Abstract
Introduction
Intrauterine growth restriction complicates 5 - 10% of pregnancies. This study aims to test the hypothesis that Chinese herbal formula, JLFC01, affects pregnancy and fetal development by modulating the pro-inflammatory decidual micro-environment.
Methods
Human decidua from gestational age-matched elective terminations or incomplete/missed abortion was immunostained using anti-CD68 + anti-CD86 or anti-CD163 antibodies. qRT-PCR and Luminex assay measured the effects of JLFC01 on IL-1β- or TNF-α-induced cytokine expression in first trimester decidual cells and on an established spontaneous abortion/intrauterine growth restriction (SA/IUGR)-prone mouse placentae. The effect of JLFC01 on human endometrial endothelial cell angiogenesis was evaluated by average area, length and numbers of branching points of tube formation. Food intake, litter size, fetal weight, placental weight and resorption rate were recorded in SA/IUGR-prone mouse treated with JLFC01. qRT-PCR, Western blot and immunohistochemistry assessed the expression of mouse placental IGF-I and IGF-IR.
Results
In spontaneous abortion, numbers of decidual macrophages expressing CD86 and CD163 are increased and decreased, respectively. JLFC01 reduces IL-1β- or TNF-α-induced GM-CSF, M-CSF, C-C motif ligand 2 (CCL2), interferon-γ-inducible protein-10 (IP-10), CCL5 and IL-8 production in first trimester decidual cells. JLFC01 suppresses the activity of IL-1β- or TNF-α-treated first trimester decidual cells in enhancing macrophage-inhibited angiogenesis. In SA/IUGR-prone mice, JLFC01 increases maternal food intake, litter size, fetal and placental weight, and reduces fetal resorption rate. JLFC01 induces IGF-I and IGF-IR expression and inhibits M-CSF, CCL2, CCL5, CCL11, CCL3 and G-CSF expression in the placentae.
Discussion
JLFC01 improves gestation by inhibiting decidual inflammation, enhancing angiogenesis and promoting fetal growth.
Keywords: Chinese herbal medicine, decidual cells, IUGR, macrophage, spontaneous abortion
Introduction
Spontaneous abortion (SA) complicates 15% of human pregnancies. Although chromosomal anomalies account for more than 50% of SA, abnormal fetal development, maternal systemic disorders and environmental insults contribute to its incidence [1]. The mechanisms causing SA remain unknown thereby precluding the development of effective prevention and treatment. Various attempts including immunological approaches [2-6] have not significantly improved the SA-associated live birth rate. Chinese herbal medicine (CHM) has been widely used in Asia for centuries. Although studies suggest that CMH prevents SA [7], evidence is insufficient to assess the effectiveness of CHM in treating SA. First appearing in Chung-Ching Chang’s “Synopsis of Golden Chamber” about 1,800 years ago, JLFC01 is derived from a traditional Chinese formula that successfully treats blood stagnation.
Intrauterine growth restriction (IUGR) complicates 5 – 10% of pregnancies [8]. Many studies indicate that preeclampsia and IUGR [9] are initiated in the first trimester as a consequence of insufficient uteroplacental blood flow to the developing fetal-placental unit and suggest a similar origin for SA. Blastocyst-derived semi-allogeneic extravillous trophoblasts (EVTs) traverse the decidua and inner third of the myometrium and interact with resident decidual cells, decidual natural killer (NK) cells and macrophages. Invading EVTs transform uterine spiral arteries into high-capacitance vessels accompanied by expression of angiogenic factors and microvascular angiogenesis [10]. The resulting increased uterine blood flow to the intervillous space is pivotal for fetal-placental development [9, 11]. Disturbances of this environment disrupt early fetal development and elicit long-term complications in affected children.
An aberrant pro-inflammatory decidual micro-environment elicits preeclampsia, SA and IUGR. In addition to mediating spiral artery transformation, decidual macrophages are critical modulators of the immune balance at the fetal-maternal interface. The function, differentiated state and responsiveness of macrophages are governed by their micro-environment and proximity to adjacent cells [12, 13]. Generally, macrophage polarization is divided into classically (M1) and alternatively (M2) activated groups [14]. Besides bridging both innate and adaptive immunity, which defend against pathogens, decidual macrophages are important mediators of implantation, placental development and cervical ripening [15]. In early pregnancy, M2 macrophages play a key role in inducing immunotolerance of the fetal semi-allograft [15]. Tight regulation of macrophage trafficking/function plays crucial roles during placentation. Under physiological steady state, macrophages isolated from first trimester decidua are polarized toward an immunotolerant M2 phenotype [16]. By contrast, pro-inflammatory M1 polarization of macrophages is linked to adverse pregnancy outcomes [17]. Our studies indicate that potent pro-inflammatory cytokines, IL-1β and TNF-α, stimulate human first trimester decidual cells (FTDCs) to secrete several chemokines that recruit NK cells [18] and monocytes [19-21]. The decidual cells then promote macrophage differentiation toward an M1 subtype via the regulation of colony-stimulating factors [13].
The current study postulates that these decidual M1 macrophages are integral to the onset of SA and proposes to treat and/or prevent SA by counteracting the resulting pro-inflammatory decidual milieu. Initial observations determined that unlike the dominant M2 immunotolerant macrophage population of normal first trimester human decidua, the decidual macrophage population accompanying SAs displays a pro-inflammatory M1 phenotype. Complementing these in situ observations, the modulating effects of JLFC01 on: 1) IL-1β- or TNF-α-induced expression of several cytokines by FTDCs; 2) FTDCs in modulating human endometrial endothelial cell (HEEC) angiogenesis-inhibiting activity of macrophages were examined. These in vitro observations were extended to include CBA/J × DBA/2J mice, an established SA/IUGR-prone model, in which the effects of JLFC01 ingestion were assessed. An aberrant decidual pro-inflammatory micro-environment [22] can also interfere with normal fetal growth by disrupting fetal programming. The insulin-like growth factors (IGFs) and their receptors are potent regulators of protein turnover, mitogenesis and differentiation [23] and implicated in fetal-placental development [24]. Abnormal IGF expression, malfunctioning IGF receptors or defective downstream signaling pathways are proposed to contribute to the development of IUGR [25]. Thus, the effects of JLFC01 ingestion were compared on placental expression of mRNA and protein levels of IGFs and their receptors in SA/IUGR-prone mice.
