Abstract
Background
The discrimination between benign and malignant adnexal masses is central to decisions regarding clinical management and surgical planning in such patients.
Purpose of Study
To determine if the RMI (RMI 2) can distinguish between benign and malignant adnexal masses.
Methods
A prospective cohort study was conducted of 58 women with an adnexal mass referred to a teaching hospital for diagnosis and management.
Results
RMI > 200 had a sensitivity of 70.5 % (95 % CI 46.87–86.72), a specificity of 87.8 % (95 % CI 74.46–94.68), a positive predictive value of 70.5%, and negative predictive value of 87.8 %. ROC showed that cut off value of 25 achieved a sensitivity and specificity of 82.35 and 43.9 %, respectively, and a cut off value of 1,000 gave a sensitivity and specificity of 58.81 and 97.56 %, respectively. The association between RMI and disease status was not statistically significant for mucinous tumors.
Conclusion
RMI is a reliable tool in differentiating benign from malignant adnexal masses. It is simple, easy to use and cost effective. However it’s predictive accuracy was less for mucinous as compared to serous epithelial ovarian cancers. The study is limited by its small sample size.
Keywords: Adnexal masses, Risk of malignancy index, Sensitivity, Specificity
Introduction
The discrimination between benign and malignant adnexal masses is central to decisions regarding clinical management and surgical planning in such patients. A standardized method for preoperative identification of probable malignant masses would allow optimization of first-line treatment for women with ovarian cancer.
Patients with malignant tumors should be referred to a gynecological oncologist, as the quality of cytoreductive surgery and surgical staging/lymph node dissection are important prognostic factors in ovarian cancer [1, 2]. Furthermore, appropriate and timely referral to a gynecological oncologist has been proven to increase survival in patients with ovarian cancer [3].
Pelvic assessment, tumor markers, and radiological investigations have been proposed in this regard, but all of the parameters when considered separately, are inadequately sensitive or specific. Various combined methods of evaluating ovarian mass have also been proposed. Risk of malignancy index (RMI) is a combined parameter which is simple, practical and highly sensitive, and more specific. RMI is calculated with a simplified regression equation obtained from the product of menopausal status score (M), ultrasonographic score (U), and absolute value of serum CA-125 [4–6].
A risk of malignancy index would be valuable for the selective referral of relevant patients to specialized oncology centers. Currently, clinical examination, ultrasound assessment, and assays of tumor markers are part of the standard work-up for an adnexal mass, although none of these indicators alone is very sensitive or specific for detecting malignancy.
The purpose of this study was to determine if the RMI (RMI 2) can distinguish between benign and malignant adnexal masses in the population of women referred to the Department of Obstetrics and Gynecology, Medical College Baroda.
Materials and Methods
Subjects with adnexal masses scheduled for surgical intervention were recruited from the outpatient Gynecology clinic of SSG Hospital Baroda. After obtaining a written consent from the patients, a full history was obtained and a general and gynecological examination was performed. Subjects then underwent a transvaginal or transabdominal ultrasound. Transabdominal scans were done using a 3.5 MHz transducer, and transvaginal scans were done with a 7.5 MHz transducer on MYLAB 50 (Esaote, Italy) color Doppler ultrasound machine.
Adnexal masses were evaluated for sonographic morphological criteria: bilaterality, solid areas, multilocularity, ascites, and metastases.
Ultrasound score was assigned as U = 1 if 0 or 1 criteria fulfilled and ultrasound score U = 4 if 2 or more criteria are fulfilled. A total score was calculated.
5 ml of venous blood was collected for Serum Ca 125 estimation. Abnormal CA-125 level is defined as serum levels >35 U/ml.
Menopausal status was noted. Menopause was defined as one or more year of amenorrhea or women who had undergone hysterectomy. Menopausal score was assigned M = 1 if premenopausal and M = 4 if postmenopausal.
