Abstract
Purpose
The study was designed to determine whether response-based therapy improves outcomes in intermediate-risk Hodgkin lymphoma. We examined patterns of first relapse in the study.
Methods
From 9/02 – 7/10, 1712 patients <22 yrs of age with stage I–IIA with bulk, I–IIAE, I–IIB, and IIIA–IVA with/without bulk were enrolled. Patients were categorized as rapid (RER) or slow early responders (SER) after 2 cycles of doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide (ABVE-PC). SER patients were randomized to 2 additional ABVE-PC cycles or augmented chemotherapy with 21Gy IFRT. RER patients were stipulated to undergo 2 additional ABVEPC cycles and were then randomized to 21Gy IFRT or no further treatment if complete response (CR) was achieved. RER without CR patients were non-randomly assigned to 21Gy IFRT. Relapses were characterized with respect to site (initial, new or both; and initial bulk or initial non-bulk), and IFRT field (in-field, out-of-field, or both). Patients were grouped by treatment assignment (SER; RER/no CR; RER/CR/IFRT; and RER/CR/no IFRT). Summary statistics were reported.
Results
At 4-year median follow-up, 244 patients had relapsed, 198 of whom were fully evaluable for review. Those who progressed on treatment (n=30) or lacked relapse imaging (n=16) were excluded. Median time to relapse was 12.8 months. Of the 198 patients, 30% were RER/no CR, 26% were SER, 26% were RER/CR/no IFRT, 16% were RER/CR/IFRT, and 2% remained uncategorized. 74% and 75% relapses involve initially bulky and non-bulky sites, respectively. First relapses rarely occurred at exclusively new or out-of-field sites. In contrast, relapses usually occurred at nodal sites of initial bulky and non-bulky disease.
Conclusion
While response-based therapy has helped define treatment for select RER patients, it has not improved outcome for SER patients or facilitated refinement of IFRT volumes or doses.
Introduction
Radiation therapy alone with extended fields was previously considered the standard treatment of pediatric Hodgkin lymphoma until the 1960’s. Treatment of lymphoma has evolved significantly since then to include combinations of multi-drug chemotherapy and involved field radiotherapy (IFRT) in order to reduce radiation-related late toxicities. The first multi-modality therapies began with the Stanford pediatric protocols [1, 2]. Cure rates improved with dose-intensified treatment regimens utilizing a combination of chemotherapy and IFRT [3–4]; however, treatment-related late toxicity, particularly as related to radiation therapy, has continued to drive the search for risk-adapted treatment regimens that are specifically tailored to an individual’s treatment response without compromising outcome [5–10].
The cooperative group designed a phase III randomized risk-adapted, response-based trial for children with intermediate-risk Hodgkin lymphoma. It constitutes the largest trial of its kind to date, with 1712 eligible patients enrolled and seven countries represented. The primary objectives of the study were twofold: to reduce therapy for subjects who demonstrated a rapid early response to initial chemotherapy in order to decrease the risk of adverse therapy-related effects, and to augment therapy for subjects who demonstrated a slow early response to initial chemotherapy in order to increase the likelihood of cure.
With four years of follow-up, the main results of the trial, presented elsewhere [11], show that IFRT may be safely omitted from a selected group of rapid early responding subjects without compromising event-free survival (EFS). At the same time, augmented chemotherapy in the group of slow early responders did not lead to improved event-free survival. EFS was 85% at four years of the total population; 86.9% for rapid early responders and 77.4% for slow early responders (p<0.001). Among a selected group of rapid early responders who had a complete early response, EFS was equivalent (87.9% vs. 84.3%, p=0.11) in the arms randomized to +/− involved field radiation. Overall survival was 97.8%, 98.5% for rapid early responders and 95.3% for slow early responders (p<0.001). With this context, the purpose of the current investigation is to examine the patterns of relapse among all subjects who were treated on trial.
