Abstract
In the modern era, contemporary management of male infertility has undergone groundbreaking changes with the introduction of new concepts, advanced testing, and therapeutic interventions. As practicing gynecologists are often the first physicians who encounter an infertile couple, it is essential that these clinicians are continuously updated about the new pearls and pitfalls of male infertility management. Semen analysis is commonly ordered by gynecologists. In 2010, the WHO released new cutoff reference values for the semen parameters adopting novel methodology, which has incited much debate. Reference values have been lowered in comparison with previous standards, with a direct clinical implication in decision-making strategies. Specialized sperm-function tests, such as sperm oxidative stress and sperm chromatin integrity assessments, became clinically available, thus offering an opportunity to better understand sperm dysfunctions concealed during routine semen analysis. Furthermore, the initial counseling of azoospermic men by an andrologically well educated gynecologist may alleviate the misconception and distress surrounding the false belief of sterility, and will clarify the available options of percutaneous and microsurgical sperm-retrieval techniques and assisted conception outcome. Regarding varicocele, which is commonly seen in infertile males, it is now clear that the best treatment option for infertile men with clinical varicocele is the microsurgical vein ligation. Natural conception is significantly improved after varicocelectomy, and recent data suggest that such treatment optimizes reproductive outcome of couples undergoing ICSI or micro-TESE sperm retrieval. Lastly, new therapeutic interventions, including oral antioxidant therapy and lifestyle modifications, have gained increasing attention, as they aid in alleviating male infertility.
Keywords: Gynecologist, Male infertility, Semen analysis, Azoospermia, Varicocele, Assisted reproductive technique, Sperm function
Introduction
Male factor infertility (MFI), alone or combined with female factor infertility, accounts for approximately 50 % of fertility problems [1]. MFI has undergone revolutionary changes in terms of diagnostic and treatment options, particularly in the last two decades. The objective of this article is to provide a better understanding of the evolving concepts in the field of male infertility to gynecologists and all health professionals involved in reproductive medicine.
Definitions
The World Health Organization (WHO) defines male factor infertility as the presence of abnormalities in the semen analysis (SA), or the presence of sexual or ejaculatory dysfunction [2]. However, a male infertility factor may be present even when the semen analysis is normal. The results of SA within normal ranges, as conventionally assessed for sperm count, motility, and morphology, have low predictive power of only 60 % to forecast the occurrence of a natural pregnancy [3].
Normal daily sperm production is about 40 million and declines with aging. The duration of spermatogenesis has been recently found to be about 60 days instead of 70 ± 4 days as had previously been described for past many years [4]. Sperm production essentially corresponds to the interactive outcome of biological, physical, and occupational factors, acting within the preceding 2 months from ejaculation. Known male infertility etiologies include reversible and correctable causes to uncorrectable ones. These etiologies may be inherited or acquired. In 37–58 % of the cases, MFI has an unknown origin, which can be idiopathic and unexplained [5–7]. Idiopathic MFI is characterized by an unexplained impairment in semen quality with no previous history of fertility problems, in association with normal findings on physical examination and endocrine laboratory testing [6]. Unexplained MFI is reserved for infertile men with semen profiles within normal ranges, and in whom female infertility factors have been ruled out. This category accounts for 6–27 % of male infertility, and it strongly depends on how exhaustive is the evaluation of the male patient [5]. Table 1 depicts the male infertility history outline, and Table 2 shows the distribution of the causes of MFI in a tertiary care center [8, 9].
Table 1.
Clinical male infertility history outline
| 1. Infertility history |
| Age of partners, time attempting to conceive |
| Contraceptive methods/duration |
| Previous pregnancy (actual partner/other partner) |
| Previous treatments |
| Treatments/evaluation of female partner |
| 2. Sexual history |
| Potency, libido, lubricant use |
| Ejaculation, timed intercourse, frequency of masturbation |
| 3. Childhood and development |
| Cryptorchidism, hernia, testicular trauma |
| Testicular torsion, infection (e.g., mumps) |
| Sexual development, puberty onset |
| 4. Personal history |
| Systemic diseases (diabetes, cirrhosis, hypertension) |
| Sexually transmitted diseases, tuberculosis, viral infections |
| 5. Previous surgeries |
| Orchidopexy, herniorraphy, orchiectomy (testicular cancer, torsion) |
| Retroperitoneal and pelvic surgery |
| Other inguinal, scrotal, and perineal surgery |
| Bariatric surgery, bladder neck surgery, transurethral resection of the prostate |
| 6. Gonadotoxin exposure |
| Pesticides, alcohol, cocaine, marijuana abuse |
| Medication (chemotherapy agents, cimetidine, sulfasalazine, nitrofurantoin, allopurinol, colchicine, thiazide, β- and α-blockers, calcium blockers, finasteride) |
| Organic solvents, heavy metals |
| Anabolic steroids, tobacco use |
| High temperatures, electromagnetic energy |
| Radiation (therapeutic, nuclear power plant workers), etc. |
| 7. Family history |
| Cystic fibrosis, endocrine diseases |
| Infertility in the family |
| 8. Current health status |
| Respiratory infection, anosmia |
| Galactorrhea, visual disturbances |
| Obesity |
Table 2.
Distribution of diagnostic categories of couples seeking infertility evaluation in a male infertility clinic*
| Category | N | % |
|---|---|---|
| Varicocele | 629 | 21.9 |
| Infectious | 72 | 2.5 |
| Hormonal | 54 | 1.9 |
| Ejaculatory dysfunction | 28 | 1.0 |
| Systemic diseases | 11 | 0.4 |
| Idiopathic | 289 | 10.0 |
| Normal/female factor | 492 | 17.1 |
| Immunologic | 54 | 1.9 |
| Obstruction | 359 | 12.5 |
| Cancer | 11 | 0.4 |
| Cryptorchidism | 342 | 11.9 |
| Genetic | 189 | 6.6 |
| Testicular failure | 345 | 11.9 |
| Total | 2,875 |
*Androfert, Brazil
Gynecologist’s Role in the Initial Male Workup
A thorough history and a preliminary SA must be obtained before referring the male partner to an urologist. Advanced male age and a longstanding history of infertility are negative factors for fecundity, similar to female infertility. Secondary male infertility is often associated with correctable causes such as varicocele, infection, and ejaculatory problems. A history of early miscarriages or fetal genetic abnormalities may suggest a male factor contribution [10, 11]. Other history components should include sexual, family, childhood, and developmental and surgical history of the infertile couple, as well as the presence of systemic medical conditions.
As paternal age increases, the conception rate decreases, and the risk of genetic defects in offspring rises. Sperm chromosomal aneuploidy increases with paternal age as well. By around age 35, both sperm DNA fragmentation and germ cell apoptosis start to rise [12–14], while semen volume, sperm morphology, and motility decline [15–17]. The risk of having a child with autosomal dominant disorders for older men is equal to that of having a child with Down syndrome for women aged >45 years [18].
A detailed history about current use of medication is also important. Antihypertensive drugs such as alpha- and beta-blockers, thiazide diuretics, and spironolactone may cause erectile and ejaculatory dysfunction. Calcium-channel blockers may negatively impact sperm-fertilizing ability by blocking the acrosome reaction. Antibiotics such as gentamicin, erythromycin, and nitrofurantoin are gonadotoxic [19]. Cimetidine, spironolactone, certain hormonal preparations, and anabolic steroids may alter the hypothalamic–pituitary gonadal axis, thus affecting spermatogenesis [20]. Cancer treatments such as radiotherapy and chemotherapy also decrease sperm production.
Obesity has been also linked with male subfertility [21, 22]. Obesity is associated with altered semen parameters [23–25]. Furthermore, reduced sperm DNA integrity is common in infertile obese men [26]. These changes are attributed to high estrogen production from fat stores and/or high accumulation of fertility-jeopardizing environmental toxins in fatty tissue. Occupational exposure to toxicants and exposure to excessive heat from sauna and hot tubs are detrimental to sperm production [20]. The use of pesticides, radiation exposure from X-ray, excessive use of cell phones, and heavy metal intoxication may also potentially affect sperm production and quality [27].
