Abstract
The crystal structure of a complex between recombinant human cyclophilin B (CypB) and a cyclosporin A (CsA) analog has been determined and refined at 1.85-A resolution to a crystallographic R factor of 16.0%. The overall structures of CypB and of cyclophilin A (CypA) are similar; however, significant differences occur in two loops and at the N and C termini. The CsA-binding pocket in CypB has the same structure as in CypA and cyclosporin shows a similar bound conformation and network of interactions in both CypB and CypA complexes. The network of the water-mediated contacts is also essentially conserved. The higher potency of the CypB/CsA complex versus CypA/CsA in inhibiting the Ca(2+)- and calmodulin-dependent protein phosphatase calcineurin is discussed in terms of the structural differences between the two complexes. The three residues Arg90, Lys113, and Ala128 and the loop containing Arg158 on the surface of CypB are likely to modulate the differences in calcineurin inhibition between CypA and CypB.
Full text
PDF
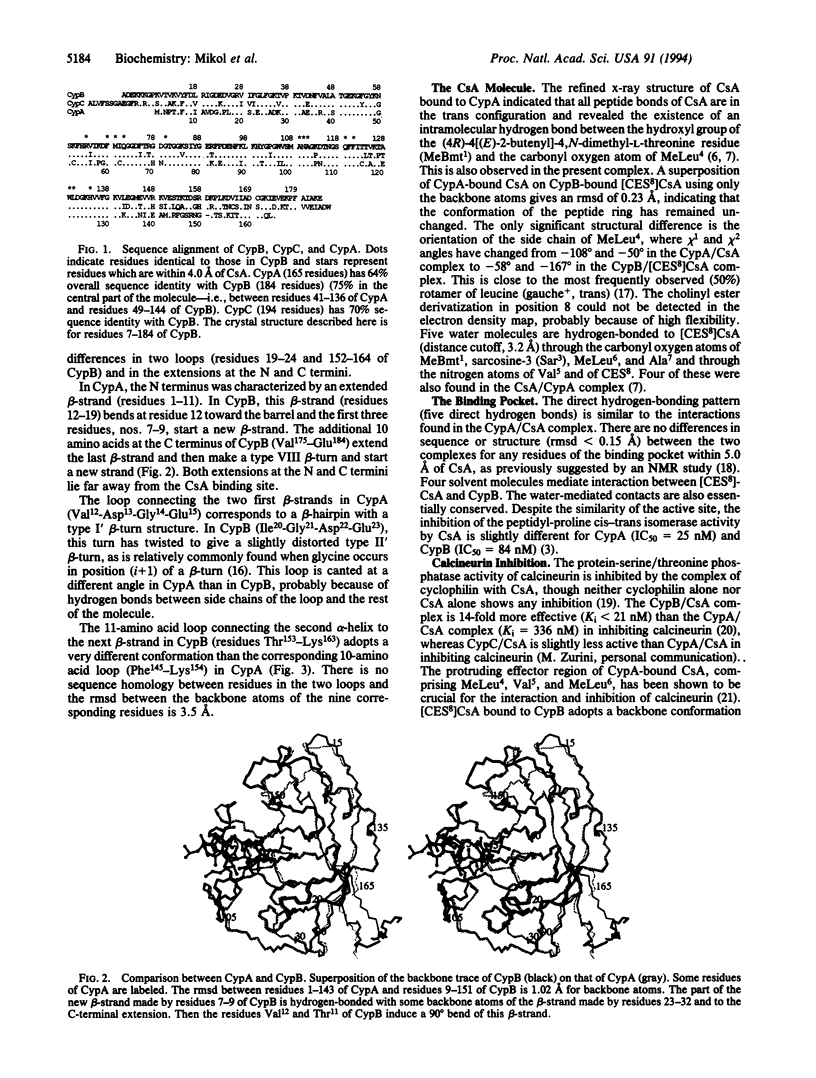

Images in this article
Selected References
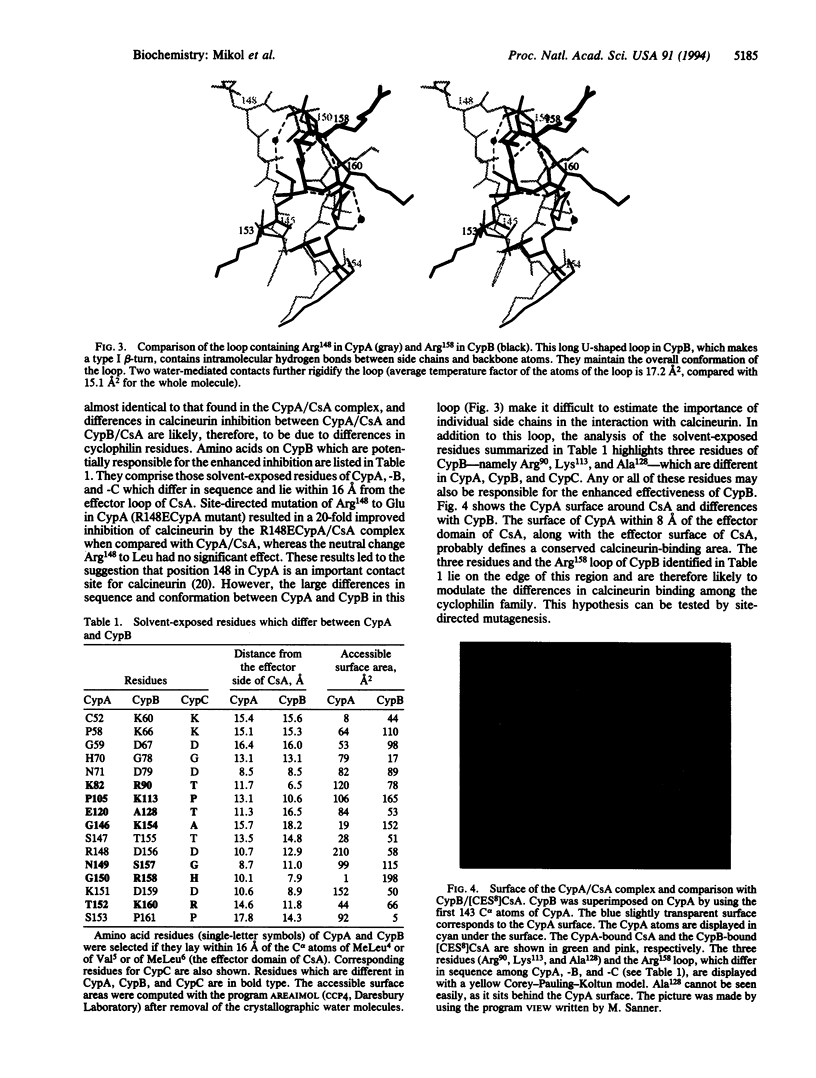
These references are in PubMed. This may not be the complete list of references from this article.
