Abstract
Organ engineering using decellularized scaffolds is a potential long-term solution to donor organ shortage. However, this technology is severely limited by small vessel thrombosis due to incompletely recellularized vessels, resulting in exposure of extracellular matrix (ECM) components to platelets and clotting factors in flowing blood. To address this limitation, we designed a polymer-ECM composite and demonstrated its potential to reduce thrombosis and facilitate re-endothelialization in a vascular graft model. Rat aortas were decellularized using a sequential combination of weak detergents followed by a nuclease treatment that resulted in 96.5±1.3% DNA removal, while ECM components and mechanical properties were well maintained. A biodegradable and biocompatible elastomer poly (1,8 octanediol citrate) (POC, 1wt%) was infused throughout the ECM at mild conditions (37°C and 45°C) and was functionalized with heparin using carbodiimide chemistry. The polymer-ECM composite significantly reduced platelet adhesion (67.4±8.2% and 82.7±9.6% reduction relative to untreated ECM using one of two processing temperatures, 37°C or 45°C, respectively); inhibited whole blood clotting (85.9±4.3% and 87.0±11.9% reduction relative to untreated ECM at 37°C or 45°C processing temperature, respectively); and supported endothelial cell—and to a lesser extent smooth muscle cell—adhesion in vitro. Taken together, this novel POC composite may provide a solution for thrombosis of small vessel conduits commonly seen in decellularized scaffolds used in tissue engineering applications.
Keywords: decellularization; organ engineering; small diameter vascular graft; poly(1,8-octanediol-co-citrate) (POC); heparin; thrombosis
1. Introduction
Organ transplantation is the only definitive treatment for end-stage organ failure. The shortage of functional donor organs, the need for immunosuppression, and the development of allo-antibodies remain major hurdles limiting extension of organ transplantation to all patients in need.[1] The concept of whole-organ tissue engineering has emerged as a promising, long-term, alternative approach for organ replacement.[2] Decellularization, the process of removing allogeneic or xenogeneic cells from donor organs also removes antigenic ligands that cause immune rejection, leaving behind the three-dimensional extracellular matrix (ECM) as an intact scaffold that is subsequently recellularized with recipient autologous cells, thus providing a template to develop whole organs from donor scaffolds and recipient cells.[3, 4]
One major challenge in whole-organ tissue engineering is thrombus formation within the microvasculature of organoids developed from decellularized scaffolds, often due to incomplete coverage of ECM proteins by endothelial cells.[5] To address this immediate hurdle in organ engineering, we developed a strategy to immobilize the anticoagulant heparin onto ECM surfaces that are exposed to blood in a method that may be applied to any ECM-based tissue engineering system. Specifically, we incorporated a biodegradable polyester elastomer, poly (1,8 octanediol citrate) (POC) to link heparin to ECM, building upon a novel strategy earlier reported by our group to biofunctionalize expanded-Polytetrafluoroethylene (ePTFE) vascular grafts with endothelial cells or immobilized heparin.[6] [7] We also report a critical modification of the post-polymerization manufacturing protocol performed at physiologic temperatures to retain matrix structure as the natural protein composition of ECM leads to a more tenuous and temperature-dependent stability compared to synthetic materials such as ePTFE. POC exhibits excellent biocompatibility, supports endothelialization, and provides carboxyl and hydroxyl groups for chemical modification of bioactive macromolecules.[8] Heparin remained active for at least 4 weeks when linked to POC coated ePTFE vascular grafts via carbodiimide chemistry.[6] [7] We hypothesize that a similar strategy can be applied to an ECM-based tissue-engineering scaffold at lower processing temperatures to improve hemocompatibility of exposed ECM.
We developed a strategy to decellularize rodent aortas as a model ECM scaffold to evaluate the ability to immobilize heparin onto ECM matrices hybridized with POC at 37°C or 45°C. The goal of this study is to develop and evaluate a polymer-ECM composite for potential use in tissue and regenerative engineering applications. We show that this strategy decreases platelet binding and clot formation, and allows recellularization. As the ECM is both easily infused with POC and readily processed under physiologic conditions that support cellular repopulation, this method can be applied to other decellularized organ systems, broadening the ultimate application of this strategy.
2. Materials and Methods
2.1. Materials
Triton X-100, sodium dodecyl sulfate (SDS, 20% stock solution) and absolute ethanol were obtained from VWR International (Radnor, PA). Deoxyribonuclease I from bovine pancreas (DNase I, ≥400U/mg), Proteinase K from Tritirachium album (≥30U/mg), Hoechst 33258 solution, urea, Trizma® hydrochloride (Tris-HCl, 99%), citric acid (99%), 1,8-Octanediol (98%), L-cysteine (≥98%), 2-(N-morpholino) ethanesulfonic acid (MES, ≥99%), N-(3 dimethylaminopropyl)-N-ethylcarbodiimide (EDC), N-hydroxysuccinimide (NHS, 98%), 1,6-Diaminohexane, Toluidine Blue O, sodium chloride, potassium chloride, magnesium chloride, calcium chloride, sodium phosphate monobasic, dextrose, apyrase, and bovine serum albumin (BSA) were obtained from Sigma-Aldrich (St. Lois, MO). Heparin sodium (200U/mg) was obtained from Celsus Laboratories (Cincinnati, OH). Quant-iT™ PicoGreen® dsDNA Assay Kit and Alexa Fluor 488 Phalloidin were obtained from Life Technologies (Carlsbad, CA) Complete mini protease inhibitors and LDH cytotoxicity detection kit were obtained from Roche Molecular Diagnostics (Pleasanton, California). BCA protein assay kits were obtained from Thermo Scientific (Rockford, IL), and Rat Fibroblast Growth Factor (FGF) ELISA kit was obtained from Bmassay (Beijing, China).
2.2. Preparation of decellularized aortas
Animal care was performed in accordance with the NIH Guide for Care and Use of Laboratory Animals, and experiments using animals were approved by the Animal Care and Use Committee of Northwestern University (NU-IACUC), Chicago, IL. Male Sprague Dawley rats (200–250 g) were used for aorta recovery. Briefly, donor rats were anesthetized, abdomen shaved, and operative field disinfected using standard surgical technique. The abdomen was entered through a midline incision. The abdominal aorta was exposed after removing internal organs, and a 3–4cm segment of the aorta was removed. All donor rats were euthanized by exsanguination at the end of the procedure and 5–10ml of blood was collected into ACD anticoagulant test tubes (BD Biosciences, Franklin Lakes, NJ) for platelet adhesion and whole-blood clotting assays described below. Recovered aortas were stored at −20°C until they were decellularized.
Rat aortas (3–4cm long in length) were washed with deionized (DI) water and then decellularized by submersion in 1% Triton X for 48 hours followed by 1.5% SDS for 48 hours. During the treatment, aortas were immersed in each solution with continuous stirring at room temperature. Decellularized aortas were incubated with 100U/ml DNase I solution at 37°C for 4 hours to removal residual DNA and subsequently washed and stored in DI water at 4°C.
2.3. Characterization of decellularized aortas
2.3.1 Imaging
Decellularized aorta and native aortas were paraffin embedded, and sectioned into 5µm thick slices using a microtome. Hematoxylin and eosin (H&E) staining, Masson’s trichrome staining and Weigert’s elastin staining were performed by the Northwestern University Mouse Histology Core. Histological sections were imaged by transmitted light microscopy with a 10× objective (Carl Zeiss AG, Germany). Aortas were further imaged with scanning electron microscopy (SEM). Briefly, all samples were fixed with 2.5% glutaraldehyde for 1 hour and dehydrated in ethanol prior to being coated in osmium tetroxide. SEM images were taken on a Hitachi 3500 N at the Northwestern University EPIC facility. Lastly, native and decellularized aortas were embedded in Optimal Cutting Temperature compound (O.C.T), frozen and sectioned (10µm thickness), and stained with Hoechst-33258 (5 µg/ml). Florescent microscopy (Nikon TE2000U, Japan) was used to image the sections under an ultra violet (UV) filter.