Risk of Malignancy Index 2 (RMI 2) as defined by Tingulstad et al. [7] was calculated. RMI 2 was calculated as a product of U × M × CA 125. Cut off level of 200 was set to differentiate between benign and malignant mass.
Additional imaging modalities such as CT scan or MRI were performed when ultrasound findings were doubtful. Specimens of the adnexal mass were sent for histopathological examination in the department of Pathology, Baroda Medical College. Histopathological results were analyzed for correlation with RMI.
Patients with following criteria were excluded: Subjects with functional cysts less than 5 cm, and subjects with evident signs of hepatic, peritoneal metastasis, or lung metastasis. Subjects were posted for surgical exploration.
RMI was correlated with surgical findings and final histopathology report. Subjects were followed up in accordance with final diagnosis.
Data were entered in an excel sheet. The t test for the means and the Chi square test was used to compare the demographic, biochemical, and ultrasonographic data of subjects with benign and malignant adnexal masses. The sensitivity, specificity, and positive predictive values of RMI with reference to a malignant or benign pelvic mass were calculated. Receiver Operating Curve (ROC) was plotted to calculate the predictive value of RMI at different cut offs from 25 to 1,000. The statistical software used was Medcalc version 12.3.0.0.
Results
Forty- one subjects (71 %) had benign, 2 (3 %) had borderline, and 15(26 %) had malignant disease. The distribution of subjects by age, menopausal status, ultrasound score, and serum Ca-125 level is shown in Table 1. The association between age and disease status was not significant. Thirty four women were premenopausal and 24 were postmenopausal. The association between ultrasound score and disease status was statistically significant at a p value of 0.0004. The values for CA 125 in the subjects with benign disease were 33 and 13 for mean and median, respectively; the corresponding values in the subjects with malignant disease were 395 and 329, respectively. This association was statistically significant at a p value of <0.0001.
Table 1.
Distribution of subjects by age, menopausal status, serum CA125 levels, and ultrasound score
| Variables | Benign (n = 41) | Malignant (n = 17) | p value |
|---|---|---|---|
| Age | No (%) | No (%) | 0.6741 |
| <30 | 9 (21.9) | 2 (11.8) | |
| 30–44 | 12 (29.2) | 5 (29.4) | |
| 45–54 | 9 (21.9) | 6 (35.3) | |
| >55 | 11 (26.8)) | 4 (23.5) | |
| Menopausal status | 1.000 | ||
| Premenopausal | 24 (58.5) | 10 (58.8) | |
| Postmenopausal | 17 (41.5) | 7 (41.2) | |
| USG score | 0.0004 | ||
| 1 | 31 (75.6) | 4 (23.5) | |
| 4 | 10 (24.4) | 13 (76.5) | |
| CA125 | <0.0001 | ||
| Mean | 33 | 395 | |
| Median | 13 | 329 | |
| Minimum | 5 | 2 | |
| Maximum | 438 | 1,000 |
RMI had a sensitivity of 70.5 % (46.87–86.72), a specificity of 87.8 % (74.46–94.68), a positive predictive value of 70.5 %, and negative predictive value of 87.8 %. Menopausal status had sensitivity of 41.1 % (21.61–63.99), specificity of 58.5 % (43.37–72.24), positive predictive value of 29.1 %, and negative predictive value of 70.5 %. Serum Ca-125 level had a sensitivity of 76.4 % (52.74–90.44), a specificity of 85.3 % (71.56–93.12), a positive predictive value of 68.4 %, and a negative predictive value of 89.7 %. Ultrasound score had a sensitivity of 76.4 % (52.74–90.44), a specificity of 75.6 % (60.66–86.17), a positive predictive value of 56.5 %, and a negative predictive value of 88.5 % (Table 2).
Table 2.
