Abstract
AIM: To evaluate the effect of glutamine on intestinal mucosa integrity, glutathione stores and acute phase response in protein-depleted rats during an inflammatory shock.
METHODS: Plasma acute phase proteins (APP), jejunal APP mRNA levels, liver and jejunal glutathione concentrations were measured before and one, three and seven days after turpentine injection in 4 groups of control, protein-restricted, protein-restricted rats supplemented with glutamine or protein powder. Bacterial translocation in mesenteric lymph nodes and intestinal morphology were also assessed.
RESULTS: Protein deprivation and turpentine injection significantly reduced jejunal villus height, and crypt depths. Mucosal glutathione concentration significantly decreased in protein-restricted rats. Before turpentine oil, glutamine supplementation restored villus heights and glutathione concentration (3.24 ± 1.05 vs 1.72 ± 0.46 μmol/g tissue, P < 0.05) in the jejunum, whereas in the liver glutathione remained low. Glutamine markedly increased jejunal α1-acid glycoprotein mRNA level after turpentine oil but did not affect its plasma concentration. Bacterial translocation in protein-restricted rats was not prevented by glutamine or protein powder supplementation.
CONCLUSION: Glutamine restored gut glutathione stores and villus heights in malnourished rats but had no preventive effect on bacterial translocation in our model.
Keywords: Acute phase response, Glutamine, Glutathione, Intestine, Malnutrition
INTRODUCTION
Sepsis and endotoxemia impair gut glutamine metabolism[1]. This impairment may contribute to the weakening of the gut mucosal barrier and to the development of bacterial translocation[1,2]. The tripeptide glutathione is an active free radical scavenging compound[3]. Glutathione has been shown to play an important role in the protection of intestinal mucosa against exogenous injury both in vitro[4,5] and in vivo[6]. Intestinal mucosa glutathione content falls markedly following a period of protein restriction[7] and also in patients with inflammatory bowel disease[8,9]. The depletion of reduced glutathione content in the mucosa could therefore favor oxidative stress within the mucosa. In addition to the liver, intestine also contributes to the systemic inflammatory response and acute phase proteins expression[10] and this intestinal acute phase response may be influenced by protein malnutrition[11,12].
Although glutamine has traditionally been recognized as a nonessential amino acid, recent studies have demon-strated that glutamine plays a major role in the response to injury[13], in the enterocyte oxidative metabolism[14], and in the maintenance of the intestinal epithelium[15,16]. Glutamine supplementation may prevent gut mucosal damage and bacterial translocation in various experimental models of gut injury[13,17]. Moreover, glutamine could with stand systemic[18] but also gut-associated[19,20] immune response, because glutamine is an important substrate for optimal lymphocyte[21] and macrophage[22] function. In addition, some reports indicate that glutamine may counteract glutathione depletion by supporting gut glutathione biosynthesis[23] and may influence cytokines production by gut mucosa in rats[24] or in humans[25,26].
Therefore, the aim of this study was to investigate the effect of glutamine supplementation on the integrity of the intestinal mucosa and its glutathione stores and on acute phase response in protein depleted rats during an inflammatory challenge.
MATERIALS AND METHODS
Animals
Guidelines for the handling and care of laboratory animals conformed to the standards established by the Animal Studies Committee of the Rouen University.
