Abstract
Introduction
Heavy drinking during adolescence is associated with increased reactivity to alcohol related stimuli and to differential neural development. Alcohol cue reactivity has been widely studied among adults with alcohol use disorders, but little is known about the neural substrates of cue reactivity in adolescent drinkers. The current study aimed to identify changes in blood-oxygen level dependent (BOLD) signal during a cue reactivity task pre- and post-monitored abstinence from alcohol.
Method
Demographically matched adolescents (16.0–18.9 years, 54% female) with histories of heavy episodic drinking (HD; n=22) and light or non-drinking control teens (CON; n=16) were recruited to participate in a month-long study. All participants completed a functional Magnetic Resonance Imaging (fMRI) scan with an alcohol cue reactivity task and substance use assessments at baseline and after 28 days of monitored abstinence from alcohol and drugs (i.e., urine toxicology testing every 48-72 hours). Repeated-measure analysis of variance (ANOVA) examined main effects of group, time, and group × time interactions on BOLD signal response in regions of interest defined by functional differences at baseline.
Results
The HD group exhibited greater (p<.01) BOLD activation than CON to alcohol cues relative to neutral cues in all regions of interest (ROIs; bilateral striatum/globus pallidus, left anterior cingulate, bilateral cerebellum, and parahippocampal gyrus extending to the thalamus/substantia nigra) across time points. Group × time effects showed that HD exhibited greater BOLD activation to alcohol cues than CON at baseline in left anterior cingulate cortex and in the right cerebellar region, but these decreased to non-significance after one month of monitored abstinence.
Conclusions
In all ROIs examined, HD exhibited greater BOLD response than CON to alcohol relative to neutral beverage picture cues at baseline, indicating heightened cue reactivity to alcohol cues in heavy drinking adolescents prior to the onset of any alcohol use diagnosis. Across the majority of these brain regions, differences in BOLD response were no longer apparent following a month of abstinence, suggesting a decrease in alcohol cue reactivity among adolescent non-dependent heavy drinkers as a consequence of abstaining from alcohol. These results highlight the malleability of adolescent brain function despite no formal intervention targeting cue reactivity. Increased understanding of the neural underpinnings of cue reactivity could have implications for prevention and intervention strategies in adolescent heavy alcohol users.
Keywords: adolescence, cue reactivity, alcohol, anterior cingulate cortex, cerebellum, heavy episodic drinking
1. INTRODUCTION
Alcohol use among adolescents is a pervasive issue and likely to have deleterious effects on health and well-being. Drinking to intoxication is associated with the most serious negative consequences (e.g., withdrawal symptoms relate to cognitive decline; Tapert and Brown 1999), and this style of drinking accelerates significantly during adolescence. Recent epidemiological data indicates that 8% of American 8th graders report having been drunk in the last year, a statistic that jumps to 43% by 12th grade. Furthermore, 22% of 12th graders report heavy episodic drinking (i.e., consuming 5 or more drinks in a row) in the last 2 weeks (Johnston, O’Malley, Miech, Bachman, & Schulenberg, 2014). Heavy episodic drinking affects not only brain functioning at an acute level, but both brain development and function over time when introduced during this critical period of brain maturation (Jacobus & Tapert, 2013). Adolescent drinkers have shown diminished performances across a range of neuropsychological functioning, including on tasks of attention (Tarter, Mezzich, Hsieh, & Parks, 1995), memory (Brown, Tapert, Granholm, & Delis, 2000), information processing (Tarter et al., 1995), visuospatial functioning (Beatty, Hames, Blanco, Nixon, & Tivis, 1996; Sher, Martin, Wood, & Rutledge, 1997), language abilities (Moss, Kirisci, Gordon, & Tarter, 1994), motor speed (Ferrett, Carey, Thomas, Tapert, & Fein, 2010) and executive functioning (Montgomery, Fisk, Murphy, Ryland, & Hilton, 2012; Moss et al., 1994).
As individuals accumulate experience with substances, they tend to develop conditioned responses to cues surrounding substance use (i.e., cue reactivity), which is often characterized by craving (Rohsenow et al., 1994). Alcohol cue reactivity has been implicated as a proxy of risk for alcohol use, as adult heavy users and alcoholics exhibit increased reactivity and craving to alcohol cues, even when sober (Cooney, Litt, Morse, Bauer, & Gaupp, 1997). Exhibiting more reactivity and reporting increased subjective craving to alcohol related stimuli are indicators of the reward value and salience these stimuli have for heavy users. As such, cue reactivity has been used to predict the probability of relapse, as it may index increased incentive value that puts individuals at risk for re-initiating use (Cooney et al., 1997; Heinz, Beck, Grüsser, Grace, & Wrase, 2009).
Brain imaging studies of cue reactivity, primarily in adults, have reliably shown increased reactivity in heavy users in areas associated with reward processing (e.g., striatal and limbic regions), and have shown differences between heavy users and light- or non-drinking controls in parietal and temporal regions (Schacht, Anton, & Myrick, 2013). Likewise, several studies link brain regions associated with addiction processes in cue reactivity responses, including reward learning (nucleus accumbens and striatum; Ihssen, Cox, Wiggett, Fadardi, and Linden, 2011; Schacht et al., 2011; Vollstädt-Klein et al., 2011), reward salience (anterior cingulate cortex [ACC] and orbitofrontal cortex [OFC]; Grüsser et al., 2004; Vollstädt-Klein et al., 2011), and decision-making (dorsolateral prefrontal cortex [DLPFC]; Grüsser et al., 2004). Beyond simply differentiating between heavy and non-users, cue-elicited BOLD response in these brain regions have also been correlated with self-report craving ratings (Myrick et al., 2004; Park et al., 2007), substantiating BOLD responses as valid indices of cue-reactivity.
Adolescent heavy drinkers with varying levels of alcohol use disorder severity have exhibited increased BOLD responses to alcohol words (Tapert, Brown, Baratta, & Brown, 2004) and pictures (Tapert et al., 2003) in similar brain regions identified in studies of adults discussed above (e.g., ACC and DLPFC). These BOLD differences in adolescents were also associated with behaviors of interest including average number of drinks per month and the self-reported desire to drink alcohol (Tapert et al., 2003). Cue reactivity represents one of the core alterations to neurobiology elicited by alcohol use (Heinz et al., 2009), and serves as a promising focus for intervention (Vollstädt-Klein et al., 2011). The presence of similar effects in adolescents as those seen in adults may represent either challenges (e.g., changes occurring early in development may be more deeply ingrained into the neural architecture) or opportunities (e.g., adolescent brains are more malleable and more likely to overcome the neural alterations associated with increased cue reactivity). Gaining a better understanding of how individuals at risk for developing alcohol use disorders react to alcohol stimuli, and monitoring how those reactions change over time, may be particularly important for developing prevention and intervention strategies aimed at reducing alcohol use disorders in adolescents and young adults.