Semen Analysis
Semen analysis is the corner stone of infertility evaluation as it provides information on the functional status of the seminiferous tubules, epididymis, and accessory sex glands. Physical properties of semen such as viscosity, color, and pH are assessed as well as semen volume and several microscopic parameters including sperm concentration, motility (percentage of motile sperm), morphology (percentage of normally shaped sperm), viability (percentage of living sperm), and number of leukocytes [28].
The semen parameters from same individuals are highly variable due to factors such as duration of ejaculatory abstinence, activity of the accessory sex glands, analytic errors, and inherent biological variability [29–31]. Clinicians should request at least two SAs following 2–5 days of ejaculatory abstinence to assess the baseline semen-quality status [32, 33].
Guidelines for evaluation, such as those issued by the American Urological Association and European Association of Urology, rely to a large extent upon the concept of abnormal SA for management [6, 34]. These recommendations understate the limitations of the SA results and do not discuss the paradigm shift that is likely to occur in referrals and management in the light of the recent changes in the WHO reference thresholds [35, 36]. Much debate has taken place thereafter, and a series of reports have questioned the validity of the newly released reference values [36–38]. Table 3 highlights the cutoff values for SA as published in consecutive WHO guidelines [35, 36]
Table 3.
Cutoff reference values for semen characteristics as published in consecutive WHO manuals
| Semen characteristics |
WHO 1980 | WHO 1987 | WHO 1992 | WHO 1999 | WHO 2010a |
|---|---|---|---|---|---|
| Volume (mL) | ND | ≥2 | ≥2 | ≥2 | 1.5 |
| Sperm count (106/mL) | 20–200 | ≥20 | ≥20 | ≥20 | 15 |
| Total sperm count (106) | ND | ≥40 | ≥40 | ≥40 | 39 |
| Total motility (% motile) | ≥60 | ≥50 | ≥50 | ≥50 | 40 |
| Progressive motilityb | ≥2c | ≥25 % | ≥25 % (grade a) | ≥25 % (grade a) | 32 % (a + b) |
| Vitality (% alive) | ND | ≥50 | ≥75 | ≥75 | 58 |
| Morphology (% normal forms) | 80.5 | ≥50 | ≥30d | (14) | 4f |
| Leukocyte count (106/mL) | <4.7 | <1.0 | <1.0 | <1.0 | <1.0 |
ND not defined, ART assisted reproductive techniques, G-band karyotype Giemsa band karyotype, CFTR cystic fibrosis transmembrane conductance regulator
aLower reference limit obtained from the lower fifth centile value
bGrade a = rapid progressive motility (> 25 μm/s); grade b = slow/sluggish progressive motility (5–25 μm/s); Normal = 50 % motility (grades a + b) or 25 % progressive motility (grade a) within 60 min of ejaculation
cForward progression (scale 0–3)
dArbitrary value
eValue not defined but strict criterion is suggested
fStrict (Tygerberg) criterion
Recommendation for treatment has been also based on the results of SAs. Current guidelines for varicocele indicate that treatment should be offered to men with clinical varicocele in the presence of abnormal semen parameters [39, 40]. Application of the new WHO reference values might lead to patients, earlier deemed to be candidates for varicocele repair, now being considered ineligible for treatment if their semen parameters are above cutoff limits. Of note, the most recent report on varicocele by the American Society for Reproductive Medicine acknowledged the limitations of the routine SA and included the presence of an abnormal sperm-function test as an indication for treatment [40].
Yet another example is sperm morphology thresholds of which were lowered to 4 % in the 2010 WHO guidelines compared with 14 % prescribed in the previous 1999 standards [41–43]. Infertility specialists recommend intracytoplasmic sperm injection (ICSI) instead of conventional IVF or intrauterine insemination (IUI) in situations when the morphology results are below 4 %, owing to the markedly lower pregnancy outcomes of these two treatment methods when using semen with low percentage of normal sperm [44, 45]. Interestingly, the results of distribution of SA of fertile men in centiles, as shown by the new WHO standards, clearly show that, although 5 % of the studied men had morphology values below the 4 % cutoff point, they still initiated an unassisted pregnancy within 12 months of unprotected intercourse [35, 36]. Physicians treating infertile couples should exercise circumspection when interpreting the results of routine SA because it is only a tool among several others for determining clinical care. The male infertility evaluation has to be complemented with a proper physical examination, a comprehensive history taking, and relevant endocrine, genetic, and other investigations [46, 47].
Current Sperm Function Tests
Before the advent of ICSI, tests which assessed antisperm antibodies [48], sperm hyperactivation and acrosome reaction, sperm binding, and penetration to the human zona pellucida were widely used both to investigate males with unexplained infertility and to predict the fertilizing potential of sperm in conventional IVF.
Our improved understanding of the molecular mechanisms controlling sperm function enabled the development of new diagnostic tests, particularly oxidative stress (OS), and nuclear DNA integrity testing [49–51]. These markers cannot be detected by routine SA but seems to better correlate with the male fertility status than the latter [52–58].
Sperm Chromatin Integrity Testing
Sperm DNA damage is the loss of DNA integrity, and it may occur at any level in vivo during spermatogenesis, spermiogenesis, epididymis transit, or in vitro when spermatozoa are prepared for assisted conception [59]. Sperm DNA damage is a broad term that accounts for many defects in the DNA structure including single or double DNA strand breaks, base deletion or modification, interstrand or intrastrand DNA crosslinkage, and protamine mispackage via defective DNA–protein crosslinking [60].
Sperm with damaged DNA although defective may still retain the ability to fertilize the ova. However, such DNA damage has been associated with several infertility phenotypes, such as unexplained and idiopathic infertility, repeated IUI and IVF failures, and recurrent miscarriage [61–67]. Furthermore, the increased risks of imprinting defects and cancer in the offspring have been linked with sperm DNA damage [68, 69].
Several assays used to measure sperm DNA damage are based on different principles and therefore differ in their ability to detect DNA damage [59, 70]. In Table 4, we summarize the principles and interpretations of the most commonly used assays. A comprehensive review about the methods to measure sperm DNA damage can be found elsewhere [51].
Table 4.
Examples of the commonly used methods for assessment of sperm DNA damage
| Assay | Principle | How results are expressed | Normal limits |
|---|---|---|---|
| Terminal deoxy nucleotide transferase-mediated dUTP nick end labeling (TUNEL) assay | Measure DNA damage by incorporating DNA probes or modified nucleotides at the site of damage | Percentage of sperm with DNA damage, represented by those with the probes incorporated to DNA breaks | <19 % for TUNEL when used to discriminate fertile from unselected infertile men with 70 % accuracy |
| Sperm chromatin structure assay (SCSA) | Measure the susceptibility of DNA to denaturation | Percentage of sperm with fragmented DNA | <30 % |
| Sperm chromatin dispersion test (SCD) | Measure the susceptibility of DNA to denaturation | Percentage of sperm with fragmented DNA | <30 % |
| Comet assay | Measure the susceptibility of DNA to denaturation | Degree of DNA fragmentation in a single spermatozoon as assessed by the percentage of DNA in the tail of the comet, tail length and intensity of staining (Comet) | Not defined |
| Aniline blue staining (AB) | Measure the level of chromatin compaction | Percentage of sperm with loose chromatin packing | Not defined |
Reactive Oxygen Species Testing
Sperm reactive oxygen species (ROS) are the byproducts of oxygen metabolism, which in small concentrations regulate physiological cellular functions such as capacitation, acrosomal reaction, hyperactivation, and the fusion with the oocyte [71]. In semen, leukocytes and spermatozoa are the two main sources for ROS. In sperm, ROS are generated by both the NADPH oxidase and NADH-dependent oxido-reductase systems at the plasma membrane and mitochondrial levels, respectively [72]. When ROS levels increase disproportionately, mainly due to the presence of superoxide, hydroxyl radicals or nitric derivatives, compared with the neutralizing capacities of intracellular and extracellular antioxidants; or when a reduction in the antioxidant capacity occurs, OS is sustained.