Patients and Methods
Patients
From September 2002 to July 2010, 1712 eligible patients younger than 22 years of age with intermediate-risk Hodgkin lymphoma defined as stage I–IIA with bulky disease, stage I–IIAE, I–IIB, and IIIA–IVA with or without bulky disease were enrolled. Bulk was defined as any contiguous nodal aggregate measuring more than 6 centimeters in longest transverse diameter on axial imaging. Mediastinal bulk was defined as a mass in the mediastinum measuring more than one-third the thoracic diameter.
Treatment
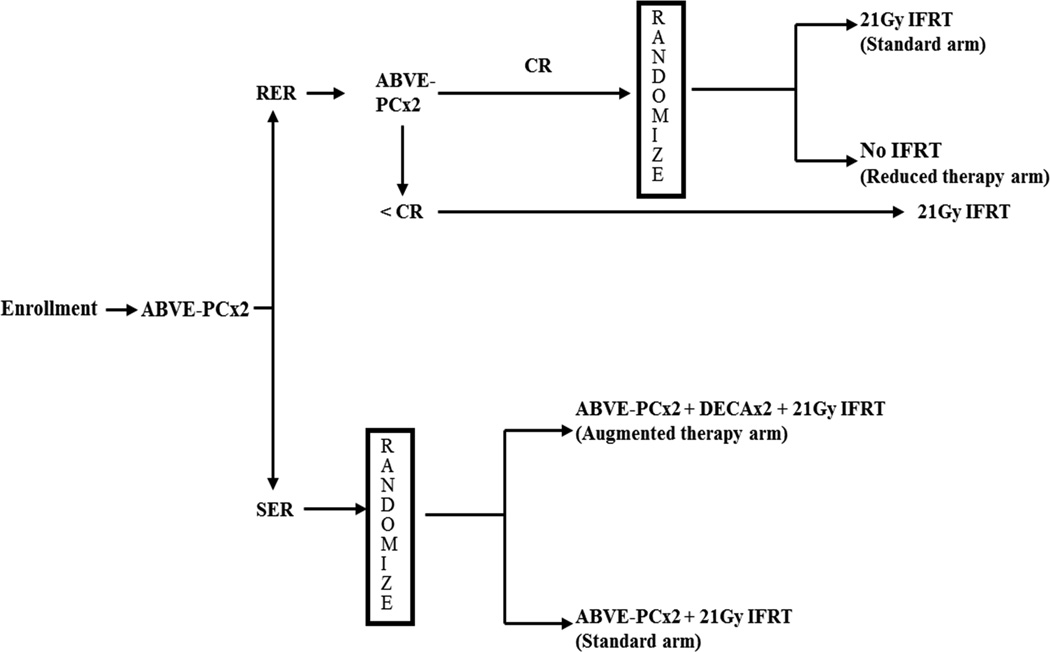
The treatment schema is outlined in Figure 1. Initial treatment consisted of two 21-day cycles of ABVE-PC chemotherapy, consisting of doxorubicin 25mg/m2/day on day 1 and 2; bleomycin 5 U/m2/day on day 1 and 10 U/m2/day on day 8; vincristine 1.4mg/m2/day on days 1 and 8; etoposide 125mg/m2/day on days 1, 2, and 3; prednisone 40mg/m2/day on days 1–7; and cyclophosphamide 800mg/m2 on day 1.
Figure 1.
Treatment protocol. Abbreviations are ABVE-PC – Doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide; RER – Rapid Early Response; SER – Slow Early Response; CR – Complete Response; IFRT – Involved Field Radiation Therapy; DECA – Dexamethasone, etoposide, cisplatin, cytarabine.
Patients were then assessed for early response to therapy by computed tomography (CT) and were categorized as having rapid early response to therapy if there was at least 60% reduction in the size of the target lesions as defined by the product of the perpendicular diameters (PPD) on CT imaging, or slow early response if there was less than 60% reduction in the PPD of the target lesions on CT imaging.