2.3.2 DNA analysis
After treatment with detergents, decellularized aortas were incubated with DNase I solution (0, 100 and 400 U/ml) for 4 hours at 37°C. Native aortas were used as controls. Native or decellularized aortas were then lyophilized, weighed to obtain dry mass, and digested overnight at 60°C with Proteinase K (15 U/ml in Tris-HCl buffer). DNA content in the digested solutions was quantified with a PicoGreen dsDNA assay kit (Life Technologies, Carlsbad, CA) as per the manufacturer’s instruction.
2.3.3 Protein analysis
Native and decellularized aortas were lyophilized, weighed and carefully grinded into small pieces. Fresh
extraction buffer (2 M urea, 50 mM Tris-HCl, 5 mg/ml heparin sodium, and complete mini protease inhibitors 1 tablet/10ml) was added to the minced aortas and incubated on a rocker at 4°C overnight. Samples were then centrifuged at 12,000g for 30min at 4°C to obtain protein extracts. The extraction and centrifugation process was repeated, and the solution containing recovered protein was added to the first time extracts. The total extract solution was then dialyzed in DI water with 3500 MWCO, and analyzed with a micro BCA protein assay and rat FGF ELISA kit for total protein content and growth factor retention, respectively.
2.3.4 Mechanical Testing
Tensile tests were performed on aortas before and after decellularization (n=3) using Instron 5544 Materials Testing Machines (Instron, Norwood, MA). Each aorta sample (~4cm length, 1.2mm diameter) was gripped on either side of the proximal and distal ends (5mm gripping length from each end), leaving a 30mm gauge length for testing, which was calibrated for each sample prior to each testing. All samples were hydrated during testing to maintain physiologic conditions and a stroke rate of 1 mm/min across 0–10% strain. A stress-strain curve was plotted for each sample and the Young’s Modulus (E) was calculated. All data were collected and analyzed with ASTM-compliant Bluehill ® Testing Software for Mechanical Testing Systems (Instron, Norwood, MA).
2.4. Polymer-ECM hybridization and heparin immobilization
2.4.1 POC synthesis
POC prepolymer synthesis using equal molar ratios of citric acid and 1,8-octanediol, was synthesized as described previously.[9] To image POC on ECM scaffolds, an auto-florescent version of POC was synthesized by adding L-Cysteine (Cys/citric acid molar ratio = 0.2, molar ratio) at the initiation of prepolymer synthesis permitting detection of the molecule by UV light. [10]
2.4.2 POC–ECM hybridization
The pre-polymer was diluted with absolute ethanol to 1% (w/w) prior to applying POC (or POC-Cys) onto decellularized aorta ECM. Decellularized aorta was firstly dehydrated with ethanol and then incubated in 1% pre-polymer solution for 30 min with continuous stirring. The pre-polymer infused ECM was then post-polymerized at 37°C or 45°C for 4 days. The hybridized POC-ECM composites were rinsed with PBS at 37°C for 3 days to remove unbound low molecular weight POC pre-polymers.
2.4.3 Heparin immobilization
POC-ECM composites were soaked in 0.1M MES buffer (pH 5.6, with 0.5M NaCl) for 1 hour. Diaminohexane (50mM in MES buffer) was reacted with POC-ECM composites in the presence of 150mM NHS and 300mM EDC for 5 hours at room temperature. POC-ECM composites were then extensively rinsed with 2M NaCl and immersed overnight at room temperature in a solution containing 5mM heparin sodium, 0.1M MES, 100mM NHS and 200mM EDC.[6]
2.5. Characterization of POC-ECM composite
2.5.1 POC Detection
Because the addition of cysteine residues to POC yields an autofluorescent POC-Cys polymer, POC-Cys was used as a surrogate for POC to image the composite scaffolds.[10] POC-Cys-ECM composites post-polymerized at 45°C or 37°C were embedded in Optimal Cutting Temperature compound (OCT) and frozen sectioned with 10 µm thickness. Unmodified decellularized aorta ECM was used as a control. Sections were imaged with florescent microscopy with UV excitation (330–380nm) for detection of POC-Cys and blue light excitation (420–495nm) for detection of ECM auto-fluorescence.
2.5.2 Heparin Detection
The presence of heparin conjugated to POC-ECM composites (heparin-POC-ECM) was detected using Toluidine Blue O staining as described previously by our group.[6] The presence of heparin is indicated by color change of Toluidine Blue O from blue to purple. Briefly, Toluidine Blue O (0.5mM, pH 10) was added to 10µm frozen sections (ECM, POC-ECM, and heparin-POC-ECM) for 30min at 37°C. After rinsing with PBS, sections were imaged with bright field microscopy with a color digital camera (Carl Zeiss, Germany). Standardized imaging settings were used across all groups to allow direct comparison for color variation among samples.
2.5.3 Water content
The water content of ECM was analyzed by measuring the swelling ratio of decellularized aortas. Briefly, decellularized aortas treated under different conditions (ECM, POC-ECM, heparin-POC-ECM, n=3 for each category) were incubated in DI water and allowed to absorb water for 24 hours at room temperature, after which the weight of each sample was recorded as Wwet. Samples were then lyophilized overnight and the weight of each dry sample obtained as Wdry. The swelling ratio was calculated as Wwet/Wdry.
2.5.4 Dimensional assessment
The inner diameters of decellularized aortas, treated under different conditions (ECM, POC-ECM, heparin-POC-ECM, n=3 for each category), were measured by firstly staining frozen sections with H&E, and imaging with digital microscopy using a 4× objective. The images were manually combined to show the entire cross-section and analyzed with ImageJ (National Institutes of Health, Bethesda, MD) to measure the inner diameter.
2.6. Thromboresistance of the POC-ECM composite
2.6.1 Platelet Adhesion
Anti-coagulated rat whole blood samples were centrifuged at 225 g for 15 minutes to obtain platelet rich plasma, which was diluted with platelet suspension buffer (137 mM sodium chloride, 2.7 mM potassium chloride, 0.4mM sodium phosphate monobasic, 5.5 mM dextrose, 10 mM HEPES, 0.1 U/ml apyrase, 0.1mM magnesium chloride, 2.5 mM calcium chloride, and 4 mg/ml BSA, pH 7.4) to 2–5×108 platelet/ml. Decellularized aortas (3~5 mm in length, n=3 for each condition) were pre-weighed, incubated in 100 µl platelet suspension at 37°C for 1 hour, rinsed with warm PBS, and lysed with 100 µl of 2% Triton-X at 37°C for 10 minutes. Release of LDH was assessed in the resulting solution with an LDH assay as per the manufacturer’s protocol. A serial dilution of a known concentration of platelets in suspensions served as the standard curve. Platelet binding is expressed as the number of adherent platelets/mg of decellularized aorta.
2.6.2 Whole blood clotting
Re-calcified whole blood clotting assay was performed on POC-ECM composites as previously described.[6] Briefly, anti-coagulated rat blood was re-calcified by adding 10% (v/v) of 0.1M CaCl2. Decellularized aorta grafts (3~5mm in length, n=3 for each condition) were pre-weighed and incubated in re-calcified blood for 1 hour at room temperature. Grafts with clotted blood were carefully removed from blood samples, blotted with Kimwipes™ and weighed again to obtain blood clot mass.
2.7. Vascular Cell Interactions with POC-ECM composites
Human umbilical vein endothelial cells (HUVECs, Lonza) and human arterial smooth muscle cells (HASMCs, Lonza) were cultured in endothelial growth medium (EGM-2, Lonza) and smooth muscle growth medium (SmGM, Lonza), respectively, under an atmosphere of 5% CO2 at 37°C. The growth medium was changed every 2–3 days and cells were harvested by exposure to trypsin/EDTA solution. Cells were suspended in growth medium and seeded into decellularized aorta ECM, POC-ECM, or heparin-POC-ECM under static cell seeding and culture conditions. 5mm-long segments of aortic grafts were used for each group. Briefly, 5,000 cells (HUVECs or HASMCs) were injected firstly from the proximal end. After 1 hour of incubation at 37°C, aortas were rotated 180°, and a subsequent 5000 cells (HUVECs or HASMCs) were injected into the distal end. The samples were incubated under static culture condition for 5 days under an atmosphere of 5% CO2 at 37°C, with fresh medium exchanged on day 3.