The aim of the current study was to elucidate neural substrates associated with cue reactivity in adolescent heavy episodic drinkers (HD) compared to non-drinking controls (CON), and to evaluate responses pre- and post-monitored abstinence. Areas of the brain associated with reward processing and decision-making were of primary interest and identified as regions of interest (ROI) a priori including the nucleus accumbens (NA), dorsal striatum and globus pallidus (DSGP), ACC, orbitofrontal cortex (OFC), and dorsolateral prefrontal cortex (DLPFC). We hypothesized that heavy drinking adolescents would show increased BOLD response to alcohol relative to neutral cues compared to non-drinking adolescents prior to prolonged abstinence (e.g., Tapert, 2004), but differences would diminish after a month of monitored abstinence, representing a decrease in the motivational salience of alcohol cues over the course of the abstinence period.
2. METHOD
2.1 Participants
Participants (16.0–18.9 years) were recruited from local high schools, colleges, and community settings via mailings and fliers (Bekman, Winward, Lau, Wagner, & Brown, 2013; Winward, Bekman, Hanson, Lejuez, & Brown, 2014). The study was advertised as an “adolescent development project,” and no information regarding alcohol or drug use criteria was described in the fliers or discussed prior to screening. Interested students responding by phone were independently screened to determine eligibility. All interested teens and their parents underwent a structured interview to confirm eligibility. In accordance with the University of California, San Diego (UCSD) Human Research Protections Program and high school district policies, written informed assent (adolescent participant) and consent (parent/legal guardian) were obtained before participation. To minimize confounds, individuals were excluded if they had: history of a psychiatric disorder; extensive marijuana (>50 lifetimes) or other drug use (>15 times); head trauma; learning disorder; neurological dysfunction or serious medical illness; family history of bipolar I or psychotic disorder; significant prenatal alcohol exposure (>7 drinks in a week or >2 drinks in a day); sensory problems; use of psychoactive medications; and substance use during the abstinence protocol.
Participants were classified as HD or CON. HD participants reported ≥100 lifetime drinking episodes, ≥3 past month heavy episodic drinking occasions (with at least 1 within 2 weeks prior to study initiation), and 1 or more recent alcohol withdrawal symptoms. CON teens reported <5 lifetime drinking episodes, no history of heavy drinking episodes (i.e., >4/5 standard alcoholic drinks on one occasion for females/males) or alcohol withdrawal symptoms, and no previous marijuana or other drug use (see Table 1 for detailed alcohol use characteristics). None of the participants met criteria for Diagnostic and Statistical Manual of Mental Disorders- Fourth edition (DSM-IV) alcohol dependence and none were seeking treatment for their alcohol use.
Table 1.
Demographic data for heavy drinking (HD) and control (CON) groups
| HD (n=22) M(SD) |
CON (n=16) M(SD) |
|
|---|---|---|
| Age | 17.93 (0.71)* | 17.42 (0.69) |
| % Female | 55% (n=12) | 44% (n=7) |
| % White | 77% (n=17) | 69% (n=11) |
| Grades Completed | 11.45 (0.80) | 11.13 (0.72) |
| Pubertal Development | Males: 17.4 (1.8) Females: 15.8 (1.5) |
Males: 16.7 (1.5) Females: 16.0 (1.5) |
| Days Drinking in past month | 6.8 (4.1)** [range 3–15] |
0.13 (0.34) [n=2, 1 time each] |
| Average drinks per occasion in past month | 5.7 (1.8)** [range 3–9] |
2.5 (0.7) [n=2, range 1–3] |
Note:
p<.05;
p<.01
A total of 51 participants were enrolled in the study (HD = 32; CON =19). Five HD participants did not complete the protocol (4 due to toxicology confirmed alcohol or drug use, 1 due to schedule conflicts/inability to fulfill protocol). An additional 8 participants’ data were excluded due to excessive artifacts in their fMRI data (i.e., >15% of trials contained artifacts; 3 CON and 5 HD), leaving a final sample of 38 adolescents (HD = 22; CON = 16). The HD group was approximately 6 months older than the CON group, but the groups did not differ significantly in pubertal development or in the distribution of males and females (see Table 1). Of the 16 CON, 3 reported ever having consumed alcohol and 2 reported drinking in the 2 months prior to the study, but none reported a heavy episodic drinking episode. The groups did not differ on the four Behavioral Inhibition System/Behavioral Activation System (BIS/BAS; Carver & White, 1994) subscales (ps > .17), indicating similar propensity for reward-responsiveness and inhibition at baseline.
2.2 Procedure
Participants were asked to remain abstinent from alcohol and drugs for the entirety of the study period (up to 5 weeks; mean = 30.5 days), and to complete in-person assessments and a functional Magnetic Resonance Imaging (fMRI) session at the beginning and end of the study period. To increase protocol adherence and minimize participant burden, study staff worked with participants to choose a 1-month period that did not conflict with birthdays, school events, vacations, or other events at which participants would normally consume alcohol. Participants were paid semi-monthly for assessment completion and received a $100 bonus if they completed all scheduled assessments and remained abstinent throughout the study period. Additionally, a weekly motivational interviewing-based protocol was used to encourage sustained abstinence among participating youth, based on prior research in our laboratory (Brown, Anderson, Schulte, Sintov, & Frissell, 2005). Participants were not included if they were actively in treatment or currently seeking treatment for substance use; therefore, eligibility was not contingent upon a teen’s expressed desire to quit drinking beyond the scope of the study. Instead, participants were motivated to adhere to the protocol by financial compensation and the opportunity to contribute to research.
2.2.1 Assessment timeline and abstinence verification
HD and CON completed full assessments (i.e., interviews and fMRI sessions) pre- and post-monitored abstinence. HD were enrolled within 2 weeks of heavy episodic drinking for the baseline assessment and then reassessed at the end of 4–5 weeks of monitored abstinence. CON were studied at the same intervals. The mean interval between baseline and follow-up MRI sessions was 30.5 days.