Seventy two adult male Sprague-Dawley rats weighing 291 ± 23 g were obtained from Charles River (L’Arbresle, France) and were allowed at least 3 d to acclimatize to laboratory conditions (constant humidity and temperature: 21°C, with a 12 h light-dark cycle) in community cages before being studied. During this time the rats were allowed ad libitum intake of water and standard rat chow (UAR A03, Epinay-sur-Orge, France). Two weeks before the inflammatory challenge (d-14), rats were housed in individual metabolism cages at 21°C with 12 h periods of dark and light cycles with a free access to food and water. Rats were assigned to 4 groups: a control group (CG) fed with a 23% protein diet (23 g casein/100 g synthetic diet, UAR, Epinay-sur-Orge, France); and 3 groups fed with an isocaloric protein-free (0% casein) diet. The composition of diets is summarized in Table 1. In two protein-restricted groups, rats were supplemented after 7 d of protein-free diet with either glutamine (3 g/100 mL) or protein powder (PolypeptalR, Novartis, 3.75 g/100 mL), and until the end of the study (Gln and PP groups, respectively). These supplements were administered in solution with the drinking water, with adequate dilution to provide isonitrogenous solutions. Equal volumes and thus isonitrogenous supplements were given to the rats of Gln and PP groups. One protein-restricted group received no supplementation later on (PR group).
Table 1.
Composition of control and protein-deficient diets1
| Constituent (g/kg) | Control diet (23% casein) | Protein-deficient diet (0% casein) |
| Protein | 2302 | 0 |
| Glucose (+ starch) | 580 | 800 |
| Lipids | 50 | 60 |
| Cellulose | 60 | 60 |
| Mineral salts3 | 70 | 70 |
| Vitamins4 | 10 | 10 |
| Total | 1000 | 1000 |
1The energy density of each diet was 18 kJ/g. In the protein-restricted (PR) group, energy was replaced with isocaloric quantities of carbohydrates (glucose + starch in equal amounts);
Containing (g/kg): L-Arginine 8.5; L-Cysteine 3.0; L-Lysine 17.4; L-Methionine 7.1; L-Tryptophane 5.0; L-Glycine 1.0;
Containing (mg/kg): phosphorus, 7750; calcium, 10 000; potassium, 6000; sodium, 4000; magnesium, 1000; manganese, 80; iron, 300; copper, 12.5; zinc, 45; cobalt, 0.09; and iodine, 0.49;
Containing (UI/kg): retinyl acetate, 19 800; cholecalciferol, 6000; and (mg/kg): thiamin, 20; riboflavin, 15; D-pantothenic acid, 70; pyridoxine, 10; inositol, 150; cyanocobalamine, 0.05; ascorbic acid, 800; dl-α-tocopherol acetate, 170; menadione sodium bisulfite, 40; nicotinic acid, 100; choline, 1360; folic acid, 5; biotin, 0.3. (ND, not detectable).
Diet regimens were maintained until sacrifice day. During the experimental period, rats were weighted weekly, and food and water consumption was monitored daily. After 14 d of regimen, rats received a subcutaneous injection of 3 mL/kg of turpentine oil (TO) to induce an acute-phase response. An experimental inflammation induced by TO may increase intestinal permeability in rats[27]. Rats were sacrificed immediately before TO injection (0 h) or 1, 3, and 7 d after TO. The number of animals ranged from 3 to 5 in each group at each time point.
Preparations
The rats were sacrificed and a ventral midline incision was made under sterile conditions and mesenteric lymph nodes were quickly removed and put into a sterile vial kept at 4°C and transferred to the bacteriology laboratory within 4 h. After excision of lymph nodes and exsanguination, the portal vein was cannulated, the supra hepatic veins were cut, and the liver was rinsed with ice cold normal saline until it appeared blood free. Then, it was rapidly excised and blotted. A sample of liver (about 1 g) was weighed, minced and homogenized in a BraunR potter homogenizer with 3 mL of normal saline for 60 s at 4°C. This homogenate was used to determine the liver content of soluble proteins. Another 1 g liver sample was similarly homogenized in 3 mL of perchloric acid (0.4 mol/L). The homogenate was centrifuged at 10 000 × g for 20 min at 4°C. The supernatant was kept at -80°C for glutathione concentration measurement. After removal of the liver, the jejunum was removed and carefully rinsed with ice-cold phosphate buffer saline to eliminate fecal material. This tissue was opened longitudinally and the mucosa was immediately scraped off and prepared as previously described for the liver[28].
