) (C).
Intraprostatic Injection of 0.1ml Gadofosveset trisodium premixed with 10% Human Serum Albumin
Finally, we reduced the dose per lobe to 0.1ml. Two dogs (weight=39kg and 26.5kg) underwent injection of 0.1ml of 50mM Gd-HSA (Gd dose=0.13μmol Gd/kg) into the prostate. The respective common iliac, retroperitoneal lymph nodes were the only visible lymph node chains (score=1) after this injection. The enhancement within the lymph nodes started in the first phase after injection and the contrast began to wash out of the lymph nodes by the third phase after injection. There was very little periprostatic contrast leakage after the injection.
Discussion
This study demonstrates the feasibility of using an FDA-approved albumin-binding gadolinium chelate, gadofosveset, to visualize the draining lymph nodes of the prostate on MRI after intraprostatic injections. Intraprostatic injections of gadofosveset mixed with 10% human serum albumin reliably depicted the draining lymph nodes even at very low volumes. Doses of 2.5ml/lobe and 2ml/lobe resulted in a large amount of periprostatic contrast material extravasation obscuring regional nodes. A smaller dose of 0.5ml/lobe injection resulted in sufficient enhancement within the lymph nodes but still considerable periprostatic leakage. The dose of 0.2ml/lobe resulted in sufficient enhancement of the lymph nodes with an imaging window of around 0-30 minutes after injection with only a small amount of periprostatic leakage. Although the dose of 0.1ml/dose revealed lymph nodes, the enhancement was faint with a limited and variable imaging window of around 0-21 minutes after injection (Table 2). Thus, in this model a dose of 0.2ml of Gd-HSA optimized SLN imaging while minimizing periprostatic leakage. Gadofosveset trisodium is known to reversibly bind HSA following ex-vivo mixing and its molecular size is expected to increase from <1nm to approximately 8.5-11nm due to the bound albumin (19). Previous studies have shown that gadofosveset trisodium premixed with HSA and injected intradermally into the hind paw of an animal resulted in rapid visualization of the lymphatics including the thoracic duct. Gadofosveset trisodium injected without prior HSA conjugation failed to reliably depict the thoracic duct as it behaved like a mixture of low and high molecular weight agents with poor depiction of nodes (17, 20). In a previous study using a porcine model of intradermal injection of a gadofosveset trisodium-HSA mixture the popliteal and inguinal lymph nodes were visualized (17). Only a small volume was needed to enhance the lymph channels and nodes. Based on that experience, a similar small dose of gadofosveset trisodium mixed with 10% HSA was used to demonstrate prostate drainage to LNs.
Table 2.
Summary of experimental results in this study.
| Dog number |
Dog weight (kg) |
Prostate size (ml) |
Injected dose (ml/lobe) |
Injection site |
Visualized lymph node chains | Visibility window (minutes) |
Periprostatic leak |
|---|---|---|---|---|---|---|---|
| 1 | 41.3 | 25 | 2.5 | Left | R common iliac, retroperitoneal | 0-30 | Large |
| 2 | 28 | 21 | 2 | Right | 1 common iliac, retroperitoneum | 0-30 | Large |
| 3 | 25.7 | 27 | 0.5 | Left | L internal iliac, bilateral common iliac |
0-21 | Moderate |
| 3 | 25.7 | 27 | 0.5 | Right | Bilateralcommon iliac | 0-30 | Moderate |
| 1 | 38.6 | 25 | 0.2 | Right | R common iliac, retroperitoneal | 0-30 | Small |
| 2 | 29.1 | 21 | 0.2 | Right | R common iliac, retroperitoneum | 0-30 | Small |
| 3 | 26.5 | 27 | 0.2 | Left | L internal iliac, L common iliac | 7-30 | Small |
| 1 | 39 | 25 | 0.1 | Right | R common iliac, retroperitoneum | 0-21 | Minimal |
| 1 | 39 | 25 | 0.1 | Left | Lcommon iliac | 0-14 | Minimal |
| 3 | 26.5 | 27 | 0.1 | Right | R common iiiac | 0-7 | none |
The optimal volume of the gadofosveset trisodium HSA mixture balanced periprostatic extravasation (due to over-injection) with under opacification of the SLNs (due to under injection). We found that a dose of 0.2ml was optimal . However, this is highly dependent on the prostate volume and will likely differ in humans. Nonetheless, this data indicates that a very small dose, much smaller (almost 100 fold) than the intravenous dose, can be used for intraprostatic SLN imaging. Additionally, other MR contrast agents (e.g. gadobenate dimeglumine) have been used for lymphatic imaging of lymphedema and may be useful in the prostate applications as well (21).