HD and CON participants’ abstinence was monitored via daily self-reports and through breath samples using a breathalyzer and urine samples obtained 3–4 times per week for the duration of their participation for a urine toxicology test to confirm abstinence from alcohol and other drugs. To prevent tampering with the urine sample, adulteration strips were used to detect diluted or altered urine, sample temperature was taken immediately upon collection, and the labeled urine sample was kept in view of research personnel at all times. Urine samples were submitted to a 13-panel drug screen, including amphetamine, barbiturates, benzodiazepines, cocaine, marijuana, MDMA (ecstasy), methadone, opiates, oxycodone, phencyclidine, and tricyclic antidepressants. LC/MS/MS analysis confirmed two alcohol metabolites, ethyl glucuronide and ethyl sulfate, which are typically detectable up to 24–72 hours following alcohol use (Jatlow et al., 2014; Wurst et al., 2006). Teens provided a urine sample every Sunday during enrollment to capture potential weekend drinking and then 2–3 additional occasions per week, which were randomly assigned and shifted based on availability of the teen so that no more than 72 hours passed between sample collection.
2.2.2 Imaging
Participants were scanned in a 3T GE CXK4 short bore Excite-2 MR system with an 8-channel phase-array head coil. A sagittally acquired spoiled gradient recalled 3d T1-weighted sequence (field of view [FOV] 24 cm, 256 × 256 × 192 matrix, .94 × .94 × 1 mm voxels, 176 slices, repetition time [TR] = 20 ms, echo time [TE] = 4.8 ms; flip angle 12°, 7:26 minutes) assisted with registration and anatomic standardization. fMRI BOLD response contrast during the alcohol cue reactivity task was measured with 3 T2*-weighted axially acquired echo-planar imaging sequences (FOV = 24 cm, 64 × 64 matrix, 3.75 × 3.75 × 3.8 mm voxels, 32 slices, TE = 30 ms, TR = 2000ms, flip angle 90°, ramped bandwidth 250 KHz; total acquisition time 8:32 minutes). Task stimuli were back projected from a laptop to a screen at the foot of the scanner bed visible via an angled mirror attached to the head coil. Picture ratings and reaction time data were logged with a response box designed for MRI studies (Current Designs, Pittsburgh, PA). Field map acquisitions employed 2 different echo times to unwarp field inhomogeneities and signal.
2.3 Measures
2.3.1 Substance use history
Teens provided self-report substance use history on the Customary Drinking and Drug Use Record (CDDR; Brown et al., 1998) and on a 45-day Timeline Follow Back (TLFB; Sobell & Sobell, 1992), including lifetime and recent tobacco, alcohol, and other drug use, withdrawal symptoms, DSM-IV abuse and dependence criteria, and other alcohol-related social and physiological problems at entry into the study.
2.3.2 Personality, psychopathology and development
At baseline, adolescent participants and their parents independently completed structured interviews to assess demographics, social and academic functioning, and personal history of Axis I psychiatric disorders to confirm eligibility for the study (Diagnostic Interview Schedule for Children; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000). In addition, participants completed the BIS/BAS (Carver & White, 1994) at baseline to characterize their incentive sensitivity. The BIS/BAS produces one index of behavioral inhibition, and 3 subscale scores under behavioral activation: Fun Seeking, Drive, and Reward Responsiveness. To characterize development beyond chronological age, the Pubertal Development Scale (PDS; Petersen, Crockett, Richards, & Boxer, 1988) was completed. Participants rated their pubertal development on a 4-point scale (1 = no development, 2 = development had barely begun, 3 = development was definitely under way, 4 = development was complete) on three characteristics (growth spurt in height, body hair, skin change/pimples). Boys rated development of facial hair and voice deepening, and girls rated breast development on the same scale, and girls indicated whether or not they had begun to menstruate (binary rating). A total score was derived based on the sum of the PDS items.
2.3.3 Alcohol expectancy and craving
At baseline, participants completed an abbreviated version of the Alcohol Expectancies Questionnaire – Adolescent (AEQ-A; Christiansen, Goldman, & Inn, 1982), to index beliefs about the anticipated effects of alcohol. This abbreviated version is made up of 21 items that load on seven expectancy factors: (1) Global positive changes, (2) Changes in social behavior, (3) Improved cognitive and motor abilities, (4) Sexual enhancement, (5) Cognitive and motor impairment, (6) Increased arousal, (7) Relaxation and tension reduction. Participants also rated alcohol craving for each day of the study on a 0 (“none”) to 4 (“very high”) scale. They were instructed to rate peak craving for each day. Average craving over the course of the month and maximum craving were computed from these ratings.
2.3.4 Alcohol Pictures Task
The alcohol pictures cue reactivity task (Pulido, Brown, Cummins, Paulus, & Tapert, 2010) was designed for neuroimaging studies and consisted of 22 stimuli in each of 4 categories: alcohol, non-alcohol, alcohol active control, and non-alcohol active controls. The active control conditions was made up of shuffled versions of the original images to control for color and brightness (see Pulido et al. 2010) for a description of the task development). The task includes 220 trials and lasts a total of 8 minutes 32 seconds. The visual stimuli from two primary conditions, alcohol and non-alcohol beverages, were presented 4 times each for 88 trials in each condition, while the shuffled pictures were presented once each. The task began with a 12-second fixation (rest) period. Each trial lasted 2000ms with an image presented for 750ms followed by a 1250ms inter-stimulus interval (blank screen). A total of 1 minute of fixation period was collected by presenting 15 fixation screens (i.e., a centered cross-hair) for 2, 4, or 6 seconds interspersed throughout the task. Participants were asked to respond whether they “Like”, feel “Neutral”, or “Dislike” each image within 2000ms using 3 buttons on a MRI-compatible button box. A practice task with non-beverage pictures was administered prior to the scanning session to ensure understanding of the task requirements.
2.4 Data Processing and Analysis
2.4.1 Image processing
Data were processed and analyzed using Analysis of Functional NeuroImages (AFNI; Cox, 1996). Motion in time series data were corrected by registering each acquisition to the maximally stable base volume with an iterated least squares algorithm (Cox & Jesmanowicz, 1999) to estimate 6 multi-directional movement parameters (Bandettini, Jesmanowicz, Wong, & Hyde, 1993; Friston, Williams, Howard, Frackowiak, & Turner, 1996). Task-related movement was evaluated by correlating the reference vector with these motion parameters for each participant’s data. Data sets with significant task-related motion were excluded from analyses. The data were also visually inspected for movement artifacts, and each repetition containing movement was removed. Any participant with >15% repetitions discarded was excluded from the final analyses (n = 6).