- Borel J. F. Pharmacology of cyclosporine (sandimmune). IV. Pharmacological properties in vivo. Pharmacol Rev. 1990 Sep;41(3):259–371. [PubMed] [Google Scholar]
- Etzkorn F. A., Chang Z. Y., Stolz L. A., Walsh C. T. Cyclophilin residues that affect noncompetitive inhibition of the protein serine phosphatase activity of calcineurin by the cyclophilin.cyclosporin A complex. Biochemistry. 1994 Mar 8;33(9):2380–2388. doi: 10.1021/bi00175a005. [DOI] [PubMed] [Google Scholar]
- Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem. 1993 Sep 15;216(3):689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kallen J., Spitzfaden C., Zurini M. G., Wider G., Widmer H., Wüthrich K., Walkinshaw M. D. Structure of human cyclophilin and its binding site for cyclosporin A determined by X-ray crystallography and NMR spectroscopy. Nature. 1991 Sep 19;353(6341):276–279. doi: 10.1038/353276a0. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Zydowsky L. D., Liu J., Walsh C. T. Crystal structure of recombinant human T-cell cyclophilin A at 2.5 A resolution. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9483–9487. doi: 10.1073/pnas.88.21.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Mikol V., Kallen J., Pflügl G., Walkinshaw M. D. X-ray structure of a monomeric cyclophilin A-cyclosporin A crystal complex at 2.1 A resolution. J Mol Biol. 1993 Dec 20;234(4):1119–1130. doi: 10.1006/jmbi.1993.1664. [DOI] [PubMed] [Google Scholar]
- Neri P., Gemmecker G., Zydowsky L. D., Walsh C. T., Fesik S. W. NMR studies of [U-13C]cyclosporin A bound to human cyclophilin B. FEBS Lett. 1991 Sep 23;290(1-2):195–199. doi: 10.1016/0014-5793(91)81258-a. [DOI] [PubMed] [Google Scholar]
- Pflügl G., Kallen J., Schirmer T., Jansonius J. N., Zurini M. G., Walkinshaw M. D. X-ray structure of a decameric cyclophilin-cyclosporin crystal complex. Nature. 1993 Jan 7;361(6407):91–94. doi: 10.1038/361091a0. [DOI] [PubMed] [Google Scholar]
- Price E. R., Zydowsky L. D., Jin M. J., Baker C. H., McKeon F. D., Walsh C. T. Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1903–1907. doi: 10.1073/pnas.88.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauber H., Eisenhaber F., Argos P. Rotamers: to be or not to be? An analysis of amino acid side-chain conformations in globular proteins. J Mol Biol. 1993 Mar 20;230(2):592–612. doi: 10.1006/jmbi.1993.1172. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L. Immunophilin-sensitive protein phosphatase action in cell signaling pathways. Cell. 1992 Aug 7;70(3):365–368. doi: 10.1016/0092-8674(92)90158-9. [DOI] [PubMed] [Google Scholar]
- Spik G., Haendler B., Delmas O., Mariller C., Chamoux M., Maes P., Tartar A., Montreuil J., Stedman K., Kocher H. P. A novel secreted cyclophilin-like protein (SCYLP). J Biol Chem. 1991 Jun 15;266(17):10735–10738. [PubMed] [Google Scholar]
- Spitzfaden C., Weber H. P., Braun W., Kallen J., Wider G., Widmer H., Walkinshaw M. D., Wüthrich K. Cyclosporin A-cyclophilin complex formation. A model based on X-ray and NMR data. FEBS Lett. 1992 Apr 6;300(3):291–300. doi: 10.1016/0014-5793(92)80866-f. [DOI] [PubMed] [Google Scholar]
- Thériault Y., Logan T. M., Meadows R., Yu L., Olejniczak E. T., Holzman T. F., Simmer R. L., Fesik S. W. Solution structure of the cyclosporin A/cyclophilin complex by NMR. Nature. 1993 Jan 7;361(6407):88–91. doi: 10.1038/361088a0. [DOI] [PubMed] [Google Scholar]
- Wilmot C. M., Thornton J. M. Analysis and prediction of the different types of beta-turn in proteins. J Mol Biol. 1988 Sep 5;203(1):221–232. doi: 10.1016/0022-2836(88)90103-9. [DOI] [PubMed] [Google Scholar]