Abstract
Although subjects with chronic kidney disease (CKD) are at markedly increased risk for cardiovascular mortality, the relationship between CKD and aortic valve calcification has not been fully elucidated. Also, few data are available on the relationship of aortic valve calcification and earlier stages of CKD. We sought to assess the relationship of aortic valve calcium (AVC) with estimated glomerular filtration rate (eGFR), traditional and novel cardiovascular risk factors, and markers of bone metabolism in the Chronic Renal Insufficiency Cohort (CRIC) Study. All patients who underwent aortic valve scanning in the CRIC study were included. The relationship between AVC and eGFR, traditional and novel cardiovascular risk factors, and markers of calcium metabolism were analyzed using both unadjusted and adjusted regression models. A total of 1964 CRIC participants underwent computed tomography for AVC quantification. Decreased renal function was independently associated with increased levels of AVC (eGFR 47.11 ml/min/1.73m2, 44.17 ml/min/1.73m2, and 39 ml/min/1.73m2, respectively, p< 0.001). This association persisted after adjusting for traditional, but not novel, AVC risk factors. Adjusted regression models identified several traditional and novel risk factors for AVC in patients with CKD. There was a difference in AVC risk factors between black and non-black patients. In conclusion, our study shows that eGFR is associated in a dose-dependent manner with AVC in patients with CKD, and this association is independent of traditional cardiovascular risk factors.
Keywords: aortic valve, calcification, cardiovascular risk factors, chronic kidney disease, CKD
Introduction
Patients with chronic kidney disease (CKD) have increased cardiovascular morbidity and mortablity, and clinical studies indicate that the prevalence and progression of aortic valve calcium (AVC) is increased in patients with end-stage renal disease.1 The prevalence of AVC in patients with earlier stages of CKD, however, is not known. The Chronic Renal Insufficiency Cohort (CRIC) study is a large prospective epidemiological study of patients with varying degree of CKD. All CRIC study participants underwent assessment for aortic valve calcium (AVC) by medical history and measurement of calcium on electron beam computed tomography (EBT). In this analysis, we examine the relation between impaired renal function and AVC and explore the association of various traditional Framingham risk factors, novel cardiovascular risk factors including inflammatory (CRP) and novel lipid biomarkers (LP(a)), as well as markers of bone metabolism with AVC in patients with CKD.
Methods
The CRIC Study population is a racially and ethnically diverse cohort of men and women aged 21 to 74 years with mild-to-moderate renal disease; approximately half of which have diabetes. The CRIC participants were recruited between May 2003 and August 2008 from seven clinical centers in the United States of America.2 The identification of subjects was facilitated through searches of laboratory databases, medical records, and referrals from health care providers. Subjects with cirrhosis, HIV infection, polycystic kidney disease, or renal cell carcinoma, as well as those on dialysis or recipients of a kidney transplant, or those taking immunosuppressant drugs were excluded from study participation. An eGFR entry criteria (20-70 mL/min/1.73m2) was used as an enrollment criteria to limit the proportion of older individuals who were recruited with age-related diminutions of GFR but otherwise non-progressive CKD. A total of 3939 CRIC participants were screened for this analysis. Of those, 2068 had baseline non-contrast CT scans with aortic valve calcium quantification, and 1964 had CT-scans between their baseline and year 3 visit. These 1964 patients were included in the unadjusted analysis, and a subset of those with non-missing eGFR calculated by the CRIC equation were used for the adjusted analyses (N=1923). Data used in the analyses were taken from first non-contrast CT scan visit with the exception of the following variables which were taken from the baseline study visit: Total metabolic equivalent (MET sum, METhrs/week), Hemoglobin A1C (%) , 24H Urine Albumin (g/24H), High Sensitivity CRP , Uric Acid (mg/dL) , Total Plasma Homocys (umol/L) , Phosphate (mg/dL) , Total Parathyroid Hormone (pg/ml) , lipoprotein(a)(mg/dl).
This study was approved by the Institutional Review Boards from each of the participating clinical centers as well as the scientific and data coordinating center. A written informed consent was obtained from all participants. This study also conformed to the Health Insurance Portability and Accountability Act (HIPAA) guidelines.
Estimated glomerular filtration rate (eGFR) was computed using the Modification of Diet in Renal Disease (MDRD) Study equation.3 CKD was defined as eGFR < 60 ml/min/1.73m2 based on the National Kidney Foundation's KDOQI (Kidney disease Outcome Quality Initiative) guidelines.