ROS can modify lipids, proteins, and DNA through a variety of oxidative mechanisms [71, 73] causing lipid peroxidation, protein carbomoylation, and oxidized DNA, respectively. Oxidative DNA modifications can sustain serious damages to DNA, such as point mutations, polymorphisms, deletions, chromosomal rearrangements, frame shifts, and single-stranded or double-stranded breaks [74].
The assays to measure ROS, their principle, methodology, clinical utility, and drawbacks are summarized in Table 5 [75–77].
Table 5.
Examples of the methods commonly used for assessing oxidative stress
| Assay | Principle | Specimen | How results are expressed | Normal limits |
|---|---|---|---|---|
| ROS by chemiluminescence | Intra- and extracellular ROS levels (mainly H2O2, O2−, and OH−) react with probes and emit photons that are measured using a luminometer. The final chemiluminescent signal is the integrated sum of the partial signals generated by every spermatozoon | Semen | ×106 counted photons per minute (cpm) per 20 × 106 sperm/mL | <0.0185 × 106 cpm/20 × 106 sperm |
| Thiobarbituric acid reactive substances (TBARS) | Malondialdehyde (MDA), a byproduct of lipid peroxidation, condenses with two equivalents thiobarbituric acid and give a fluorescent red derivative that can be assayed spectrophotometrically. Absorbance at 532 nm is recorded | Semen and seminal plasma | nmoL MDA/ 10 × 107 sperm, nmol MDA mL−1 seminal plasma, or nmoL MDA/total seminal plasma | 0.0287 ± 0.0162 nmol/108 sperm and 0.65 ± 0.17 nmol/mL-1 seminal plasma |
| Seminal total antioxidant capacity (TAC) by enhanced chemiluminescence | Capacity of the antioxidants in a given sample to prevent ABTS oxidation is proportional to their Concentration. Suppression of absorbance at 750 nm is measured and compared with that of standard Trolox, a water-soluble tocopherol analog | Seminal plasma | Molar Trolox equivalents | >2,000 micromoles of Trolox |
Genetic Conditions Associated with Male Infertility
Approximately 6 % of infertile men have chromosomal abnormalities; the rate is even higher (~16 %) in men with azoospermia [78]. Sex chromosomal aneuploidy (Klinefelter syndrome [KS]; 47, XXY) is the most common chromosomal disorder in infertile men and is generally associated with hypotrophic or atrophic testicles, elevated serum FSH levels and azoospermia or severe oligozoospermia. In men with KS presenting with azoospermia, sperm are present in approximately 20–50 % of cases on testicular exploration, and pregnancy rates associated with ICSI range from 30 to 50 % [79]. Men with KS can have biological offspring with a normal karyotype because germ cells are usually euploid (46, XY) and thus can form normal, haploid gametes [80].
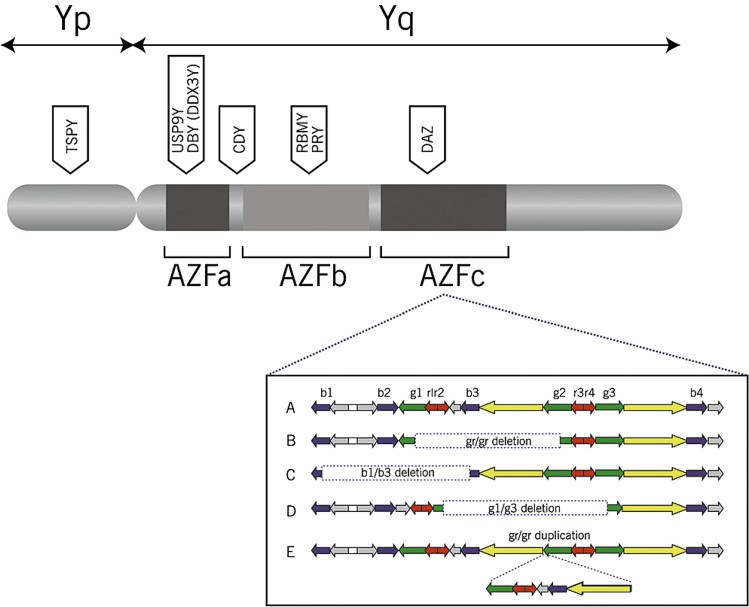
The long arm (q) of Y-chromosome contains genes regulating spermatogenesis [81]. The Y-chromosome region related to infertility is called azoospermia factor locus (AZF). This locus can harbor complete or partial microscopic deletions, isolated or in combination, in subregions called AZFa, AZFb, and AZFc (Fig. 1).
Fig. 1.
Image of Y chromosome. Reprinted with permission from: O’Flynn O’Brien KL Varghese AC, Agarwal A. The genetic causes of male factor infertility: a review. Fertil Steril. 2010 Jan;93 [1]:1–12
Yq chromosome microdeletions (YCMDs) are found in 15 % of men with azoospermia and in 6 % of men presenting with severe oligozoospermia (<1 million/mL) [78, 82, 83]. For sperm counts between 1 and 5 million/mL, the detection rate drops down to 1.7 % [84]. YCMD affecting the AZFc region usually results in severe oligozoospermia or azoospermia. Patients with AZFa microdeletions generally present with germ cell aplasia on testicular histopathology, while most patients with AZFb microdeletions present with maturation arrest [85, 86]. To test for YCMD, peripheral blood is obtained, and polymerase chain reaction is used to amplify the long arm of the Y-chromosome, which will identify deletions of the AZF regions. YCMD screening may also predict the chance of sperm retrieval (SR) for candidates of assisted conception. The findings of complete AZFa and/or AZFb microdeletions normally preclude a sperm-retrieval attempt as there is no evidence that testicular sperm can be found irrespective of the retrieval method. However, in cases with AZFc microdeletion, sperm can be retrieved in 50–71 % of patients [84]. Clinical pregnancy rates are virtually the same as those of idiopathic azoospermic patients [87]. However, the offspring of a father with YCMD will inherit the same genetic trait. The main indications for genetic testing in male infertility and the tests used to assess such conditions are highlighted in Table 6.
Table 6.
Main indications for genetic testing in male infertility
| Indications | Recommended tests |
|---|---|
| Men with infertility of unknown etiology and sperm concentration <10 million/mL who are candidates for ART | Y-chromosome microdeletion and G-band karyotype |
| Nonobstructive azoospermia in a male considering testicular sperm retrieval for ART | Y-chromosome microdeletion and G-band karyotype |
| Azoospermic or oligozoospermic men with absence of at least one vas deferens at physical examination | CFTR gene mutation |
| Azoospermic men with signs of normal spermatogenesis (e.g., obstructive azoospermia of unknown origin) | CFTR gene mutation |
| History of recurrent miscarriage or personal/familiar history of genetic syndromes | G-band karyotype |
ART assisted reproductive techniques
G-band karyotype giemsa band karyotype
CFTR cystic fibrosis transmembrane conductance regulator
The most common genetic sperm-transport disorder is the congenital bilateral absence of the vas deferens (CBAVD). Approximately 80 % of men presenting with CBAVD have mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, located on the long arm of chromosome 7. Depending on the extension of the mutation, cystic fibrosis can manifest in a full clinical presentation (an autosomic recessive potentially fatal disease) or in a mild form, i.e., CBAVD, which affects approximately 1.3 % of infertile men [88]. The female partner should also be tested for the CFTR mutation; she may be a carrier (approximately 4 % risk). Such testing should be done before the man’s sperm is used for assisted conception because the cystic fibrosis gene can be transmitted to offspring [87].
Varicocele
Varicocele is an elongated, dilated, and tortuous testicular vein in the spermatic cord. It is identified in 7 and 10–25 % of prepubertal and postpubertal males, respectively [89, 90]. Higher prevalence in elderly males and in men with secondary MFI suggests it to be a progressive disease [91, 92].
Recent data suggest that varicocele causes infertility by inducing ultrastructural testicular changes and OS, with implications for the seminal antioxidant capacity and sperm chromatin integrity [93–95]. Abnormalities of semen parameters in patients with varicocele are variable and involve sperm count, motility, and morphology [96, 97]. Men with varicocele were also shown to have lower testosterone levels, as well as reduced testicular size on the same side of varicose vessels compared to those without varicocele [98, 99].