Analytical methods
Total reduced glutathione concentration in the super-natant was determined according to a modified spectro-photometric glutathione reductase assay as previously[28]. Glutathione assay was performed twice, with a variation coefficient of less than 10%. Results were expressed as µmoles glutathione per g wet weight tissue. Intracellular glutamine and glutamate levels were determined in the supernatant of jejunal homogenates after protein precipitation by using an amino acid analyzer (Biotronik LC3000; Eppendorf).
For measurement of villus height, additional 1 cm samples of jejunum were rinsed with ice-cold saline and fixed in a 10% formol solution during 24-72 h. The samples were coded and further handled in blinded fashion by the same observer. The samples were cut longitudinally in 3 pieces and embedded in paraffin. The sections (40 μm) were placed on a slide and then stained with hematoxylin. To minimize the variability of measurements, about 20 villi were studied in jejunal samples, and for each villus, epithelial thickness, chorion or lamina propria height and crypt depths were measured in duplicate.
In the bacteriology laboratory, lymph nodes were weighed in sterile conditions and then homogenized in a Teflon potter homogenizer with sterilized NaCl (10% weight/volume). The homogenate was then cultured in aerobic atmosphere on 3 different medium: horse blood Columbia, CLED (Biomérieux, Marcy l'Etoile, France) and nalidixic acid (Pasteur Diagnostics, Marnes-la-Coquette, France) agar plates, during 48 h. Colony counts were expressed as colony forming units per gram of organ tissue (CFU/g tissue), and culture was considered positive for (CFU/g tissue) > 100.
α-1 AGP and α2-macroglobulin plasma concentrations were determined by rocket immunoelectrophoresis as previously described[12] using rat anti-α-1 AGP or anti-α2-macroglobulin (UER Sciences Pharmaceutiques et Biologiques, Chatenay Malabray, France) antibodies.
RT-PCR
Total RNAs were extracted from the liver or intestinal mucosa by a modified-extraction method as previously described[26]. The quality and quantity of total RNA were determined by spectrophotometry using the absorbance at A260/A280 nm. The integrity was also controlled by visualization of 18S and 28S ribosomal bands. RT-PCR was performed as previously reported[26]. The RT products were amplified by PCR using sense and antisense primers (Eurogentec) specific for α-1 AGP and glycerladehyde-3 phosphate deshydrogenase (GAPDH) used as an internal standard: α-1 AGP, 5'-GCAGCTTTCCGAGACCCCGT-3' and 5'-CATGCCCACATCTTTGACAG-3'; GAPDH, 5'-AAAGGGTCATCATCTCCGCC-3' and 5'-GTGGAGGAATGGGAGTTGCT-3'. The relative quantification of the autoradiogram bands represents an integrated area under the curve of densitometric tracing, estimated as the ratio of targeted gene to GAPDH.
Statistical analysis
Values are expressed as the mean ± SD. Data were analyzed by analysis of variance, and differences between means were determined using Scheffe’s multiple comparison test. The incidence of bacterial translocation was compared by corrected χ² test. Significance was defined as P < 0.05.
RESULTS
Dietary intake and weight of animals
Before starting the specialized regimens, there was no significant body weight difference between the groups (Table 2). Control group’s body weight rose during the whole period before TO injection, slightly diminished 24 h after TO (not significant) and then remained unchanged (Figure 1). In contrast, in the three other groups, the animals lost about 60 g of their body weight during the first week of protein deprivation, and about 10 g during the second week, Turpentine oil did not induce an additional loss of weight and no significant difference was observed between these 3 groups (Figure 1).
Table 2.