Predictive values of RMI, menopausal status, serum Ca-125 levels, and ultrasound score of malignant and benign adnexal masses
| Variable | Malignant (n = 17) | Benign (n = 41) | Sensitivity (%) (95 % CI) | Specificity (%) (95 % CI) | PPV | NPV |
|---|---|---|---|---|---|---|
| RMI > 200 | 12 (70.5) | 5 (12.2) | 70.5 (46.87–86.72) | 87.8 (74.46–94.68) | 70.5 | 87.8 |
| RMI < 200 | 5 (29.4) | 36 (87.8) | ||||
| Menopausal | 7 (41.2) | 17 (41.4) | 41.1 (21.61–63.99) | 58.5 (43.37–72.24) | 29.1 | 70.5 |
| Pre menopausal | 10 (58.8) | 24 (58.5) | ||||
| CA125 > 35 | 13 (76.4) | 6 (14.6) | 76.4 (52.74–90.44) | 85.3 (71.56–93.12) | 68.4 | 89.7 |
| CA125 < 35 | 4 (23.5) | 35 (85.3) | ||||
| USG score1 | 13 (76.4) | 10 (24.3) | 76.4 (52.74–90.44) | 75.6 (60.66–86.17) | 56.5 | 88.5 |
| USG score4 | 4 (23.5) | 31 (75.6) |
PPV positive predictive value, NPV negative predictive value, RMI risk of malignancy index
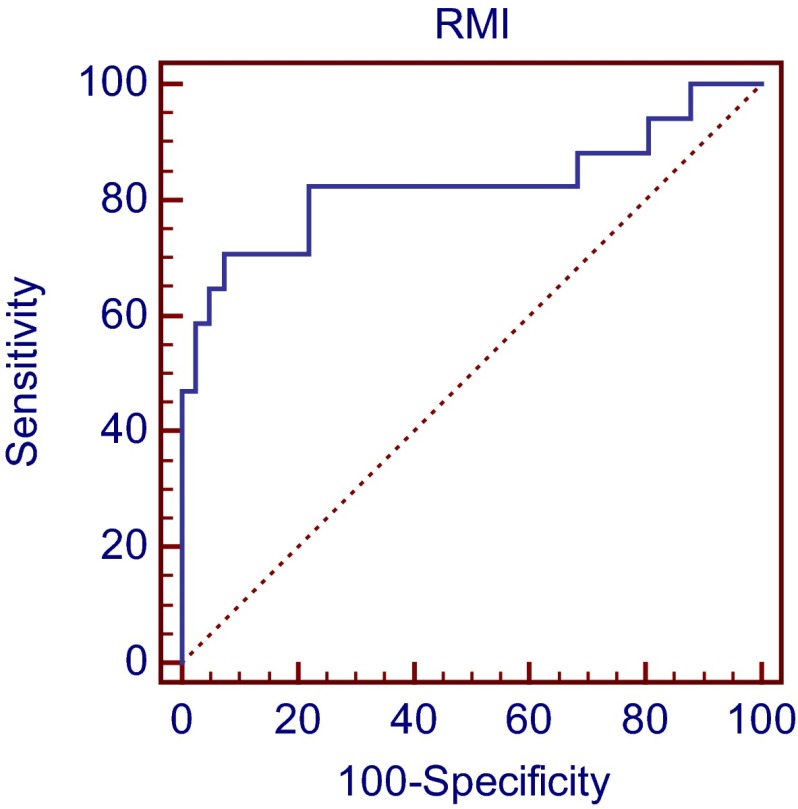
A Receiver Operating Curve (ROC) (Fig. 1) was plotted. The sensitivity and specificity for the different cut off values for RMI are given in Table 3. A cut off value of 25 achieved a sensitivity and specificity of 82.35 and 43.9 %, respectively, meaning that 82.35 % of ovarian cancer cases have an RMI of more than 25. A cut off value of 1,000 gave a sensitivity and specificity of 58.81 and 97.56 %, respectively, meaning that 97.56 % of benign cases have a RMI less than 1,000. Similarly at cut off value of 25, the likelihood of having malignant disease is 1.46, while at cut off level of 1,000 the likelihood is 24.11 times higher.
Fig. 1.
