Abstract
While picornaviruses can cause diseases in many mammals, little is known of their host range for replication in non-mammalian vertebrates. Here, a picornavirus in liver and kidney tissues from diseased Sulawesi tortoises (Indotestudo forsteni) was genetically characterized. Tortoise rafivirus A (ToRaV-A, KJ415177) represents a potential new genus in the family Picornaviridae, for which we propose the name “Rafivirus”. Our finding confirms the susceptibility of reptiles to picornaviruses.
Picornaviridae is a diverse viral family with positive, single-stranded RNA genomes. More than one hundred years after the discovery of the first picornavirus, foot-and-mouth disease virus in 1898, the family now includes at least 46 species grouped into 26 genera, with a rapidly growing number of new tentative species. The family Picornaviridae belongs to the order Picornavirales and an even more diverse, recently proposed “picorna-like superfamily”, which consists of positive-strand RNA viral families from animals, plants, insects and even algae [11]. Sequence analyses of RNA polymerases and helicases has suggested that the picorna-like superfamily had already diversified in a “big bang” manner before the radiation of eukaryotic hosts, as members of some of the major subdivisions can infect highly diverse eukaryotes (e.g., members of the family Partitiviridae can infects fungi, plants, excavates, and chromalveolates) [11]. Although it is therefore reasonable to expect that picornaviruses can infect members of all vertebrate classes, picornaviruses were only recently identified in ray-finned fish (class Actinopterygii) [2, 12, 20]. While picornaviruses of mammalian hosts are best known, the only published picornaviruses of reptiles are found in birds (Dinosauria). Picorna-like viruses have been reported in non-avian reptiles such as snakes [3] and tortoises [5, 6, 10, 14], but their genomic features and phylogenies are yet to be characterized. Here, we confirm that the host range of the picornaviruses includes non-avian reptiles in the order Testudines by genetically characterizing a divergent picornavirus from tortoise tissues.
Over one hundred Sulawesi tortoises (Indotestudo forsteni) in the United States showed signs of severe disease, including anorexia, lethargy, mucosal ulcerations, palatine erosions of the oral cavity, nasal and ocular discharge, and diarrhea [22]. A reptile adenovirus, Sulawesi tortoise adenovirus 1, was found to be associated with the outbreak [22, 23]. During unbiased metagenomic sequencing from a tortoise spleen sample, we also detected sequences from a highly divergent picornavirus co-infecting the same animal. Briefly, deep sequencing using Illumina Miseq and the 454 Genome Sequencer FLX platform was performed on enriched viral particles from the tissue according to previously described protocols in which both viral DNA and RNA were randomly amplified and sequenced [16, 18]. Sequences were analyzed by a customized pipeline in which de novo assembly was performed, and the resulting contigs and the unassembled singlets were compared against the GenBank database using BLASTx and BLASTn [16, 17]. Over 1,800 Miseq sequences and 30 pyrosequences were mapped to a picornavirus coding sequence. Finally, we performed rapid amplification of cDNA ends (RACE) and Sanger dideoxy sequencing to obtain the sequence of the remaining untranslated region (UTR).