Samples were fixed with 4% paraformaldehyde solution, counter-stained with Hoechst (5 µg/ml) and Phalloidin (200 U/ml), and imaged with fluorescent microscopy on day 5 after seeding. An MTT assay was also used to assess cell viability on cells seeded onto grafts (n=3 for each condition), as per the manufacturer’s instructions. A Pico-Green assay was used to indirectly assess the number of cells adherent to each scaffold (n=3 for each condition), also as per the manufacturer’s instructions. The results from both MTT assay and Pico-Green assay were normalized to the mass of each sample.
2.8 Statistical Analysis
All statistical data are expressed as mean±standard deviation. Data were analyzed on SigmaStat (San Jose, CA) using a one-way ANOVA with a Tukey-Kramer post-test. For all comparisons, p<0.05 was considered statistically significant.
3. Results
3.1 Decellularization
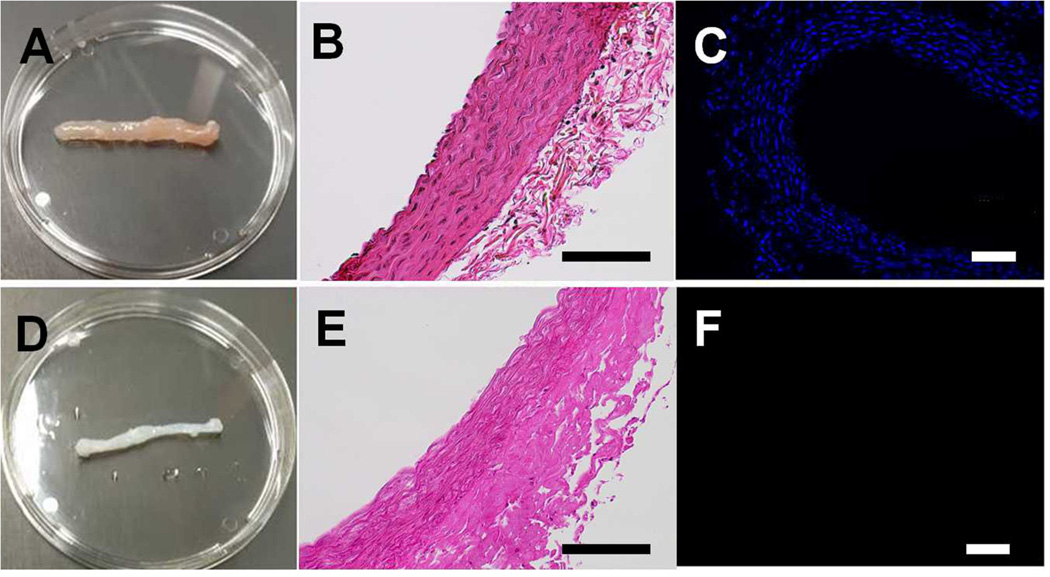
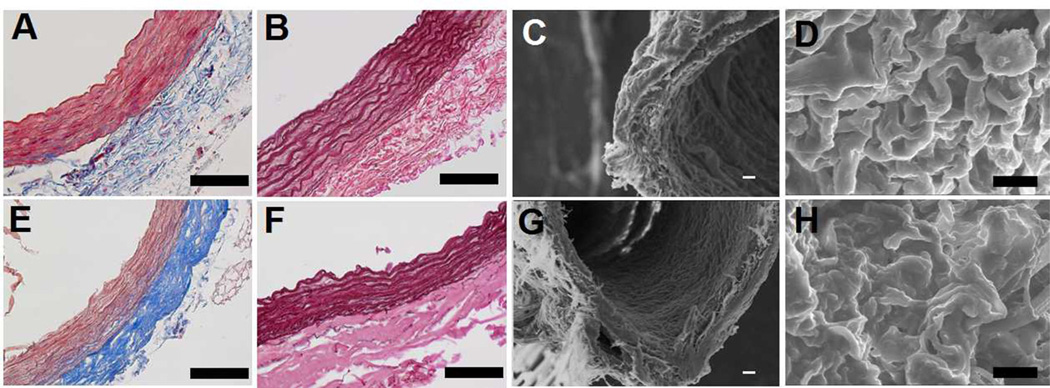
Aortas recovered from Sprague Dawley rats (200–250 mg) (Fig. 1A) were decellularized by submersion in 1% Triton-X 100 for 48 hours followed by 1.5% SDS for 48 hours and 100 U/ml DNAse to remove residual DNA. Decellularized aortas maintained original dimensions (Fig. 1D) despite a 42.0±6.1% reduction in dry mass due to removal of the cellular components. Removal of cells from the scaffold was confirmed with H&E (Fig. 1B, E) and DAPI staining (Fig. 1C, F) that revealed complete loss of cell nuclei within the wall of decellularized aortas compared to native tissue. Retention of critical ECM proteins (e.g. collagen and elastin) needed for maintenance of structural and mechanical properties was confirmed by Masson’s trichrome stain for collagen (Fig. 2A, E) and Werigert’s stain for elastin (Fig. 2B, F). SEM images revealed a surface topography of densely-connected fibrous structures in decellularized arteries, similar to native arteries (Fig. 2C, D, G, H).
Figure 1.
Macroscopic view (A, D), H&E staining (B, E) and DAPI staining (C, F) of native (A, B, C) and decellularized (D, E, F) rat aorta. Scale bar=200µm.
Figure 2.
Masson’s trichrome staining (A, E), Weigert’s elastin staining (B, F) and SEM imaging with low (C, G) and high (D, H, lumen surface) magnifications showed the retention of ECM before (A, B, C, D) and after (E, F, G, H) rat aorta decellularization. Scale bar=200µm.
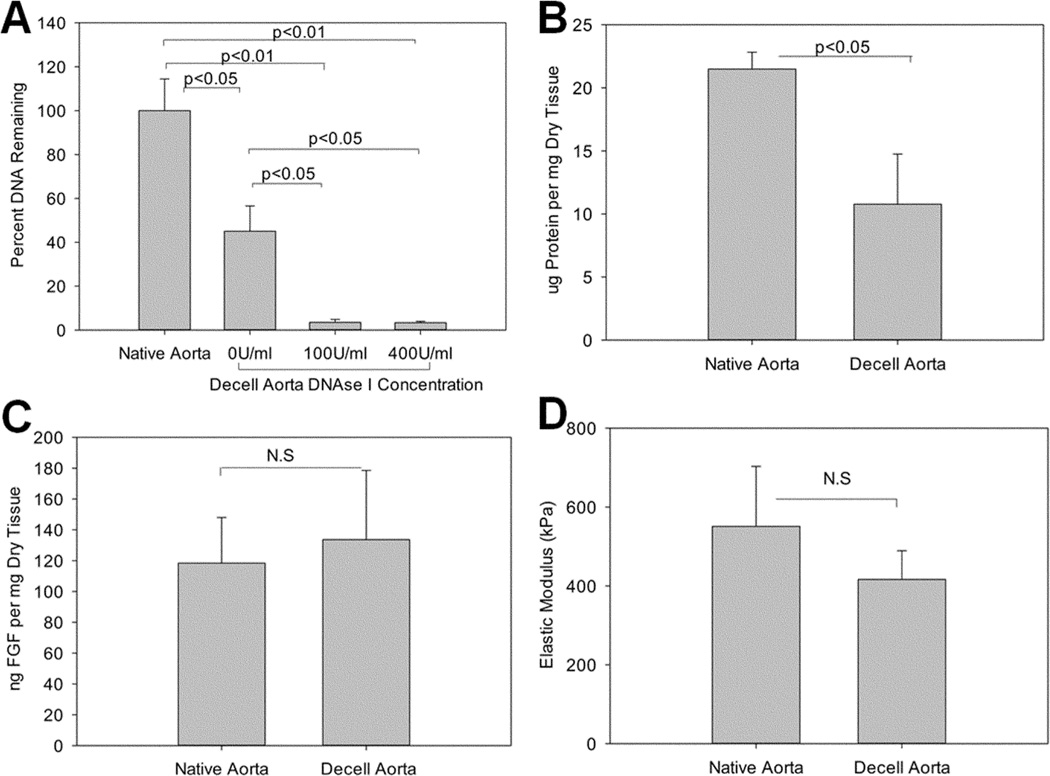
Decellularization of rodent aortas by Triton X and SDS led to a 54.93±11.48% reduction in DNA content immediately after detergent treatment. Residual DNA was further degraded and removed by 100 U/ml DNase I to yield a total removal of 96.5±1.3% DNA; however, additional DNase I (400 U/ml) did not further improve DNA removal (Fig. 3A). Protein analysis revealed ~50% total protein loss during decellularization (21.49±1.33 ug/mg in native aorta vs. 10.78±3.97 ug/mg in decell aorta), largely due to removal of cellular components (Fig. 3B); however FGF, an extracellular growth factor that binds to ECM, was well retained after decellularization (118.43±29.54 ng/mg in native aorta vs. 133.63±44.88 ng/mg in decell aorta, p=0.3494, Fig. 3C). Tensile strength of decellularized aortas was not significantly affected by decellularization (416.5±72.6 kPa and 551.1±152.2 kPa, for decellularized and native aorta, respectively p=0.239) (Fig. 3D). Therefore, retention of the ECM alone was sufficient to preserve mechanical properties after decellularization.