Methods
Immunofluorescent staining of decidua for macrophage markers
Decidua was obtained under Institutional Review Board (IRB) approval at Mackay Memorial Hospital, Taipei, Taiwan. Gestational age (GA)-matched tissue was obtained from elective terminations of normal pregnancies between 6 to 12 weeks of gestation without uterine contraction, vaginal bleeding or evidence of fetal demise. Upon diagnosis of missed/incomplete abortion, decidual basalis was evacuated within 24h from patients without infection or systemic diseases. Serial sections of OCT-embedded specimens were immunostained with mouse anti-human CD68 (1:25, Dako, Carpinteria, CA) followed by Rhodamine–conjugated donkey anti-mouse antibody (1:50, EMD Millipore, Billerica, MA). Sections were then incubated with rabbit anti-human CD163 (1:250, Sigma-Aldrich, St. Louise, MO) or CD86 (1:200, GeneTex, Irvine, CA) followed by corresponding FITC–conjugated secondary antibody (1:100) and 4′,6′-diamidino-2-phenylindole (1:500,000, Sigma-Aldrich). Morphometric analysis of cell numbers used Axiovision 3.1 software (Carl Zeiss, Oberkochen, Germany). Five randomly selected fields from each section (three sections/tissue) were examined. Cell numbers per field (3 × 106 pixel2) were counted and calculated as the mean of 15 fields for each tissue. A total of 15 cases per group were examined.
Cell isolation and culture
FTDCs were isolated and cultured as previously described [20]. Briefly, decidua from elective termination of 6-12 weeks gestation was obtained under IRB approval at The Ohio State University (OSU) and Beth Israel Medical Center, New York, NY. Cells were purified using Ficoll-Hipaque Plus (GE Healthcare, Piscataway, NJ). CD45 staining confirmed the absence of leukocytes. Cultured FTDCs were found to be vimentin-positive and cytokeratin-negative and displayed morphological changes and enhanced prolactin and plasminogen activator inhibitor-1 as well as inhibited interstitial collagenase and stromelysin-1 expression with prolonged tissue factor expression during incubation with a progestin. Confluent FTDCs were primed with estradiol (10−8M) + medroxyprogesterone acetate (10−7M) for 7d and pre-treated with 1.25 μg/ml of a Chinese herbal formula, JLFC01, for 24h then incubated with 1 ng/ml of IL-1β or TNF-α (R&D Systems, Minneapolis, MN) with JLFC01. JLFC01 was manufactured from herbs and processed in stainless steel extractors at a low temperature (below 100°C) in order to preserve the activity of essential ingredients and generate a water decoction. This procedure follows the sequence described in canonical Chinese medicine book since the sequence of the herbs processed within a decoction determines the efficacy of the formula. The extracted liquid is then spray dried to form a powder. This procedure follows good manufacturing practice (GMP) guidelines. In addition, both the presence and levels of heavy metal and microbes are also assessed. Conditioned medium (CM) supernatants were collected. Monocytes were isolated from peripheral blood of healthy reproductive age female donors using Ficoll-Hipaque and purified using anti-CD14-paramagnetic beads according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA).
For HEECs [26], blood vessels in the endometrium obtained from hysterectomy for myomas was dissected, then, minced and digested with type VII collagenase/dispase/DNase I followed by filtration through a 70-μm cell strainer. The cells was labeled with biotinylated UEA-1 (Ulex europaeus) lectin, and then, separated from non-labeled cells by panning on activated surface/AIS MicroCELLector flasks coated with streptavidin. Cells were cultured in EBM-2 medium supplemented with 15% fetal calf serum.
Cell proliferation assay
Cell proliferation was examined using a CellTiter 96 One Solution Cell Proliferation Assay (Promega, Madison, WI). Briefly, FTDCs were treated with vehicle, 0.08, 0.16, 0.31, 0.63, 1.25, 2.5, 5 or 10 μg/ml of JLFC01 for 48h. Absorbance was detected at 490 nm after adding MTS reagent.
Luminex Assay
Luminex assays (Bio-Rad, Hercules, CA) measured GM-CSF, M-CSF, C-C motif ligand 2 (CCL2), interferon-γ-inducible protein-10 (IP-10), CCL5 and IL-8 levels in CM from FTDC cultures as well as M-CSF, CCL11, CCL2, CCL3, CCL5 and G-CSF levels in mouse placenta lysates. Data acquisition and analyses were completed with the Bio-Plex 200 system using Bio-Plex Manager Software v6. Bicinchoninic acid protein assay (Thermo scientific, Rockford, IL) measured total cell protein levels.
Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted using total RNA purification plus kit (Norgen Bioteck, ON, Canada). Reverse transcription used SuperScript™III First-Strand Synthesis System from Invitrogen. Specific primer sets for human GM-CSF, M-CSF, CCL2, IP-10, CCL5, IL8 or β-actin and mouse M-CSF, CCL11, CCL2, CCL3, CCL5, G-CSF or β-actin (Integrated DNA technologies, Coralville, IA) (Supplementary Table 1) were used for qPCR using SYBR® green-based detection. Quantitation of unknowns was determined and adjusted to quantitative expression of β-actin in the specific samples. Melting curve analysis determined the specificity of the amplified products and the absence of primer-dimer formation.
Angiogenesis assays
CM from FTDCs was treated with anti-IL-1β or anti-TNF-α neutralizing antibody (R & D Systems) followed by protein G-sepharose (Sigma-Aldrich, St. Louise, MO) treatment. Macrophages were first cultured with CM from FTDCs ± JLFC01 ± IL-1β or TNF-α for 2d and then co-cultured with HEECs for 8h. To test whether JLFC01 had a direct effect on angiogenesis, macrophages were incubated with CM from FTDCs without JLFC01 treatment for 2d followed by co-culture with HEECs pretreated ± 1.25 μg/ml JLFC01 for 8h. The culture wells were coated with growth factor-reduced Matrigel (BD Biosciences, San Jose, CA). Tube formation was assessed by measuring average area, length and number of branching points using AngioTool software (NCI) [27].
Mouse studies
Studies were performed under OSU Institutional Animal Care and Use Committee approval. Eight-week-old virgin female CBA/J mice were treated with 0.3 gm/kg body weight/d of JLFC01 dissolved in ddH2O, compared to 1 – 2 gm/d for human, based on the manufacturer’s recommendation or ddH2O by oral gavage for 7d before mated with 10-week-old male DBA/2J mice (The Jackson Laboratory, Bar Harbor, ME). Sighting a vaginal plug was designated as gestational day 0 (GD0). The treatment was continued until sacrifice on GD15. Maternal food intake, litter size, placental and fetal weight, and the numbers of fetal resorption were recorded.