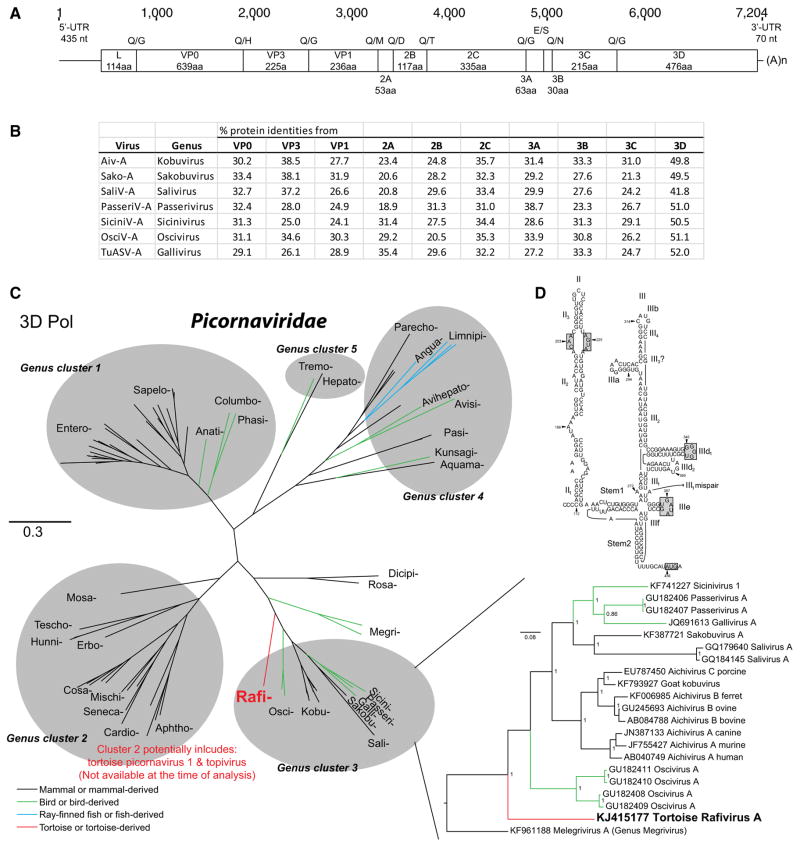
The genome of this reptilian picornavirus, tortoise rafivirus A (ToRaV-A; GenBank accession KJ415177), is comprised of 8,204 nucleotides and exhibits a typical picornaviral genome organization, in the form of 5′ UTRIRES-IV [L/VP0-VP3-VP1/2A-2B-2C/3A-3B-3C-3D] 3′ UTR-poly(A) (Fig. 1). The 5′ UTR of ToRaV-A is 435 nt long. The predicted in-frame AUG initiation codon (GCAUA436UGA) is at position 436–438. Based upon the predicted secondary RNA structure of the 5′ UTR-IRES, ToRaV-A has a potential Hepacivirus/Pestivirus-like (HP) type-IV IRES with pseudoknot and conserved motifs in domain II and in domain III (Fig. 1).
Fig. 1.
Genomic and phylogenetic analysis of the tortoise rafivirus A (ToRaV-A). A) Schematic depiction of the ToRaV-A genome organization. Size of the predicted proteins and cleavage sites are indicated. UTR, untranslated region; (A)n, poly(A) tail. B) Pairwise amino acid sequence identities between tortoise rafivirus A (ToRaV-A) and the closest genome representatives of Picornaviridae. Aiv-A, aichivirus A; Sako-A, sakobuvirus A; SaliV-A, salivirus A; PasseriV-A, passerivirus A or turdivirus 1; SiciniV-A, sicinivirus A or 1; OsciV-A, oscivirus A or turdivirus 2; TuASV-A, gallivirus A. Protein sequences were aligned with MAFFT [7], and sequence identities were calculated using a species demarcation tool [9, 15]. C) 3D polymerase phylogeny of the family Picornaviridae, including the newly described tortoise rafivirus A of the proposed genus “Rafivirus” (shown as “Rafi-”). The Bayesian phylogeny was generated with MrBayes [16], where 1,000,000 generations were sampled every 50 steps. Genus-level representatives are presented on the left, while species-level representatives are presented on the right with a focus on genus cluster 3. The scale bar represents evolutionary distance in substitutions per site. D) Predicted RNA secondary structure of the 5′ UTR-IRES of tortoise rafivirus A. The type-IV IRES has been annotated as proposed previously [4, 21]. Domains are labeled II and III; individual helical segments are labeled II1, II2, III1, and III2, etc.; and individual hairpins are labeled IIIa and IIIb, etc. to maintain the continuity of the current nomenclature. The positions of conserved domains and the polyprotein AUG start codon are indicated by shaded boxes
Phylogenetically, rafivirus is related to a cluster of picornaviruses that includes mammalian picornaviruses (kobuviruses, saliviruses, and sakobuviruses), and avian picornaviruses (passeriviruses, galliviruses and osciviruses), based on a Bayesian inference analysis of the 3D RNA-dependent RNA polymerase (genus cluster 3; Fig. 1c). As described below, rafivirus also shared similar GC content, phylogeny, and presence of L protein with the other members of this cluster [19]. Both structural (VP0, VP3 and VP1) and non-structural proteins (2A-3D) were highly divergent from known picornaviral proteins, sharing only 19–39 % identity with the closest picornaviral protein sequences. The more conserved 3D polymerase protein shared 41–52 % identity (Fig. 1). This reflects the distant evolutionary relationship of the tortoise rafivirus to other mammalian and avian picornaviruses within this genus cluster (Fig. 1).