Figure 3.
DNA analysis (A) showed over 95% removal of DNA in the presence of DNase I. Protein analysis showed decrease of overall proteins (B) but retention of extracellular growth factor FGF(C). Elastic Modulus (D) showed no significant change after decellularization. N.S=No significant difference.
3.2 POC-ECM hybridization and heparin immobilization
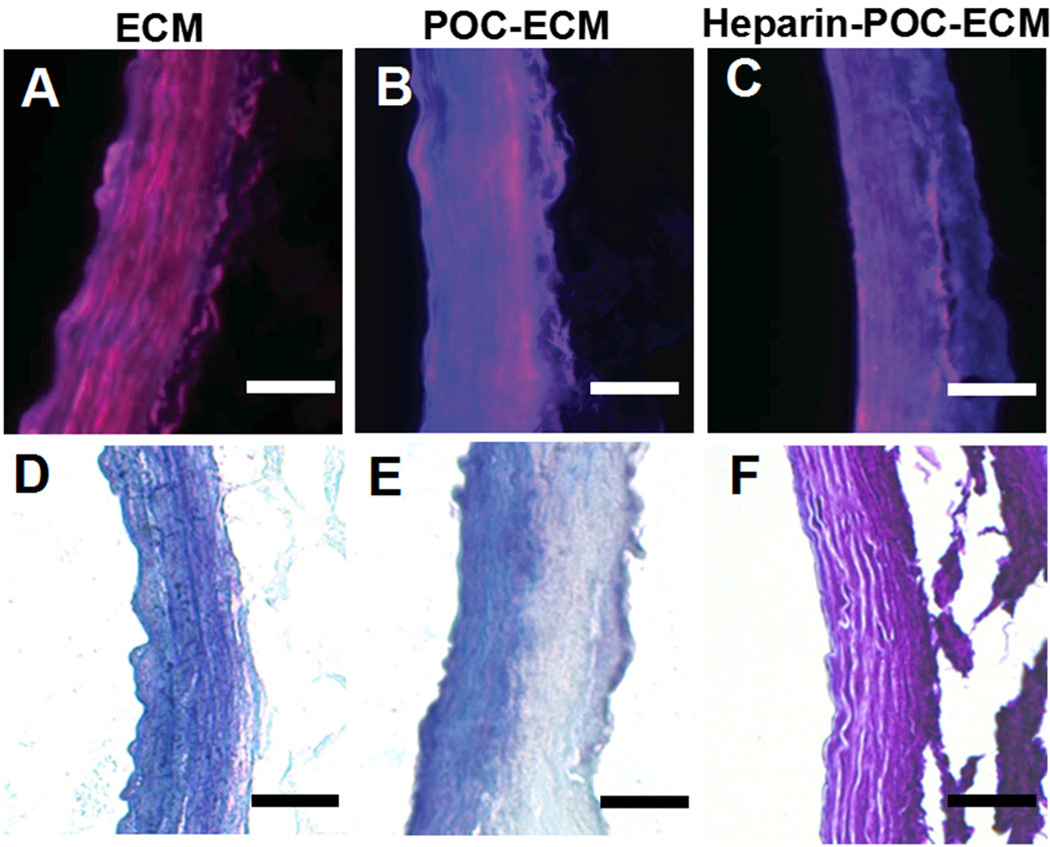
POC was successfully hybridized onto the ECM, providing binding sites for heparin conjugation. The presence of POC on ECM grafts was confirmed via the characteristic blue fluorescence of POC-Cys (Fig. 4.BC). POC was detectable on the ECM as per fluorescence microscopy images for at least 4 months during incubation in PBS at 37°C. The presence of heparin on POC-ECM composites was confirmed by its purple hue after Toluidine blue staining (Fig. 4F), which also remained detectable after 4 weeks. Importantly, the ECM showed no significant change in diameter or water absorption (Table 1) after POC hybridization and heparin immobilization.
Figure 4.
Fluorescent microscopy images (A, B, C) and Toluidine blue staining images (D, E, F) of decellularized aorta ECM (A, D), POC-ECM (B, E) and heparin-POC-ECM (C, E). Blue fluorescence was observed in the presence of POC (B, C) due to auto-fluorescence of the cysteine residue incorporated in to POC (POC-Cys). A purple hue was observed in the presence of heparin conjugation with Toluidine blue staining (F). All images were taken immediately after POC hybridization and heparin immobilization. Scale bar=100 µm.
Table 1.
Characterization of Decellularize Aorta with Varying Modifications
| Inner Diameter (mm) | Swelling Ratio | |||
|---|---|---|---|---|
| 37°C | 45°C | 37°C | 45°C | |
| ECM | 1.23±0.33 | 1.41±0.36 | 3.13±0.04 | 3.56±0.79 |
| POC-ECM | 1.20±0.13 | 1.16±0.14 | 3.48±0.38 | 4.21±1.10 |
| Heparin-POC-ECM | 1.20±0.05 | 1.00±0.26 | 3.03±0.49 | 3.76±0.42 |
No significant difference was found among 3 different ECM groups and between 2 different processing temperatures.