Rapid early responders underwent two additional cycles of ABVE-PC chemotherapy, after which a second evaluation of response was performed with CT and functional imaging (positron emission tomography [PET] or gallium scan). Rapid early responders with at least 80% reduction in the PPD of each of the target lesions and PET- or gallium-negative were classified as complete responders and randomized to 21Gy IFRT or no further treatment. Rapid early responders without complete response were non-randomly assigned to receive 21Gy IFRT.
Patients who were slow early responders after the initial two cycles of ABVE-PC chemotherapy were randomized to two additional ABVE-PC chemotherapy cycles followed by 21Gy IFRT, or two additional cycles of ABVE-PC chemotherapy plus augmented chemotherapy with two 21-day cycles of dexamethasone 10mg/m2 on day 1 and 2; etoposide 100mg/m2 on day 1 and 2, cytarabine 3000mg/m2 on day 1 and 2; and cisplatin 90mg/m2 on day 1. Augmented chemotherapy was also followed by 21Gy IFRT.
Radiation therapy
Involved field radiation therapy was given with a total dose of 21Gy over 14 fractions of 1.5 Gy per day, with balanced anterior and posterior fields, beginning approximately three but no more than four weeks after the last chemotherapy cycle. Treatment volumes included the initial sites of disease. The gross tumor volume included any lymph node measuring more than 1.5 centimeters in a single axis on CT. The clinical target volume (CTV) was defined as the anatomical nodal region of involvement. For instance, any cervical node involvement would mandate treatment to the entire ipsilateral but not bilateral cervical chain. Radiotherapy for mediastinal involvement would include the post-chemotherapy mediastinal width plus the bilateral hila. The axillae were excluded unless initially involved. When para-aortic nodes were involved, the spleen was also included in the field. However, splenic radiation was done without inclusion of para-aortic nodes if they were not involved. The planning target volume was a 1 centimeter margin around the CTV. Whole organ radiotherapy was used for parenchymal metastases to lung and liver as well as for extensive pericardial involvement. Whole organ doses in those cases were limited to 10.5Gy for lung and heart, and 15Gy for the liver using partial transmission blocking. Quality control of response assessments and IFRT parameters was performed centrally in real-time at the Quality Assurance Review Center (QARC) by study radiologists and radiation oncologists.
Analysis
Relapses were reported to the cooperative group’s central office. Relapse imaging and records were sent to QARC. Each relapse case was analyzed with respect to the site or sites of relapse by reviewing the imaging at the time of the diagnosis of the relapse. The initial site or sites of involvement and anatomic sites included in the radiation field were recorded for comparison. All relapses were characterized with respect to the anatomic site(s) of initial disease and at relapse, presence of bulky disease, radiotherapy field, date of relapse, presence of B symptoms, stage, histology, gender, age, and rapid- or slow-early response status. Patients were grouped by response assessments and treatment assignments. Relapses were categorized by site (initial site, new site, or both) and whether it occurred in a nodal vs. extranodal site; by IFRT field (in-field, out-of-field, or both); by presence of bulk at the initial disease site; and by treatment assignment (slow early responders, rapid early responders without complete response, rapid early responders randomized to IFRT, and rapid early responders randomized to no IFRT). Summary statistics were reported.
Results
A total of 244 patients with documented recurrence were eligible for further evaluation, comprising 14% of the total study population. Of these, 30 patients were diagnosed with progression of disease on therapy and 16 patients had incomplete imaging or other documentation precluding their inclusion in the analysis. The remaining 198 post-therapy relapses were fully evaluated. The characteristics of these patients are shown in Table 1. The median time interval from study enrollment to relapse diagnosis was 12.8 months (range 3.9 to 82.2 months).
Table 1.