A reference vector representing the changing stimuli conditions over the course of the task was used to deconvolve the time series data (Ward, 2000), which was then convolved with a hemodynamic response model (Bandettini et al., 1993). This process yielded a functional image in which every voxel contained a fit coefficient representing the change in signal across stimulus conditions. Standardization transformations were made for each high-resolution anatomical image (Talairach & Tournoux, 1988), and then were resampled into 3 mm3 isotropic voxels, and spatially smoothed using a 5 mm full-width half-maximum Gaussian filter. The contrast of interest for analyses was BOLD response to alcohol pictures relative to non-alcohol beverage pictures. A greater BOLD response contrast (i.e., fit coefficient > 0) was interpreted as more cue reactivity to alcohol images compared to non-alcohol beverage pictures.
2.4.2 Functional ROI Analyses
Masks for the a priori ROIs associated with reward processing and decision-making were created using the Talairach atlas in AFNI. Each ROI was created bilaterally, yielding 10 total ROIs (bilateral NA, DSGP, OFC, ACC and DLPFC). The NA and ACC regions were selected using the regions pre-defined within AFNI, while the DSGP region consisted of the caudate, putamen, and globus pallidus, the OFC region included Brodmann’s areas 11, 12, and 47, and the DLPFC consisted of Brodmann’s areas 9 and 46. First, differences between HD and CON at baseline were assessed using the AFNI program 3dttest++. AlphaSim (Ward, 2000) was used to determine the number of contiguous voxels required within each ROI to meet an α < .05 criterion across the entire ROI. That is, instead of simply extracting data for the entire ROI, we statistically tested for clusters within each ROI that differed between HD and CON. Second, subclusters within ROIs showing significant BOLD percent change differences between HD and CON at baseline were used to create “functional ROIs” to extract precisely the same volumes from the baseline and follow-up scans. The functional ROIs were, therefore, used to extract the fMRI data from both the baseline and follow-up scan sessions for subsequent repeated measures ANOVAs outside of AFNI.
Since the design of the current study examined between-subject differences in BOLD response rather than simply within subject differences, it is possible that regions other than those identified in our ROIs could explain additional variance (cf., Schacht et al., 2013). Therefore, an exploratory whole-brain analysis using 3dttest++ was conducted to determine additional brain regions that show BOLD percent change response differences between groups at baseline. AlphaSim (Ward, 2000) was again used to determine the number of contiguous voxels needed to meet the criterion level of α < .01 across each cluster evaluated (30 voxels; 810 μL). In the same way as the ROI analysis, significant clusters from this analysis were then used as functional ROIs to extract the same volumes from baseline and follow-up scan data.
The data were extracted and transferred to SPSS Statistics 21 (IBM, 2013), where subsequent analyses were conducted. Group × Time repeated measures ANOVAs were conducted on BOLD percent change contrast values (baseline and follow-up). Independent samples t-tests (α = .05, 2-tailed) evaluated whether key demographic variables (i.e., age and pubertal development) differed between groups at baseline. Pearson correlations examined the relationship between BOLD response coefficients and other variables of interest for both time points.
3. RESULTS
3.1 Baseline Characteristics
The fMRI data revealed that HD exhibited greater cue reactivity than CON in subclusters of 3 of the a priori ROIs at baseline: DSGP bilaterally and in the left ACC (clusters > 2052 μL, corrected p < .05; see Table 2). The exploratory, whole-brain analysis revealed an additional 3 functional ROIs in which HD exhibited more BOLD response to alcohol vs. non-alcohol beverage pictures when compared to CON: (a) left cerebellum; (b) right cerebellum; and (c) left parahippocampal gyrus extending to thalamus/substantia nigra (clusters > 1134 μL, corrected p < .01; see Table 2). The significant subclusters of the ROIs as well as the areas identified by the whole brain analysis served as functional ROIs to extract data from the follow-up MRI for subsequent repeated measures ANOVAs testing whether differences in BOLD response at baseline persisted after a month of monitored abstinence.
Table 2.
Regions of BOLD cue reactivity difference between heavy drinkers (n=22) and normal controls (n=16).
| Anatomical Region | Brodmann Area | Volume μL | Talairach coordinates a
|
||
|---|---|---|---|---|---|
| x | y | z | |||
| L anterior cingulate cortex | 24, 32, 33 | 2052 | 4.5 | −40.5 | −6.5 |
| R dorsal striatum and globus pallidus | - | 2187 | −28.5 | 7.5 | −3.5 |
| L dorsal striatum and globus pallidus | - | 4050 | 13.5 | 1.5 | −0.5 |
|
| |||||
| L Cerebellum | - | 4752 | 10.5 | 76.5 | −21.5 |
| R Cerebellum | - | 4077 | −31.5 | 49.5 | −18.5 |
| Parahippocampal gyrus, extending to thalamus/substantia nigra | 36 | 1134 | 10.5 | 28.5 | −3.5 |
Notes: R = right; L = left
Coordinates refer to location of peak group difference at baseline
As expected, HD reported significantly higher alcohol expectancies than CON in all categories except cognitive and motor slowing (see Table 3). Behavioral data from the Alcohol Pictures Task indicated that HD liked a significantly larger proportion of the alcohol pictures than CON (55% vs. 24%, p < .01), though reaction times did not differ between groups (676 ms vs. 629 ms, ns). CON reported no alcohol craving throughout the study (i.e., all CON rated “none” on each day), while only 3 HD reported no craving throughout the study1. A total of 10 HD reported experiencing “high” or “very high” craving at some point during the study.
Table 3.
Alcohol Expectancies for heavy drinking (HD) and control (CON) groups
| HD (n=22) M(SD) |
CON (n=16) M(SD) |
|
|---|---|---|
| Global positive changes | 8.6 (2.6)** | 6.1 (2.9) |
| Changes in social behavior | 11.1 (2.2)** | 7.1 (2.5) |
| Improved cognitive and motor abilities | 5.0 (1.4)* | 3.8 (1.2) |
| Sexual enhancement | 11.1 (1.9)** | 8.1 (2.8) |
| Cognitive and motor impairment | 13.1 (1.2) | 12.3 (1.7) |
| Increased arousal | 11.1 (2.1)** | 8.7 (3.1) |
| Relaxation and tension reduction | 11.2 (2.2)* | 9.5 (2.6) |
Note:
p<.05;
p<.01.
3.2 Group × Time Effects
Main effects of group were present for three of the functional ROIs (e.g., bilateral DSGP and in the left ACC); while main effects of time were not significant (ps > .05). Right and left DSGP, showed no interaction effects, and the left ACC showed a marginal Group × Time interaction (p = .07; η2 = .09; see Table 4). This marginal difference was probed with follow-up t-tests, which indicated that HD and CON groups differed at baseline with HD exhibiting greater BOLD response, but the difference between HD and CON decreased to non-significant levels at follow-up (see Figure 1).