3.3 Thrombo-resistance
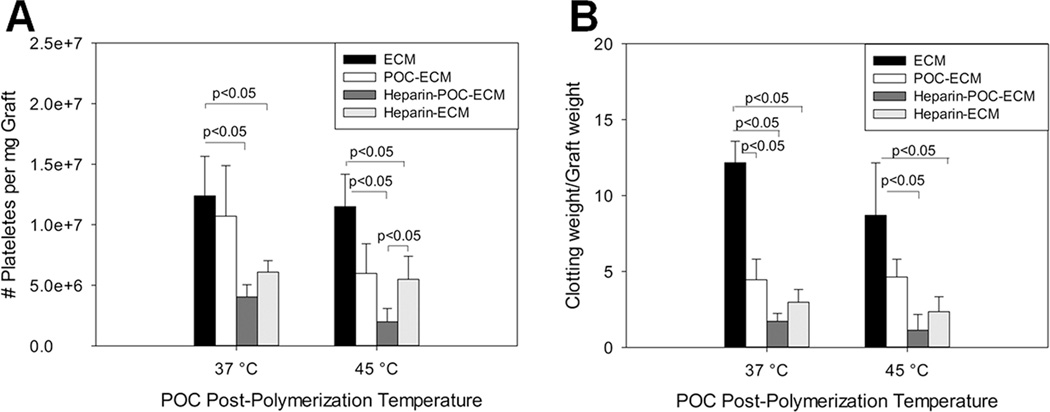
ECM is a highly thrombogenic surface, illustrating the difficulty in developing an animal model of a tissue engineered organ due to small vessel thrombosis. When the aorta ECM was exposed to platelet rich plasma, a large number of platelets adhered to it (Fig. 5A, 12.39±3.26 at 37°C processing temperature and 11.50±2.66 million platelets/mg at 45°C processing temperature). POC-ECM composites without immobilized heparin exhibited a trend toward a reduced number of bound platelets, but did not reach statistical significance (Fig. 5A, 10.71±4.18 million platelets/mg POC-ECM at 37°C, 13.6±33.7% reduction, p=0.612 vs. ECM; and 5.98±2.45 million platelets/mg POC-ECM at 45°C, 48.0±21.3% reduction, p=0.057 vs. ECM). However, after heparin immobilization onto POC-ECM, platelet adhesion was reduced by 60–80% on heparin-POC-ECM composites relative to ECM (Fig. 5A, 4.04±1.01 million platelets/mg heparin-POC-ECM at 37°C, 67.4±8.2% reduction, p=0.0132 vs. ECM; 1.98±1.10 million platelets/mg heparin-POC-ECM at 45°C, 82.8±9.6% reduction, p<0.01 vs. ECM). Direct conjugation of heparin onto ECM (heparin-ECM) also resulted in reduction in platelet binding of ~50% (Fig. 5A, 6.08±0.95 million platelets/mg heparin-ECM at 37°C, 50.9±7.7% reduction, p=0.0323 vs. ECM; 5.49±1.91 million platelets/mg heparin-ECM at 45°C, 54.9±16.6% reduction, p=0.0336 vs. ECM). Taken together, these findings confirm that POC hybridization improves thromboresistance, likely via contribution of additional carboxylic groups to the scaffold matrix, allowing for enhanced heparin conjugation and overall decrease in platelet binding.
Figure 5.
Platelet adhesion (A) and re-calcified whole blood clotting (B) assays assessed on decellularized aortas alone (ECM) or treated with POC (POC-ECM), heparin (heparin-ECM), or POC and heparin (POC-Heparin) using either 37°C or 45°C for the post-polymerization processing temperature.
A synergistic effect between POC hybridization and heparin immobilization was likewise observed in a reduction of the clot burden produced by the addition of re-calcified whole blood to the vascular scaffold. When ECM is exposed to re-calcified rat whole blood, thrombi formed on the matrix within 1 hour (Fig. 5B, 12.16±1.41 and 8.69±3.45 mclotting/mgraft for ECM at 37°C or 45°C, respectively). POC hybridization to ECM at 37°C or 45°C resulted in a ~50% decrease in thrombi formation on POC-ECM, which may be due to accumulation of Ca2+ at the POC surface, where POC may act as a metal chelator (Fig. 5B, 4.45±1.36 mclotting/mgraft for POC-ECM at 37°C, 63.4±11.2% reduction, p=0.0024 vs. ECM; 4.63±1.18 mclotting/mgraft for POC-ECM at 45°C, 46.7±13.6% reduction, p=0.0386 vs. ECM). Heparin immobilization onto POC-ECM led to a >85% reduction in clot formation relative to ECM alone (Fig. 5B, 1.71±0.52 mclotting/mgraft for heparin-POC-ECM at 37°C, 85.9±4.3% reduction, p<0.001 vs. ECM; 1.13±1.04 mclotting/mgraft for heparin-POC-ECM at 45°C, 87.0±11.9% reduction, p=0.0221 vs. ECM), which is consistent with the trend in thrombo-resistance to platelet adhesion reported above. Direct conjugation of heparin onto ECM also resulted in ~70% decrease in blood clotting formation, which did not reach the high degree of thrombo-resistance observed for heparin-POC-ECM composites (Fig. 5B, 2.97±0.84 mclotting/mgraft for heparin-ECM at 37°C, 65.5±6.9% reduction, p<0.001 vs. ECM; 2.35±0.98 clotting/mgraft for heparin-ECM at 45°C, 73.0±11.3% reduction, p=0.0375 vs. ECM).
3.4 Vascular cell interactions with POC-ECM composites
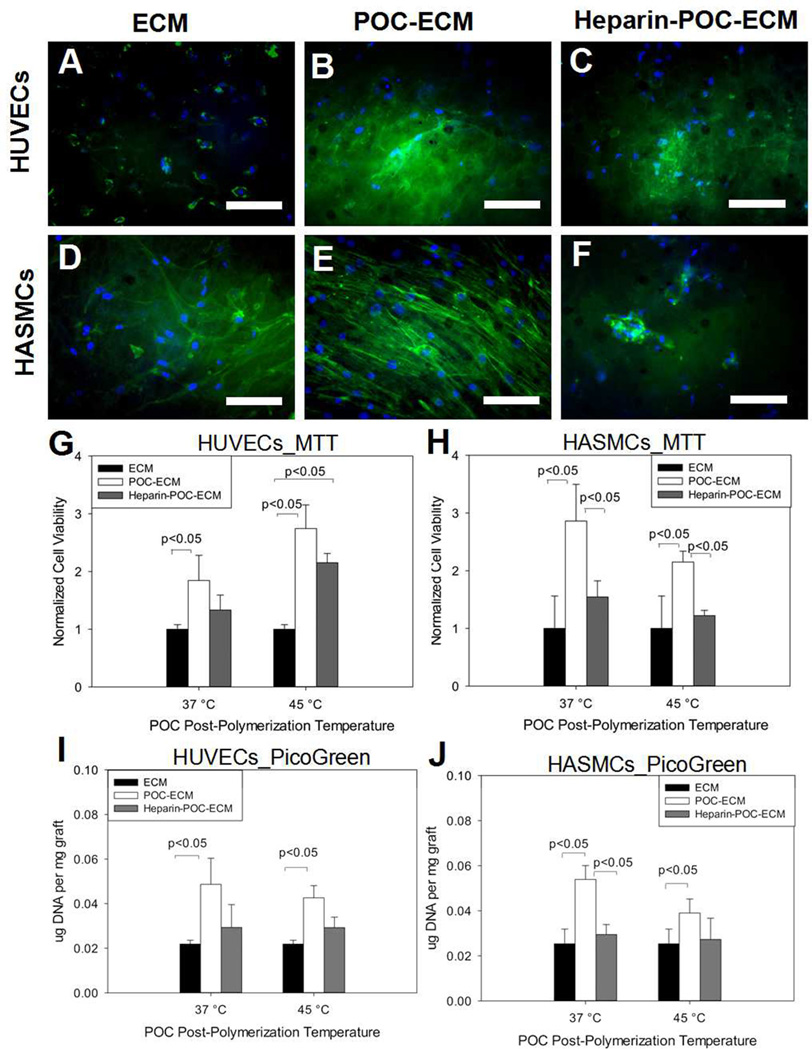
Both HUVECs and HASMCs attach to and spread on all ECM-based scaffolds that were generated (Fig. 6A, D). However, a higher number of adherent cells were observed for both cell types (Fig. 6, B, E) on POC-ECM compared to ECM alone, and these findings correlated with increased cell viability and adhesion (Fig. 6G–J). Heparin immobilization (heparin-POC-ECM) led to a slight decrease in the number of adherent cells for both HUVECs and HASMCs (Fig. 6, C, F), this was perhaps due to the high density of negative charges on heparin.
Figure 6.