The concept of SLN imaging in prostate cancer has been validated in several studies, mainly in Europe. The most popular method is to adapt conventional lymphoscintigraphy used in breast cancer to the setting of prostate cancer. Holl et al. reported on a cohort of 2020 patients, who received 1.2ml of 99mTc-nanocolloid intraprostatically and demonstrated a sensitivity of 98% for SLNs with a high percentage bearing metastases (22). In order to combine a preoperative SLN method with an intraoperative method, Rousseau et al. injected 0.3ml of 99mTc-sulphur colloid in each prostate lobe of 93 prostate cancer patients and used a gamma probe to detect SLNs resulting in a sensitivity of 93.5% for malignant nodes (23). Others have used a combination of optical and radionuclide methods. For instance, Van der Poel et al. injected 0.4ml mixture of indocyanine green-99mTc-NanoColloid in eleven patients, and reported successful visualization of sentinel nodes both before (with scintigraphy) and during (with scintigraphy and optical imaging) surgery (24). One disadvantage of lymphoscintigraphy is the radiation exposure to both patient and operator which, while admittedly low, is nevertheless undesirable. Gadofosveset-albumin could be a viable substitute requiring no additional FDA clearance. It is conceivable that indocyanine green (ICG) could be added to the mixture to allow for optical detection of SLNs intraoperatively as it similarly binds reversibly with albumin.
A major issue with lymphoscintigraphy is false positive diagnoses due to spurious radiation from the bladder or periprostatic tissues. The gamma probe used to detect radioactivity in nodes is not very directional and high concentrations of radionuclide can lead to false readings (13, 14). Lymphoscintigraphy has limited spatial resolution and smaller SLNs may be missed. In contrast, MRI has much higher spatial resolution and may account for why much smaller volumes were needed.
There are several limitations to this study. Injections were made into the left or right lobe of the prostate as these animals did not have tumors and therefore, the detected nodes were not tested for malignancy. Ideally, one would inject the contrast directly into the tumor to most accurately depict the lymphatic drainage. This is made possible by a commercially available transrectal MR biopsy guidance system that can be adapted for this purpose. Considering the high accuracy of multi-parametric MRI to localize prostate cancer lesions, with MR guidance it may be possible to directly visualize the tumor and then inject the agent intratumorally to better map the draining LNs. Additionally, this study used only a small number of animals, several of whom had repeated injections (range of interval between repeat injections were 3 to 8 weeks), as it was considered a pilot feasibility trial; nonetheless, the results were reproducible and consistent. No ill effects were noted in dogs despite repeated intraprostatic injections.
As a future perspective, we envision this agent being used in the following manner: Prior to surgery a prostate MRI will be obtained and a tumor identified. This may require imaging guided biopsy for histologic confirmation. Following the decision to perform prostate surgery, the patient would return to the MR suite and obtain an MRI in the prone position with the intrarectal needle guide (same as used in our study) inserted. With prior knowledge of the location of the tumor, an injection needle would be inserted into the tumor and a volume of approximately 0.2ml of gadofosveset-HSA would be injected. It is unclear at this time whether prior mixing with HSA will be necessary in humans and this will require further testing. Once injected, the T1W MRI would commence and SLNs would be identified allowing for surgical planning. As mentioned, in a future iteration, the mixture could be mixed with ICG for intrasurgical visualization with a near infrared camera. Otherwise, the nodes would be located by the surgeon, removed and evaluated for metastatic disease, prior to further resection.