Immunohistochemistry of mouse placental IGF-I & IGF-IR
Deparaffinized formalin-fixed sections were incubated with goat serum (LabVision, Fremont, California), then, rabbit anti-IGF-I (1:2,400, Aviva System Biology, San Diego, CA), Anti-IGF-IR antibody or IgG isotype (1:2,400, Cell Signaling Technology, Beverly, MA). Biotinylated goat anti-rabbit antibody (1:400, Vector Laboratories, Burlingame, CA) was added. The antigen–antibody complex was detected using a streptavidin–avidin–biotin–peroxidase kit and 3,3-Diaminobenzidine tetrahydrochloride dihydrate (Vector Laboratories) with hematoxylin counterstaining.
Statistics
The variance and normality of data from immunofluorescent staining, qRT-PCR, Luminex assay, angiogenesis assay, mouse studies, densitometry and H-scores of immunohistochemistry were first examined. Then, the statistical significance of results with equal variance was examined by t-test assuming equal variance. The results with unequal variance that either passed or failed normality test were then evaluated by t-test assuming unequal variance or the Mann-Whitney rank sum test, respectively. Generalized estimating equation assessed statistical significance of mouse food intake. A p < 0.05 was considered significant.
Results
Macrophages polarize toward M1 subtype in first trimester decidua from SA
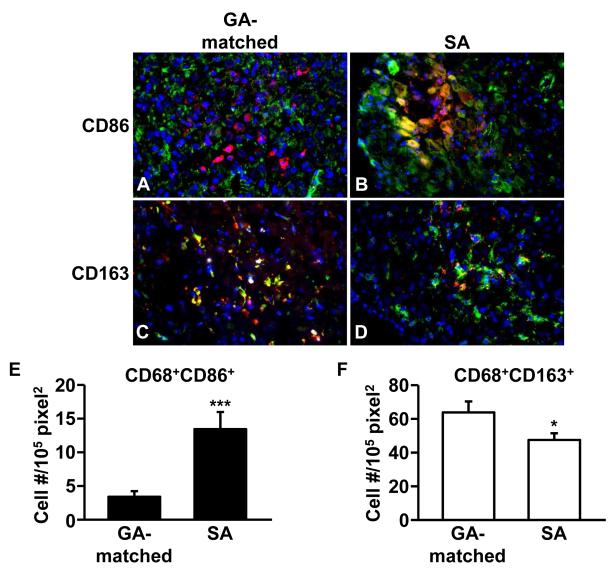
Co-localization of anti-CD68 (macrophage marker, red) with either anti-CD86 (M1 marker, green) or anti-CD163 (M2 marker, green) is indicated by yellow-orange immunofluorescence. Figure 1 indicates that FTDCs also express the CD86 and CD163. Compared to GA-matched controls (Figure 1A, C), SA-derived decidua displays significant higher numbers of CD86-positive pro-inflammatory M1 macrophages (Figure 1B, E) and lower numbers of tolerogenic CD163-positive M2 macrophages (Figure 1D, F) (n=15).
Figure 1. Macrophages polarized toward M1 subtype in the decidua from patients with SA.
Representative photomicrographs of immunofluorescent staining of M1 macrophage markers, (A, B), CD86 (green) and M2 macrophage marker, (C, D) CD163 (green), in decidua from GA-matched elective terminations and patients with SA. These tissues were co-stained with anti-CD68 antibody (red). Arrows indicate macrophages expressing CD86 or CD163 (yellow or orange). (E) CD68+CD86+ cells (F) CD68+CD163+ cells. Cell numbers/field were calculated as the mean of 15 fields (3 sections/tissue, 5 fields/section). The results are reported as mean ± SEM. n=15; * p < 0.05, *** p < 0.005; magnification: 400x; Scale bar: 50 μm.
JLFC01 inhibits IL-1β- or TNF-α-induced cytokine production in FTDCs
MTS assay shows that FTDC proliferation was suppressed by JLFC01 at concentrations greater than 1.25 μg/ml (Supplementary Figure 1). Therefore, to eliminate these potential confounding anti-proliferation effects in subsequent in vitro experiments, FTDCs were pre-incubated with 1.25 μg/ml of JLFC01 for 24h and then treated with IL-1β or TNF-α for an additional 24h.
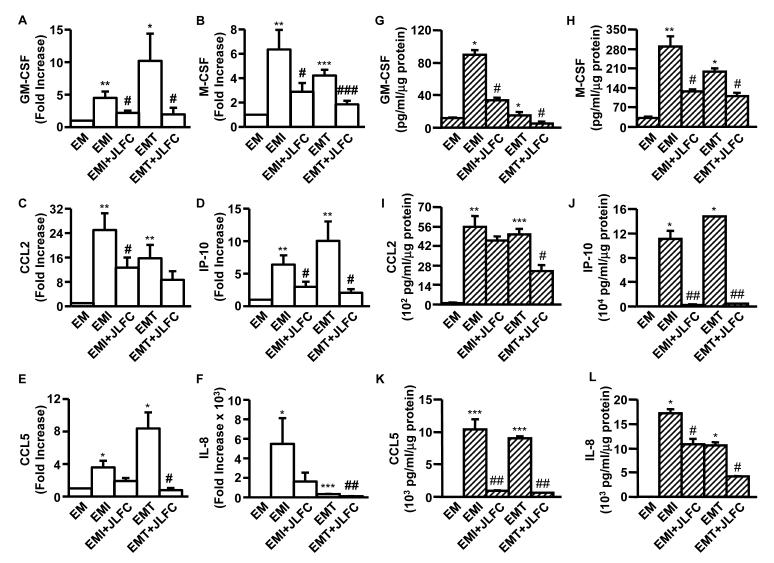
Figure 2 shows profound enhancement by either IL-1β or TNF-α of mRNA expression of several pro-inflammatory cytokines was blunted during co-incubation with JLFC01 (n=3 – 11, Supplementary Table 2). Specifically, IL-1β and TNF-α induced GM-CSF expression by 4.5-and 10.19-fold, respectively. This induction was correspondingly reduced by 51% and 81% by JLFC01 treatment (Figure 2A). M-CSF expression enhanced by IL-1β (6.34-fold) or TNF-α (4.21-fold) was suppressed by 55% and 56%, respectively, by JLFC01 (Figure 2B). Figure 2C demonstrates that IL-1β elicited a 24.99-fold and TNF-α up-regulated a 15.74-fold increase of CCL2 expression. JLFC01 treatment decreased this activation by 49% (by IL-1β) and 45% (by TNF-α) though inhibition of TNF-α-induced CCL2 expression only attained borderline statistical significance. For IP-10 expression, Figure 2D indicates a 6.39- and 10.04-fold increase by IL-1β and TNF-α, respectively. JLFC01 correspondingly inhibited IL-1β- and TNF-α-stimulated IP-10 expression by 53% and 79%. The expression of CCL5 and IL-8 was promoted in response to IL-1β by 3.58- and 5483.77-fold, respectively, whereas JLFC01 suppression of this induction only attained borderline statistical significance. However, JLFC01 significantly repressed TNF-α-induced CCL5 (8.37-fold) and IL-8 (326.60-fold) expression by 91% and 63%, respectively (Figure 2E, F).