In both sequence and phylogenetic analysis, ToRaV-A is distinct from A) a recently reported picornavirus-like virus in juvenile tortoises (tortoise picornavirus 1 in Testudo graeca and Geochelone elegans) [6] (Nick Knowles, unpublished data) and B) the recently described topivirus from Testudo graeca, Geochelone sulcata, and Pyxis arachnoides tortoises [1] (Neither sequence was publically available at the time of writing.) The genome sequences of both tortoise picornavirus 1 and topivirus clustered with members of the genus Cardiovirus and related genera in cluster 2 [1, 10], while the ToRaV-A genome described here clustered with members of the genus Kobuvirus and related genera in cluster 3 (Fig. 1). Our finding suggests that ToRaV-A is a prototype for a picornavirus genus. In homage to a fictitious character of the reptilian family Testudinidae (Raphael) in the popular culture, we propose the genus name “Rafivirus”.
The ToRaV-A genome encodes a 2,233-amino-acid (aa)-long polyprotein that is cleaved into smaller proteins. An L protein is present but lacks the GxCG motif (where x represents a non-conserved amino acid) responsible for chymotrypsin-like protease activity in some other picornaviruses. An internal cleavage site that cleaves VP0 into VP4-VP2 could not be identified. The N-terminus of VP0 contains a GxxxT (GANIT) myristoylation site. The 2A protein does not contain an H-box/NC motif. The 2C protein contains the conserved NTPase motif GxxGxGKS (GLPGCGKS), and the helicase motif DDxxQ (DDLGQ) which resembles those within the genus cluster 3 (DD[L/I/V]GQ) [19]. The 3C protein, which encodes a protease, contains an H-D-C catalytic triad and a conserved active site motif GxCG (GMCG) but lacks the RNA-binding motif KFRDI. All of the above genomic features, with the exception of the H-D-C catalytic triad instead of H-E-C, are common to members of cluster 3 (Fig. 1).
Recombination occurs frequently between closely related picornaviruses, facilitated by template switching during genome replication between two picornaviruses that share high nucleotide sequence similarity [8]. No recombination was detected between ToRaV-A and currently available picornavirus genome sequences from mammals, birds and fishes, as the ToRaV-A coding sequence does not share detectable nucleotide sequence identity with other picornaviruses (using BLASTn). Since recombination rarely occurs between divergent picornavirus from different genera [13], recombination analysis of ToRaV-A may be improved when more related picornaviruses of Testudines are sequenced.
To determine the prevalence of ToRaV-A in the outbreak population, a qPCR assay targeting the 3D sequence was performed on tissues from animals involved in the Sulawesi tortoise disease outbreak [22]. To prepare the target standards, DNA from a known AgAdV1-positive sample was amplified by PCR and gel extracted, the DNA concentration was determined using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Inc.), and a qPCR standard curve was generated by analysis of tenfold serial dilutions ranging from 10 to 106 copies. Forward primer TortPicornaF 5′-TGAACAAGGGAAAACCAGGA-3′, reverse primer TortPicornaR 5′-CGTCCAAAAATCATC CTTCC-3′, and probe TortPicornaProbe 5′-[6-FAM]TGTT GATGCAGCTAATTTGCCCTCT[BHQ1a]-3′ were used in one-step RT-PCR reactions (4× TaqMan Fast Virus 1-Step Master Mix, Life Technologies). Each 20-μL qPCR reaction was run in triplicate using a standard fast protocol. Paired liver and kidney samples were tested from six tortoises in the outbreak, as well as liver from one additional tortoise. To confirm the specificity of the assay, a bovine enterovirus sample and a feline sakobuvirus sample were also tested. The presence of ToRaV-A RNA was detected in 6 of 7 livers and 2 of 6 kidney samples tested (Table 1). ToRaV-A loads appear to be higher in the liver than in kidneys of tortoises. In a previous study, the majority of the tortoises tested were positive for adenovirus [22]. Our data suggest that many of these tortoises are also infected with ToRaV-A. While histologic and ultrastructural findings support a significant role for adenovirus in pathology, more epidemiological and experimental data are required to determine the relative roles of these two viruses in this Sulawesi tortoise disease outbreak. Although infectious diseases are often studied as a single agent causing disease, co-infections are very common and can significantly modulate disease; infection with multiple agents is often synergistic in causing disease [24]. Previous reports of picornavirus-like particles in tortoises have all been based on virus isolation [5, 6, 10, 14]. In this study, neither this picornavirus nor the tortoise adenovirus was successfully cultured despite multiple attempts. Our discovery of ToRaV-A highlights the utility of metagenomic analysis to obtain a more complete picture of possible pathogens in an outbreak, as well as culture-independent identification and genome sequencing of this virus.