Physical examination with the patient standing in a warm room is currently the preferred method for varicocele diagnosis and has a sensitivity and specificity of around 70 % compared with other diagnostic tools [100, 101]. The most widely used classification is as follows:
Grade 3: visible and palpable at rest (Fig. 2)
Grade 2: palpable at rest
Grade 1: palpable during Valsalva maneuver
Subclinical: not palpable or visible at rest or under Valsalva maneuver but detectable by Doppler ultrasound
Fig. 2.

Image of grade III varicocele. Reprinted with permission from Clinics (São Paulo) 2011, Esteves, Miyaoka, Agarwal. An update on the clinical assessment of the infertile male, vol. 66, issue 4, pages 691–700
Whenever physical examination is inconclusive or difficult to perform, imaging studies are recommended. Color Doppler ultrasound (CDU) has been shown to be the best diagnostic tool. Using a cutoff value of 3 mm for vein diameter, CDU has a sensitivity of about 50 % and specificity of 90 % compared with physical examination [102].
Current recommendations propose varicocele treatment for couples with documented infertility, whose male partner has a clinical varicocele and at least one abnormal semen parameter. Men not attempting to achieve conception but who fit into this description and have a desire for future fertility are also candidates for treatment [6, 103]. After varicocelectomy, the chances of natural conception increase 2.8-fold [104], and varicocele repair is more cost effective than ART [105].
In azoospermic men with favorable testicular histopathology, clinical varicocele repair may lead to sperm appearance in the ejaculate [106]. As such, IVF/ICSI can be performed without the need to surgically retrieve sperm. Sperm-retrieval success rates seems to be increased in azoospermic men with treated varicocele compared with untreated ones [107]. Live birth rates were also shown to be significantly higher in men who had the varicocele treated before ICSI (46.2 %) compared to those undergoing ICSI in the presence of a clinical varicocele (31.4 %) [108].
Azoospermia
Azoospermia is defined by the complete absence of sperm cells in the ejaculate after centrifugation without implying an underlying cause [109]. It affects approximately 1 % of the male population. Men diagnosed with azoospermia are broadly categorized as having a mechanical obstruction along the seminal tract (obstructive) or an intrinsic testicular impairment of sperm production (nonobstructive azoospermia).
In obstructive azoospermia (OA), the blockage is located between the epididymis and the ejaculatory duct. Causes of OA include CBAVD, infection, and vasectomy. Surgical reconstructive procedures are available for select patients with OA (e.g., vasectomy reversal), and surgical SR is usually successful in noncorrectable cases [110, 111].
Nonobstructive azoospermia (NOA) is caused by genetic factors, prior testicular toxic exposures such as radiation or chemotherapy, trauma, infection, and idiopathic reasons. While about 50 % of NOA patients have mature spermatozoa in their testicles, no reliable predictive factors exist to prospectively distinguish which patients may have sperm that can be surgically retrieved except in cases of YCMD as indicated above [112].
Given its untreatable nature, men with NOA seeking fertility should rely on SR and ICSI for achieving biological offspring. Among the SR methods, microsurgical testicular sperm extraction (micro-TESE) is considered to be the best option for SR in men with NOA (retrieval rates ranging from 40 to 60 %). Micro-TESE also provides the opportunity for both preserving testicular vasculature and minimizing the amount of extracted parenchyma [112, 113].
ICSI is associated with lower fertilization rates per injected oocyte as well as clinical pregnancy and delivery rates when testicular spermatozoa from men with NOA are used in comparison to epididymal/testicular sperm from men with OA [113–115]. Once a live birth is achieved, newborn parameters of infants conceived were not significantly different among the groups, and no major differences are noted in the offspring’s neonatal profile [113]. If there is no worthy sperm for fertilization, then the couple must consider adoption or donor insemination.
Prescription of Antioxidants
Fair evidence suggests that antioxidant therapy has a beneficial role in MFI. However, it is also essential to encourage lifestyle modifications, which helps in reducing the generation of free radicals. For example, increased intake of vegetables and fruits, reduction in excess weight, smoking cessation, and moderation in alcohol consumption are advised [116]. Exposures to various environmental pollutants and/or radiation should be avoided or reduced. Chemical gonadotoxins, including pesticides found in vegetables and industrial waste, can increase the formation of free radicals due to the unstable chemical compounds found in these products. Radiation exposures from cell phones and laptop computers, can produce OS by inducing cellular chemical changes through the electromagnetic waves emitted from the devices, and decrease semen quality [117].
Antioxidant therapy, including vitamins E and C, carotenoids, zinc, and selenium, enhances total antioxidant capacity of body fluids including semen resulting in scavenging of excess free radicals [118–129]. Improvements in sperm motility, sperm DNA fragmentation, fertilization capacity, and odds of normal sperm count were observed in most studies [120–123], albeit, in a few of them, no advantage was documented [124, 130]. A Cochrane meta-analysis on the use of oral antioxidants in male infertility found that these agents significantly improved pregnancy rates and live births and decreased sperm DNA damage [131]. Nevertheless, improvements in semen parameters are not well evident [131] (Table 7). These observations support the concept that antioxidants can improve sperm function by improving sperm DNA integrity and fertilizing capabilities.
Table 7.
Results of Cochrane review 2011 [131]
| Semen parameter | No. of studies No. of patients |
Average duration of treatment | Mean difference (MD) ± SD, 95 % CI | Pooled cumulative grade of response |
|---|---|---|---|---|
| Total motile sperm | 10 trials 514 patients |
3 months or less | MD 11.72, 95 % CI 6.94 to 16.49; P < 0.00001 |
+ Very low |
| 7 trials 963 patients |
6 months | MD 4.19, 95 % CI 3.81 to 4.56; P < 0.00001 |
+ Very low |
|
| 3 trials 332 patients |
9 months | MD 1.38, 95 % CI 0.81 to 1.95; P < 0.00001 |
+ Very low |
|
| Sperm Count | 7 trials 320 patients |
3 months or less | MD 6.04, 95 % CI −5.42 to 17.50; P = 0.30 |
No effect |
| 6 trials 825 patients |
6 months | MD 5.25, 95 % CI 4.43 to 6.08; P < 0.00001 |
+ Very low |
|
| 3 trials 332 patients T181, C151 |
9 months | MD 1.61, 95 % CI 0.61 to 2.61; P = 0.002 |
+ Very low |
|
| Sperm DNA fragmentation index (DFI) | 1 trial 64 patients T32, C32 |
2 months | MD -13.80, 95 % CI −17.50 to −10.10; P < 0.00001 |
Decrease DFI |
| Pregnancy rate | 15 trials 964 couples |
4.5 months | OR 4.18, 95 % CI 2.65 to 6.59; P < 0.00001 |
+ + + + High |
| Live birth per couple | 3 trials 214 couples |
4 months | OR 3.94, 95 % CI 1.14 to 13.55; P = 0.03 |
+ + + Moderate |
Counseling of Male Infertile Patients
Infertile men are often anxious, and feel guilty regarding their inability to induce a pregnancy [132]. Proper counseling of both partners should be one of the top priorities of the treating physician, and in this sense, the gynecologist plays a crucial role. The following list contains practical recommendations for couples with male factor infertility, who are attempting to conceive:
All commercially available lubricants decrease sperm motility and increase sperm DNA damage. Hydroxylethylcellulose-based lubricant was shown to be relatively less detrimental to sperm than other substances [133]. Saliva and vegetable oil also decrease sperm motility [134, 135].
Male partners should stop smoking, and limit alcohol use to 3–4 units/week, and abstain from illicit drug use [136, 137].
Prescribe exercise and weight loss for overweight or obese men. A healthy BMI ranges from 20 to 27 [137–139].
Advise the male partner to avoid any situations that can increase scrotal temperature such as sitting in a hot tubs/sauna, and placing portable computers directly on the lap. If occupational exposure to a hot work environment is unavoidable, the patient can take proper precautionary measures to minimize testicular heat exposure [93].
Abnormal findings in SA require thorough physical examination and further laboratory investigation. This workup should be discussed with the patient, and it should be explained that referral to an urologist/andrologist is recommended. In the presence of azoospermia in SA, the couple should not be discouraged and should be informed that treatment modalities are available.