Figure 2. LFC01 inhibits IL-1β- (EMI) or TNF-α- (EMT) induced GM-CSF, M-CSF, CCL2, IP-10, CCL5 and IL-8 expression in FTDCs.
(A – F) The mRNA expression was measured by qRT-PCR. (G – L) The protein levels of each individual cytokines in CM from leukocyte-free FTDCs were measured by multiplex Luminex assay and normalized to total cell protein. The results are reported as mean ± SEM. n=3 - 11; *: vs. EM, #: vs. EMI or EMT. * or # p < 0.05, ** or ## p < 0.01, *** or ### p < 0.005
In parallel incubations, protein expression levels of GM-CSF, M-CSF, CCL2, IP-10, CCL5 and IL-8 were measured by Multiplex assays (Figure 2G – L, n=3 – 11, Supplementary Table 2). In FTDCs, IL-1β induced GM-CSF, M-CSF, CCL2, IP-10, CCL5 and IL-8 production by 7.5-, 8.78-, 52.9-, 734.9, 545.9- and 186.2-fold, respectively. JLFC01 suppressed this induction by 38%, 98%, 62%, 56%, 17% and 91%, respectively. Similarly, GM-CSF, M-CSF, CCL2,, IP-10, CCL5 and IL-8 production was up-regulated by TNF-α by 1.3-, 6-, 47.6-, 982-, 474- and 115-fold with JLFC01 treatment suppressing this enhancement by 69%, 544%, 52%, 97%, 93% and 61%, respectively.
JLFC01 suppresses the activity of IL-1β- or TNF-α-treated FTDCs in enhancing angiogenesis-inhibiting activity of macrophages
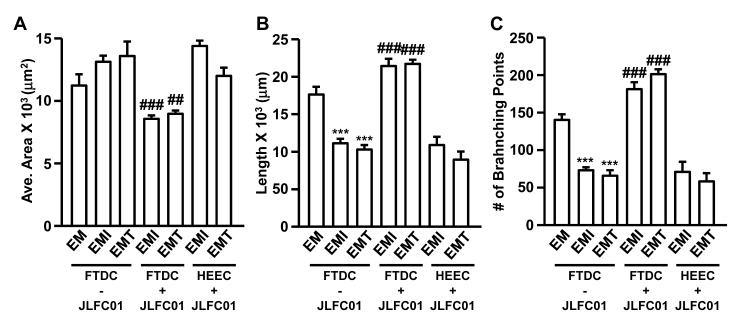
To test potential interactions among different cell types, macrophage-regulated angiogenesis was determined by first incubating macrophages with CM from IL-1β- or TNF-α-stimulated FTDCs ± JLFC01 followed by co-culturing with HEECs in an angiogenesis assay. Direct effects of JLFC01 on HEECs were also assessed by co-culturing macrophages treated with CM from IL-1β- or TNF-α-stimulated FTDCs and JLFC01-treated HEECs in an angiogenesis assay (n=4). Figure 3A indicates average tube area was increased by macrophages incubated with CM derived from FTDCs treated with IL-1β or TNF-α, while JLFC01 blocked the effects. Consistently, other angiogenesis measurements, i.e. tube length (Figure 3B) and number of branching points (Figure 3C), were reduced by macrophages incubated with CM from FTDCs treated with IL-1β or TNF-α. Co-incubation of FTDCs with JLFC01 reversed this effect. However, JLFC01 treatment of HEECs did not affect any of the three parameters (Figure 3A, B, C) in HEECs co-cultured with macrophages pre-incubated with CM from IL-1β- or TNF-α-stimulated FTDCs.
Figure 3. JLFC01 suppresses the activity of IL-1β- or TNF-α-treated FTDCs in enhancing angiogenesis-inhibiting activity of macrophages.
HEECs treated with or without JLFC01 co-cultured with macrophages incubated with CM from IL-1β- or TNF-α-treated FTDCs in the presence or absence of JLFC01 were grown on Matrigel for 8h. Angiogenesis was assessed by measuring (A) average area (μm2), (B) length (μm) and (C) numbers of branching points (5 random fields/well). The results are reported as mean ± SEM. n=4; *: vs. EM, #: vs. EMI or EMT. ## p < 0.01, *** or ### p < 0.005
JLFC01 prevents placental pro-inflammatory cytokine production, miscarriage and improves fetal growth and maternal food intake in SA/IUGR-prone mice
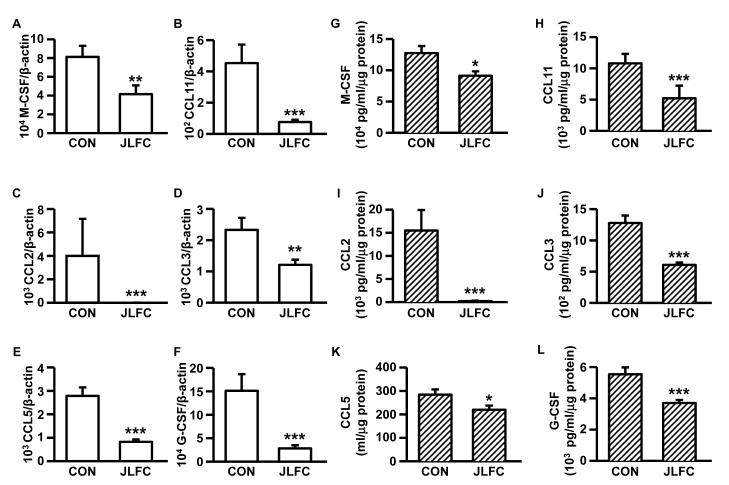
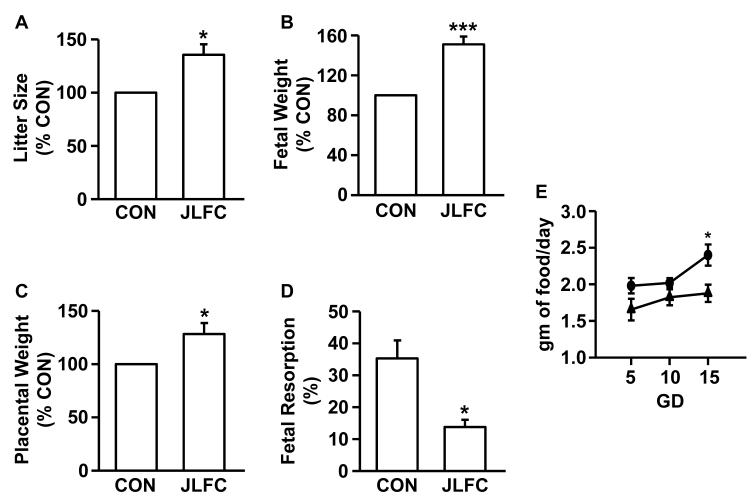
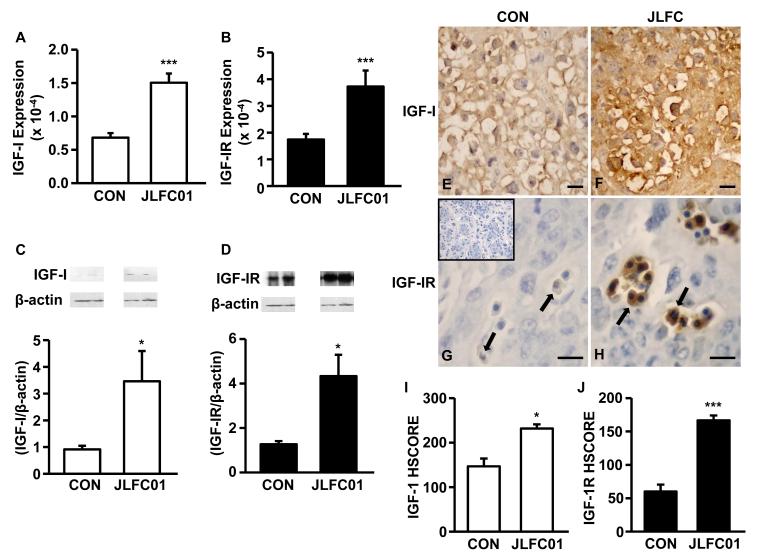