Table 1.
Copies of tortoise rafivirus A (ToRaV-A) detected by qPCR in paired liver and kidney samples of Sulawesi tortoises.
| Animal | Liver | Kidney |
|---|---|---|
| 1 | 3,326 | – |
| 2 | 6,930 | – |
| 3 | 53 | – |
| 4 | 24,919 | 7,063 |
| 5 | 16,043 | 3,503 |
| 6 | – | – |
| 7 | 8,610 | n/a |
“–” indicates not detectable; n/a indicates not tested
The recent addition of ray-finned fish, and now tortoises, to the host range of picornaviruses provides a broader perspective on picornaviral evolution. Fishes are ancestral to all vertebrates; whereas terrestrial vertebrates, including sauropsids and mammals, evolved from a more recent common ancestor [25]. However, phylogenetic analyses of the picornaviral 3D polymerase protein from these disparate hosts do not yield a topology similar to that of their hosts. Phylogenetic analysis placed picornaviruses from tortoises in at least two genus clusters (Fig. 1). ToRaV-A is genetically more closely related to other mammalian and avian picornaviruses in genus cluster 3 than other tortoise picornaviruses in genus cluster 2. This is consistent with a previous analysis of mammalian and avian picornaviruses in which host-virus co-phylogeny was not supported [13]. On a higher level, sequence analysis has suggested that the major clades of the “picorna-like superfamily” were already present before the radiation of eukaryotes [11]. If this proposed evolutionary history is true, one can expect ancestral vertebrate species to have already been infected by highly divergent picornaviruses during the radiation of the tetrapods – this hypothesis offers a potential explanation for the current intertwined distribution of picornavirus in birds and mammals (Fig. 1). While the three currently available fish picornavirus RdRp sequences cluster together, this may be due to the still small amount of viral sampling that has been done from these animals. Further sequencing of fish and reptile picornaviruses may yet reveal lineages in all of the five major clusters of the family Picornaviridae.
Nucleotide sequence accession numbers
The genome sequence of ToRaV-A has been submitted to GenBank under accession no. KJ415177/NC_023988.
Acknowledgments
This work was supported by NHLBI grant R01 HL105770 to E.L.D and the Blood Systems Research Institute and grants from the Hungarian Scientific Research Fund (OTKA K83013 and OTKA K111615).
Contributor Information
Terry Fei Fan Ng, Email: terryfeifan@gmail.com, Blood Systems Research Institute, San Francisco, CA, USA. Department of Laboratory Medicine, University of California at San Francisco, San Francisco, CA, USA.
James F. X. Wellehan, College of Veterinary Medicine, University of Florida, Gainesville, FL, USA
James K. Coleman, College of Veterinary Medicine, University of Florida, Gainesville, FL, USA
Nikola O. Kondov, Blood Systems Research Institute, San Francisco, CA, USA
Xutao Deng, Blood Systems Research Institute, San Francisco, CA, USA. Department of Laboratory Medicine, University of California at San Francisco, San Francisco, CA, USA.
Thomas B. Waltzek, College of Veterinary Medicine, University of Florida, Gainesville, FL, USA
Gábor Reuter, Regional Laboratory of Virology, ÁNTSZ Regional Institute of State Public Health Service, Pécs, Hungary.