Receiver operator characteristic curve showing relation between sensitivity and specificity in differentiating between benign and malignant adnexal masses
Table 3.
The sensitivity, specificity, and the likelihood ratio for malignancy given a positive or negative result for different levels of RMI
| RMI | Sensitivity | Specificity | Positive LR | Negative LR |
|---|---|---|---|---|
| 25 | 82.35 | 43.9 | 1.46 | 0.40 |
| 50 | 82.35 | 63.41 | 2.25 | 0.27 |
| 75 | 82.35 | 75.61 | 3.37 | 0.23 |
| 100 | 82.35 | 78.05 | 3.75 | 0.22 |
| 125 | 82.35 | 78.05 | 3.75 | 0.22 |
| 150 | 76.47 | 78.05 | 3.48 | 0.30 |
| 175 | 70.59 | 82.93 | 4.13 | 0.35 |
| 200 | 70.59 | 87.8 | 5.78 | 0.33 |
| 225 | 70.59 | 90.24 | 7.23 | 0.32 |
| 250 | 70.59 | 90.24 | 7.23 | 0.32 |
| 500 | 64.71 | 95.12 | 13.26 | 0.37 |
| 1,000 | 58.81 | 97.56 | 24.11 | 0.42 |
LR likelihood ratio, RMI risk of malignancy index
As shown in Table 4, 16 out of 58 subjects had RMI > 200, of which 11 (64.7 %) subjects had malignant tumor, 1 had borderline tumor, and 5 (12.2 %) subjects had benign tumor. Forty one subjects had RMI < 200, of which 36 (87.8 %) had benign tumor, 1 had borderline, and 4 (23.5 %) had malignant tumor. Table 5 classifies these data further by histological tumor type. The association between RMI and disease status was not statistically significant for mucinous tumors. While for serous tumors the association between RMI and disease status was highly significant at p value of 0.0003 and that for other tumors (as discussed in Table 1) was also statistically significant at p value of 0.0043.
Table 4.
Distribution of subjects by RMI less than/more than 200
| RMI | Benign (n = 41) (%) | Borderline (n = 2) (%) | Malignant (n = 15) (%) |
|---|---|---|---|
| RMI < 200 | 36 (87.8) | 1 (50.0) | 4 (23.5) |
| RMI > 200 | 5 (12.2) | 1 (50.0) | 11 (64.7) |
RMI risk of malignancy index
Values in parentheses are percentages
Table 5.
RMI versus histologic type
| RMI | Histology type | |||||||
|---|---|---|---|---|---|---|---|---|
| Mucinous (n = 15) | Serous (n = 27) | Other (n = 16) | ||||||
| Benign | Malignant | Borderline | Benign | Malignant | Borderline | Benign | Malignant | |
| No (%) | No (%) | No (%) | No (%) | No (%) | No (%) | No (%) | No (%) | |
| <200 | 8 (53.3) | 3 (20.0) | 1 (6.7) | 14 (51.8) | 1 (3.7) | 0 | 14 (87.5) | 0 |
| >200 | 3 (20.0) | 0 | 0 | 2 (3.5) | 9 (33.3) | 1 (3.7) | 0 | 2 (12.5) |
| p value: 0.6615 | p value: 0.0003 | p value: 0.0043 | ||||||
RMI risk of malignancy index
Values in parentheses are percentages
CT/MRI was able to detect 8 of 10 malignant masses and 11 of 13 benign masses in 23 subjects, which gave a sensitivity of 80 % (49.02–94.33) and specificity of 84.61 % (57.77–95.67). The association between CT/MRI findings and HP reports was statistically significant at p value of 0.0075.
Discussion
The aim of this observational study over a period of 1 year was to evaluate the role of RMI2 in distinguishing benign from malignant adnexal masses. Fifty eight consecutively admitted subjects were recruited over the study period of 1 year. Seventeen adnexal masses were detected to be malignant on final histopathology, including two borderline tumors. All of the 15 subjects with malignant disease had undergone primary cytoreductive surgery, none had received neo adjuvant chemotherapy, 7 subjects received adjuvant chemo therapy, and 8 were lost to follow up.