Body weight, total energy and nitrogen intake
| CG | PR | Gln | PP | |
| Body weight (g) | 295 ± 9 | 288 ± 31 | 284 ± 30 | 296 ± 8 |
| Energy intake (kcal/d. 100 g body weight) | ||||
| 1st wk | 34 ± 2 | 20 ± 5a | 22 ± 7a | 19 ± 7a |
| 2nd wk | 30 ± 4 | 30 ± 4 | 28 ± 10 | 28 ± 10 |
| Nitrogen intake (g/100 g body weight) | 0.28 ± 0.02 | 0.00 | 0.05 ± 0.03 | 0.04 ± 0.01 |
Body weight (g) before the beginning of the study, total energy intake (powder or powder + supplementation in the drinking water) expressed in kcal/d per 100 g of body weight, and total nitrogen intake (g/100 g body weight) in CG (control group), protein-restricted group (PR) and protein-restricted groups supplemented with glutamine (Gln) or a protein powder (PP).
P < 0.05 vs CG.
Figure 1.
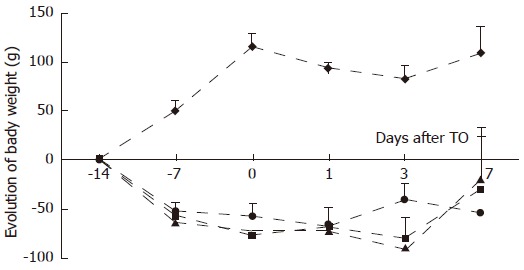
Evolution of body weight from initial weight as a function of time during the feeding period and at each time point studied after turpentine oil (TO) injection (arrow) for control (CG; ), protein-restricted (PR; ), glutamine (Gln; ) and protein-powder (PP; ) groups. Values are means ± SD. P < 0.05, between CG and other groups from d-7 until d7.
The mean daily energy intake during the first week of the feeding period was reduced in all the groups fed with 0% protein diets (Table 2). During the second week of the feeding period, the energy intake (powder or powder + supplementation in the drinking water, see the methods section) was not significantly different between the groups (Table 2). During the supplementation period, nitrogen intake of Gln and PP groups represented about 18% and 14% (expressed in g N/d per 100 g body weight, respectively) of the nitrogen intake of CG (Table 3). When the rats were injected with TO, a significant (about 50%) reduction of food intake was observed in all groups, with no significant difference between groups, and food intake normalized 24 h later.
Table 3.
Positive cultures (colony forming units per gram of organ tissue (CFU) > 100) from cultured lymph nodes/total cultures for (CG ), protein-restricted (PR), glutamine-(Gln) and protein powder (PP) supplemented groups
| d 0 | d 1 | d 3 | |
| CG | 0/4 | 2/4 | 1/4 |
| PR | 1/5 | 1/5 | 1/5 |
| Gln | 2/6 | 4/7 | 2/5 |
| PP | 3/5 | 3/4 | 3/4 |
Intestinal morphology
The morphometric data of the four groups are displayed on Figure 2. After 14 d of 0% protein diet, both the villus and lamina propria heights in the jejunum (446 ± 80 vs 590 ± 43 μm and 388 ± 65 vs 518 ± 39 μm respectively; P < 0.05) were significantly decreased in comparison to CG. The jejunal crypt depths were also decreased, although not significantly. There was no significant difference for crypt depths and lamina propria height before injection of TO between Gln and PP-supplemented groups. In contrast, villus heights were maintained in Gln group but not in PP group (Figure 2A). One day after TO injection, a significant decrease was observed in jejunal villus height, crypt depth and lamina propria for CG group (421 ± 36 μm vs 590 ± 43 μm, 130 ± 4 μm vs 282 ± 13 μm, 386 ± 40 μm vs 517 ± 39 μm respectively; P <0.05). In the other groups, morphometrics did not change after TO. Three days after injury, morphometric parameters were restored only in CG group. There was no difference between the 3 protein restricted groups (PR, Gln, PP; Figure 2).
Figure 2.