All CRIC participants included in this analysis underwent baseline non-contrast CT scans, which were analyzed for both coronary artery and aortic valve calcium. Spatial resolution for each system was 1.15 mm3 for multi-detector detector row CT (0.68 × 0.68 × 2.50 mm) and 1.38 mm3 for electron-beam CT (0.68 × 0.68 × 3.00 mm). Full details concerning the equipment, scanning methods, and CT quality control in CRIC, including results of CAC associations with GFR have been reported previously.4
All scans were sent to a central CRIC CT reading center (Harbor-UCLA Research and Education Institute, Los Angeles, CA). Calcium strongly attenuates x-rays, appears bright on CT scans, and is readily differentiated from surrounding tissue. All scans were analyzed with a commercially available software package (Neo Imagery Technologies, City of Industry, California). An attenuation threshold of 130 Hounsfield units and a minimum of 3 contiguous pixels were utilized for identification of a calcific lesion. Each focus exceeding the minimum criteria was scored using the algorithm developed by Agatston et al,5 calculated by multiplying the lesion area by a density factor derived from the maximal Hounsfield unit (Hu) within this area. The density factor was assigned in the following manner: 1 for lesions with peak attenuation of 130-199 Hu, 2 for lesions with peak attenuation of 200-299 Hu, 3 for lesions with peak attenuation of 300-399 Hu, and 4 for lesions with peak attenuation >400 Hu. Consistent with prior methodology,6-8 lesion was classified as total aortic valve calcium (AVC) if it resided within the aortic valve leaflets or aortic annulus, aortic sinuses, aortic wall at the level of the aortic valve, and if there were 3 contiguous pixels of at least 130 Hounsfield units in brightness. Single lesion measurements were then summed to give a total Agatston score. If no lesions reached threshold values, the Agatston score was recorded as zero.
The study population was divided into three groups based on the presence and severity of AVC, where total AVC of 0 is no disease, AVC = 0-100 represents mild-to-moderate disease, and ≥100 represents severe disease. Demographic and baseline characteristics were described using mean (standard deviation) and/or median (inter-quartile range) for continuous variables and frequency (percent) for categorical variable. ANOVA or Wilcoxin rank sum test were used to compare the distribution of continuous variables across the three AVC categories. Chi-square test was used for categorical variables. The mean and median AVC scores as well as the percent of participants in each AVC category were reported for different CKD stages. Multinomial logistic regression models were fit to adjust for traditional AVC risk factors (Model 1), novel AVC risk factors (including CRP, LP(a), uric acid, and homocysteine, (Model 2) and bone metabolism risk factors (model 3) in a sequential fashion. Subgroup analyses by race (black versus non-black) were done in the final model (Model 3) in which all risk factors were included. A statistical significance threshold of α=0.05 was used.
Results
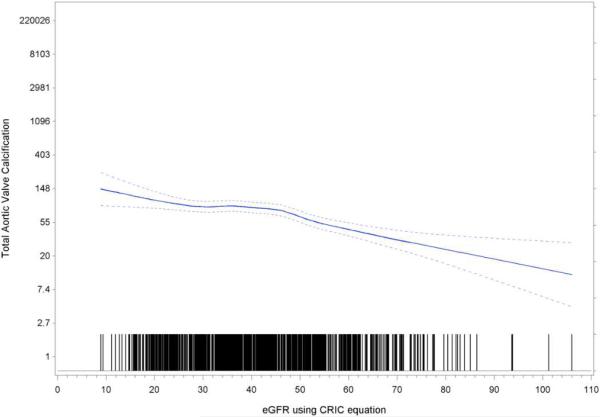
Table 1 summarizes demographics and baseline characteristics from the CRIC database participants who underwent baseline CT with calcium scoring of their aortic valves (n=1964). The participants were grouped based on the presence and severity of AVC. Increasing age, BMI, waist circumference, blood pressure, cholesterol, hemoglobin A1c, and decreased physical activity, as well as a history of diabetes, hypertension or cardiovascular disease, were all independently associated with increased AVC in this unadjusted analysis. Novel cardiovascular risk factors such as hs-CRP, uric acid, total plasma homocysteine, and Lp(a) were also associated with increased AVC. The average eGFR of the cohort was 44.6 ml/min/1.73 m2, and decreased eGFR was associated with increased AVC (p<0.0001). Figure 1 illustrates this inverse relationship between eGFR and aortic valve calcification across the study population.
Table 1.
Baseline characteristics of study participants by degree of aortic valve calcification.