Table 4.
BOLD Percent Signal Change Contrast between Alcohol and Neutral Pictures for Baseline and Follow-up
| Anatomical Region | Heavy Drinkers BOLD Value | Controls BOLD Value | t-test p-values | η2 of Group × Time Interaction |
|---|---|---|---|---|
| L ACC BL | 1.56 (2.2) | −2.51 (4.2) | <.001 | .09a |
| L ACC FU | 1.33 (2.7) | −0.37 (2.7) | .07 | |
| R DSGP BL | 0.00 (1.5) | −1.60 (1.5) | .002 | ns |
| R DSGP FU | 0.11 (1.4) | −0.47 (1.3) | .21 | |
| L DS BL | 0.02 (1.5) | −1.56 (1.5) | .003 | ns |
| L DS FU | 0.02 (1.2) | −0.72 (1.3) | .08 | |
|
| ||||
| L Cerebellum BL | 1.19 (2.1) | −1.88 (1.5) | <.001 | ns |
| L Cerebellum FU | 0.96 (2.7) | −0.20 (1.8) | .15 | |
| R Cerebellum BL | 0.60 (1.8) | −1.77 (1.2) | <.001 | .14* |
| R Cerebellum FU | −0.48 (2.6) | −0.84 (1.4) | .62 | |
| Parahip. BL | 1.00 (1.9) | −1.50 (1.1) | <.001 | ns |
| Parahip. FU | −0.02 (2.5) | −0.78 (2.2) | .33 | |
Notes: R = right; L = left; BL = baseline; FU = follow-up. ACC = anterior cingulate cortex; DSGP = dorsal striatum and globus pallidus; Parahip. = Parahippocampal gyrus extending to thalamus/substantia nigra. Values are Mean(SD). Positive BOLD values indicate greater response to alcohol pictures vs. neutral beverage pictures; negative BOLD values indicate less response to alcohol pictures compared to neutral beverages.
p = .07;
p < .05
Figure 1.
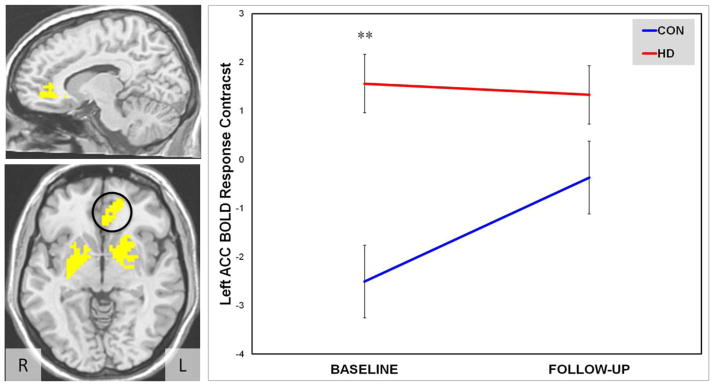
Left ACC BOLD response contrast depicting Group × Time interaction between Heavy Drinkers (HD, n=22) and controls (CON, n=16) from baseline to 4-week follow-up.
Note: Axial slice depicts masks of three functional ROIs (left ACC, left and right DSGP) in radiological view with left ACC delimited. Sagittal slice depicts only the left ACC.
** p < .01 for Group main effect.
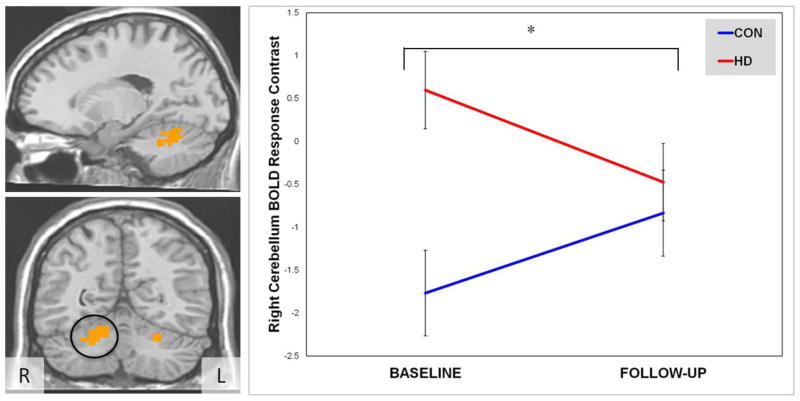
In the whole brain analysis, there were main effects of group for each of the 3 regions (bilateral cerebellum and left parahippocampal gyrus extending to thalamus/substantia nigra), but main effects of time were not significant (ps > .05). A Group × Time interaction was present in the right cerebellum (F(1,36) = 5.06, p < .05; η2 = .14; see Figure 2). Again, follow-up t-tests revealed that significant differences between HD and CON at baseline diminished to non-significant levels after the one month of monitored abstinence (see Table 4). In both instances, the interaction effects were driven by a decrease in the BOLD response contrast difference between HD and CON.
Figure 2.
Right cerebellum BOLD response contrast depicting Group × Time interaction between Heavy Drinkers (HD, n=22) and controls (CON, n=16) from baseline to 4-week follow-up.
Note: Sagittal and coronal slices depict significant right cerebellum cluster from whole brain analysis, with right cerebellum delimited in coronal slice.
* p < .05 for Group × Time interaction.
3.3 Comparisons to other measures
Additional variables that may be related to alcohol cue reactivity as indexed by BOLD response were also examined to determine if relationships changed over the course of the study. The percentage of alcohol pictures rated as “like” was positively correlated with left ACC BOLD response at baseline (r = .40, p < .05), and the percentage of alcohol pictures rated as “disliked” was negatively correlated with left ACC and left DSGP BOLD response at baseline (r = −.45 and −.35, ps < .05), but these relationships were not significant at follow-up. In addition, average alcohol picture rating was significantly correlated with BOLD response in right cerebellum at baseline (r = .34, p < .05), but no significant correlations existed between ratings and BOLD response in the functional ROIs at follow-up.
HD who reported higher average alcohol craving over the course of the study tended to show greater BOLD response to alcohol pictures in the left ACC ROI at follow-up (r = .51, p < .05). In contrast, HD who reported a higher maximum alcohol craving through the month rated an increased proportion of alcohol pictures as “disliked” at follow-up (r = .57, p < .05).