Characteristics of relapsed patients at diagnosis
| Characteristic | N | % of Relapsed |
|---|---|---|
| Median age | 15.6 years | |
| Male | 100 | 51 |
| RER | 142 | 72 |
| SER | 52 | 26 |
| Non-categorized response | 4 | 2 |
| IA Bulk | 4 | 2 |
| IB | 1 | 1 |
| IIA Bulk | 64 | 32 |
| IIB | 54 | 27 |
| IAE, IIAE | 2 | 1 |
| IIIA, IIIAE | 32 | 16 |
| IVA | 41 | 21 |
| B-symptoms | 55 | 28 |
| Bulk | 162 | 82 |
RER – Rapid early responder. SER – Slow early responder.
Relapse location
The mediastinum was the most common nodal site of relapse above the diaphragm, with 63% of the evaluable relapsed patients experiencing a relapse in this location. The second and third most common sites of relapse were the supraclavicular fossa and the neck, representing 39% and 32% of relapses, respectively. The least common site of relapse was in Waldeyer’s ring. Below the diaphragm, the para-aortic lymph node region represented the most common site of relapse, with 12% of relapses occurring in this nodal region. Relapses in the spleen were also rare, representing just 3% of all relapses. Outside of nodal regions the lung was the most common site of extra-nodal relapse representing 23% of all relapses, followed by bone, representing 9%. Pulmonary involvement causing a pleural or pericardial effusion at relapse was rare, representing only 1.5 – 2% of relapses. Disease sites at relapse are described in Table 2.
Table 2.
Disease sites at first relapse
| Site above diaphragm | % | Number of Relapses |
|---|---|---|
| Mediastinum | 63 | 125 |
| Left supraclavicular fossa | 25 | 50 |
| Right supraclavicular fossa | 20 | 40 |
| Left neck | 23 | 46 |
| Right neck | 16 | 32 |
| Left axilla | 13 | 26 |
| Right axilla | 11 | 20 |
| Left hilum | 13 | 26 |
| Right hilum | 14 | 28 |
| Paratracheal | 15 | 30 |
| Pericardial | 13 | 26 |
| Subcarinal | 11 | 22 |
| Internal mammary chain | 9 | 17 |
| Supradiaphragmatic | 6 | 11 |
| Waldeyer's ring | 1.5 | 3 |
| Sites below diaphragm | ||
| Paraaortic | 12 | 23 |
| Porta hepatis | 6 | 12 |
| Spleen | 3 | 6 |
| Mesenteric | 2.5 | 5 |
| Right iliac chain | 2 | 4 |
| Left iliac chain | 1.5 | 3 |
| Right inguino-femoral | 1 | 2 |
| Left inguino-femoral | 3 | 6 |
| Extranodal sites | ||
| Lung | 23 | 46 |
| Bone | 9 | 17 |
| Chest wall | 6 | 11 |
| Pericardial effusion | 2 | 4 |
| Pleural effusion | 1.5 | 3 |
| Liver | 1.5 | 3 |
| Bone marrow | 0.5 | 1 |
Relapse in new versus previously involved sites
Relapses were evaluated with respect to involvement of a site of initial disease presentation or a new site. First relapses uncommonly occurred at new anatomic sites only, regardless of treatment strata. Rather, they occurred within the original anatomic sites of disease found at initial presentation or at both previously involved and new sites concurrently. In the Slow Early Response group, 98% of first relapses occurred in previously involved and concurrent previously involved and new sites. Similarly, in the Rapid Early Response group of patients who achieved a complete response and were randomized to the no-IFRT arm, 98% of the first relapses occurred in previously involved sites or concurrently in previously involved and new sites, rather than in new sites alone. First relapses according to new vs. previously involved site are further described in Table 3.
Table 3.