| All with AVC Measured (n=1964) | AVC and AVRING both =0 (n=1023) | AVC + AVRING >0 - 100 (n=515) | AVC + AVRING >100 (n=426) | value | |
|---|---|---|---|---|---|
| Participant Age | 58.45 (11.45) | 53.16 (11.73) | 62.26 (7.90) | 66.53 (7.00) | <.0001 |
| Female | 918 (47%) | 466 (46%) | 262 (51%) | 190 (45%) | 0.0864 |
| Male | 1046 (53%) | 557 (54%) | 253 (49%) | 236 (55%) | . |
| Black | 672 (34%) | 342 (33%) | 192 (37%) | 138 (32%) | 0.2167 |
| Not Black | 1292 (66%) | 681 (67%) | 323 (63%) | 288 (68%) | . |
| Self-reported history of CVD | 494 (25%) | 169 (17%) | 149 (29%) | 176 (41%) | <.0001 |
| Current Smoker | 194 (10%) | 111 (11%) | 43 (8%) | 40 (9%) | 0.2791 |
| Body Mass Index (kg/m^2) | 31.13 (6.70) | 30.57 (6.87) | 31.73 (6.58) | 31.72 (6.32) | 0.0007 |
| Waist Circumference (cm) | 103.71 (15.83) | 101.77 (16.32) | 105.01 (15.53) | 106.81 (14.31) | <.0001 |
| Total METs (METhrs/week) | 209.93 (145.91) | 228.87 (161.28) | 198.37 (125.59) | 178.24 (120.95) | <.0001 |
| Diabetes Mellitus | 917 (47%) | 384 (38%) | 281 (55%) | 252 (59%) | <.0001 |
| Glucose (mg/dL) | 113.80 (47.85) | 110.24 (46.95) | 118.78 (51.33) | 116.36 (44.95) | 0.0020 |
| Hemoglobin A1C (%) at Baseline | 6.50 (1.52) | 6.33 (1.55) | 6.73 (1.52) | 6.64 (1.36) | <.0001 |
| Systemic Hypertension Diagnosis | 1703 (87%) | 816 (80%) | 478 (93%) | 409 (96%) | <.0001 |
| Systolic Blood Pressure (mmHg) | 126.59 (21.25) | 123.63 (20.35) | 127.75 (21.99) | 132.28 (21.21) | <.0001 |
| Diastolic Blood Pressure (mmHg) | 70.55 (12.45) | 73.22 (12.35) | 68.64 (12.11) | 66.44 (11.57) | <.0001 |
| Pulse Pressure | 56.04 (18.56) | 50.41 (16.47) | 59.10 (18.05) | 65.88 (18.99) | <.0001 |
| High Cholesterol Diagnosis | 1698 (86%) | 834 (82%) | 463 (90%) | 401 (94%) | <.0001 |
| Low-density Lipoprotein (mg/dL) | 103.07 (34.82) | 105.58 (34.81) | 100.84 (34.99) | 99.76 (34.23) | 0.0041 |
| High-density Lipoprotein (mg/dL) | 48.97 (15.76) | 49.27 (16.06) | 50.16 (16.99) | 46.80 (13.12) | 0.0039 |
| Triglycerides (mg/dL) | 154.61 (105.91) | 155.52 (112.37) | 151.24 (97.79) | 156.50 (99.39) | 0.7003 |
| eGFR using CRIC equation | 44.60 (17.58) | 47.11 (18.94) | 44.18 (16.31) | 39.03 (13.97) | <.0001 |
| 24H Urine Protein (g/24H) | 1.07 (2.16) | 1.15 (2.24) | 0.94 (2.01) | 1.05 (2.16) | 0.2369 |
| Median (IQR) | 0.17 (0.07 - 0.93) | 0.18 (0.07 - 1.02) | 0.15 (0.06 - 0.67) | 0.18 (0.07 - 0.94) | 0.0697 |
| 24H Urine Albumin (g/24H) | 0.74 (1.76) | 0.84 (1.93) | 0.60 (1.39) | 0.69 (1.74) | 0.0481 |
| Median (IQR) | 0.05 (0.01 - 0.56) | 0.06 (0.01 - 0.65) | 0.04 (0.01 - 0.44) | 0.05 (0.01 - 0.41) | 0.3405 |
| High Sensitivity CRP (mg/dL) | 4.64 (7.64) | 4.18 (7.28) | 4.85 (7.29) | 5.48 (8.77) | 0.0100 |
| Median (IQR) | 2.22 (0.94 - 5.23) | 1.94 (0.87 - 4.69) | 2.37 (0.96 - 5.97) | 2.64 (1.14 - 6.04) | 0.0001 |
| Uric Acid (mg/dL) | 7.17 (1.88) | 7.01 (1.89) | 7.21 (1.87) | 7.51 (1.85) | <.0001 |
| Total Plasma Homocysteine (umol/L) | 14.35 (5.62) | 13.54 (5.03) | 14.17 (4.86) | 16.55 (7.06) | <.0001 |
| CBC Hemoglobin (g/dL) | 12.88 (1.79) | 13.10 (1.83) | 12.69 (1.69) | 12.57 (1.72) | <.0001 |
| Calcium (mg/dL) | 9.31 (0.54) | 9.30 (0.56) | 9.30 (0.50) | 9.33 (0.54) | 0.5973 |
| Phosphate (mg/dL) | 3.70 (0.67) | 3.66 (0.68) | 3.75 (0.67) | 3.73 (0.66) | 0.0206 |
| Total Parathyroid Hormone (pg/ml) | 69.47 (70.19) | 70.12 (68.65) | 63.15 (49.15) | 75.53 (91.58) | 0.0259 |