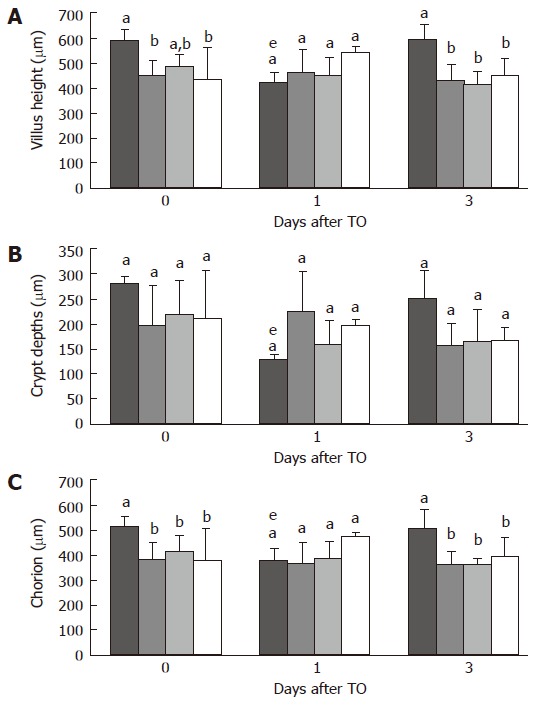
Morphometrics of jejunal villus height (A), jejunal crypt depths (B), and jejunal lamina propria (C), immediately before turpentine oil (TO) injection and 1, and 3 d after TO. Rats received either control ( ), protein-restricted (PR; ), glutamine (Gln; ), or protein-powder (PP; ) supplemented diets. Values are means ± SD. Means without a common letter (a, b or c) differ. eP < 0.05 vs d 0.
Bacterial translocation
For the CG, no viable bacteria were detectable in cultured mesenteric lymph nodes before TO injection (Table 3). In contrast, some positive cultures were observed for lymph nodes from PR animals as well as from Gln or PP-supplemented groups. After TO induced inflammation, bacterial translocation was also noted in several animals; however, no significant difference between groups was observed (Table 3).
Glutathione and glutamine concentrations
The glutathione concentration in liver and jejunum is displayed in Figure 3. In both tissues, glutathione concentration significantly decreased after protein restriction (jejunum: 1.72 ± 0.46 vs 4.24 ± 1.40 μmol/g tissue; liver: 2.59 ± 0.38 vs 7.3 ± 0.38 μmol/g tissue, both P < 0.05). Glutathione concentration was restored after glutamine supplementation in the jejunum (3.24 ± 1.05 vs 1.72 ± 0.46 μmol/g tissue, P < 0.05), while it remained low despite supplementation with protein powder (Figure 3A). In the liver, glutamine supplementation had no significant effect on glutathione concentration (Figure 3B).
Figure 3.
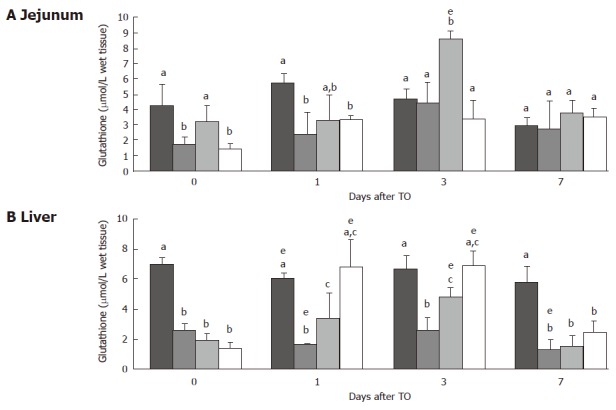
Jejunal (A) and liver (B) glutathione (μmol/g tissue) immediately before turpentine oil (TO) injection and 1, 3 and 7 d after TO. Rats received either control ( ), protein-restricted (PR; ), glutamine (Gln; ), or protein-powder (PP; ). Values are means ± SD. Means without a common letter (a, b or c) differ. eP < 0.05 vs d 0.