If a palpable varicocele is present, then advise that surgical repair may be an option to improve fertility.
Be aware that elderly infertile men usually have chronic medical diseases, which should be identified and treated because they can negatively affect fertility.
Compliance with ethical requirements and Conflict of interest
This article complies with the ethical standards as per the Journal's policies. Each author indicates that there is no financial interest or conflict of any kind to disclose. In addition, this article does not contain any studies with animals performed by any of the authors.
Dr. Ashok Agarwal
is the Director of the Andrology Center Head of Research at the Center for Reproductive Medicine. He holds these positions at Cleveland Clinic, where he is a Professor at the Lerner College of Medicine of Case Western Reserve University; and, since 1993, Senior Staff in the Glickman Urological and Kidney Institute, Obstetrics-Gynecology; and in the Women’s Health Institute, Anatomic Pathology, and Immunology. Ashok is active in basic and clinical research, and his laboratory has trained more than 500 basic scientists and clinical researchers from the United States and over 50 countries. His current research interests are identifying biological markers of oxidative stress, DNA damage, and apoptosis using proteomic research tools and bioinformatics analysis as well as preserving fertility in patients with cancer.
Contributor Information
Ashok Agarwal, Phone: +(216) 444-9485, Email: agarwaa@ccf.org.
Alaa Hamada, Email: ala1977hh@gmail.com.
Sandro C. Esteves, Email: s.esteves@androfert.com.br
References
- 1.Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989) Hum Reprod. 1991;6(6):811–816. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 2.Rowe PJCF, Hargreave TB, et al. WHO Manual for the Standardized Investigation and Diagnosis of the Infertile Male. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- 3.van der Steeg JW, Steures P, Eijkemans MJ, et al. Role of semen analysis in subfertile couples. Fertil Steril. 2011;95(3):1013–1019. doi: 10.1016/j.fertnstert.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Misell LM, Holochwost D, Boban D, et al. A stable isotope-mass spectrometric method for measuring human spermatogenesis kinetics in vivo. J Urol. 2006;175(1):242–246. doi: 10.1016/S0022-5347(05)00053-4. [DOI] [PubMed] [Google Scholar]
- 5.Moghissi KS, Wallach EE. Unexplained infertility. Fertil Steril. 1983;39(1):5–21. doi: 10.1016/s0015-0282(16)46750-6. [DOI] [PubMed] [Google Scholar]
- 6.Dohle GRDT, Giwercman A, et al., 2011). Guidelines of male infertility. [online]. (2010) [cited 2014 July]. http://www.uroweb.org/gls/pdf/Male%20Infertility%202010.pdf.
- 7.Rowe PJ, Comhaire F, Hargreave TG, et al. WHO manual for the standardized investigation and diagnosis of the infertile couple. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 8.Witt MA, Lipshultz LI. Varicocele: a progressive or static lesion? Urology. 1993;42(5):541–543. doi: 10.1016/0090-4295(93)90268-f. [DOI] [PubMed] [Google Scholar]
- 9.Spira A. Epidemiology of human reproduction. Hum Reprod. 1986;1(2):111–115. doi: 10.1093/oxfordjournals.humrep.a136353. [DOI] [PubMed] [Google Scholar]
- 10.Puscheck EE, Jeyendran RS. The impact of male factor on recurrent pregnancy loss. Curr Opin Obstet Gynecol. 2007;19(3):222–228. doi: 10.1097/GCO.0b013e32813e3ff0. [DOI] [PubMed] [Google Scholar]
- 11.Kim JW, Lee WS, Yoon TK, et al. Chromosomal abnormalities in spontaneous abortion after assisted reproductive treatment. BMC Med Genet. 2010;11:153. doi: 10.1186/1471-2350-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80(6):1420–1430. doi: 10.1016/j.fertnstert.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Sloter E, Nath J, Eskenazi B, et al. Effects of male age on the frequencies of germinal and heritable chromosomal abnormalities in humans and rodents. Fertil Steril. 2004;81(4):925–943. doi: 10.1016/j.fertnstert.2003.07.043. [DOI] [PubMed] [Google Scholar]
- 14.Thacker PD. Biological clock ticks for men, too: genetic defects linked to sperm of older fathers. JAMA. 2004;291(14):1683–1685. doi: 10.1001/jama.291.14.1683. [DOI] [PubMed] [Google Scholar]
- 15.Rolf C, Behre HM, Nieschlag E. Reproductive parameters of older compared to younger men of infertile couples. Int J Androl. 1996;19(3):135–142. doi: 10.1111/j.1365-2605.1996.tb00451.x. [DOI] [PubMed] [Google Scholar]
- 16.Sloter E, Schmid TE, Marchetti F, et al. Quantitative effects of male age on sperm motion. Hum Reprod. 2006;21(11):2868–2875. doi: 10.1093/humrep/del250. [DOI] [PubMed] [Google Scholar]
- 17.Kuhnert B, Nieschlag E. Reproductive functions of the ageing male. Hum Reprod Update. 2004;10(4):327–339. doi: 10.1093/humupd/dmh030. [DOI] [PubMed] [Google Scholar]
- 18.Wyrobek AJ, Aardema M, Eichenlaub-Ritter U, et al. Mechanisms and targets involved in maternal and paternal age effects on numerical aneuploidy. Environ Mol Mutagen. 1996;28(3):254–264. doi: 10.1002/(SICI)1098-2280(1996)28:3<254::AID-EM9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 19.Schlegel PN, Chang TS, Marshall FF. Antibiotics: potential hazards to male fertility. Fertil Steril. 1991;55(2):235–242. doi: 10.1016/s0015-0282(16)54108-9. [DOI] [PubMed] [Google Scholar]
- 20.Nudell DM, Monoski MM, Lipshultz LI. Common medications and drugs: how they affect male fertility. Urol Clin North Am. 2002;29(4):965–973. doi: 10.1016/s0094-0143(02)00079-4. [DOI] [PubMed] [Google Scholar]
- 21.Corona G, Mannucci E, Schulman C, et al. Psychobiologic correlates of the metabolic syndrome and associated sexual dysfunction. Eur Urol. 2006;50(3):595–604. doi: 10.1016/j.eururo.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 22.Ramlau-Hansen CH, Thulstrup AM, Nohr EA, et al. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22(6):1634–1637. doi: 10.1093/humrep/dem035. [DOI] [PubMed] [Google Scholar]
- 23.Jensen TK, Andersson AM, Jorgensen N, et al. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril. 2004;82(4):863–870. doi: 10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 24.Martini AC, Tissera A, Estofan D, et al. Overweight and seminal quality: a study of 794 patients. Fertil Steril. 2010;94(5):1739–43. [DOI] [PubMed]
- 25.Hammoud AO, Wilde N, Gibson M, et al. Male obesity and alteration in sperm parameters. Fertil Steril. 2008;90(6):2222–2225. doi: 10.1016/j.fertnstert.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Smit M, Romijn JC, Wildhagen MF, et al. Sperm chromatin structure is associated with the quality of spermatogenesis in infertile patients. Fertil Steril. 2010;94(5):1748–52. [DOI] [PubMed]
- 27.Agarwal A, Deepinder F, Sharma RK, et al. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil Steril. 2008;89(1):124–128. doi: 10.1016/j.fertnstert.2007.01.166. [DOI] [PubMed] [Google Scholar]
- 28.Esteves SC, Hamada A, Agarwal A. Evaluation and diagnosis of male infertility. In: Dubey AK, editor. Infertility: diagnosis, management & IVF. Jaypee Brothers Medical Publisher; 2012. p. 29–56.