| Median (IQR) | 50.00 (33.00 - 81.00) | 50.00 (32.40 - 80.00) | 47.00 (32.90 - 76.50) | 54.45 (34.00 - 89.00) | 0.0532 |
| Serum 25(OH)-Vitamin D (ng/mL) | 26.14 (14.35) | 25.93 (14.14) | 26.82 (14.85) | 25.77 (14.22) | 0.6191 |
| Median (IQR) | 23.85 (14.30 - 35.50) | 23.75 (15.20 - 34.95) | 24.50 (14.10 - 36.95) | 23.80 (14.00 - 34.60) | 0.6654 |
| Lipoprotein(a) (mg/dl) | 36.70 (40.73) | 34.51 (40.34) | 38.65 (40.58) | 39.59 (41.64) | 0.0452 |
| Median (IQR) | 20.70 (7.40 - 54.40) | 16.95 (7.10 - 47.80) | 23.65 (8.20 - 61.00) | 23.00 (7.90 - 61.30) | 0.0116 |
Data was taken from the non-contrast CT visit, if available, or from the initial baseline visit. Data taken at the baseline visit include Total metabolic equivalents, phosphate, total parathyroid hormone, lipoprotein(a), plasma homocysteine, high-sensitivity C-reactive protein, and 24-hour albumin.
Figure 1.
Mean aortic valve calcification by eGFR illustrates the inverse relationship between aortic valve calcification and eGFR. Dashed lines represent 95% confidence intervals.
Table 2 summarizes the graded relationship between stage of CKD and increased AVC (p=0.0082). Patients with more significant renal impairment had greater prevalence of AVC and more severe AVC with a higher burden of calcium. A clear difference in prevalence and severity of calcium was noted between patients with stage 3A and stage 3B CKD.
Table 2.
Prevalence and severity of aortic valve calcification by eGFR.
| eGFR (ml/min/1.73m2) at EBT Visit | ||||||
|---|---|---|---|---|---|---|
| All with EBT (n = 1923) | <30 (n = 418) | 30-<45 (n = 622) | 45-<60 (n = 507) | >60 (n = 376) | p-value | |
| TOTAL AVC | . | |||||
| Median (IQR) | 81.32 (20.35 - 246.27) | 113.56 (34.58 - 292.53) | 100.74 (29.16 - 320.18) | 64.96 (13.81 - 194.30) | 32.01 (10.75 - 112.17) | <.0001 |
| N (%) | . | |||||
| 0 | 1002 (52.1%) | 205 (49%) | 274 (44.1%) | 264 (52.1%) | 259 (68.9%) | <.0001 |
| >0to 100 | 507 (26.4%) | 101 (24.2%) | 173 (27.8%) | 148 (29.2%) | 85 (22.6%) | . |
| >100 | 414 (21.5%) | 112 (26.8%) | 175 (28.1%) | 95 (18.7%) | 32 (8.5%) | . |
eGFR from the EBT visit was used. Total aortic valve calcification (AVC) includes aortic valve and aortic valve ring calcification.
Multinomial regression models were used to examine the relation of AVC and CKD with traditional and novel cardiovascular risk factors and markers of bone metabolism in a sequential manner (Table 3). Model 1 examined eGFR and traditional cardiovascular risk factors, where eGFR was independently associated with severity of AVC. It also identified age, race, history of cardiovascular disease, diabetes, systolic blood pressure, high cholesterol, and low HDL as independent factors associated with more severe AVC. Of these risk factors, age and hyperlipidemia had the strongest association with AVC. A second model added novel cardiovascular risk factors to Model 1. When adjusting for these novel risk factors, eGFR was no longer independently associated with AVC burden. Furthermore, CRP and plasma homocysteine were additional risk factors that were independently associated with AVC. In this analysis, Lp(a) and uric acid were not independently associated with AVC. From Model 2, a third model incorporated markers of bone metabolism identified in the unadjusted analysis, including calcium, phosphate, and parathyroid hormone. eGFR and these markers were not independently associated with AVC.
Table 3.