Nick J. Knowles, The Pirbright Institute, Woking, Surrey, UK
Eric Delwart, Blood Systems Research Institute, San Francisco, CA, USA. Department of Laboratory Medicine, University of California at San Francisco, San Francisco, CA, USA.
References
- 1.Farkas S, Ihász K, Fehér E, Bartha D, Jakab F, Gál J, Bányai K, Marschang R. Sequencing and phylogenetic analysis identifies candidate members of a new picornavirus genus in terrestrial tortoise species. Arch Virol. 2014:1–6. doi: 10.1007/s00705-014-2292-z. [DOI] [PubMed] [Google Scholar]
- 2.Fichtner D, Philipps A, Groth M, Schmidt-Posthaus H, Granzow H, Dauber M, Platzer M, Bergmann SM, Schrudde D, Sauerbrei A, Zell R. Characterization of a novel picornavirus isolate from a diseased European eel (Anguilla anguilla) J Virol. 2013;87:10895–10899. doi: 10.1128/JVI.01094-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heldstab A, Bestetti G. Virus associated gastrointestinal diseases in snakes. J Zoo Anim Med. 1984;15:118–128. [Google Scholar]
- 4.Hellen CU, de Breyne S. A distinct group of hepacivirus/pestivirus-like internal ribosomal entry sites in members of diverse picornavirus genera: evidence for modular exchange of functional noncoding RNA elements by recombination. J Virol. 2007;81:5850–5863. doi: 10.1128/JVI.02403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heuser W, Kaleta E, Giesow K, Keil GM, Knowles NJ. Genome sequence of virus “X”, a picornavirus isolated from a spur-thighed tortoise (Testudo graeca). EUROPIC 2010: XVI Meeting of the European Study Group on the Molecular Biology of Picornaviruses; St. Andrews. 2010. p. 147. [Google Scholar]
- 6.Heuser W, Pendl H, Knowles NJ, Keil G, Herbst W, Lierz M, Kaleta EF. Soft plastron, soft carapace with skeletal abnormality in juvenile tortoises. Histopathology and isolation of a novel picornavirus from Testudo graeca and Geochelone elegans. Tieraerztliche Praxis. 2014 (in press) [PubMed] [Google Scholar]
- 7.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 8.Jarvis TC, Kirkegaard K. Poliovirus RNA recombination: mechanistic studies in the absence of selection. EMBO J. 1992;11:3135–3145. doi: 10.1002/j.1460-2075.1992.tb05386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katoh K, Kuma K-i, Toh H, Miyata T. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005;33:511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles NJ, Hovi T, Hyypiä T, King AMQ, Lindberg M, Pallansch MA, Palmenberg AC, Simmonds P, Skern T, Stanway G, Yamashita T, Zell R. Picornaviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus taxonomy: classification and nomenclature of viruses: ninth report of the International Committee on Taxonomy of Viruses. Elsevier; San Diego: 2012. pp. 855–880. [Google Scholar]
- 11.Koonin EV, Wolf YI, Nagasaki K, Dolja VV. The Big Bang of picorna-like virus evolution antedates the radiation of eukaryotic supergroups. Nat Rev Microbiol. 2008;6:925–939. doi: 10.1038/nrmicro2030. [DOI] [PubMed] [Google Scholar]
- 12.Lange J, Groth M, Fichtner D, Granzow H, Keller B, Walther M, Platzer M, Sauerbrei A, Zell R. Virus isolate from carp: genetic characterization reveals a novel picornavirus with two aphthovirus 2A-like sequences. J Gen Virol. 2014;95:80–90. doi: 10.1099/vir.0.058172-0. [DOI] [PubMed] [Google Scholar]