- 29.Alvarez C, Castilla JA, Martinez L, et al. Biological variation of seminal parameters in healthy subjects. Hum Reprod. 2003;18(10):2082–2088. doi: 10.1093/humrep/deg430. [DOI] [PubMed] [Google Scholar]
- 30.Keel BA. Within- and between-subject variation in semen parameters in infertile men and normal semen donors. Fertil Steril. 2006;85(1):128–134. doi: 10.1016/j.fertnstert.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 31.Castilla JA, Alvarez C, Aguilar J, et al. Influence of analytical and biological variation on the clinical interpretation of seminal parameters. Hum Reprod. 2006;21(4):847–851. doi: 10.1093/humrep/dei423. [DOI] [PubMed] [Google Scholar]
- 32.Carlsen E, Petersen JH, Andersson AM, et al. Effects of ejaculatory frequency and season on variations in semen quality. Fertil Steril. 2004;82(2):358–366. doi: 10.1016/j.fertnstert.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Pozo MC, Mendiola J, Serrano M, et al. Proposal of guidelines for the appraisal of SEMen QUAlity studies (SEMQUA) Hum Reprod. 2013;28(1):10–21. doi: 10.1093/humrep/des355. [DOI] [PubMed] [Google Scholar]
- 34.American Urological Association: The optimal evaluation of the infertile male: AUA best practice statement [revised… 2010 [cited 2014 July]; http://www.auanet.org/common/pdf/education/clinical-guidance/Male-Infertility-d.pdf.
- 35.Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 36.Esteves SC, Zini A, Aziz N, et al. Critical appraisal of World Health Organization’s new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012;79(1):16–22. doi: 10.1016/j.urology.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Barratt CL, Mansell S, Beaton C, et al. Diagnostic tools in male infertility-the question of sperm dysfunction. Asian J Androl. 2011;13(1):53–58. doi: 10.1038/aja.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haidl G. New WHO-reference limits-revolution or storm in a teapot? Asian J Androl. 2011;13(2):208–211. doi: 10.1038/aja.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Male Infertility Best Practice Policy Committee of the American Urological Association; Practice Committee of the American Society for Reproductive Medicine Report on varicocele and infertility. Fertil Steril. 2004;82(Suppl 1):S142–5. [DOI] [PubMed]
- 40.Male Infertility Best Practice Policy Committee of the American Urological Association; Practice Committee of the American Society for Reproductive Medicine Report on varicocele and infertility. Fertil Steril. 2008;90(5 Suppl):S247–9. [DOI] [PubMed]
- 41.Kruger TF, Acosta AA, Simmons KF, et al. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49(1):112–117. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction, 4th ed. Cambridge: Cambridge University Press; 1999.
- 43.World Health Organization. WHO laboratory manual for the examination and processing of human semen. Geneva: World Health Organization; 2010.
- 44.Coetzee K, Kruge TF, Lombard CJ. Predictive value of normal sperm morphology: a structured literature review. Hum Reprod Update. 1998;4(1):73–82. doi: 10.1093/humupd/4.1.73. [DOI] [PubMed] [Google Scholar]
- 45.Van Waart J, Kruger TF, Lombard CJ, et al. Predictive value of normal sperm morphology in intrauterine insemination (IUI): a structured literature review. Hum Reprod Update. 2001;7(5):495–500. doi: 10.1093/humupd/7.5.495. [DOI] [PubMed] [Google Scholar]
- 46.Esteves SC, Hamada A, Kondray V, et al. What every gynecologist should know about male infertility: an update. Arch Gynecol Obstet. 2012;286(1):217–229. doi: 10.1007/s00404-012-2274-x. [DOI] [PubMed] [Google Scholar]
- 47.Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. [corrected]. Clinics (Sao Paulo). 2011;66(4):691–700. [DOI] [PMC free article] [PubMed]
- 48.Ross C, Morriss A, Khairy M, et al. A systematic review of the effect of oral antioxidants on male infertility. Reprod Biomed Online. 2010;20(6):711–23. [DOI] [PubMed]
- 49.Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004;81(5):1289–1295. doi: 10.1016/j.fertnstert.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 50.Aitken RJ, Baker MA, Sawyer D. Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reprod Biomed Online. 2003;7(1):65–70. doi: 10.1016/s1472-6483(10)61730-0. [DOI] [PubMed] [Google Scholar]
- 51.Esteves SC, Sharma RK, Gosalvez J, et al. A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int Urol Nephrol. 2014;46(6):1037–1052. doi: 10.1007/s11255-014-0715-0. [DOI] [PubMed] [Google Scholar]
- 52.Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93(4):1027–1036. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 53.Bungum M, Bungum L, Giwercman A. Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl. 2011;13(1):69–75. doi: 10.1038/aja.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agarwal A, Nallella KP, Allamaneni SS, et al. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2004;8(6):616–627. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 55.Esteves SC, Agarwal A. Novel concepts in male infertility. Int Braz J Urol. 2011;37(1):5–15. doi: 10.1590/s1677-55382011000100002. [DOI] [PubMed] [Google Scholar]
- 56.Bungum M, Humaidan P, Spano M, et al. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004;19(6):1401–1408. doi: 10.1093/humrep/deh280. [DOI] [PubMed] [Google Scholar]
- 57.Duran EH, Morshedi M, Taylor S, et al. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod. 2002;17(12):3122–3128. doi: 10.1093/humrep/17.12.3122. [DOI] [PubMed] [Google Scholar]
- 58.Hull MG, Glazener CM, Kelly NJ, et al. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed). 1985;291(6510):1693–1697. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shamsi MB, Imam SN, Dada R. Sperm DNA integrity assays: diagnostic and prognostic challenges and implications in management of infertility. J Assist Reprod Genet. 2011;28(11):1073–1085. doi: 10.1007/s10815-011-9631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aitken RJ, De Iuliis GN, McLachlan RI. Biological and clinical significance of DNA damage in the male germ line. Int J Androl. 2009;32(1):46–56. doi: 10.1111/j.1365-2605.2008.00943.x. [DOI] [PubMed] [Google Scholar]
- 61.Kumar S. Occupational, environmental and lifestyle factors associated with spontaneous abortion. Reprod Sci. 2011;18(10):915–930. doi: 10.1177/1933719111413298. [DOI] [PubMed] [Google Scholar]
- 62.Spano M, Bonde JP, Hjollund HI, et al. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril. 2000;73(1):43–50. doi: 10.1016/s0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 63.Venkatesh S, Singh A, Shamsi MB, et al. Clinical significance of sperm DNA damage threshold value in the assessment of male infertility. Reprod Sci. 2011;18(10):1005–1013. doi: 10.1177/1933719111401662. [DOI] [PubMed] [Google Scholar]
- 64.Larson-Cook KL, Brannian JD, Hansen KA, et al. Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil Steril. 2003;80(4):895–902. doi: 10.1016/s0015-0282(03)01116-6. [DOI] [PubMed] [Google Scholar]
- 65.Host E, Lindenberg S, Ernst E, et al. DNA strand breaks in human spermatozoa: a possible factor, to be considered in couples suffering from unexplained infertility. Acta Obstet Gynecol Scand. 1999;78(7):622–5. [PubMed]
- 66.Saleh RA, Agarwal A, Nelson DR, et al. Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril. 2002;78(2):313–318. doi: 10.1016/s0015-0282(02)03219-3. [DOI] [PubMed] [Google Scholar]
- 67.Check JH, Graziano V, Cohen R, et al. Effect of an abnormal sperm chromatin structural assay (SCSA) on pregnancy outcome following (IVF) with ICSI in previous IVF failures. Arch Androl. 2005;51(2):121–124. doi: 10.1080/014850190518125. [DOI] [PubMed] [Google Scholar]
- 68.Aitken RJ, Koopman P, Lewis SE. Seeds of concern. Nature. 2004;432(7013):48–52. doi: 10.1038/432048a. [DOI] [PubMed] [Google Scholar]
- 69.Zini A, Meriano J, Kader K, et al. Potential adverse effect of sperm DNA damage on embryo quality after ICSI. Hum Reprod. 2005;20(12):3476–3480. doi: 10.1093/humrep/dei266. [DOI] [PubMed] [Google Scholar]
- 70.Gosálvez J, López-Fernández C, Fernández JL. Sperm chromatin dispersion (SCD) test: technical aspects and clinical applications. In: Zini AAA (ed) Sperm DNA damage: biological and clinical applications in male infertility and assisted reproduction, 1st ed. New York: Springer; (2011). p. 151–70.