Odds ratios from the adjusted multinomial regression models including traditional (Model 1), novel (Model 2), and bone metabolism (Model 3) risk factors of AVC. For the following continuous variables, the odds ratio is per standard deviation of the value: eGFR, age, waist circumference, total METs at baseline, LDL, HDL, and phosphate. The natural log of one plus the following continuous variables was used: hs-CRP, Lipoprotein a, total PTH, 24 hours urine protein. The following variables were from baseline measurements: Total Met, hsCRP, uric acid, homocysteine, Lipoprotein a, and total PTH.
| Unadjusted Model (n=1923) | Model 1: Traditional CV risk factors (n=1691) | Model 2: Nouel CV risk factors (n=1470) | Model 3: Markers of bone metabolism (n=1442) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total AVC | Total AVC | Total AVC | Total AVC | |||||||||
| >0-100 | 100+ | p | >0-100 | 100+ | p | >0-100 | 100+ | p | >0 - 100 | 100+ | p | |
| eGFR using CRIC equation | 0.84 (0.76, 0.94) | 0.60 (0.53, 0.69) | <.001 | 1.08 (0.92, 1.27) | 0.79 (0.65, 0.97) | 0.015 | 1.14 (0.94, 1.40) | 0.97 (0.76, 1.24) | 0.297 | 1.12 (0.90, 1.39) | 0.95 (0.72, 1.24) | 0.41 |
| Participant Age | 2.87 (2.37, 3.48) | 6.04 (4.64, 7.85) | <.001 | 3.03 (2.45, 3.74) | 6.33 (4.72, 8.50) | <.001 | 3.15 (2.53, 3.93) | 6.85 (5.04, 9.30) | <.001 | |||
| Black | 0.90 (0.66, 1.22) | 0.56 (0.39, 0.80) | 0.005 | 0.65 (0.46, 0.94) | 0.36 (0.24, 0.55) | <.001 | 0.66 (0.45, 0.95) | 0.38 (0.25, 0.59) | <.001 | |||
| Female | 1.21 (0.90, 1.65) | 0.95 (0.67, 1.36) | 0.3 | 1.20 (0.85, 1.69) | 1.08 (0.72, 1.62) | 0.59 | 1.14 (0.79, 1.63) | 1.02 (0.67, 1.57) | 0.775 | |||
| Cardio-Vascular Disease | 1.41 (1.04, 1.93) | 2.17 (1.56, 3.03) | <.001 | 1.26 (0.89, 1.77) | 2.00 (1.39, 2.90) | <.001 | 1.18 (0.84, 1.67) | 1.92 (1.32, 2.79) | 0.002 | |||
| Current Smoker | 0.99 (0.63, 1.56) | 1.33 (0.80, 2.21) | 0.485 | 0.91 (0.55, 1.50) | 0.94 (0.51, 1.72) | 0.927 | 0.91 (0.55, 1.52) | 0.89 (0.48, 1.66) | 0.911 | |||
| Body Mass Index (kg/m^2) | 1.01 (0.97, 1.06) | 1.01 (0.96, 1.06) | 0.807 | 1.01 (0.97, 1.06) | 1.00 (0.95, 1.05) | 0.813 | 1.01 (0.97, 1.06) | 1.00 (0.95, 1.05) | 0.779 | |||
| Waist Circumference (cm) | 0.98 (0.74, 1.29) | 1.08 (0.79, 1.48) | 0.801 | 0.93 (0.69, 1.25) | 1.01 (0.72, 1.43) | 0.85 | 0.92 (0.68, 1.25) | 1.01 (0.71, 1.43) | 0.822 | |||
| Total MET sum (METhrs/week) | 0.99 (0.86, 1.14) | 0.96 (0.80, 1.15) | 0.883 | 1.03 (0.89, 1.19) | 0.96 (0.79, 1.17) | 0.793 | 1.04 (0.89, 1.20) | 0.95 (0.78, 1.17) | 0.72 | |||
| Diabetes | 1.90 (1.42, 2.55) | 1.86 (1.34, 2.60) | <.001 | 2.01 (1.46, 2.77) | 1.82 (1.26, 2.64) | <.001 | 1.89 (1.36, 2.63) | 1.87 (1.27, 2.74) | <.001 | |||
| Systolic BP (mmHg) | 1.00 (1.00, 1.01) | 1.01 (1.00, 1.02) | 0.009 | 1.01 (1.00, 1.02) | 1.02 (1.01, 1.03) | 0.001 | 1.01 (1.00, 1.02) | 1.02 (1.01, 1.03) | 0.001 | |||
| High Cholesterol | 1.66 (1.11, 2.49) | 2.44 (1.41, 4.23) | 0.002 | 1.98 (1.26, 3.09) | 2.54 (1.41, 4.59) | <.001 | 2.04 (1.29, 3.22) | 2.55 (1.39, 4.66) | <.001 | |||
| Low-density Lipoprotein (mg/dL) | 0.99 (0.85, 1.14) | 1.16 (0.99, 1.37) | 0.11 | 0.96 (0.82, 1.13) | 1.16 (0.96, 1.39) | 0.127 | 0.96 (0.82, 1.13) | 1.13 (0.93, 1.36) | 0.242 | |||
| High-density Lipoprotein (mg/dL) | 1.13 (0.98, 1.30) | 0.92 (0.77, 1.10) | 0.051 | 1.11 (0.95, 1.30) | 0.93 (0.76, 1.13) | 0.148 | 1.12 (0.95, 1.31) | 0.94 (0.77, 1.15) | 0.185 | |||
| 24-hour urine protein | 1.05 (0.79, 1.40) | 0.92 (0.66, 1.27) | 0.704 | 1.01 (0.73, 1.40) | 0.93 (0.63, 1.35) | 0.886 | 0.96 (0.68, 1.34) | 0.98 (0.66, 1.45) | 0.966 | |||