JLFC01 ingestion: i) inhibits placental expression of M-CSF (Fig. 4A & G), CCL11 (Fig. 4B & H), CCL2 (Fig. 4C & I), CCL3 (Fig. 4D & J), CCL5 (Fig. 4E & K) and G-CSF (Fig. 4F & L) (mRNA: 1.96 x, 5.96x, 618.39x, 1.93x, 3.37x and 5.36x, respectively, n= 5 - 8; protein: 1.40x, 2.07x, 74.69x, 2.11x, 1.29x and 1.50x, respectively, n=18 - 20); ii) increased litter size (Figure 5A), fetal (Figure 5B) and placental weight (Figure 5C) by 35.56%, 50.92% (from 1.86 to 2.80 gm) and 28.20% (from 356.5 to 438.7 mg), respectively, accompanied by a 40.44% fetal resorption rate reduction (Figure 5D); iii) increased food intake by 19.6%, 10.8% and 27.8% at GD5, GD10 and GD15, respectively (Figure 5E) (n=3). Potential mechanisms responsible for increased fetal growth accompanying JLFC01 ingestion were examined by placental IGF-I and IGF-IR expression. Steady state mRNA levels of IGF-I and IGF-IR are up-regulated by JLFC01 treatment by 2.2- and 2.1-fold, respectively (Figure 6A & B), with Western blot revealing parallel effects on IGF-I and IGF-IR protein expression (Figure 6C & D) (n=3). Immunohistochemistry demonstrates that IGF-I (Figure 6F & I) and IGF-IR (Figure 6H & J) expression in the placentae are consistently enhanced by JLFC01 ingestion (n=3).
Figure 4. LFC01 inhibits M-CSF, CCL11, CCL2, CCL3, CCL5 and G-CSF expression in placentae from mice with SA/IUGR.
(A – F) The mRNA expression was measured by qRTPCR. (G – L) The protein levels of each individual cytokines in placentae from mice with SA/IUGR were measured by multiplex Luminex assay and normalized to total cell protein. The results are reported as mean ± SEM. n=5 – 8 for qRT-PCR; n=18 – 20 for Luminex. * p < 0.05, ** p < 0.01, *** p < 0.005
Figure 5. LFC01 reduces spontaneous abortion and improves fetal growth.
(A) litter size, (B) fetal weight, (C) placental weight, (D) fetal resorption and (E) maternal food intake at gestational day (GD) 5, 10 and 15 of CBA/J mice treated with JLFC01. The results are reported as mean ± SEM. n=3; * p < 0.05, *** p < 0.005
Figure 6. LFC01 increases IGF-I and IGF-1R expression in the placenta of CBA/J mice.
Placental IGF-I and IGF-IR expression was evaluated by (A, B) qRT-PCR, (C, D) Western blot with densitometry. The expression of IGF-I and IGF-IR in placentae from CBA/J mice treated in the absence (E, G) or presence (F, H) of JLFC01 was evaluated by immunohistochemistry (400x). IGF-I and IGF-IR immunoreactivity was semi-quantitatively evaluated using the following intensity categories: 0, no staining; +, weak but detectable staining; ++, moderate or distinct staining; and +++, intense staining. (I, J) A histological score (HSCORE) was calculated using the formula HSCORE = ∑(Pi × i), where i represents the intensity scores, and Pi is the corresponding percentage of the cells. Five fields/slide were evaluated by 2 investigators blinded to the tissue source. The results of densitometry and immunohistochemistry are reported as mean ± SEM. n=3; * p < 0.05; *** p < 0.005. Inset: IgG control; Scale bar: 50 μm.
Discussion
Successful pregnancy and adequate fetal growth require maintenance of homeostasis and immune balance at the maternal-fetal interface. Conditions disrupting this equilibrium are implicated in eliciting shallow placentation accompanied by SA, IUGR and preeclampsia. These human pregnancy complications are associated with induction of depression, stress, anxiety and lower self-esteem in affected couples, while imposing a financial burden on affected families and society [28]. The pathogenesis of SA and IUGR are multi-factorial with the underlying mechanisms poorly understood. Immunological [2], nutritional [29], hormonal [30], and surgical [31] interventions are ineffective in either preventing or treating SA and IUGR.