- 71.Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol. 2012;9(12):678–690. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 72.Aitken RJ, Buckingham D, West K, et al. Differential contribution of leucocytes and spermatozoa to the generation of reactive oxygen species in the ejaculates of oligozoospermic patients and fertile donors. J Reprod Fertil. 1992;94(2):451–462. doi: 10.1530/jrf.0.0940451. [DOI] [PubMed] [Google Scholar]
- 73.Dalle-Donne I, Rossi R, Giustarini D, et al. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329(1–2):23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 74.Aitken RJ, Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction. 2001;122(4):497–506. doi: 10.1530/rep.0.1220497. [DOI] [PubMed] [Google Scholar]
- 75.Hamada A, Esteves SC, Agarwal A. Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol. 2013;10(1):26–37. doi: 10.1038/nrurol.2012.198. [DOI] [PubMed] [Google Scholar]
- 76.Desai N, Sharma R, Makker K, et al. Physiologic and pathologic levels of reactive oxygen species in neat semen of infertile men. Fertil Steril. 2009;92(5):1626–1631. doi: 10.1016/j.fertnstert.2008.08.109. [DOI] [PubMed] [Google Scholar]
- 77.Mahfouz R, Sharma R, Sharma D, et al. Diagnostic value of the total antioxidant capacity (TAC) in human seminal plasma. Fertil Steril. 2009;91(3):805–811. doi: 10.1016/j.fertnstert.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 78.Foresta C, Moro E, Ferlin A. Y chromosome microdeletions and alterations of spermatogenesis. Endocr Rev. 2001;22(2):226–239. doi: 10.1210/edrv.22.2.0425. [DOI] [PubMed] [Google Scholar]
- 79.Schiff JD, Palermo GD, Veeck LL, et al. Success of testicular sperm extraction [corrected] and intracytoplasmic sperm injection in men with Klinefelter syndrome. J Clin Endocrinol Metab. 2005;90(11):6263–6267. doi: 10.1210/jc.2004-2322. [DOI] [PubMed] [Google Scholar]
- 80.Sciurano RB, Luna Hisano CV, Rahn MI, et al. Focal spermatogenesis originates in euploid germ cells in classical Klinefelter patients. Hum Reprod. 2009;24(9):2353–2360. doi: 10.1093/humrep/dep180. [DOI] [PubMed] [Google Scholar]
- 81.Tiepolo L, Zuffardi O. Localization of factors controlling spermatogenesis in the nonfluorescent portion of the human Y chromosome long arm. Hum Genet. 1976;34(2):119–124. doi: 10.1007/BF00278879. [DOI] [PubMed] [Google Scholar]
- 82.Pryor JL, Kent-First M, Muallem A, et al. Microdeletions in the Y chromosome of infertile men. N Engl J Med. 1997;336(8):534–539. doi: 10.1056/NEJM199702203360802. [DOI] [PubMed] [Google Scholar]
- 83.Viswambharan N, Suganthi R, Simon AM, et al. Male infertility: polymerase chain reaction-based deletion mapping of genes on the human chromosome. Singapore Med J. 2007;48(12):1140–1142. [PubMed] [Google Scholar]
- 84.Stahl PJ, Masson P, Mielnik A, et al. A decade of experience emphasizes that testing for Y microdeletions is essential in American men with azoospermia and severe oligozoospermia. Fertil Steril. 2010;94(5):1753–6. [DOI] [PubMed]
- 85.Choi JM, Chung P, Veeck L, et al. AZF microdeletions of the Y chromosome and in vitro fertilization outcome. Fertil Steril. 2004;81(2):337–341. doi: 10.1016/j.fertnstert.2003.06.030. [DOI] [PubMed] [Google Scholar]
- 86.Hopps CV, Mielnik A, Goldstein M, et al. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod. 2003;18(8):1660–1665. doi: 10.1093/humrep/deg348. [DOI] [PubMed] [Google Scholar]
- 87.Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. Clinics (Sao Paulo). 2011;66(4):691–700. [DOI] [PMC free article] [PubMed]
- 88.Jequier AM, Ansell ID, Bullimore NJ. Congenital absence of the vasa deferentia presenting with infertility. J Androl. 1985;6(1):15–19. [PubMed] [Google Scholar]
- 89.Callam MJ. Epidemiology of varicose veins. Br J Surg. 1994;81(2):167–173. doi: 10.1002/bjs.1800810204. [DOI] [PubMed] [Google Scholar]
- 90.Akbay E, Cayan S, Doruk E, et al. The prevalence of varicocele and varicocele-related testicular atrophy in Turkish children and adolescents. BJU Int. 2000;86(4):490–493. doi: 10.1046/j.1464-410x.2000.00735.x. [DOI] [PubMed] [Google Scholar]
- 91.Canales BK, Zapzalka DM, Ercole CJ, et al. Prevalence and effect of varicoceles in an elderly population. Urology. 2005;66(3):627–631. doi: 10.1016/j.urology.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 92.Raman JD, Walmsley K, Goldstein M. Inheritance of varicoceles. Urology. 2005;65(6):1186–1189. doi: 10.1016/j.urology.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 93.Jung A, Schuppe HC. Influence of genital heat stress on semen quality in humans. Andrologia. 2007;39(6):203–215. doi: 10.1111/j.1439-0272.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 94.Twigg J, Fulton N, Gomez E, et al. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod. 1998;13(6):1429–1436. doi: 10.1093/humrep/13.6.1429. [DOI] [PubMed] [Google Scholar]
- 95.Saleh RA, Agarwal A, Sharma RK, et al. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril. 2003;80(6):1431–1436. doi: 10.1016/s0015-0282(03)02211-8. [DOI] [PubMed] [Google Scholar]
- 96.Nevoux P, Mitchell V, Chevallier D, et al. Varicocele repair: does it still have a role in infertility treatment? Curr Opin Obstet Gynecol. 23(3):151–7. [DOI] [PubMed]
- 97.The influence of varicocele on parameters of fertility in a large group of men presenting to infertility clinics. World Health Organization. Fertil Steril. 1992;57(6):1289–93. [PubMed]
- 98.Jarow JP. Effects of varicocele on male fertility. Hum Reprod Update. 2001;7(1):59–64. doi: 10.1093/humupd/7.1.59. [DOI] [PubMed] [Google Scholar]
- 99.Cayan S, Akbay E, Bozlu M, et al. The effect of varicocele repair on testicular volume in children and adolescents with varicocele. J Urol. 2002;168(2):731–734. doi: 10.1016/s0022-5347(05)64735-0. [DOI] [PubMed] [Google Scholar]
- 100.Trum JW, Gubler FM, Laan R, et al. The value of palpation, varicoscreen contact thermography and colour Doppler ultrasound in the diagnosis of varicocele. Hum Reprod. 1996;11(6):1232–1235. doi: 10.1093/oxfordjournals.humrep.a019362. [DOI] [PubMed] [Google Scholar]
- 101.Gat Y, Bachar GN, Zukerman Z, et al. Physical examination may miss the diagnosis of bilateral varicocele: a comparative study of 4 diagnostic modalities. J Urol. 2004;172(4 Pt 1):1414–1417. doi: 10.1097/01.ju.0000138540.57137.5f. [DOI] [PubMed] [Google Scholar]
- 102.Chiou RK, Anderson JC, Wobig RK, et al. Color Doppler ultrasound criteria to diagnose varicoceles: correlation of a new scoring system with physical examination. Urology. 1997;50(6):953–956. doi: 10.1016/S0090-4295(97)00452-4. [DOI] [PubMed] [Google Scholar]
- 103.Male Infertility Best Practice Policy Committee of the American Urological Association; Practice Committee of the American Society for Reproductive Medicine Report on varicocele and infertility. Fertil Steril. 2006;86(5 Suppl 1):S93–5. [DOI] [PubMed]
- 104.Marmar JL, Agarwal A, Prabakaran S, et al. Reassessing the value of varicocelectomy as a treatment for male subfertility with a new meta-analysis. Fertil Steril. 2007;88(3):639–648. doi: 10.1016/j.fertnstert.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 105.Meng MV, Greene KL, Turek PJ. Surgery or assisted reproduction? A decision analysis of treatment costs in male infertility. J Urol. 2005;174(5):1926–1931. doi: 10.1097/01.ju.0000176736.74328.1a. [DOI] [PubMed] [Google Scholar]
- 106.Esteves SC, Glina S. Recovery of spermatogenesis after microsurgical subinguinal varicocele repair in azoospermic men based on testicular histology. Int Braz J Urol. 2005;31(6):541–548. doi: 10.1590/s1677-55382005000600005. [DOI] [PubMed] [Google Scholar]
- 107.Inci K, Hascicek M, Kara O, et al. Sperm retrieval and intracytoplasmic sperm injection in men with nonobstructive azoospermia, and treated and untreated varicocele. J Urol. 2009;182(4):1500–1505. doi: 10.1016/j.juro.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 108.Esteves SC, Oliveira FV, Bertolla RP. Clinical outcome of intracytoplasmic sperm injection in infertile men with treated and untreated clinical varicocele. J Urol. 2010;184(4):1442–6. [DOI] [PubMed]
- 109.Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Male Reproduction and Urology. Evaluation of the azoospermic male. Fertil Steril. 2008;90(5 Suppl):S74–7. [DOI] [PubMed]
- 110.Pisipati S, Pearcy R. The role of urological surgery in male infertility. Hum Fertil (Camb). 2010;13(4):233–41. [DOI] [PubMed]
- 111.Pavlovich CP, Schlegel PN. Fertility options after vasectomy: a cost-effectiveness analysis. Fertil Steril. 1997;67(1):133–141. doi: 10.1016/s0015-0282(97)81870-5. [DOI] [PubMed] [Google Scholar]
- 112.Tournaye H. Update on surgical sperm recovery—the European view. Hum Fertil (Camb). 2010;13(4):242–6. [DOI] [PubMed]
- 113.Esteves SC, Prudencio C, Seol B, et al. Comparison of sperm retrieval and reproductive outcome in azoospermic men with testicular failure and obstructive azoospermia treated for infertility. Asian J Androl. 2014;16(4):602–606. doi: 10.4103/1008-682X.126015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prudencio C, Seol B, Esteves SC…; (. Reproductive potential of azoospermic men undergoing intracytoplasmic sperm injection is dependent on the type of azoospermia. Fertil Steril. 2010;94(S):232–3.