Higher positive alcohol expectancies were related to greater BOLD response to alcohol pictures in a number of alcohol expectancy subscales at baseline (see Table 5). For example, Sexual Enhancement expectancies were positively correlated with BOLD response in the left ACC and in all three areas identified by the whole brain analysis, and Changes in Social Behavior expectancies were positively correlated with left ACC and left and right cerebellum ROIs. These relationships decreased to non-significant levels after 1 month, perhaps indicating decreased salience of alcohol expectancies in responses to alcohol cues after a month of abstaining from alcohol among HD participants.
Table 5.
Intercorrelations between alcohol expectancies at baseline and BOLD responses at baseline and follow-up
| Glo. Pos. | Soc. | Cog. + | Sex + | Cog. − | Arouse | Relax | |
|---|---|---|---|---|---|---|---|
| L ACC – BL | .22 | .48* | .12 | 35* | .03 | .48* | .36* |
| L ACC – FU | .07 | .24 | .19 | .25 | .25 | .17 | .14 |
| R DSGP – BL | .19 | .18 | .44* | .18 | .16 | .20 | .08 |
| R DSGP – FU | .30 | .14 | .31 | .20 | .27 | .21 | .12 |
| L DS – BL | .11 | .24 | .09 | .28 | .07 | .22 | .02 |
| L DS – FU | .33* | .18 | .44* | .18 | .16 | .20 | .08 |
|
| |||||||
| L Cerebellum – BL | .30 | .42* | .21 | .54* | .18 | .56* | .31 |
| L Cerebellum – FU | .36* | .08 | .25 | .06 | −.09 | .18 | .11 |
| R Cerebellum – BL | .15 | .36* | .02 | .34* | .24 | .29 | .11 |
| R Cerebellum – FU | .14 | .08 | .18 | .03 | .11 | .09 | −.05 |
| Parahip. – BL | .13 | .31 | .12 | .33* | .22 | .35* | .14 |
| Parahip. – FU | .14 | .14 | .35* | .04 | .07 | .11 | −.01 |
Notes: R = right; L = left; BL = baseline; FU = follow-up; ACC = anterior cingulate cortex; DSGP = dorsal striatum and globus pallidus; Parahip. = Parahippocampal Gyrus extending to thalamus/substantia nigra; Glo. Pos. = Global Positive Changes; Soc. = Changes in Social Behavior; Cog. + = Improved Cognitive and Motor Abilities; Sex + = Sexual Enhancement; Cog. – = Cognitive and Motor Impairment; Arouse = Increased Arousal; Relax = Relaxation and Tension Reduction.
p < .05
4. DISCUSSION
The current study investigated the neural substrates underlying alcohol cue reactivity in adolescent heavy drinkers (HD) compared to non-drinking controls (CON), and whether a period of monitored abstinence from alcohol would decrease cue reactivity differences between groups. Results highlighted differences between the groups in brain regions associated with reward processing and decision making at baseline that largely diminished after the month of abstinence. HD showed greater BOLD response to alcohol pictures in the left ACC and bilateral DSGP, as well as in areas within the left and right cerebellum and a region spanning from the parahippocampal gyrus to the thalamus/substantia nigra, indicating that heavy drinking adolescents exhibit heightened cue reactivity prior to the onset of a diagnosable alcohol use disorder. The group differences present at baseline diminished to non-significant levels after a month of abstinence from alcohol.
The brain regions identified in the current study coincide with several areas that have been associated with cue reactivity in adult samples with alcohol use disorders (e.g., ACC; Schacht et al., 2013), and across several other substances (e.g., cerebellar regions; Kühn and Gallinat, 2011). The current study was designed to identify brain regions that differed functionally between HD and CON when observing alcohol cues, and does not necessarily reflect the areas of peak activation or cue reactivity. The results, therefore, could serve as areas of interest for examination in future studies of adolescents as these areas may be developmentally important to maintenance or exacerbation of substance use.
The differences observed between HD and CON responses systematically decrease after the month of monitored abstinence, which could result from a number of underlying factors. First, as seems to be the case in the right cerebellum, HD could be exhibiting decreased BOLD to alcohol cues in the task. This difference could be signaling decreased cue reactivity in fronto-cerebellar connections that have been shown to be altered in those at risk for alcohol use (Herting, Fair, & Nagel, 2011) and in adult alcoholics (Rogers, Parks, Nickel, Katwal, & Martin, 2012). Thus, the baseline differences in the right cerebellum may reflect some altered appetitive cue processing that diminishes over the course of the month of abstaining from alcohol.
Another factor that may be important for the present results, is the possibility that normal controls exhibited some habituation to the alcohol stimuli, while the heavy drinkers processed them in a more consistent manner over time. This could explain results in the left ACC, where drinkers’ BOLD response did not decrease over time (see Figure 2). The ACC is implicated in a number of roles including reward learning and decision making (Bush et al., 2002; Kennerley, Behrens, & Wallis, 2011; Kennerley, Walton, Behrens, Buckley, & Rushworth, 2006), which could signal that HD are not exhibiting the same level of habituation or learning from repeated exposure to the alcohol pictures task. This interpretation could be supported by the fact that HD who reported more craving over the course of the abstinent period exhibited higher BOLD response at follow-up. HD who reported a higher maximum alcohol craving through the month rated an increased proportion of alcohol pictures as “disliked” at follow-up (r = .57, p < .05), perhaps indicating that stronger craving during the study led to subjective aversion for alcohol related stimuli in the fMRI session despite the appetitive cue reactivity response highlighted in the left ACC (e.g., a form of “frustrative non-reward”, Cloninger, 1987).
On the other hand, the relationship between alcohol expectancies and BOLD response over time seems to support the decreased salience of alcohol cues in several brain areas, including the left ACC. That is, at baseline, individuals who reported expecting more positive outcomes from alcohol consumption exhibited greater cue reactivity to alcohol pictures, but at follow-up expectancies were largely unrelated to BOLD response (see Table 5). Therefore, it is possible that individuals habituated (i.e., exhibited decreased cue reactivity) to the alcohol cues after the one-month abstinence period because the rewarding effects of alcohol were less salient.
It is important to highlight that while the HD in the current study drank heavily, they were not treatment seeking and the monitored abstinence protocol did not involve a treatment component beyond motivational interviewing focused on protocol adherence. Thus, the changes in cue reactivity could be attributed to decreased salience of alcohol cues following ceased alcohol involvement during the course of the study. Cue reactivity is assumed to develop due to continued pairing of cues with alcohol consumption, and in non-dependent users like the current HD sample, the association between cues and consumption may be degraded simply by avoiding alcohol for a one-month period. Unlike studies examining the effect of targeted treatment on cue reactivity that have employed forms of cue retraining in alcoholics (Braus et al., 2001; Vollstädt-Klein et al., 2011), the current study depicts changes that occur without targeted intervention.