Relapses at new vs. previously involved sites, according to treatment group
| SER N (%) |
RER, no CR N (%) |
RER, CR, IFRT N (%) |
RER, CR, no IFRT N (%) |
Non-categorized N (%) |
|
|---|---|---|---|---|---|
| Initial Site only | 21 (40) | 21 (36) | 10 (31) | 27 (53) | 0 (0) |
| Initial & New Site | 30 (58) | 31 (53) | 18 (56) | 23 (45) | 4 (100) |
| New Site only | 1 (2) | 7 (12) | 4 (13) | 1 (2) | 0 (0) |
SER: Slow early responder; RER, no CR: Rapid early responder without complete response; RER, CR, IFRT: Rapid early responder with complete response randomized to involved-field radiotherapy treatment arm; RER, CR, no IFRT: Rapid early responder with complete response randomized to the no radiation treatment arm.
Relapse in irradiated vs. non-irradiated sites
Relapses were analyzed according to their occurrence within an irradiated field or external to the irradiated field. Out-of-field relapses were uncommon across all irradiated treatment groups. In-field relapses, in contrast, were common across all irradiated treatment groups particularly in the slow early responder group. Thirty patients did not receive IFRT because they were complete responders randomized to the no-IFRT treatment arm. The remainder of the relapsed patients received 21Gy IFRT according to the study guidelines. A summary of in-field vs. out-of-field relapses is found in Table 4.
Table 4.
Relapse in vs out of radiation field
| Field | SER N (%) |
RER, no CR N (%) |
RER, CR, IFRT N (%) |
Non-categorized N (%) |
|---|---|---|---|---|
| In-field Only | 27 (52) | 24 (41) | 15 (47) | 2 (50) |
| In- & Out-of field | 23 (44) | 24 (41) | 13 (41) | 2 (50) |
| Out-of-field Only | 2 (4) | 11 (19) | 4 (13) | 0 |
SER: Slow early responder; RER, no CR: Rapid early responder without complete response; RER, CR, IFRT: Rapid early responder with complete response randomized to involved-field radiotherapy treatment arm.
Relapse according to site of initial bulk disease
Relapses were analyzed according to their occurrence in initially bulky or non-bulky sites of disease. Of note, it was possible that any patient could relapse in either a bulky site, a non-bulky site, or both bulky and non-bulky sites. In cases of the latter, patients were counted in both categories. First relapses were found in initially bulky sites as well as in initially non-bulky sites, regardless of treatment group. The proportion of relapses at initially non-bulky sites was highest in the rapid early responding group with incomplete treatment response. Relapses according to bulk are described in Table 5.
Table 5.
Relapse according to presence of bulk disease
| Bulk | RER, no CR N (%) |
SER N (%) |
RER, CR, IFRT N (%) |
RER, CR, no IFRT N (%) |
Non-categorized N (%) |
|---|---|---|---|---|---|
| In Sites of Initial Bulk Disease (n=120) | 26 (59) | 40 (80) | 20 (77) | 31 (82) | 3 (75) |
| In Sites of Initial Non-bulk Disease (n=148) | 44 (75) | 36 (71) | 20 (65) | 43 (83) | 5 (100) |
RER, no CR: Rapid early responder without complete response; SER: Slow early responder; RER, CR, IFRT: Rapid early responder with complete response randomized to involved-field radiotherapy treatment arm; RER, CR, no IFRT: Rapid early responder with complete response randomized to the no radiation treatment arm.
Discussion
This study is the largest phase III study to date in pediatric Hodgkin lymphoma designed to evaluate the outcome of early response-based therapy in intermediate-risk patients with dual objectives. These objectives were 1) to reduce late effects while preserving excellent cure rates among the favorable risk patients, defined as those with rapid early response after two chemotherapy cycles followed by complete response after chemotherapy completion, and 2) to simultaneously improve cure rates in the higher risk patients, defined as those with slow early response after two chemotherapy cycles. While several prior pediatric cooperative group trials have demonstrated the prognostic significance and adjustment of therapy based upon early response assessments [7–10, 12–14], this study is the first randomized study to compare standard and response-based treatment arms in order to risk-stratify select groups of patients for omission of radiotherapy or augmentation of systemic therapy.