JLFC01 used in this study was modified from a traditional formula, Guizhi Fuling Wan, containing the same herbs described in canonical Chinese medicine books. However, the dosages and extracting procedures vary among different pharmaceutical companies and can potentially affect the efficacy. Therefore, JLFC01 was chosen as the name of this preparation manufactured by Brion Research Institute to avoid confusion. Despite centuries long use in Asia of JLFC01 to effectively treat SA with minimal side effects, the mechanisms and components in the herbs responsible for its actions are understudied and consequently not understood. To elucidate this question, the current study integrates in vitro observations on primary human cells with in vivo observations on an SA/IUGR-prone mouse model. The latter demonstrates that JLFC01 induces novel effects by improving growth of the fetal-placental unit and increasing maternal food intake, thus complementing its historical effectiveness in preventing SA. Initial assessment by immunofluorescent staining revealed that, consistent with a local pro-inflammatory milieu, first trimester decidual macrophages are polarized toward an M1 subtype in SA. Previously, our laboratory found that stimulation of FTDCs with TNF-α- or IL-1β enhances macrophage-induced EVT apoptosis as well as decidual macrophage and dendritic cell recruitment/activation [13, 20, 21]. These observations implicate TNF-α- and IL-1β-stimulated decidual cells in the pathogenesis of pregnancy complications [13]. In the current study, incubation of FTDCs with JLFC01 inhibited both TNF-α- and IL-1β-induced production of cytokines associated with monocyte and NK cell recruitment and activation, suggesting that JLFC01 improves pregnancy outcomes and fetal growth by exerting broad anti-inflammatory activities. Consistently, JLFC01 treatment suppressed mouse placental production of pro-inflammatory cytokines, suggesting an association with its roles in improving pregnancy and fetal growth.
EVT invasion of the decidua and underlying myometrium acting in concert with robust angiogenesis are critical to successful placentation. In addition to leukocyte recruitment and activation, specific cytokines up-regulated by IL-1β or TNF-α in FTDCs were shown to exert mixed angiogenic effects. Specifically, GM-CSF, M-CSF and IL-8 promote [32], whereas IP-10 suppresses [33], angiogenesis and endothelial cell proliferation. Moreover, IL-8 inhibits [34], while CCL2 and IP-10 [33, 35] increase, endothelial cell apoptosis. The net effect of JLFC01 on secreted angiogenesis-regulating cytokines by FTDCs under the modulating influence of IL-1β or TNF-α has yet to be determined. Moreover, in early pregnancy, decidual macrophages and FTDCs are positioned to affect angiogenesis [36, 37]. The current use of primary HEECs to test angiogenesis found that CM derived from IL-1β- or TNF-α-treated FTDCs enhances the angiogenesis-inhibiting activity of macrophages. However, treatment of FTDCs with JLFC01 reverses this effect, suggesting that JLFC01 improves placentation by promoting decidual angiogenesis. The current study used blood drawn from non-pregnant healthy reproductive age women. Ideally, blood, first trimester decidual cells and HEECs should all be obtained from the same woman receiving elective termination. However, both ethical proscriptions and varying locations of sample collection present a major practical obstacle in obtaining blood as well as sufficient tissue for the isolation of decidual cells and HEECs from the same patient. Thus, in recruiting the donors for blood and tissue, cells were isolated from individuals with similar physiological characteristics using the same inclusion/exclusion criteria.
In addition to implantation, JLFC01 may aid pregnancy by directly targeting the embryo and/or by modifying fetal programming to improve fetal growth. Critical windows of developmental plasticity are initiated pre-conceptually and extend through early postnatal life [38]. Fetal programming encompasses the role of developmental plasticity in response to environmental and nutritional signals during early life and its potential adverse consequences in later life [38]. The maternal contribution to the nutritional, hormonal and metabolic fetal environment is crucial to fetal growth [39]. Disturbances in related mechanisms can impede early fetal development with potential long-term outcomes and pregnancy complications [39]. Increasing evidence indicates an association of such fetal growth pathologies as IUGR and small-for-gestational-age fetuses with changes in placental transporter function [39]. Previous attention has generally focused on the molecular mechanisms underlying programming effects with the goal of development of targeted therapy in the population at risk [40].
Decreased maternal serum IGF-I levels are suggested to be associated with poor placental function, but not low birth weight [41]. Both IGF-I and IGF-II play crucial roles in fetal-placental growth throughout pregnancy [42]. However, previous studies provide conflicting results. Specifically, IGF-I and IGF-II expression levels are increased in term placentae associated with IUGR [43], whereas immunohistochemistry studies revealed conserved distribution of the IGF-I receptor in placentae from normal pregnancies and pregnancies associated with IUGR [44]. Although inconsistent with results from other studies [43], the increase of placental IGF-I and IGF-1R accompanying increased fetal and placental weight by JLFC01 in the SA/IUGR-prone mice used in the current study indicates an association of IGF-I with fetal growth that is regulated by JLFC01. These conflicting observations suggest a need to test the direct effects of JLFC01 on the embryo. Moreover, the current study found that JLFC01 improves maternal food intake in pregnant mice prone to SA/IUGR, thus, mandating further investigations of the regulation of nutrient transport machinery at the maternal-fetal interface by JLFC01.
In summary, the centuries-long use of JLFC01 to treat such diverse ailments as blood stagnation and threatened abortion attest to its unprecedented safety record. This study confirms its protective effects during gestation and reveals a novel effect on fetal development. The current findings also suggest mechanisms that maintain pregnancy and improve fetal growth. Further studies are required to uncover critical regulatory mechanisms by which JLFC01 prevents SA by focusing on key processes necessary to maintain pregnancy at the implantation site, i.e. angiogenesis, macrophages polarization, and fetal programming and the active components of JLFC01. In addition to FTDCs, HEECs and macrophages, other cell types in the decidua, including various immune cells, vascular smooth muscle cells, EVTs, and glandular cells may also be targets for JLFC01, indicating the need to test the effects of JLFC01 on these cell types.
Supplementary Material
Supplementary Figure 1 Proliferation assay for first trimester decidual cells treated with JLFC01. First trimester decidual cells primed with estradiol and medroxyprogesterone for 7 days were treated with various concentrations of JLFC01 for 48h. The results are reported as mean ± SEM; n=3.