After TO injection, glutathione concentration in the jejunum peaked on d 1 and d 3 for CG and PR animals, respectively (Figure 3A). In the Gln group, but not in the PP group, a marked jejunal glutathione concentration peak was also observed on d 3 (Figure 3A, P < 0.05 Gln vs PR). Glutathione in the liver was decreased on d 1, and later increased on d 3 in CG and PR animals (Figure 3B, P < 0.05 d 1 vs d 0 for CG , and P < 0.05 on d 3 vs d 1 for PR). In both groups of supplemented rats, liver glutathione rose markedly on d 1 and peaked on d 3 after TO injection, and returned to initial values on d 7 (Figure 3B). Protein concentrations were similar and did not vary significantly in the both organs.
Intracellular glutamine concentration before inflamma-tion was significantly increased in the Gln group in comparison to CG (2.36 ± 2.93 vs 1.05 ± 0.23 μmol/g tissue; P < 0.05), while it was not affected by protein deprivation alone or by supplementation with protein powder. After TO, no significant difference was observed between groups and intracellular glutamate was not modified by any diet nor by TO induced inflammation (data not shown).
Acute phase response
Before TO, α-1 AGP was detected to a low level in the plasma but no difference was observed between the groups (Figure 4A). In contrast, α2-MG was increased in plasma from Gln rats (Figure 4B). After TO, plasma α-1 AGP and α2-MG increased in all groups to a similar extent.
Figure 4.
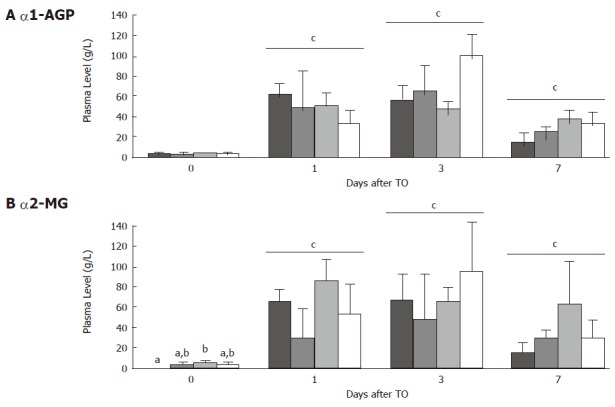
Plasma levels (g/L) for alpha-1 acid glycoprotein (A) and alpha-2 macroglobulin (B) immediately before turpentine oil (TO) injection and 1, 3 and 7 d after TO. Rats received either control ( ), protein-restricted (PR; ), glutamine (Gln; ), or protein-powder (PP; ) supplemented diets. Values are means ± SD. Means without a common letter (a, b) differ. cP < 0.05 vs d 0.
Jejunal α-1 AGP mRNA (Figure 5) was not constitu-tively expressed in any groups. TO injection induced a weak increase of α-1 AGP mRNA level in CG group only at d 1. This increase was prolonged in PR group until d 7. However, the peak response of α-1 AGP mRNA was higher in the two supplemented groups (Gln and PP, P < 0.05, Figure 5). The highest α-1 AGP mRNA peak response was observed in the Gln group at d 1, and was significantly higher than that observed in PR and PP groups (P < 0.05). Jejunal α2-MG mRNA level remained not affected by protein restriction, Gln or PP supplementation and TO challenge (data not shown).
Figure 5.
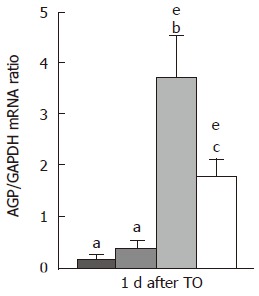
Jejunal mRNA level for alpha-1 acid glycoprotein (AGP/GAPDH mRNA ratio) one day after turpentine oil injection. Rats received either control ( ), protein-restricted (PR; ), glutamine (Gln; ), or protein-powder (PP; ) supplemented diets. Values are means ± SD. Means without a common letter (a, b or c) differ. eP < 0.05 vs d 0.