HUVECs (A, B, C) and HASMCs (D, E, F) adhesion onto decellularized aorta ECM (A, D), POC-ECM (B, E) and heparin-POC-ECM (C, F). Green fluorescence (phalloidin) stained for actin, while blue fluorescence (Hoechst) stained for cell nuclei. All images were taken for scaffolds processed at 45°C. Cell viability was analyzed via MTT assay for HUVECs (G) and HASMCs (H) on decellularized aortas with varying modification conditions. In addition, cell adhesion was quantified via PicoGreen assay for HUVECs (I) and HASMCs (J). Scale bar=100µm.
We evaluated cell viability on all scaffolds via the MTT assay; all results were normalized to the viability of cells grown on an ECM scaffold. POC-ECM composites exhibited the highest number of viable HUVECS (Fig. 6G, 1.85±0.44 at 37°C, p=0.0299 vs. ECM; 2.74±0.41 at 45°C, p=0.0019 vs. ECM). After heparin immobilization, the viability of adherent cells on heparin-POC-ECM decreased to 1.33±0.25 for 37°C (p=0.1004 vs. ECM), while heparin-POC-ECM post-polymerized at 45°C retained a significantly higher level of viable HUVECs compared to ECM alone (1.85±0.44, p=0.0004 vs. ECM). In addition to the MTT cell viability assay, a Pico-Green study was also used to indirectly quantify the number of cells adherent to each scaffold. A significantly higher level of HUVECs were detected on POC-ECM compared to ECM alone at both 37°C and 45°C processing temperatures (Fig. 6 I, p=0.017 POC-ECM vs ECM at 37°C, and p=0.0034 POC-ECM vs ECM at 45°C). However, no significant change in cell adhesion was detected with heparin conjugation at either processing temperature. (Fig. 6 I, p=0.279 heparin-POC-ECM vs ECM at 37°C, and p=0.0627 POC-ECM vs ECM at 45°C)
POC-ECM composites also showed the highest number of viable HASMCs adherent to the scaffolds (Figure 6.H, 2.86±0.64 at 37°C, p=0.0190 vs. ECM; 2.15±0.19 at 45°C, p=0.0280 vs. ECM). However after heparin immobilization, HASMCs viability on heparin-POC-ECM decreased to the baseline level, similar to that on ECM scaffolds (1.54±0.28 at 37°C, p=0.2065 vs. ECM; 1.22±0.09 at 45°C, p=0.5350 vs. ECM). The decrease in HASMC viability after heparin immobilization onto POC-ECM is likely caused by the effect of heparin on inhibiting smooth muscle cell proliferation,[11] which may be beneficial as a tissue engineering therapy by inhibiting intimal hyperplasia that typically limits long-term patency.
Pico-Green assays yielded results similar to the ones found through the MTT assay. A significantly higher number of HASMCs were detected on POC-ECM compared to ECM at both 37°C and 45°C processing temperatures (Fig. 6J, p=0.0202 POC-ECM vs ECM at 37°C, and p=0.0475 POC-ECM vs ECM at 45°C). After heparin conjugation to POC-ECM, a relative decrease in cell adhesion was observed at 37°C processing temperature but not at 45°C (p=0.0403 heparin-POC-ECM vs POC-ECM at 37°C, and p=0.0601 heparin-POC-ECM vs heparin-ECM at 45°C). No significant difference was observed between heparin-POC-ECM and untreated ECM. (p=0.312 Heparin-POC-ECM vs ECM at 37°C, and p=0.172 Heparin-POC-ECM vs ECM at 45°C).
4. Discussion
In this study, we describe a method to: 1) develop a decellularized ECM-based vascular scaffold that maintains the original three-dimensional structure and mechanical properties of the native aorta as a model to study the thrombo-resistance of novel anticoagulant additives, and 2) create a heparinized polymer-ECM composite matrix with reduced thrombogenicity compared to the native ECM matrix and with sufficient cell biocompatibility, equivalent or improved to untreated ECM. Taken together, this strategy addresses a major current limitation in decellularized scaffold technology, which is the tendency for thrombi formation and blockage of small vessels due to exposed ECM proteins upon interaction with whole blood. [3] Using the decellularized aorta as a model vascular conduit, we further show that the polymer-ECM complex allows for proliferation of HUVECS and, to a lesser extent HASMCs, indicating that future applications of this strategy may incorporate autologous cells to form a functional vasculature within tissue-engineered organs. Both the polymer and ECM are biodegradable, allowing for tissue remodeling over time as the matrix is gradually replaced with ECM derived from the new, recellularized scaffold. The capacity for POC to support cell seeding and tissue remodeling in vivo has been demonstrated in a bladder regeneration animal model whereby POC films seeded with mesenchymal stem cells and hematopoietic stem cells gave rise to histologically normal bladder tissue.[12] [13]
We developed a strategy to decellularize the rat aorta as a proof of concept model for testing a polymer-ECM composite for a tissue-engineering scaffold. A number of decellularization protocols have been reported to decellularize tissues, including the use of ionic or non-ionic detergents (e.g. sodium deoxycholate, SDS and Triton X), enzymes (e.g. trypsin, dispase and nuclease), osmotic pressure change or a combination of several strategies.[14] We incorporated some of the methods and optimized our protocol to ensure efficient elimination of cellular components without disrupting the ECM. Our protocol included a series of mild detergents (low concentration of Triton X-100 and SDS) to remove cells and demonstrate that DNase rinse is necessary to remove residual DNA. Our protocol resulted in over 95% DNA removal, which is improved compared to some [15] and comparable to many of the reported protocols for artery decellularization.[16] Although the duration of the decellularization process is 5 days, the mild conditions used during the process allows for maintenance of ECM structural integrity and mechanical strength and also preserves extracellular growth factors (such as FGF), while effectively eliminating cells and cell-based components. We chose FGF as a proof-of-principle to demonstrate that matrix-bound extracellular growth factors are retained within the decellularized scaffolds. Moreover, FGF promotes angiogenesis in small arterial vessels, and could be important in recruiting vascular cells to a scaffold in vivo. [17]
Our rationale behind integrating POC with the ECM is to exploit the advantages of both synthetic and natural biomaterials. Combining a synthetic polymer with decellularized tissue ECM has been previously explored for several tissue engineering applications, including bones[18], heart valves[19], and vascular grafts[20]. In the latter study, decellularized and cross-linked porcine aortic grafts were coated with poly(D,L-lactide) (PDLLA) that eluted the anticoagulant drug (lepirudin) from the polymer coating.[20] However, while the lepirudin elution reduced clot burden and improved patency, the polymer coating led to significant intimal hyperplasia and inhibited cellular repopulation.
In comparison, we use a biocompatible elastomer, POC, as an intermediate material to increase the carboxylate-biding sites to link heparin to the ECM-based scaffold. Previously, our lab has used this biodegradable polymer to coat ePTFE to improve the hemocompatibility of synthetic vascular grafts.[6] However, in that study elevated POC concentrations and high annealing temperatures were used to coat ePTFE vascular grafts (10% POC and post-polymerization annealing at 80°C), which are not suitable for the delicate proteinaceous ECM because high polymer concentration and supra-physiologic postpolymerization temperature leads to ECM denaturation and ECM stiffness. Masson’s trichrome staining for 10% POC-coated decellularized aorta at 80°C post-polymerization temperature demonstrate absence of collagen staining, confirming the degradation or denaturation of ECM proteins. We therefore optimized hybridization conditions using a lower 1% concentration of POC and post-polymerization temperatures of 37°C or 45°C. Both post-polymerization temperatures used in this study led to successful hybridization of POC onto ECM to form composite scaffolds, and the subsequent immobilization of heparin, without denaturing the ECM. However, the 45°C processing temperature led to a lower number of adherent platelets (Fig. 5A) and a higher number of viable HUVECs (Fig. 6G) when compared to composites processed at 37°C. Therefore, we recommend that future applications using this strategy should apply the 45°C POC post-polymerization temperature as the optimal strategy for POC-ECM hybridization for tissue engineering applications.