RMI was more accurate than any individual criterion in distinguishing malignant from benign masses. The high false-positive rate of ultrasound, especially in premenopausal women, is often cited as the main limitation of its use in screening for ovarian cancer [8]. Raised serum CA 125 levels are also found in association with benign ovarian cysts, endometriosis, and pelvic infection in addition to cancers of the endometrium, fallopian tube, breast, and colon. RMI translates the morphological description of the pelvic mass into objective numerical data, reducing the bias attributable to the examiner’s subjectivity. This is more effective than any of the other parameters on their own, that is, ultrasound, CA125 level, menopausal status [9].
In our study, RMI had a sensitivity of 70.5 % (46.87–86.72), a specificity of 87.8 % (74.46–94.68), a positive predictive value of 70.5 %, and negative predictive value of 87.8 %. The sensitivity of RMI 2 in our study is lower as compared to that reported in other studies [10, 11]. This could be due to the following factors: small sample size, higher number of cases with benign and low stage disease, and also a substantial number of mucinous tumors. Secondly, we have also used transvaginal ultrasound in most cases and this could have impacted the difference in sensitivity.
In this study, we have used RMI 2. Morgante et al. [5] in 1999 found that RMI 2 was more reliable in discriminating benign and malignant ovarian disease than RMI 1. In a study by Van Trappen et al. [12], analysis of 123 patients managed sequentially, using RMI cut off values of ≥25 and <1,000 and then US and MRI provided a sensitivity of 94 % and a specificity of 90 %.
In our study, a ROC analysis has shown that at a cut off value of 25 the likelihood of having malignant disease is 1.46, while at cut off level of 1,000 the likelihood is 24.11 times higher. RCOG guidelines [13] use the RMI to triage women as low (RMI < 25), moderate (25–250), or high (above >250) risk. For tumors classified as low risk, the proposed management is expectant management or laparoscopic surgery by a generalist in a gynecology unit. If at moderate risk, laparoscopic surgery in a cancer unit by a surgeon with a special interest is suggested. If at high risk, referral of the woman to a cancer center for a full staging procedure by a subspecialist gynecological oncologist is advised. A recent study by Van Calster et al. has found that the IOTA protocol was more accurate for triage than the RCOG protocol [14].
The association between RMI and disease status was not statistically significant for mucinous tumors. While for serous tumors the association between RMI and disease status was highly significant at p value of 0.0003. This association by histological tumor type has not been reported in the literature we have reviewed on this subject.
In conclusion, RMI 2 is a simple scoring system utilizing currently available tests. The ultrasound component of the score incorporates features that should be easily seen using either transabdominal or transvaginal scanning. RMI accurately differentiated between benign and malignant adnexal masses with a sensitivity of 70.5 % (46.87–86.72), a specificity of 87.8 % (74.46–94.68), a positive predictive value of 70.5 %, and negative predictive value of 87.8 %. The threshold RMI score for referral could depend on the facilities available. A higher RMI with lower sensitivity but better specificity could be used when availability of specialist care is limited. However, RMI was not reliable in predicting malignancy when the tumor was mucinous.
Acknowledgments
Compliance with ethical standards and Conflict of interest
Informed Consent in studies with human subjects: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Institutional and National) and with the Helsinki declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study. Dr. Rujuta Javdekar and Dr. Nandita Maitra declare that they have no conflict of interest.
Dr. Rujuta Javdekar
completed M. S. from the department of Obstetrics and Gynecology, Medical College, Baroda. She is presently working as Assistant Professor at the PDU Medical College in Rajkot.
Contributor Information
Rujuta Javdekar, Email: ruju_star@yahoo.co.in.
Nandita Maitra, Email: n.maitra03@gmail.com.