DISCUSSION
In the present study, we measured the effect of glutamine supplementation on gut barrier, glutathione content and acute phase response in severely protein-restricted rats during TO injection. Our results indicate that glutamine restored gut glutathione content and regulated intestinal acute phase response without influencing bacterial translocation in protein-restricted rats.
In the present study, weight gain was severely impaired in rats fed a protein-free diet (PR group). Growth retardation has been reported as a consequence of feeding a poor-protein diet without taking into consideration the anorexia associated with the consumption of low protein diets[29]. In our study, the mean daily energy intake during the first week of the feeding period was also diminished in all the groups fed with 0% protein diets (see results section). Thus, as both diets were isocaloric, this suggests that the absence of proteins may have affected the palatability of the diets or influenced the central regulation of appetite[30]. Neither glutamine nor protein powder supplementation restored a growth rate similar to that of animals fed the normal-protein diet. However, during the second week, the variations in the energy intake between the 4 groups did not reach significance. It may appear not physiological to have used a protein-free diet. However, it is not rare that patients remain with only fluids and glucose for several days in postoperative situations while undergoing inflammatory stress. In addition, this diet was appropriate to test the pharmacological effect of glutamine alone, apart from that of other amino acids.
Inflammation was induced by a subcutaneous injection of 3 mL/kg of body weight of TO. Previous studies have shown that this experimental model elicits hormonal and metabolic changes similar to those observed during the response to injury and infection[12,17,27]. This includes the production of the acute-phase protein α-2 macroglobulin[12,27] but does not lead to the anorexia commonly associated with administration of endotoxin, gavage with bacteria or other injury models[27]. The injection of TO had only a marginal effect upon the rate of weight gain in normally fed animals following the injection. This observation confirms similar findings observed in pigs[31].
The gastrointestinal tract is a major organ of glutamine utilization[32]. In various models of stress-induced injury (cancer, radiation, and chemotherapy) or malnutrition, glutamine supply maintains or restores the normal morphometric values of the small bowel mucosa and supports gut function[33]. However, glutamine deprivation in an otherwise complete diet prior to TO injury had only a marginal detrimental effect on the structural integrity of the small intestinal mucosa[17]. This may be due to the fact that animals were not otherwise protein-depleted, and were thus able to maintain an adequate glutamine endogenous de novo synthesis from several other amino acids i.e. branched-chain amino acids[34].
In our study, the histological assessment of the mucosa in the jejunum was carried out on 4 rats from each dietary group before and 1 and 3 d after TO injection. Protein restriction induced a marked, significant decrease of jejunal villus and chorion height (Figure 2). In contrast, in Gln group, villus height was only modestly reduced (not significantly different from CG). This is consistent with a beneficial effect of glutamine supplementation on enterocyte proliferation rate as reported in human mucosa in vitro[35]. It may also be explained by a prolongation of cell life[20] or apoptosis inhibition[36,37].
Bacterial translocation occurred in several animals in all protein-restricted rats. This may be due to the passage of viable bacteria through paracellular pathways, as a consequence of altered tight junction selectivity secondary to severely impaired metabolism. None of the supplements had any significant preventive effect on bacterial translocation, which is at variance with some other reports in enterally[1] or parenterally fed rats supplemented with glutamine[19]. This lack of preventive effect may be due to the fact that other factors involved in gut barrier function, such as mucosal immune cells or IgA secretion, had been impaired by severe protein restriction[1,19].