The primary endpoint of the study was event-free survival. Results, presented previously [11], demonstrated no difference in event-free survival between the group of rapid early responders who experienced complete response and were randomized to no further therapy as compared to the group randomized to standard IFRT. Similarly, results failed to show a difference in event-free survival between the slow early responding patients who were randomized to the augmented chemotherapy regimen compared with those who received standard chemotherapy.
This sub-analysis is a retrospective investigation of first relapses from the study. It constitutes the largest prospectively collected dataset of relapses available for analyzing patterns of failure in pediatric intermediate risk Hodgkin lymphoma. All radiographic response assessments and radiation treatment records were reviewed centrally, thus making the relapse dataset particularly robust and high-quality for analysis.
We compared the sites of relapse and found that, similar to findings published by Dhakal et al and Krasin et al [15, 16] that the mediastinum was the most common site of relapse particularly when bulky disease was present. Lung was found to be the most common extranodal site of relapse in previously published literature as well [15, 17, 18].
In our relapse cohort, first relapses rarely occurred outside the irradiated field only. This finding is corroborated by a randomized cooperative group trial of 829 children (CCG 5942) and two smaller single-institutional studies of combined modality therapy in early and advanced stage Hodgkin lymphoma that similarly reported recurrences generally developed within irradiated sites [8, 15, 16]. Our findings imply that there is little rationale for expanding fields even in the higher risk patients and that treating involved fields is sufficient, particularly when combined modality treatment is employed as is the standard of care today. Much older studies demonstrated recurrences tended to develop outside the radiation fields, but it is noted that patients on these studies were treated with radiotherapy alone [19, 20].
Moreover, relapses on this study more commonly occurred in previously involved disease sites. This finding is also supported in previously published literature [8, 15, 21]. In the chemotherapy alone arm of CCG-5942, 32/34 relapses included initial sites and for the chemo-RT arm, 10/12 relapsed included the initial sites. In AHOD0031, this pattern was particularly noticeable in the slow early responding patients. Our data demonstrated 94% of relapses occurred in initial disease sites. These would have been included in an involved-field treatment plan as well as an involved-node treatment plan, raising the possibility for consideration of further refined radiotherapy treatment strategies, such as involved node radiation, in carefully selected patients with favorable early responses in future protocols with this question as a specific aim in order to further reduce risks of radiation-related late effects, as well as potential dose escalation (rather than field enlargement) to involved sites among patients who have an initially less favorable response or who have very bulky disease. The involved node concept is currently being applied in adult patients with early stage Hodgkin lymphoma [22–24]. Future trials that explore tailored radiotherapy dose escalation may be especially relevant in the slow early responders since augmented chemotherapy did not lead to improved outcome in this study.
Relapses occurred at both initially bulky as well as non-bulky disease sites, implying that irradiation of bulk disease only while omitting radiation to non-bulk disease in patients may not be an optimal treatment strategy. These findings contrast the results of Krasin et al [16] in which the risk of local failure was fivefold higher for patients with bulky mediastinal disease.
Limitations of our analysis of the relapse cohort in the study relate firstly to the relatively small number of relapses comprising each treatment group, which precludes the ability to find statistically significant differences of relapse characteristics among the various treatment groups. Secondly, the population of intermediate-risk patients was heterogeneous in terms of stage, with lower risk and higher risk patients being considered ‘intermediate risk’. Thirdly, information regarding initial disease sites and radiotherapy fields of the non-relapsed patients was not available for comparison, though a comprehensive cataloging of the non-relapsed patients’ initial disease sites is currently underway at the Quality Assurance Review Center. Finally, follow-up from the study is approximately four years. Longer term follow-up may be necessary to ensure the stability of our findings.