The number of HD participants who were unable to complete the protocol was a limitation of the current study. While on the one hand, getting over 80% of a heavy drinking adolescent sample to avoid alcohol consumption for a month is a success, 15% (n=4) of the HD sample did not complete the protocol due to the restrictions on alcohol consumption and an additional HD participant dropped out without identified use. This proportion of HD who did not complete the protocol could serve as an interesting comparison group in subsequent studies if they were followed throughout the month despite breaking the protocol. Given that cue reactivity has been shown to relate to subsequent relapse in alcoholic samples (Cooney et al., 1997; Heinz et al., 2009), following these individuals and comparing their cue reactivity to the abstaining HDs at the end of the one-month follow-up could provide additional insight into factors associated with continued drinking in adolescents.
Several factors limit the generalizability of the current results. First, while powerful enough to detect differences between the groups, the study contained relatively modest sample sizes and may have been underpowered to detect additional differences between groups or over time, and particularly for interaction effects. The limited time course of the study (~30 days) may also have been insufficiently lengthy to detect some changes, and since there was no follow-up beyond the 1 month period it is unclear whether or how long the differences at follow-up persisted or whether cue reactivity was reinstated if and when the HD began drinking again after study completion. In addition, there are a number of other factors that could affect cue reactivity and propensity for relapse that were not measured in the current study, including level of exposure to alcohol in teen’s environment in their homes, homes of peers, or through media exposure (Wechsler, Kuo, Lee, & Dowdall, 2000; Komro, Maldonado-Molina, Tobler, Bonds, & Muller, 2007; Anderson, de Bruijn, Angus, Gordon, & Hastings, 2009). Each of these factors will be important for future studies on cue reactivity in adolescent heavy drinkers to consider.
4.1 Conclusion
In summary, the present study identified a number of brain regions associated with increased cue reactivity in non-dependent heavy drinking adolescents compared to non-drinking controls. Furthermore, results indicate that a period of one-month of abstaining from alcohol consumption may alter cue reactivity in adolescents. These results suggest the malleability of adolescent brains and their cue reactivity profiles, and may provide insight for development of prevention and intervention strategies targeting non-dependent adolescent drinkers. Future studies with additional power may also be able to evaluate whether additional components (e.g., attentional retraining) exert an additive effect to the decreases in cue reactivity seen with monitored abstinence alone.
Highlights.
Adolescent heavy drinkers’ BOLD cue-reactivity was compared to controls
Adolescents were followed for 1 month of monitored abstinence and then reassessed
Heavy drinkers’ greater BOLD response initially decreased after alcohol free month
Acknowledgments
Funding Support: R21 AA017321 (PI: Brown), T32 AA013525 (PI: Riley; Fellow: Brumback), U01 AA021692 (PI: Tapert), U01 AA02169 (PI: Brown), R01 AA13419 (PI: Tapert), R21 AA19748 (PI: Pulido), F32 AA021610 (PI: Squeglia), and F32 DA032188 (PI: Jacobus)
Financial Support
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Numbers: R21 AA017321 (PI: Brown), T32 AA013525 (PI: E. Riley; Fellow: Brumback), U01 AA021692 (PI: Tapert), U01 AA021695 (PI: Brown), R01 AA13419 (PI: Tapert), F32 AA021610 (PI: Squeglia), and the National Institute on Drug Abuse: F32 DA032188 (PI: Jacobus). The National Institutes of Health had no role in the study design, collection, analysis or interpretation of the data. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Special thanks to the Adolescent Brain Imaging Project lab and the participating schools in the San Diego Unified School District and their families.
Footnotes
Craving data were not available for 3 of the 22 HD due to experimenter error and participant non-compliance (e.g., attempting to report daily craving retrospectively over a week period).
Contributors
T. Brumback conducted the statistical analyses and wrote the first draft of the manuscript. L. Squeglia & J. Jacobus provided guidance and feedback on the statistical analyses and on the written manuscript. C. Pulido conducted the initial analyses of the fMRI data and provided editorial comments on the written manuscript. S. Brown & S. Tapert designed the study and wrote the protocol and provided significant editorial on the manuscript. All authors contributed to and have approved the final manuscript.
Conflicts of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson P, de Bruijn A, Angus K, Gordon R, Hastings G. Impact of Alcohol Advertising and Media Exposure on Adolescent Alcohol Use: A Systematic Review of Longitudinal Studies. Alcohol and Alcoholism. 2009;44(3):229–243. doi: 10.1093/alcalc/agn115. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional mri of the human brain. Magnetic Resonance in Medicine. 1993;30(2):161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Hames KA, Blanco CR, Nixon SJ, Tivis LJ. Visuospatial perception, construction and memory in alcoholism. Journal of Studies on Alcohol. 1996;57(2):136–143. doi: 10.15288/jsa.1996.57.136. [DOI] [PubMed] [Google Scholar]
- Bekman NM, Winward JL, Lau LL, Wagner CC, Brown SA. The Impact of Adolescent Binge Drinking and Sustained Abstinence on Affective State. Alcoholism: Clinical and Experimental Research. 2013;37(8):1432–1439. doi: 10.1111/acer.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grüsser S, Hermann D, Ruf M, Flor H, Heinz A. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. Journal of Neural Transmission. 2001;108(7):887–894. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- Brown SA, Anderson KG, Schulte MT, Sintov ND, Frissell KC. Facilitating youth self-change through school-based intervention. Addictive Behaviors. 2005;30(9):1797–1810. doi: 10.1016/j.addbeh.2005.07.003. doi: http://dx.doi.org/10.1016/j.addbeh.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcoholism-Clinical and Experimental Research. 2000;24(2):164–171. doi: 10.1097/00000374-200002000-00005. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proceedings of the National Academy of Sciences. 2002;99(1):523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67(2):319. [Google Scholar]
- Christiansen BA, Goldman MS, Inn A. Development of alcohol-related expectancies in adolescents: Separating pharmacological from social-learning influences. Journal of Consulting and Clinical Psychology. 1982;50(3):336–344. doi: 10.1037//0022-006x.50.3.336. doi: http://dx.doi.org/10.1037/0022-006X.50.3.336. [DOI] [PubMed] [Google Scholar]