Spontaneous abortion decidua displays a pro-inflammatory micro-environment.
JLFC01 inhibits inflammation in first trimester decidual cells.
JLFC01 suppresses inflammation in SA/IUGR-prone mouse placentae.
JLFC01 may improve gestation by enhancing angiogenesis at fetal-maternal interface.
JLFC01 also promotes fetal growth and potentially modulates fetal programming.
Acknowledgments
The authors report no conflict of interest. However, the authors were advised by Brion Research Institute not to reveal the herbal components included in the formulation.
We thank the Brion Research Institute for providing JLFC01 and assistance of Dr. Pai-Lien Chen at Family Health International in statistical data analysis. This work was supported by grant 5R01HD056123 from NICHD, NIH (S.J.H.). The authors also gratefully acknowledge the editorial assistance provided by Mr. John Shapiro at The Ohio State University.
Glossary
- CCL
C-C motif ligand
- CHM
Chinese herbal medicine
- CM
conditioned media
- EVTs
extravillous trophoblasts
- FTDCs
first trimester decidual cells
- GA
gestational age
- GD
gestational day
- HEEC
human endometrial endothelial cell
- IP-10
interferon-γ-inducible protein-10
- IRB
institutional review board
- IUGR
intrauterine growth restriction
- NK
natural killer
- OSU
The Ohio State University
- SA
spontaneous abortion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Reviews in obstetrics and gynecology. 2009;2(2):76–83. [PMC free article] [PubMed] [Google Scholar]
- [2].Blois S, Alba Soto CD, Olmos S, Chuluyan E, Gentile T, Arck PC, Margni RA. Therapy with dendritic cells influences the spontaneous resorption rate in the CBA/J × DBA/2J mouse model. American journal of reproductive immunology. 2004;51(1):40–8. doi: 10.1046/j.8755-8920.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- [3].Taylor CG, Faulk WP, McIntyre JA. Prevention of recurrent spontaneous abortions by leukocyte transfusions. Journal of the Royal Society of Medicine. 1985;78(8):623–7. doi: 10.1177/014107688507800804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chavez DJ, McIntyre JA, Colliver JA, Faulk WP. Allogeneic matings and immunization have different effects on nulliparous and multiparous mice. Journal of immunology. 1987;139(1):85–8. [PubMed] [Google Scholar]
- [5].Christiansen OB, Mathiesen O, Husth M, Rasmussen KL, Ingerslev HJ, Lauritsen JG, Grunnet N. Placebo-controlled trial of treatment of unexplained secondary recurrent spontaneous abortions and recurrent late spontaneous abortions with i.v. immunoglobulin. Human reproduction. 1995;10(10):2690–5. doi: 10.1093/oxfordjournals.humrep.a135769. [DOI] [PubMed] [Google Scholar]
- [6].Porter TF, LaCoursiere Y, Scott JR. Immunotherapy for recurrent miscarriage. The Cochrane database of systematic reviews. 2006;(2):CD000112. doi: 10.1002/14651858.CD000112.pub2. [DOI] [PubMed] [Google Scholar]
- [7].Li L, Dou L, Leung PC, Wang CC. Chinese herbal medicines for threatened miscarriage. The Cochrane database of systematic reviews. 2012;5:CD008510. doi: 10.1002/14651858.CD008510.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Froen JF, Gardosi JO, Thurmann A, Francis A, Stray-Pedersen B. Restricted fetal growth in sudden intrauterine unexplained death. Acta obstetricia et gynecologica Scandinavica. 2004;83(9):801–7. doi: 10.1111/j.0001-6349.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- [9].Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69(1):1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- [10].Klauber N, Rohan RM, Flynn E, D’Amato RJ. Critical components of the female reproductive pathway are suppressed by the angiogenesis inhibitor AGM-1470. Nature medicine. 1997;3(4):443–6. doi: 10.1038/nm0497-443. [DOI] [PubMed] [Google Scholar]
- [11].Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2(9):656–63. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- [12].Smarason AK, Gunnarsson A, Alfredsson JH, Valdimarsson H. Monocytosis and monocytic infiltration of decidua in early pregnancy. J Clin Lab Immunol. 1986;21(1):1–5. [PubMed] [Google Scholar]
- [13].Wu ZM, Yang H, Li M, Yeh CC, Schatz F, Lockwood CJ, Di W, Huang SJ. Pro-inflammatory cytokine-stimulated first trimester decidual cells enhance macrophage-induced apoptosis of extravillous trophoblasts. Placenta. 2012;33(3):188–94. doi: 10.1016/j.placenta.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73(2):209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- [15].Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies. American journal of reproductive immunology. 2010;63(6):460–71. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- [16].Gustafsson C, Mjosberg J, Matussek A, Geffers R, Matthiesen L, Berg G, Sharma S, Buer J, Ernerudh J. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One. 2008;3(4):e2078. doi: 10.1371/journal.pone.0002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Renaud SJ, Graham CH. The role of macrophages in utero-placental interactions during normal and pathological pregnancy. Immunol Invest. 2008;37(5):535–64. doi: 10.1080/08820130802191375. [DOI] [PubMed] [Google Scholar]
- [18].Lockwood CJ, Huang SJ, Chen CP, Huang Y, Xu J, Faramarzi S, Kayisli O, Kayisli U, Koopman L, Smedts D, Buchwalder LF, Schatz F. Decidual cell regulation of natural killer cell-recruiting chemokines: implications for the pathogenesis and prediction of preeclampsia. Am J Pathol. 2013;183(3):841–56. doi: 10.1016/j.ajpath.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huang SJ, Chen CP, Schatz F, Rahman M, Abrahams VM, Lockwood CJ. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J Pathol. 2008;214(3):328–36. doi: 10.1002/path.2257. [DOI] [PubMed] [Google Scholar]
- [20].Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, Niven-Fairchild T, Tang C, Abrahams VM, Lockwood CJ. Regulation of Chemokine Production in Response to Pro-inflammatory Cytokines in First Trimester Decidual Cells. J Repdrod Immunol. 2006;72(1-2):60–73. doi: 10.1016/j.jri.2006.03.002. [DOI] [PubMed] [Google Scholar]