| High Sensitivity CRP | 1.17 (0.97, 1.42) | 1.36 (1.10, 1.69) | 0.019 | 1.17 (0.97, 1.42) | 1.34 (1.08, 1.67) | 0.03 | ||||||
| Uric Acid (mg/dL) | 1.01 (0.93, 1.10) | 1.06 (0.96, 1.18) | 0.444 | 1.01 (0.92, 1.10) | 1.08 (0.97, 1.20) | 0.329 | ||||||
| Total Plasma Homocysteine | 1.01 (0.98, 1.04) | 1.08 (1.04, 1.12) | <.001 | 1.01 (0.98, 1.05) | 1.08 (1.04, 1.12) | <.001 | ||||||
| Lipoprotein(a)(mg/dl) | 1.12 (1.00, 1.27) | 1.10 (0.96, 1.27) | 0.139 | 1.13 (0.99, 1.27) | 1.10 (0.95, 1.27) | 0.15 | ||||||
| Calcium (mg/dL) | 0.77 (0.56, 1.06) | 1.02 (0.71, 1.48) | 0.186 | |||||||||
| Phosphate (mg/dL) | 1.20 (1.01, 1.42) | 1.06 (0.86, 1.30) | 0.104 | |||||||||
| Total Parathyroid Hormone (pg/ml) | 0.82 (0.63, 1.06) | 0.83 (0.61, 1.11) | 0.262 | |||||||||
African Americans are at lower risk of developing severe aortic stenosis.9 In our analyses, race was independently associated with AVC in all adjusted models with black patients having decreased odds ratio of presence and severity of AVC. Furthermore, we performed an analysis to test for interactions between race and all covariates in Model 3. In this model, interactions between race and gender, history of CVD, the presence of diabetes, systolic blood pressure, and LDL were all statistically significant. These findings, coupled with the existing evidence, led us to perform a stratification analysis by race.
Risk factors were therefore examined separately for black and non-black patients in Table 4. Although eGFR was not independently associated with severity of AVC in both analyses, age, high systolic blood pressure, homocysteine levels, and the presence of diabetes were strong predictors of AVC severity in both groups. Additionally, a history of cardiovascular disease, high cholesterol and LDL, and high CRP levels were risk factors for more severe AVC in non-black patients but not in black patients.
Table 4.
Odds ratio of aortic valve calcification associated with traditional, novel, and bone metabolism risk factors by race.
| Total AVC >0 - 100 | Total AVC 100+ | P-value | ||||
|---|---|---|---|---|---|---|
| Black (n=502) | Not Black (n=940) | Black(n=502) | Not Black (n=940) | Black | Not Black | |
| eGFR using CRIC equation | 1.00 (0.70, 1.43) | 1.22 (0.91, 1.63) | 0.93 (0.59, 1.45) | 0.90 (0.63, 1.30) | 0.941 | 0.208 |
| Participant Age | 3.76 (2.49, 5.68) | 2.86 (2.18, 3.74) | 7.36 (4.20, 12.89) | 6.90 (4.68, 10.19) | <.001 | <.001 |
| Female | 1.42 (0.75, 2.68) | 1.00 (0.63, 1.59) | 2.29 (1.09, 4.82) | 0.72 (0.41, 1.28) | 0.092 | 0.462 |
| Cardio-Vascular Disease | 1.42 (0.82, 2.46) | 1.12 (0.69, 1.81) | 1.23 (0.66, 2.29) | 2.55 (1.54, 4.23) | 0.459 | <.001 |
| Current Smoker | 1.19 (0.57, 2.48) | 0.72 (0.33, 1.55) | 1.12 (0.47, 2.65) | 0.61 (0.23, 1.60) | 0.892 | 0.525 |
| Body Mass Index (kg/m^2) | 1.02 (0.95, 1.10) | 1.00 (0.94, 1.06) | 0.99 (0.91, 1.08) | 0.98 (0.91, 1.05) | 0.744 | 0.785 |
| Waist Circumference (cm) | 0.90 (0.55, 1.48) | 1.00 (0.67, 1.49) | 1.07 (0.62, 1.85) | 1.17 (0.73, 1.89) | 0.826 | 0.764 |
| Total MET (METhrs/week) | 1.02 (0.81, 1.29) | 1.06 (0.86, 1.30) | 1.01 (0.75, 1.34) | 0.92 (0.69, 1.22) | 0.98 | 0.625 |
| Diabetes | 2.03 (1.16, 3.55) | 1.93 (1.25, 2.98) | 0.82 (0.42, 1.60) | 2.76 (1.66, 4.58) | 0.01 | <.001 |
| Systolic BP (mmHg) | 1.00 (0.98, 1.01) | 1.02 (1.01, 1.03) | 1.03 (1.01, 1.04) | 1.01 (1.00, 1.03) | 0.001 | 0.007 |
| High Cholesterol | 1.30 (0.59, 2.88) | 2.48 (1.39, 4.44) | 1.89 (0.71, 5.00) | 3.52 (1.56, 7.95) | 0.437 | <.001 |