- Cloninger C. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236(4800):410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. Journal of Abnormal Psychology. 1997;106(2):243–250. doi: 10.1037//0021-843x.106.2.243. doi: http://dx.doi.org/10.1037/0021-843X.106.2.243. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. doi: http://dx.doi.org/10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42(6):1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Ferrett HL, Carey PD, Thomas KGF, Tapert SF, Fein G. Neuropsychological performance of South African treatment-naive adolescents with alcohol dependence. Drug and Alcohol Dependence. 2010;110(1–2):8–14. doi: 10.1016/J.Drugalcdep.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RSJ, Turner R. Movement-Related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MlN, Ruf M, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175(3):296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grüsser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addiction Biology. 2009;14(1):108–118. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naïve youth with a family history of alcoholism. Neuroimage. 2011;54(4):2582–2589. doi: 10.1016/j.neuroimage.2010.10.030. doi: http://dx.doi.org/10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihssen N, Cox WM, Wiggett A, Fadardi JS, Linden DEJ. Differentiating Heavy from Light Drinkers by Neural Responses to Visual Alcohol Cues and Other Motivational Stimuli. Cerebral Cortex. 2011;21(6):1408–1415. doi: 10.1093/cercor/bhq220. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF. Neurotoxic Effects of Alcohol in Adolescence. Annual Review of Clinical Psychology. 2013;9(1):703–721. doi: 10.1146/annurev-clinpsy-050212-185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatlow PI, Agro A, Wu R, Nadim H, Toll BA, Ralevski E, O’Malley SS. Ethyl Glucuronide and Ethyl Sulfate Assays in Clinical Trials, Interpretation, and Limitations: Results of a Dose Ranging Alcohol Challenge Study and 2 Clinical Trials. Alcoholism: Clinical and Experimental Research. 2014:n/a–n/a. doi: 10.1111/acer.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use: 2013 Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan; 2014. [Google Scholar]
- Kennerley SW, Behrens TEJ, Wallis JD. Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci. 2011;14(12):1581–1589. doi: 10.1038/nn.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TEJ, Buckley MJ, Rushworth MFS. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9(7):940–947. doi: 10.1038/nn1724. doi: http://www.nature.com/neuro/journal/v9/n7/suppinfo/nn1724_S1.html. [DOI] [PubMed] [Google Scholar]
- Komro KA, Maldonado-Molina MM, Tobler AL, Bonds JR, Muller KE. Effects of home access and availability of alcohol on young adolescents’ alcohol use. Addiction. 2007;102(10):1597–1608. doi: 10.1111/j.1360-0443.2007.01941.x. [DOI] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs – a quantitative meta-analysis of cue-reactivity brain response. European Journal of Neuroscience. 2011;33(7):1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Montgomery C, Fisk JE, Murphy PN, Ryland I, Hilton J. The effects of heavy social drinking on executive function: a systematic review and meta-analytic study of existing literature and new empirical findings. Human Psychopharmacology-Clinical and Experimental. 2012;27(2):187–199. doi: 10.1002/Hup.1268. [DOI] [PubMed] [Google Scholar]
- Moss HB, Kirisci L, Gordon HW, Tarter RE. A Neuropsychologic Profile of Adolescent Alcoholics. Alcoholism-Clinical and Experimental Research. 1994;18(1):159–163. doi: 10.1111/J.1530-0277.1994.Tb00897.X. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29(2):393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Park MS, Sohn JH, Suk JA, Kim SH, Sohn S, Sparacio R. Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol and Alcoholism. 2007;42(5):417–422. doi: 10.1093/alcalc/agl117. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/bf01537962. [DOI] [PubMed] [Google Scholar]
- Pulido C, Brown SA, Cummins K, Paulus MP, Tapert SF. Alcohol cue reactivity task development. Addictive Behaviors. 2010;35(2):84–90. doi: 10.1016/j.addbeh.2009.09.006. doi: http://dx.doi.org/10.1016/j.addbeh.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers BP, Parks MH, Nickel MK, Katwal SB, Martin PR. Reduced Fronto-Cerebellar Functional Connectivity in Chronic Alcoholic Patients. Alcoholism: Clinical and Experimental Research. 2012;36(2):294–301. doi: 10.1111/j.1530-0277.2011.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Sirota AD, Niaura RS, Colby SM, Abrams DB. Cue reactivity as a predictor of drinking among male alcoholics. Journal of Consulting and Clinical Psychology. 1994;62(3):620–626. doi: 10.1037//0022-006x.62.3.620. doi: http://dx.doi.org/10.1037/0022-006X.62.3.620. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addiction Biology. 2013;18(1):121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Stability of fMRI striatal response to alcohol cues: A hierarchical linear modeling approach. Neuroimage. 2011;56(1):61–68. doi: 10.1016/j.neuroimage.2011.02.004. doi: http://dx.doi.org/10.1016/j.neuroimage.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, Differences From Previous Versions, and Reliability of Some Common Diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Martin ED, Wood PK, Rutledge PC. Alcohol use disorders and neuropsychological functioning in first-year undergraduates. Experimental and Clinical Psychopharmacology. 1997;5(3):304–315. doi: 10.1037/1064-1297.5.3.304. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Theime; 1988. [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addictive Behaviors. 2004;29(1):33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of general psychiatry. 2003;60(7):727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Mezzich AC, Hsieh YC, Parks SM. Cognitive capacity in female adolescent substance-abusers. Drug and Alcohol Dependence. 1995;39(1):15–21. doi: 10.1016/0376-8716(95)01129-m. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Bühler M, Kiefer F. Effects of Cue-Exposure Treatment on Neural Cue Reactivity in Alcohol Dependence: A Randomized Trial. Biological Psychiatry. 2011;69(11):1060–1066. doi: 10.1016/j.biopsych.2010.12.016. doi: http://dx.doi.org/10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000 Retrieved from http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim.
- Wechsler H, Kuo M, Lee H, Dowdall GW. Environmental correlates of underage alcohol use and related problems of college students. American Journal of Preventive Medicine. 2000;19(1):24–29. doi: 10.1016/s0749-3797(00)00163-x. doi: http://dx.doi.org/10.1016/S0749-3797(00)00163-X. [DOI] [PubMed] [Google Scholar]
- Winward JL, Bekman NM, Hanson KL, Lejuez CW, Brown SA. Changes in Emotional Reactivity and Distress Tolerance Among Heavy Drinking Adolescents During Sustained Abstinence. Alcoholism: Clinical and Experimental Research. 2014;38(6):1761–1769. doi: 10.1111/acer.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst FM, Dresen S, Allen JP, Wiesbeck G, Graf M, Weinmann W. Ethyl sulphate: a direct ethanol metabolite reflecting recent alcohol consumption. Addiction. 2006;101(2):204–211. doi: 10.1111/j.1360-0443.2005.01245.x. [DOI] [PubMed] [Google Scholar]