References
- 1.Giede KC, Kieser K, Dodge J, et al. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol. 2005;99(2):447–461. doi: 10.1016/j.ygyno.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 2.ACOG Committee Opinion number 280 The role of the generalist obstetrician gynecologist in the early detection of ovarian cancer. Gynecol Oncol. 2002;2002(100):1413–1416. doi: 10.1016/s0029-7844(02)02630-3. [DOI] [PubMed] [Google Scholar]
- 3.Kirwan JM, Tinchello DG, Herodd JJ, et al. Effect of delays in primary care referral on survival of women with epithelial ovarian cancer. BMJ. 2002;324(7330):148–151. doi: 10.1136/bmj.324.7330.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akturk E, Karaka RE, Alanbay I, et al. Comparison of four malignancy risk indices in the detection of malignant ovarian masses. J Gynecol Oncol. 2011;22(3):177–182. doi: 10.3802/jgo.2011.22.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgante G, Marca AI, Ditto A, et al. Comparison of two malignancy risk indices based on serum CA-125, ultrasound score and menopausal status in the diagnosis of ovarian masses. Br J Obstet Gynecol. 1999;1999(106):524–527. doi: 10.1111/j.1471-0528.1999.tb08318.x. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs I, Oram D, Fairbanks J, et al. A risk of malignancy index incorporating CA-125, ultrasound and menopausal status for the accurate pre-operative diagnosis of ovarian cancer. Br J Obstet Gynecol. 1990;1990(97):922–927. doi: 10.1111/j.1471-0528.1990.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 7.Tingulstad S, Hagen B, Skjeldestad EF, et al. The Risk-of- Malignancy Index to evaluate potential ovarian cancers in local hospitals. Obstet Gynecol. 1999;1999(93):448–452. [PubMed] [Google Scholar]
- 8.Fung MF, Bryson P, Johnston M, et al. Screening postmenopausal women for ovarian cancer. A systematic review. J Obstet Gynecol Can. 2004;24(8):717–728. doi: 10.1016/s1701-2163(16)30643-0. [DOI] [PubMed] [Google Scholar]
- 9.Skates SJ, Mai P, Horrick NK, et al. Large prospective study of ovarian cancer screening in high risk women Ca 125 cut point defined by menopausal status. Cancer Prev Res. 2011;4(9):1401–1408. doi: 10.1158/1940-6207.CAPR-10-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies AP, Jacobs I, Woolas R, et al. The adnexal mass: benign or malignant? Evaluation of a risk of malignancy index. Br J Obstet Gynaecol. 1993;1993(100):927–931. doi: 10.1111/j.1471-0528.1993.tb15109.x. [DOI] [PubMed] [Google Scholar]
- 11.Manjunath AP, Pratapkumar, Sujatha K, et al. Comparison of three risk of malignancy indices in evaluation of pelvic masses. Gynecol Oncol. 2001;2001(82):225–229. doi: 10.1006/gyno.2001.6122. [DOI] [PubMed] [Google Scholar]
- 12.van Trappen PO, Rufford BD, Mills TD, et al. Differential diagnosis of adnexal masses: risk of malignancy index, ultrasonography, magnetic resonance imaging, and radioimmunoscintigraphy. Int J Gynecol Cancer. 2007;17:61–67. doi: 10.1111/j.1525-1438.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- 13.Rufford BD, Jacobs IJ. Green-top Guideline No. 34. Ovarian cysts in postmenopausal women. London, UK: Royal College of Obstetricians and Gynaecologists, 2003. http://www.rcog.org.uk/files/rcog-corp/GTG3411022011.pdf.
- 14.Van Calster B, Timmerman D, Valentin L, et al. Triaging women with ovarian masses for surgery: observational diagnostic study to compare RCOG guidelines with an international Ovarian Tumour Analysis (IOTA) group protocol. BJOG. 2012;2012(119):662–671. doi: 10.1111/j.1471-0528.2012.03297.x. [DOI] [PubMed] [Google Scholar]