It is noteworthy that a number of studies have used EDC/NHS conjugation chemistry to immobilize heparin directly onto vascular grafts or other ECM-based scaffolds to improve blood biocompatibility. [21] [22] [23] [24] In comparison, we report the first study that explores hybridization of a biodegradable polymer to the ECM as an intermediary for heparin binding. One of the major advantages of using POC prior to heparin immobilization is its ability to improve endothelial cell adhesion to the resulting POC-ECM composite scaffold (Fig. 6), which is important in vascular tissue engineering as well as other organ engineering applications. POC supports endothelial cell adhesion and proliferation on a variety of surfaces.[6] [7] [8] [25] Additionally, POC can be readily modified to slowly release targeted levels of nitric oxide, a natural molecule that inhibits platelet adhesion and promotes endothelialization.[26] [27] Moreover, POC hybridization provides additional functional groups for chemical derivatization to increase heparin immobilization density. The importance of the POC component is evident by the ability of heparin-POC-ECM to significantly reduced degree of blood clotting compared to direct heparin conjugation to the ECM (heparin-ECM) (Fig. 5).
Lastly, POC has also been shown to have significant antioxidant activity,[28] which may contribute to its observed anti-inflammatory properties.[29] Therefore, the use of POC on the ECM of decellularized tissues or organs could potentially decrease inflammatory responses in vivo by masking any residual antigen and/or attenuate oxidative stress in tissues.
Scaffolds made from composite synthetic polymers and/or ECM proteins must support the function of vascular cells, including both endothelial and smooth muscle cells, to minimize vessel thrombosis.[30] A healthy endothelial cell lining is important for proper functioning of the vasculature such as preventing coagulation, regulating delivery of inflammatory cells, and controlling formation of new blood vessels.[31] Ideally, a vascular graft should support healthy and viable endothelial cell lining but inhibit over-proliferation of smooth muscle cells, which causes intimal hyperplasia and leads to low graft patency.[32] We show that the POC-ECM composite scaffold supports endothelial cell proliferation and recellularization. HUVECs seeded onto POC-ECM or heparin-POC-ECM at 45°C processing temperature showed a 2.74±0.41 fold or a 2.15±0.16 fold increase, respectively, in cell viability compared to cells seeded on the untreated ECM scaffold. The composite scaffold may either be used to recruit endothelial progenitor cells in circulating blood after implantation, or be used to seed endothelial cells from the recipient prior to implantation. On the other hand, viability and adhesion of HASMC to heparin-POC-ECM was comparable to untreated ECM, suggesting equivalent biocompatibility to HASMCs.
Our strategy using POC to coat ECM scaffolds opens up a broader application of this methodology to further modify ECM-based scaffolds with mitogens, adhesion proteins or growth factors to enhance cell adhesion, proliferation, and viability. For example, incorporating growth factors such as vascular endothelial growth factor (VEGF) [33], stromal cell-derived factor-1α (SDF-1α) [34] or granulocyte-colony stimulating factor (G-CSF) [35] into decellularized arterial scaffolds showed increased endothelial cell recruitment in various animal models, including canine carotid artery interposition model [33], rat carotid artery interposition model [34], and rat abdominal aorta interposition model [35]. Autologous recellularization with endothelial progenitor cells isolated from canine[36] or sheep[37] peripheral blood prior to implanting into canine/sheep carotid artery interposition models also led to decreased intimal hyperplasia and improved graft patency. New strategies to develop endogenous cell sources, such as induced pluripotent stem cell (iPSC)-derived endothelial cells [38], provide a novel approach to reconstruct tissue engineered vascular grafts, especially in the case of chronic cardiovascular diseases, where the number and viability of circulating endothelial progenitor cells are impaired.[39] We demonstrate that a POC-ECM composite vascular graft exhibits excellent biocompatibility towards endothelial cells and thus may be used as a substrate for recellularization to develop a tissue engineered small diameter vascular graft. Furthermore, this strategy could potentially be applied to the vasculature of other tissue-engineered organ systems where the decellularization process is used to produce an acellular ECM scaffold template for organ engineering.
5. Conclusion
We describe a method to generate a polymer-ECM composite for organ/tissue engineering applications to reduce thrombogenicity of decellularized scaffolds. The decellularization strategy removes the cellular components, while the ECM structure is well preserved. Modification of decellularized vascular grafts with POC and heparin results in a significant improvement in graft anti-thrombotic properties and vascular cell biocompatibility. Therefore, this polymer-ECM composite scaffold has great potential for use as a small-diameter vascular graft or may be applied more broadly to reduce the thrombogenicity in the vasculature of engineered organs developed using a similar decellularization strategy.
Acknowledgment
This work was supported by the Dixon Translational Research Grants Innovation Award from the Northwestern Memorial Foundation and the Northwestern University Chemistry of Life Processes Chairman’s Innovation Award given to Drs. Jason Wertheim and Guillermo Ameer. This work was also supported in part by grant 5R01EB017129-02 from the National Institute of Biomedical Imaging and Bioengineering. Dr. Jason Wertheim acknowledges support from the Robert R. McCormick Foundation, NIDDK K08DK101757, and the Excellence in Academic Medicine Act through the Illinois Department of Healthcare and Family Services. Bin Jiang acknowledges support from the American Heart Association’s Mid-Western Association Postdoctoral Fellowship. The authors would also like to thank the Northwestern University Microsurgical Core for performing animal surgeries. This work was supported by the Northwestern University Mouse Histology and Phenotyping Laboratory and a Cancer Center Support Grant (NCI CA060553). Imaging work was performed at the Northwestern University Cell Imaging Facility generously supported by NCI CCSG P30CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
POC is a patented polymer that is licensed to VesselTek BioMedical, LLC. Dr. Guillermo Ameer has ownership interests in VesselTek Biomedical LLC.
References
- 1.Wertheim JA, Petrowsky H, Saab S, Kupiec-Weglinski JW, Busuttil RW. Major Challenges Limiting Liver Transplantation in the United States. American Journal of Transplantation. 2011;11:1773–1784. doi: 10.1111/j.1600-6143.2011.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soto-Gutierrez A, Wertheim JA, Ott HC, Gilbert TW. Perspectives on whole-organ assembly: moving toward transplantation on demand. The Journal of clinical investigation. 2012;122:3817–3823. doi: 10.1172/JCI61974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Arenas-Herrera J, Ko I, Atala A, Yoo J. Decellularization for whole organ bioengineering. Biomedical Materials. 2013;8:014106. doi: 10.1088/1748-6041/8/1/014106. [DOI] [PubMed] [Google Scholar]
- 5.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nature medicine. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 6.Hoshi RA, Van Lith R, Jen MC, Allen JB, Lapidos KA, Ameer G. The blood and vascular cell compatibility of heparin-modified ePTFE vascular grafts. Biomaterials. 2013;34:30–41. doi: 10.1016/j.biomaterials.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen JB, Khan S, Lapidos KA, Ameer GA. Toward Engineering a Human Neoendothelium with Circulating Progenitor Cells. STEM CELLS. 2010;28:318–328. doi: 10.1002/stem.275. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Motlagh D, Allen JB, Webb AR, Kibbe MR, Aalami O, et al. Modulating Expanded Polytetrafluoroethylene Vascular Graft Host Response via Citric Acid-Based Biodegradable Elastomers. Advanced Materials. 2006;18:1493–1498. [Google Scholar]