| Low-density Lipoprotein (mg/dL) | 1.18 (0.90, 1.54) | 0.85 (0.69, 1.05) | 1.00 (0.72, 1.38) | 1.23 (0.96, 1.57) | 0.404 | 0.013 |
| High-density Lipoprotein (mg/dL) | 1.23 (0.94, 1.61) | 1.08 (0.87, 1.33) | 0.85 (0.61, 1.18) | 0.98 (0.75, 1.29) | 0.065 | 0.714 |
| 24-hour Urine Protein | 0.94 (0.52, 1.72) | 0.92 (0.60, 1.41) | 1.14 (0.57, 2.27) | 0.89 (0.54, 1.48) | 0.879 | 0.889 |
| High Sensitivity CRP | 1.06 (0.79, 1.44) | 1.26 (0.97, 1.65) | 1.08 (0.76, 1.54) | 1.53 (1.13, 2.08) | 0.883 | 0.022 |
| Uric Acid (mg/dL) | 1.03 (0.89, 1.19) | 0.97 (0.86, 1.10) | 1.09 (0.92, 1.29) | 1.08 (0.93, 1.25) | 0.618 | 0.344 |
| Total Plasma Homocysteine | 1.00 (0.94, 1.05) | 1.03 (0.98, 1.07) | 1.09 (1.03, 1.14) | 1.07 (1.02, 1.13) | 0.001 | 0.017 |
| Lipoprotein(a) (mg/dl) | 1.19 (0.92, 1.53) | 1.12 (0.97, 1.30) | 1.23 (0.89, 1.70) | 1.06 (0.90, 1.26) | 0.29 | 0.319 |
| Calcium (mg/dL) | 0.76 (0.45, 1.30) | 0.79 (0.51, 1.20) | 1.00 (0.55, 1.83) | 0.97 (0.59, 1.58) | 0.546 | 0.494 |
| Phosphate (mg/dL) | 1.18 (0.89, 1.56) | 1.25 (1.00, 1.56) | 0.98 (0.69, 1.39) | 1.10 (0.84, 1.44) | 0.443 | 0.143 |
| Total Parathyroid Hormone (pg/ml) | 0.82 (0.54, 1.25) | 0.83 (0.58, 1.19) | 0.69 (0.42, 1.15) | 0.93 (0.62, 1.39) | 0.337 | 0.599 |
For the following continuous variables, the odds ratio is per standard deviation of the value: eGFR, age, waist circumference, total metabolic equivalent (MET), LDL, HDL, and phosphate. The natural log of one plus the following continuous variables was used: hs-CRP, Lipoprotein a, total PTH, 24 hours urine protein. The following variables were from baseline measurements: Total METs, hsCRP, uric acid, homocysteine, Lipoprotein a, and total PTH.
Discussion
To our knowledge this study is the first to show a statistically significant correlation between non-end stage CKD and AVC and to identify associations between traditional and novel cardiovascular risk factors and derangements in bone metabolisms and AVC in patients with early stages of CKD. In addition to confirming the relationship between traditional cardiovascular risk factors and increased AVC in patients with CKD, the unadjusted analysis also identified decreased eGFR as independently associated with AVC. Furthermore, there was a graded relationship between stage of CKD and increased AVC and a clear difference in prevalence and severity of AVC noted between patients with stage 3A and stage 3B CKD, reinforcing the heterogeneity of cardiovascular morbidity in patients with eGFR 30-60. Derangements in bone metabolism, including increased PTH and Ph levels, were also associated with AVC, as were novel cardiovascular risk factors including hs-CRP, total plasma homocysteine, Lp(a), and uric acid, which are elevated in patients with CKD.10
The adjusted regression models further examined the relationship between traditional, novel, and bone metabolism risk factors of AVC. When adjusted for traditional cardiovascular risk factors, eGFR remained independently associated with AVC. However, when novel cardiovascular risk factors such as homocysteine and CRP are included in the model, eGFR was no longer independently associated with AVC. Similarly, markers of bone metabolism were also not independently associated with AVC when novel cardiovascular risk factors were included in the analysis. These results raise the interesting hypothesis such as that the association between CKD and AVC may be related to increased homocysteine and CRP, reflecting altered methionine metabolism or methylation state and a systemic inflammatory milieu in these patients.