Conclusion
This study was instrumental in defining risk-stratified treatment based upon early response assessments. The strategy has successfully allowed omission of involved-field radiotherapy in selected rapid early responding patients without compromising event-free survival, but it has not led to improvements in outcome for the slow early responding patients. Analysis of first relapses with four-year follow up from the study demonstrated that relapses more commonly occurred within the previously irradiated field, within previously involved sites of disease, and at not only initially bulky sites. The implications of this analysis are twofold: first, further tailored radiotherapy such as involved node therapy in selected lower risk patients and dose escalation in selected higher risk patients may be considerations for future cooperative group studies, and second, given the frequency of bulky as well as non-bulky site relapses, irradiation of solely bulky sites may not be an optimal treatment strategy.
Summary.
We examined the patterns of first relapse on the phase III Study of Response-Based Therapy for Intermediate-Risk Hodgkin Lymphoma (AHOD0031) from the Children’s Oncology Group. Therapy was titrated using CT and functional imaging-based response assessments with radiotherapy omitted in select patients. Four years after study completion, 198 patients were diagnosed with a fully evaluable first relapse. Relapses were assessed with respect to anatomic location, previous site involvement, radiation field, presence of bulk disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None.
References
- 1.Donaldson SS, Glatstein E, Rosenberg SA, et al. Pediatric hodgkin's disease. II. Results of therapy. Cancer. 1976;37(5):2436–2447. doi: 10.1002/1097-0142(197605)37:5<2436::aid-cncr2820370537>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson SS, Link MP. Combined modality treatment with low-dose radiation and MOPP chemotherapy for children with Hodgkin's disease. J Clin Oncol. 1987;5(5):742–749. doi: 10.1200/JCO.1987.5.5.742. [DOI] [PubMed] [Google Scholar]
- 3.Hunger SP, Link MP, Donaldson SS. ABVD/MOPP and low-dose involved field radiotherapy in pediatric Hodgkin's Disease: the Stanford Experience. J Clin Oncol. 1994;12(10):2160–2166. doi: 10.1200/JCO.1994.12.10.2160. [DOI] [PubMed] [Google Scholar]
- 4.Dieckmann K, Pötter R, Hofmann J, et al. Does bulky disease at diagnosis influence outcome in childhood Hodgkin's disease and require higher radiation doses? Results from the German- Austrian Pediatric Multicenter Trial DAL-HD-90. Int J Radiat Oncol Biol Phys. 2003;56(3):644–652. doi: 10.1016/s0360-3016(03)00125-1. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson SS, Link MP, Weinstein HJ, et al. Final results of a prospective clinical trial with VAMP and low-dose involved-field radiation for children with low-risk Hodgkin's disease. J Clin Oncol. 2007;25(3):332–337. doi: 10.1200/JCO.2006.08.4772. [DOI] [PubMed] [Google Scholar]
- 6.Dorffel W, Lüders H, Rühl U, et al. Preliminary results of the multicenter trial GPOH-HD 95 for the treatment of Hodgkin's disease in children and adolescents: analysis and outlook. Klin Padiatr. 2003;215(3):139–145. doi: 10.1055/s-2003-39372. [DOI] [PubMed] [Google Scholar]
- 7.Kelly KM, Sposto R, Hutchinson R, et al. BEACOPP chemotherapy is a highly effective regimen in children and adolescents with high-risk Hodgkin lymphoma: a report from the Children's Oncology Group. Blood. 2011;117(9):2596–2603. doi: 10.1182/blood-2010-05-285379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nachman JB, Sposto R, Herzog P, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin's disease who achieve a complete response to chemotherapy. J Clin Oncol. 2002;20(18):3765–3771. doi: 10.1200/JCO.2002.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: the results of P9425. Blood. 2009;114(10):2051–2059. doi: 10.1182/blood-2008-10-184143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolden SL, Chen L, Kelly KM, et al. Long-term results of CCG 5942: a randomized comparison of chemotherapy with and without radiotherapy for children with Hodgkin's lymphoma--a report from the Children's Oncology Group. J Clin Oncol. 