- 9.Yang J, Motlagh D, Webb AR, Ameer GA. Novel biphasic elastomeric scaffold for small-diameter blood vessel tissue engineering. Tissue engineering. 2005;11:1876–1886. doi: 10.1089/ten.2005.11.1876. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Zhang Y, Gautam S, Liu L, Dey J, Chen W, et al. Development of aliphatic biodegradable photoluminescent polymers. Proceedings of the National Academy of Sciences. 2009;106:10086–10091. doi: 10.1073/pnas.0900004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart EM, Liu X, Clark GM, Kapsa RMI, Wallace GG. Inhibition of smooth muscle cell adhesion and proliferation on heparin-doped polypyrrole. Acta Biomaterialia. 2012;8:194–200. doi: 10.1016/j.actbio.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 12.Sharma AK, Hota PV, Matoka DJ, Fuller NJ, Jandali D, Thaker H, et al. Urinary bladder smooth muscle regeneration utilizing bone marrow derived mesenchymal stem cell seeded elastomeric poly (1,8-octanediol-co-citrate) based thin films. Biomaterials. 2010;31:6207–6217. doi: 10.1016/j.biomaterials.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 13.Sharma AK, Bury MI, Fuller NJ, Marks AJ, Kollhoff DM, Rao MV, et al. Cotransplantation with specific populations of spina bifida bone marrow stem/progenitor cells enhances urinary bladder regeneration. Proceedings of the National Academy of Sciences. 2013;110:4003–4008. doi: 10.1073/pnas.1220764110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahl SL, Koh J, Prabhakar V, Niklason LE. Decellularized native and engineered arterial scaffolds for transplantation. Cell transplantation. 2003;12:659–666. [PubMed] [Google Scholar]
- 16.Wilshaw S-P, Rooney P, Berry H, Kearney JN, Homer-Vanniasinkam S, Fisher J, et al. Development and Characterization of Acellular Allogeneic Arterial Matrices. Tissue Engineering Part A. 2011;18:471–483. doi: 10.1089/ten.tea.2011.0287. [DOI] [PubMed] [Google Scholar]
- 17.Parsons-Wingerter P, Elliott KE, Clark JI, Farr AG. Fibroblast growth factor-2 selectively stimulates angiogenesis of small vessels in arterial tree. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:1250–1256. doi: 10.1161/01.atv.20.5.1250. [DOI] [PubMed] [Google Scholar]
- 18.Sun X-j, Peng W, Yang Z-l, Ren M-l, Zhang S-c, Zhang W-g, et al. Heparin-Chitosan-Coated Acellular Bone Matrix Enhances Perfusion of Blood and Vascularization in Bone Tissue Engineering Scaffolds. Tissue Engineering Part A. 2011;17:2369–2378. doi: 10.1089/ten.TEA.2011.0027. [DOI] [PubMed] [Google Scholar]
- 19.Stamm C, Khosravi A, Grabow N, Schmohl K, Treckmann N, Drechsel A, et al. Biomatrix/Polymer Composite Material for Heart Valve Tissue Engineering. The Annals of Thoracic Surgery. 2004;78:2084–2093. doi: 10.1016/j.athoracsur.2004.03.106. [DOI] [PubMed] [Google Scholar]
- 20.Heidenhain C, Weichert W, Schmidmaier G, Wildemann B, Hein M, Neuhaus P, et al. Polymer coating of porcine decellularized and cross-linked aortic grafts. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2010;94:256–263. doi: 10.1002/jbm.b.31650. [DOI] [PubMed] [Google Scholar]
- 21.Conklin B, Richter E, Kreutziger K, Zhong D-S, Chen C. Development and evaluation of a novel decellularized vascular xenograft. Medical engineering & physics. 2002;24:173–183. doi: 10.1016/s1350-4533(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 22.Wissink MJB, Beernink R, Pieper JS, Poot AA, Engbers GHM, Beugeling T, et al. Immobilization of heparin to EDC/NHS-crosslinked collagen. Characterization and in vitro evaluation. Biomaterials. 2001;22:151–163. doi: 10.1016/s0142-9612(00)00164-2. [DOI] [PubMed] [Google Scholar]
- 23.Wang X-N, Chen C-Z, Yang M, Gu YJ. Implantation of Decellularized Small-caliber Vascular Xenografts With and Without Surface Heparin Treatment. Artificial Organs. 2007;31:99–104. doi: 10.1111/j.1525-1594.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- 24.Liao D, Wang X, Lin PH, Yao Q, Chen C. Covalent linkage of heparin provides a stable anti-coagulation surface of decellularized porcine arteries. Journal of cellular and molecular medicine. 2009;13:2736–2743. doi: 10.1111/j.1582-4934.2008.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motlagh D, Allen J, Hoshi R, Yang J, Lui K, Ameer G. Hemocompatibility evaluation of poly(diol citrate) in vitro for vascular tissue engineering. Journal of Biomedical Materials Research Part A. 2007;82A:907–916. doi: 10.1002/jbm.a.31211. [DOI] [PubMed] [Google Scholar]
- 26.Zhao H, Serrano MC, Popowich DA, Kibbe MR, Ameer GA. Biodegradable nitric oxide-releasing poly (diol citrate) elastomers. Journal of Biomedical Materials Research Part A. 2010;93:356–363. doi: 10.1002/jbm.a.32536. [DOI] [PubMed] [Google Scholar]
- 27.Jen MC, Serrano MC, van Lith R, Ameer GA. Polymer-Based Nitric Oxide Therapies: Recent Insights for Biomedical Applications. Advanced Functional Materials. 2012;22:239–260. doi: 10.1002/adfm.201101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Lith R, Gregory EK, Yang J, Kibbe MR, Ameer GA. Engineering biodegradable polyester elastomers with antioxidant properties to attenuate oxidative stress in tissues. Biomaterials. 2014;35:8113–8122. doi: 10.1016/j.biomaterials.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregory EK, Webb AR, Vercammen JM, Flynn ME, Ameer GA, Kibbe MR. Periadventitial atRA citrate-based polyester membranes reduce neointimal hyperplasia and restenosis after carotid injury in rats. American Journal of Physiology-Heart and Circulatory Physiology. 2014;307:H1419–H1429. doi: 10.1152/ajpheart.00914.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang B, Akar B, Waller TM, Larson JC, Appel AA, Brey EM. Design of a composite biomaterial system for tissue engineering applications. Acta Biomaterialia. 2014;10:1177–1186. doi: 10.1016/j.actbio.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 31.Michiels C. Endothelial cell functions. Journal of cellular physiology. 2003;196:430–443. doi: 10.1002/jcp.10333. [DOI] [PubMed] [Google Scholar]
- 32.Kapadia MR, Popowich DA, Kibbe MR. Modified prosthetic vascular conduits. Circulation. 2008;117:1873–1882. doi: 10.1161/CIRCULATIONAHA.107.714170. [DOI] [PubMed] [Google Scholar]
- 33.Zhou M, Liu Z, Wei Z, Liu C, Qiao T, Ran F, et al. Development and Validation of Small-diameter Vascular Tissue From a Decellularized Scaffold Coated With Heparin and Vascular Endothelial Growth Factor. Artificial organs. 2009;33:230–239. doi: 10.1111/j.1525-1594.2009.00713.x. [DOI] [PubMed] [Google Scholar]
- 34.Yu J, Wang A, Tang Z, Henry J, Li-Ping Lee B, Zhu Y, et al. The effect of stromal cell-derived factor-1α/heparin coating of biodegradable vascular grafts on the recruitment of both endothelial and smooth muscle progenitor cells for accelerated regeneration. Biomaterials. 2012;33:8062–8074. doi: 10.1016/j.biomaterials.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou M, Liu Z, Li K, Qiao W, Jiang X, Ran F, et al. Beneficial effects of granulocyte-colony stimulating factor on small-diameter heparin immobilized decellularized vascular graft. Journal of Biomedical Materials Research Part A. 2010;95A:600–610. doi: 10.1002/jbm.a.32864. [DOI] [PubMed] [Google Scholar]
- 36.Zhou M, Liu Z, Liu C, Jiang X, Wei Z, Qiao W, et al. Tissue engineering of small-diameter vascular grafts by endothelial progenitor cells seeding heparin-coated decellularized scaffolds. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100:111–120. doi: 10.1002/jbm.b.31928. [DOI] [PubMed] [Google Scholar]
- 37.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nature medicine. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belair DG, Whisler JA, Valdez J, Velazquez J, Molenda JA, Vickerman V, et al. Human Vascular Tissue Models Formed from Human Induced Pluripotent Stem Cell Derived Endothelial Cells. Stem cell reviews. 2014 doi: 10.1007/s12015-014-9549-5. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circulation research. 2001;89:e1–e7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]