- 115.Verza S, Jr, Esteves SC. Sperm defect severity rather than sperm Source is associated with lower fertilization rates after intracytoplasmic sperm injection. Int Braz J Urol. 2008;34(1):49–56. doi: 10.1590/s1677-55382008000100008. [DOI] [PubMed] [Google Scholar]
- 116.Gosalvez J, Rodriguez-Predreira M, Mosquera A, et al. Characterisation of a subpopulation of sperm with massive nuclear damage, as recognised with the sperm chromatin dispersion test. Andrologia. 2014;46(6):602–609. doi: 10.1111/and.12118. [DOI] [PubMed] [Google Scholar]
- 117.Adams JA, Galloway TS, Mondal D, et al. Effect of mobile telephones on sperm quality: A systematic review and meta-analysis. Environ Int. 2014;70C:106–112. doi: 10.1016/j.envint.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 118.Moslemi MK, Tavanbakhsh S. Selenium-vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. Int J Gen Med. 2011;4:99–104. [DOI] [PMC free article] [PubMed]
- 119.Keskes-Ammar L, Feki-Chakroun N, Rebai T, et al. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch Androl. 2003;49(2):83–94. [DOI] [PubMed]
- 120.Omu AE, Al-Azemi MK, Kehinde EO, et al. Indications of the mechanisms involved in improved sperm parameters by zinc therapy. Med Princ Pract. 2008;17(2):108–116. doi: 10.1159/000112963. [DOI] [PubMed] [Google Scholar]
- 121.Scott R, MacPherson A, Yates RW, et al. The effect of oral selenium supplementation on human sperm motility. Br J Urol. 1998;82(1):76–80. doi: 10.1046/j.1464-410x.1998.00683.x. [DOI] [PubMed] [Google Scholar]
- 122.Paradiso Galatioto G, Gravina GL, Angelozzi G, et al. May antioxidant therapy improve sperm parameters of men with persistent oligospermia after retrograde embolization for varicocele? World J Urol. 2008;26(1):97–102. doi: 10.1007/s00345-007-0218-z. [DOI] [PubMed] [Google Scholar]
- 123.Greco E, Iacobelli M, Rienzi L, et al. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl. 2005;26(3):349–353. doi: 10.2164/jandrol.04146. [DOI] [PubMed] [Google Scholar]
- 124.Rolf C, Cooper TG, Yeung CH, et al. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: a randomized, placebo-controlled, double-blind study. Hum Reprod. 1999;14(4):1028–1033. doi: 10.1093/humrep/14.4.1028. [DOI] [PubMed] [Google Scholar]
- 125.Gupta NP, Kumar R. Lycopene therapy in idiopathic male infertility—a preliminary report. Int Urol Nephrol. 2002;34(3):369–372. doi: 10.1023/a:1024483520560. [DOI] [PubMed] [Google Scholar]
- 126.Comhaire FH, El Garem Y, Mahmoud A, et al. Combined conventional/antioxidant “Astaxanthin” treatment for male infertility: a double blind, randomized trial. Asian J Androl. 2005;7(3):257–262. doi: 10.1111/j.1745-7262.2005.00047.x. [DOI] [PubMed] [Google Scholar]
- 127.Patel SR, Sigman M. Antioxidant therapy in male infertility. Urol Clin North Am. 2008;35(2):319–30, x. [DOI] [PubMed]
- 128.Chia SE, Ong CN, Chua LH, et al. Comparison of zinc concentrations in blood and seminal plasma and the various sperm parameters between fertile and infertile men. J Androl. 2000;21(1):53–57. [PubMed] [Google Scholar]
- 129.Fuse H, Kazama T, Ohta S, et al. Relationship between zinc concentrations in seminal plasma and various sperm parameters. Int Urol Nephrol. 1999;31(3):401–408. doi: 10.1023/a:1007190506587. [DOI] [PubMed] [Google Scholar]
- 130.Lloyd DR, Carmichael PL, Phillips DH. Comparison of the formation of 8-hydroxy-2’-deoxyguanosine and single- and double-strand breaks in DNA mediated by fenton reactions. Chem Res Toxicol. 1998;11(5):420–427. doi: 10.1021/tx970156l. [DOI] [PubMed] [Google Scholar]
- 131.Showell MG, Brown J, Yazdani A, et al. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2011 Jan 19;(1):CD007411. [DOI] [PubMed]
- 132.Zhang LH, Qiu Y, Wang KH, et al. Measurement of sperm DNA fragmentation using bright-field microscopy: comparison between sperm chromatin dispersion test and terminal uridine nick-end labeling assay. Fertil Steril. 2010;94(3):1027–32. [DOI] [PubMed]
- 133.Agarwal A, Deepinder F, Cocuzza M, et al. Effect of vaginal lubricants on sperm motility and chromatin integrity: a prospective comparative study. Fertil Steril. 2008;89(2):375–379. doi: 10.1016/j.fertnstert.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 134.Tulandi T, Plouffe L, Jr, McInnes RA. Effect of saliva on sperm motility and activity. Fertil Steril. 1982;38(6):721–723. doi: 10.1016/s0015-0282(16)46700-2. [DOI] [PubMed] [Google Scholar]
- 135.Kutteh WH, Chao CH, Ritter JO, et al. Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud. 1996;41(4):400–404. [PubMed] [Google Scholar]
- 136.Kamel RM. Management of the infertile couple: an evidence-based protocol. Reprod Biol Endocrinol. 2010;8:21. [DOI] [PMC free article] [PubMed]
- 137.Anderson K, Nisenblat V, Norman R. Lifestyle factors in people seeking infertility treatment—a review. Aust N Z J Obstet Gynaecol. 2010;50(1):8–20. [DOI] [PubMed]
- 138.Du Plessis SS, Cabler S, McAlister DA, et al. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol. 2010;7(3):153–61. [DOI] [PubMed]
- 139.Brown HL. Preconceptional considerations and counseling for the infertile couple. N C Med J. 2009;70(5):463–465. [PubMed] [Google Scholar]