Significant ethnic differences in risk for AVC and in the risk factor profile associated with were also noted. Black race was associated with lower amounts of AVC, which is interesting in light of evidence that African Americans are at lower risk of developing severe aortic stenosis.9 Several risk factors such as hypertension, diabetes, age, and homocysteine level were associated with increased AVC in both black and non-black patients. However, there were several risk factors that were unique to non-black patients, including a history of cardiovascular disease and high cholesterol, LDL, and CRP levels. These correlations raise interesting hypotheses about the mechanism of valve calcification in different populations and suggest heterogeneity in the pathogenesis of AVC. AVC may not be a stereotyped single process, but instead may represent different pathologic mechanisms that result in the similar end of aortic valve sclerosis and calcification with ensuing stenosis. Furthermore, there is evidence that there may be racial differences in calcification of the aortic valve leaflets versus the surrounding structures, such as the aortic valve ring and adjacent aortic root walls.11 These were combined into a single variable in our analysis, but as resolution of CT improves, further studies into the risk factors for and pathophysiology of calcification in these areas are warranted.
The study has several limitations including the use of eGFR rather than direct measure of GFR. However, eGFR is an accepted and commonly used surrogate for GFR in clinical practice. The cross-sectional design does not provide insight data on disease progression or clinical outcomes. Though this study revealed a correlation between CKD and AVC and identified several relevant risk factors that may contribute to the pathophysiology of AVC in CKD patients, future studies are needed to determine the rate of progression of AVC in patients with CKD and the role of different risk factors in progression. This study focused on AVC as detected by CT, however the calcium load on the valve is not always predictive of clinical stenosis or disease progression. Using echocardiography to understand the functional result of AVC will yield a deeper understanding of the relationship between calcification and disease in patients with CKD.
In summary, we found that decreased renal function was independently associated with increased levels of AVC in an unadjusted model. This association persisted after adjusting for traditional, but not novel, AVC risk factors. Adjusted regression models identified several traditional and novel risk factors for AVC in patients with CKD, and showed that there was a difference in AVC risk factors between black and non-black patients.
Acknowledgments
Funding for the CRIC Study was obtained under a cooperative agreement from National Insti tute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021,U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) V 2014.07.28 UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maher ER, Young G, Smyth-Walsh B, Pugh S, Curtis JR. Aortic and mitral valve calcification in patients with end-stage renal disease. Lancet. 1987;2:875–877. doi: 10.1016/s0140-6736(87)91370-5. [DOI] [PubMed] [Google Scholar]
- 2.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT. Chronic Renal Insufficiency Cohort Study I. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol : JASN. 2003;14:S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 4.Budoff MJ, Rader DJ, Reilly MP, Mohler ER, 3rd, Lash J, Yang W, Rosen L, Glenn M, Teal V, Feldman HI. Relationship of estimated GFR and coronary artery calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2011;58:519–526. doi: 10.1053/j.ajkd.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 6.Budoff MJ, Takasu J, Katz R, Mao S, Shavelle DM, O'Brien KD, Blumenthal RS, Carr JJ, Kronmal R. Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol. 2006;13:166–172. doi: 10.1016/j.acra.2005.09.090. [DOI] [PubMed] [Google Scholar]
- 7.Linefsky J, Katz R, Budoff M, Probstfield J, Owens D, Takasu J, Shavelle D, Ouyang P, Psaty B, O'Brien KD. Stages of systemic hypertension and blood pressure as correlates of computed tomography-assessed aortic valve calcium (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2011;107:47–51. doi: 10.1016/j.amjcard.2010.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasir K, Katz R, Al-Mallah M, Takasu J, Shavelle DM, Carr JJ, Kronmal R, Blumenthal RS, O'Brien K, Budoff MJ. Relationship of aortic valve calcification with coronary artery calcium severity: the Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomogr. 2010;4:41–46. doi: 10.1016/j.jcct.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Patel DK, Green KD, Fudim M, Harrell FE, Wang TJ, Robbins MA. Racial differences in the prevalence of severe aortic stenosis. J Am Heart Assoc. 2014;3:e000879. doi: 10.1161/JAHA.114.000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med. 2004;140:9–17. doi: 10.7326/0003-4819-140-1-200401060-00006. [DOI] [PubMed] [Google Scholar]
- 11.Nasir K, Katz R, Takasu J, Shavelle DM, Detrano R, Lima JA, Blumenthal RS, O'Brien K, Budoff MJ. Ethnic differences between extra-coronary measures on cardiac computed tomography: multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. 2008;198:104–114. doi: 10.1016/j.atherosclerosis.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]