2012;30(26):3174–3180. doi: 10.1200/JCO.2011.41.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman DL, Wolden S, Constine L, et al. AHOD0031: a phase III study of dose intensive therapy for intermediate risk Hodgkin Lymphoma: a report from the Children's Oncology Group. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.52.5410. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kung FH, Schwartz CL, Ferree CR, et al. POG 8625: a randomized trial comparing chemotherapy with chemoradiotherapy for children and adolescents with Stages I, IIA, IIIA1 Hodgkin Disease: a report from the Children's Oncology Group. J Pediatr Hematol Oncol. 2006;28(6):362–368. doi: 10.1097/00043426-200606000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Tebbi C, Mendenhall NP, London WB, et al. Response-dependent and reduced treatment in lower risk Hodgkin lymphoma in children and adolescents, results of P9426: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2012;59(7):1259–1265. doi: 10.1002/pbc.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiner MA, Leventhal B, Brecher ML, et al. Randomized study of intensive MOPP-ABVD with or without low-dose total-nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin's disease in pediatric patients: a Pediatric Oncology Group study. J Clin Oncol. 1997;15(8):2769–2779. doi: 10.1200/JCO.1997.15.8.2769. [DOI] [PubMed] [Google Scholar]
- 15.Dhakal S, Leventhal B, Brecher ML, et al. Patterns and timing of initial relapse in patients subsequently undergoing transplantation for Hodgkin's lymphoma. Int J Radiat Oncol Biol Phys. 2009;75(1):188–192. doi: 10.1016/j.ijrobp.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 16.Krasin MJ, Rai SN, Kun LE, et al. Patterns of treatment failure in pediatric and young adult patients with Hodgkin's disease: local disease control with combined-modality therapy. J Clin Oncol. 2005;23(33):8406–8413. doi: 10.1200/JCO.2004.00.8763. [DOI] [PubMed] [Google Scholar]
- 17.Brice P, Bastion Y, Divine M, et al. Analysis of prognostic factors after the first relapse of Hodgkin's disease in 187 patients. Cancer. 1996;78(6):1293–1299. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1293::AID-CNCR18>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 18.Healey EA, Tarbell NJ, Kalish LA, et al. Prognostic factors for patients with Hodgkin disease in first relapse. Cancer. 1993;71(8):2613–2620. doi: 10.1002/1097-0142(19930415)71:8<2613::aid-cncr2820710828>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Horwich A, Specht L, Ashley S. Survival analysis of patients with clinical stages I or II Hodgkin's disease who have relapsed after initial treatment with radiotherapy alone. Eur J Cancer. 1997;33(6):848–853. doi: 10.1016/s0959-8049(96)00518-7. [DOI] [PubMed] [Google Scholar]
- 20.Roach M, Brophy N, Cox R, et al. Prognostic factors for patients relapsing after radiotherapy for early-stage Hodgkin's disease. J Clin Oncol. 1990;8(4):623–629. doi: 10.1200/JCO.1990.8.4.623. [DOI] [PubMed] [Google Scholar]
- 21.Shahidi M, Kamangari N, Ashley S, et al. Site of relapse after chemotherapy alone for stage I and II Hodgkin's disease. Radiother Oncol. 2006;78(1):1–5. doi: 10.1016/j.radonc.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Girinsky T, van der Maazen R, Specht L, et al. Involved-node radiotherapy (INRT) in patients with early Hodgkin lymphoma: concepts and guidelines. Radiother Oncol. 2006;79(3):270–277. doi: 10.1016/j.radonc.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Maraldo MV, Aznar MC, Vogelius IR, et al. Involved node radiation therapy: an effective alternative in early-stage hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2013;85(4):1057–1065. doi: 10.1016/j.ijrobp.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 24.Paumier A, Ghalibafian M, Beaudre A, et al. Involved-node radiotherapy and modern radiation treatment techniques in patients with Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2011;80(1):199–205. doi: 10.1016/j.ijrobp.2010.09.007. [DOI] [PubMed] [Google Scholar]