In conclusion, we demonstrate that gadofosveset trisodium (premixed with 10% HSA) can be injected intraprostatically to image lymph nodes draining the prostate on T1W MRI. Accurate intraprostatic injections, potentially targeting an intraprostatic lesion are possible using an in-gantry MRI biopsy guidance system. Since all the components of the study, the gadofosveset trisodium, human serum albumin and MRI biopsy guidance platform are FDA approved; it should be relatively easy to adapt this off-label method into future clinical trials for men with prostate cancer. It is hoped that successful tumor targeted SLN MRI could result in fewer and less extensive pelvic lymph node dissections with commensurate improvements in adverse events, while maintaining or augmenting the prognostic yield.
Acknowledgements
We would like to acknowledge valuable assistance of Randy Clevenger, Gayle Nugent, Shawn Koslov and Art Zetts from the National Heart Lung Blood Institute Animal Surgery and Resources during our experiments.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement:
No competing financial interests exist.
References
- 1.Tiguert R, Gheiler EL, Tefilli MV, et al. Lymph node size does not correlate with the presence of prostate cancer metastasis. Urology. 1999;53:367–371. doi: 10.1016/s0090-4295(98)00518-4. [DOI] [PubMed] [Google Scholar]
- 2.Oyen RH, Van Poppel HP, Ameye FE, Van de Voorde WA, Baert AL, Baert LV. Lymph node staging of localized prostatic carcinoma with CT and CT-guided fine-needle aspiration biopsy: prospective study of 285 patients. Radiology. 1994;190:315–322. doi: 10.1148/radiology.190.2.8284375. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Bader P, Burkhard FC, Markwalder R, Studer UE. Is a limited lymph node dissection an adequate staging procedure for prostate cancer? J Urol. 2002;168:514–8. doi: 10.1016/s0022-5347(05)64670-8. [DOI] [PubMed] [Google Scholar]
- 5.Briganti A, Blute ML, Eastham JH, et al. Pelvic lymph node dissection in prostate cancer. Eur Urol. 2009;55:1251–1265. doi: 10.1016/j.eururo.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Clark T, Parekh DJ, Cookson MS, et al. Randomized prospective evaluation of extended versus limited lymph node dissection in patients with clinically localized prostate cancer. J Urol. 2003;169:145–147. doi: 10.1016/S0022-5347(05)64055-4. discussion 147-148. [DOI] [PubMed] [Google Scholar]
- 7.Hövels A, Heesakkers R, Adang E, Jager G, Strum S, Hoogeveen Y, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin. Radiol. 2008;63:387–95. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Thoeny HC, Froehlich JM, Triantafyllou M, Huesler J, Bains LJ, Vermathen P, et al. Metastases in normal-sized pelvic lymph nodes: detection with diffusion-weighted MR imaging. Radiology. 2014;273:125–35. doi: 10.1148/radiol.14132921. [DOI] [PubMed] [Google Scholar]
- 9.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N. Engl. J. Med. 2003;348:2491–9. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 10.Schöder H, Herrmann K, Gönen M, Hricak H, Eberhard S, Scardino P, et al. 2-[18F]fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin. Cancer Res. 2005;11:4761–9. doi: 10.1158/1078-0432.CCR-05-0249. [DOI] [PubMed] [Google Scholar]
- 11.Jeschke S, Beri A, Grüll M, et al. Laparoscopic radioisotope-guided sentinel lymph node dissection in staging of prostate cancer. Eur Urol. 2008;53:126–132. doi: 10.1016/j.eururo.2007.03.064. [DOI] [PubMed] [Google Scholar]