- 13.Lewis-Rogers N, Crandall KA. Evolution of Picornaviridae: an examination of phylogenetic relationships and cophylogeny. Mol Phylogenet Evol. 2010;54:995–1005. doi: 10.1016/j.ympev.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Marschang RE. Viruses infecting reptiles. Viruses. 2011;3:2087–2126. doi: 10.3390/v3112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muhire B, Martin DP, Brown JK, Navas-Castillo J, Moriones E, Zerbini FM, Rivera-Bustamante R, Malathi VG, Briddon RW, Varsani A. A genome-wide pairwise-identity-based proposal for the classification of viruses in the genus Mastrevirus (family Geminiviridae) Arch Virol. 2013;158:1411–1424. doi: 10.1007/s00705-012-1601-7. [DOI] [PubMed] [Google Scholar]
- 16.Ng TF, Marine R, Wang C, Simmonds P, Kapusinszky B, Bodhidatta L, Oderinde BS, Wommack KE, Delwart E. High variety of known and new RNA and DNA viruses of diverse origins in untreated sewage. J Virol. 2012;86:12161–12175. doi: 10.1128/JVI.00869-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng TF, Driscoll C, Carlos MP, Prioleau A, Schmieder R, Dwivedi B, Wong J, Cha Y, Head S, Breitbart M, Delwart E. Distinct lineage of vesiculovirus from big brown bats, United States. Emerg Infect Dis. 2013;19:1978–1980. doi: 10.3201/eid1912.121506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng TF, Kondov NO, Hayashimoto N, Uchida R, Cha Y, Beyer AI, Wong W, Pesavento PA, Suemizu H, Muench MO, Delwart E. Identification of an astrovirus commonly infecting laboratory mice in the US and Japan. PLoS ONE. 2013;8:e66937. doi: 10.1371/journal.pone.0066937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng TF, Mesquita JR, Nascimento MSJ, Kondov NO, Wong W, Reuter G, Knowles NJ, Vega E, Esona MD, Deng X, Vinjé J, Delwart E. Feline fecal virome reveals novel and prevalent enteric viruses. Veterinary Microbiol. 2014;171:102–111. doi: 10.1016/j.vetmic.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phelps NB, Mor SK, Armien AG, Batts W, Goodwin AE, Hopper L, McCann R, Ng TF, Puzach C, Waltzek TB, Delwart E, Winton J, Goyal SM. Isolation and molecular characterization of a novel picornavirus from baitfish in the USA. PLoS ONE. 2014;9:e87593. doi: 10.1371/journal.pone.0087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reuter G, Boldizsár A, Pankovics P. Complete nucleotide and amino acid sequences and genetic organization of porcine kobuvirus, a member of a new species in the genus Kobuvirus, family Picornaviridae. Arch Virol. 2009;154:101–108. doi: 10.1007/s00705-008-0288-2. [DOI] [PubMed] [Google Scholar]
- 22.Rivera S, Wellehan JFX, McManamon R, Innis CJ, Garner MM, Raphael BL, Gregory CR, Latimer KS, Rodriguez CE, Diaz-Figueroa O, Marlar AB, Nyaoke A, Gates AE, Gilbert K, Childress AL, Risatti GR, Frasca S. Systemic adenovirus infection in Sulawesi tortoises (Indotestudo forsteni) caused by a novel siadenovirus. J Veterinary Diagn Investig. 2009;21:415–426. doi: 10.1177/104063870902100402. [DOI] [PubMed] [Google Scholar]
- 23.Schumacher VL, Innis CJ, Garner MM, Risatti GR, Nordhausen RW, Gilbert-Marcheterre K, Wellehan JFX, Childress AL, Frasca S. Sulawesi tortoise adenovirus-1 in two impressed tortoises (Manouria impressa) and a Burmese star tortoise (Geochelone platynota) J Zoo Wildl Med. 2012;43:501–510. doi: 10.1638/2011-0228R.1. [DOI] [PubMed] [Google Scholar]
- 24.Sinha A, SenGupta S, Guin S, Dutta S, Ghosh S, Mukherjee P, Mukhopadhyay AK, Ramamurthy T, Takeda Y, Kurakawa T, Nomoto K, Nair GB, Nandy RK. Culture-independent real-time PCR reveals extensive polymicrobial infections in hospitalized diarrhoea cases in Kolkata, India. Clin Microbiol Infect. 2013;19:173–180. doi: 10.1111/j.1469-0691.2011.03746.x. [DOI] [PubMed] [Google Scholar]
- 25.Werneburg I, Sánchez-Villagra MR. Timing of organogenesis support basal position of turtles in the amniote tree of life. BMC Evol Biol. 2009 doi: 10.1186/1471-2148-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]