- [21].Huang SJ, Zenclussen AC, Chen CP, Basar M, Yang H, Arcuri F, Li M, Kocamaz E, Buchwalder L, Rahman M, Kayisli U, Schatz F, Toti P, Lockwood CJ. The Implication of Aberrant GM-CSF Expression in Decidual Cells in the Pathogenesis of Preeclampsia. Am J Pathol. 2010;177(5):2472–82. doi: 10.2353/ajpath.2010.091247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li M, Huang SJ. Innate immunity, coagulation and placenta-related adverse pregnancy outcomes. Thrombosis research. 2009;124(6):656–62. doi: 10.1016/j.thromres.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Annunziata M, Granata R, Ghigo E. The IGF system. Acta diabetologica. 2011;48(1):1–9. doi: 10.1007/s00592-010-0227-z. [DOI] [PubMed] [Google Scholar]
- [24].Gicquel C, Le Bouc Y. Hormonal regulation of fetal growth. Hormone research. 2006;65(Suppl 3):28–33. doi: 10.1159/000091503. [DOI] [PubMed] [Google Scholar]
- [25].Laviola L, Perrini S, Belsanti G, Natalicchio A, Montrone C, Leonardini A, Vimercati A, Scioscia M, Selvaggi L, Giorgino R, Greco P, Giorgino F. Intrauterine growth restriction in humans is associated with abnormalities in placental insulin-like growth factor signaling. Endocrinology. 2005;146(3):1498–505. doi: 10.1210/en.2004-1332. [DOI] [PubMed] [Google Scholar]
- [26].Schatz F, Soderland C, Hendricks-Munoz KD, Gerrets RP, Lockwood CJ. Human endometrial endothelial cells: isolation, characterization, and inflammatory-mediated expression of tissue factor and type 1 plasminogen activator inhibitor. Biol Reprod. 2000;62(3):691–7. doi: 10.1095/biolreprod62.3.691. [DOI] [PubMed] [Google Scholar]
- [27].Zudaire E, Gambardella L, Kurcz C, Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS One. 2011;6(11):e27385. doi: 10.1371/journal.pone.0027385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Serrano F, Lima ML. Recurrent miscarriage: psychological and relational consequences for couples. Psychology and psychotherapy. 2006;79(Pt 4):585–94. doi: 10.1348/147608306x96992. [DOI] [PubMed] [Google Scholar]
- [29].Rumbold A, Middleton P, Pan N, Crowther CA. Vitamin supplementation for preventing miscarriage. The Cochrane database of systematic reviews. 2011;(1):CD004073. doi: 10.1002/14651858.CD004073.pub3. [DOI] [PubMed] [Google Scholar]
- [30].Morley LC, Simpson N, Tang T. Human chorionic gonadotrophin (hCG) for preventing miscarriage. The Cochrane database of systematic reviews. 2013;1:CD008611. doi: 10.1002/14651858.CD008611.pub2. [DOI] [PubMed] [Google Scholar]
- [31].Szabo I, Szilagyi A. Management of threatened abortion. Early pregnancy : biology and medicine : the official journal of the Society for the Investigation of Early Pregnancy. 1996;2(4):233–40. [PubMed] [Google Scholar]
- [32].Feder LS, Laskin DL. Regulation of hepatic endothelial cell and macrophage proliferation and nitric oxide production by GM-CSF, M-CSF, and IL-1 beta following acute endotoxemia. J Leukoc Biol. 1994;55(4):507–13. [PubMed] [Google Scholar]
- [33].Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. The Journal of experimental medicine. 1995;182(1):219–31. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. Journal of immunology. 2003;170(6):3369–76. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- [35].Zhang X, Liu X, Shang H, Xu Y, Qian M. Monocyte chemoattractant protein-1 induces endothelial cell apoptosis in vitro through a p53-dependent mitochondrial pathway. Acta biochimica et biophysica Sinica. 2011;43(10):787–95. doi: 10.1093/abbs/gmr072. [DOI] [PubMed] [Google Scholar]
- [36].Croy BA, Chen Z, Hofmann AP, Lord EM, Sedlacek AL, Gerber SA. Imaging of vascular development in early mouse decidua and its association with leukocytes and trophoblasts. Biol Reprod. 2012;87(5):125. doi: 10.1095/biolreprod.112.102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Khaliq A, Li XF, Shams M, Sisi P, Acevedo CA, Whittle MJ, Weich H, Ahmed A. Localisation of placenta growth factor (PIGF) in human term placenta. Growth factors. 1996;13(3-4):243–50. doi: 10.3109/08977199609003225. color plates I-II,pre bk cov. [DOI] [PubMed] [Google Scholar]
- [38].Srinivasan M, Patel MS. Metabolic programming in the immediate postnatal period. Trends in endocrinology and metabolism: TEM. 2008;19(4):146–52. doi: 10.1016/j.tem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- [39].Jansson T, Powell TL. IFPA 2005 Award in Placentology Lecture. Human placental transport in altered fetal growth: does the placenta function as a nutrient sensor? -- a review. Placenta. 2006;27(Suppl A):S91–7. doi: 10.1016/j.placenta.2005.11.010. [DOI] [PubMed] [Google Scholar]
- [40].Longtine MS, Nelson DM. Placental dysfunction and fetal programming: the importance of placental size, shape, histopathology, and molecular composition. Seminars in reproductive medicine. 2011;29(3):187–96. doi: 10.1055/s-0031-1275515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Holmes RP, Holly JM, Soothill PW. A prospective study of maternal serum insulin-like growth factor-I in pregnancies with appropriately grown or growth restricted fetuses. British journal of obstetrics and gynaecology. 1998;105(12):1273–8. doi: 10.1111/j.1471-0528.1998.tb10005.x. [DOI] [PubMed] [Google Scholar]
- [42].Fowden AL. The insulin-like growth factors and feto-placental growth. Placenta. 2003;24(8-9):803–12. doi: 10.1016/s0143-4004(03)00080-8. [DOI] [PubMed] [Google Scholar]
- [43].Abu-Amero SN, Ali Z, Bennett P, Vaughan JI, Moore GE. Expression of the insulin-like growth factors and their receptors in term placentas: a comparison between normal and IUGR births. Molecular reproduction and development. 1998;49(3):229–35. doi: 10.1002/(SICI)1098-2795(199803)49:3<229::AID-MRD2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- [44].Holmes R, Porter H, Newcomb P, Holly JM, Soothill P. An immunohistochemical study of type I insulin-like growth factor receptors in the placentae of pregnancies with appropriately grown or growth restricted fetuses. Placenta. 1999;20(4):325–30. doi: 10.1053/plac.1998.0387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Proliferation assay for first trimester decidual cells treated with JLFC01. First trimester decidual cells primed with estradiol and medroxyprogesterone for 7 days were treated with various concentrations of JLFC01 for 48h. The results are reported as mean ± SEM; n=3.