- 12.Beri A, Janetschek G. Technology insight: radioguided sentinel lymph node dissection in the staging of prostate cancer. Nat Clin Pract Urol. 2006;3:602–610. doi: 10.1038/ncpuro0625. [DOI] [PubMed] [Google Scholar]
- 13.Ganswindt U, Schilling D, Müller AC, Bares R, Bartenstein P, Belka C. Distribution of prostate sentinel nodes: a SPECT-derived anatomic atlas. Int J Radiat Oncol Biol Phys. 2011;79:1364–1372. doi: 10.1016/j.ijrobp.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Jeschke S, Nambirajan T, Leeb K, Ziegerhofer J, Sega W, Janetschek G. Detection of early lymph node metastases in prostate cancer by laparoscopic radioisotope guided sentinel lymph node dissection. J Urol. 2005;173:1943–1946. doi: 10.1097/01.ju.0000158159.16314.eb. [DOI] [PubMed] [Google Scholar]
- 15.Lauffer RB, Parmelee DJ, Ouellet HS, et al. MS-325: a small-molecule vascular imaging agent for magnetic resonance imaging. Acad Radiol. 1996;3(Suppl 2):S356–358. doi: 10.1016/s1076-6332(96)80583-6. [DOI] [PubMed] [Google Scholar]
- 16.Nakajima T, Turkbey B, Sano K, et al. MR lymphangiography with intradermal gadofosveset and human serum albumin in mice and primates. J Magn Reson Imaging. 2013 doi: 10.1002/jmri.24395. in press. doi: 10.1002/jmri.24395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turkbey B, Kobayashi H, Hoyt RF, Jr, et al. Magnetic Resonance Lymphography of the Thoracic Duct after Interstitial Injection of Gadofosveset Trisodium: A Pilot Dosing Study in a Porcine Model. Lymphat Res Biol. 2014;12:32–36. doi: 10.1089/lrb.2013.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hambrock T, Fütterer JJ, Huisman HJ, et al. Thirty-two-channel coil 3T magnetic resonance-guided biopsies of prostate tumor suspicious regions identified on multimodality 3T magnetic resonance imaging: technique and feasibility. Invest Radiol. 2008;43:686–694. doi: 10.1097/RLI.0b013e31817d0506. [DOI] [PubMed] [Google Scholar]
- 19.Kiselev MA, Gryzunov IuA, Dobretsov GE, Komarova MN. Size of a human serum albumin molecule in solution. Biofizika. 2001;46:423–427. [PubMed] [Google Scholar]
- 20.Ikomi F, Hanna GK, Schmid-Schönbein GW. Mechanism of colloidal particle uptake into the lymphatic system: basic study with percutaneous lymphography. Radiology. 1995;196:107–113. doi: 10.1148/radiology.196.1.7784553. [DOI] [PubMed] [Google Scholar]
- 21.Lu Q, Bui D, Liu NF, Xu JR, Zhao XH, Zhang XF. Magnetic resonance lymphography at 3T: a promising noninvasive approach to characterise inguinal lymphatic vessel leakage. Eur J Vasc Endovasc Surg. 2012;43:106–11. doi: 10.1016/j.ejvs.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Holl G, Dorn R, Wengenmair H, Weckermann D, Sciuk J. Validation of sentinel lymph node dissection in prostate cancer: experience in more than 2,000 patients. Eur J Nucl Med Mol Imaging. 2009;36:1377–1382. doi: 10.1007/s00259-009-1157-2. [DOI] [PubMed] [Google Scholar]
- 23.Rousseau C, Rousseau T, Bridji B, et al. Laparoscopic sentinel lymph node (SLN) versus extensive pelvic dissection for clinically localized prostate carcinoma. Eur J Nucl Med Mol Imaging. 2012;39:291–299. doi: 10.1007/s00259-011-1975-x. [DOI] [PubMed] [Google Scholar]
- 24.van der Poel HG, Buckle T, Brouwer OR, Valdés Olmos RA, van Leeuwen FW. Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate cancer patients: clinical proof of concept of an integrated functional imaging approach using a multimodal tracer. Eur Urol. 2011;60:826–833. doi: 10.1016/j.eururo.2011.03.024. [DOI] [PubMed] [Google Scholar]