Glutathione plays an important role in detoxification reactions with xenobiotics and oxygen radicals[3] and is also required in large amounts in catabolic states, not only by hepatic and intestinal cells but also by inflammatory cells; thus, impaired mucosal glutathione content increases the susceptibility to oxidative tissue injury[3,5]. Glutathione requires glutamate for its biosynthesis and the main intracellular source of glutamate is derived from glutamine[38]. Some authors reported that glutamine supplementation may increase glutathione concentration both in the liver[39] and in the gut[23,40,41]. In both tissues studied in the present study (jejunum and liver), glutathione concentration decreased markedly in PR rats before TO injection. After the inflammatory shock, liver glutathione slightly decreased in CG and PR groups, and later increased up to values not different from initial values; in contrast, it was markedly increased in Gln and PP groups. The transient initial decrease in CG and PR animals may reflect the short-term reduction of solid food intake after TO, since liver glutathione is very sensitive to food deprivation. Contrastingly, after TO, rats in all groups markedly increased their water intake (data not shown) until d 3; this resulted in an increased intake of both glutamine and protein powder. Accordingly, an increase of liver glutathione was observed after inflammatory shock on d 3 and may have been supported by the increased intake of precursors from glutamine or protein powder. The significant decrease in jejunal glutathione of severely protein-restricted rats in the present study is in accordance with previous results in rats fed a 3% protein diet[31]. Both a decreased synthetic rate of glutathione in the mucosa[23,42] and/or a leakage in the lumen may have contributed to this decreased concentration of glutathione. At most time points, glutamine but not protein powder restored glutathione concentrations in the jejunum and this effect was significant on d 0 and d 3. In contrast, glutathione in the liver was best restored with the amino acid mixture (PP). The specific beneficial effect of glutamine on jejunal glutathione probably reflects the high capacities of uptake, as reflected by intracellular glutamine concentration and of glutamine utilization in the proximal small intestine[43] for immediate glutathione synthesis. In addition, beneficial effects of glutamine on villus height and GSH content in jejunal mucosa could be related to each other since a negative correlation between GSH content and apoptosis of epithelial cells has been reported[44]. Despite being provided with isonitrogenous drinking solutions, rats in the PP group drank somewhat less water than those in the Gln group, with consequently a lower nitrogen intake in the PP group than in the Gln group. Despite this, jejunal glutathione content in the glutamine group was even higher than in normally fed rats (Figure 3B). Thus, the supply of free glutamine via the oral route may have a distinct kinetic advantage as far as mucosal glutathione synthesis is concerned.
Before the induction of inflammatory shock, the mRNA for α-1 AGP was not expressed in the jejunal mucosa, which is in accordance with data in cultured rat intestinal epithelial cells[45]. In response to TO challenge, mRNA expression for α-1 AGP increased in jejunal mucosa in normally fed rats. However, protein restriction and glutamine-supplementation enhanced jejunal α-1 AGP response without altering plasma APP concentrations. The α-1 AGP has been reported to have an anti-inflammatory effect by increasing the production of IL-1 receptor antagonist[46], by limiting the migration of leukocytes through endothelium[47] and by reducing complement- and neutrophil-mediated intestinal injury[48]. Thus, an enhanced intestinal α-1 AGP production after supplementation with glutamine may contribute to the local protective effects of glutamine, together with the reduction of pro-inflammatory cytokine production[49], as well as the improvement of protein metabolism[50].
In summary, our results indicate that glutamine supports glutathione stores and may support the intestinal acute phase response in the jejunum of malnourished rats during inflammatory shock. Since a marked glutathione depletion has been observed during inflammatory bowel diseases, specially when combined with malnutrition[8], the beneficial effects of glutamine-supplemented diets on antioxidative capacities and gut integrity in inflammatory conditions with associated malnutrition should be evaluated.
ACKNOWLEDGMENTS
The authors are grateful to Damien Genty and Corinne Bodenant for their skilful contribution to morphometric analyses, to Claudine Dauguet for their helpful technical assistance and to Jean-François Menard for his help in the statistical analysis.
Footnotes
S- Editor Liu Y L- Editor Alpini GD E- Editor Chen GJ
References
- 1.Souba WW, Herskowitz K, Klimberg VS, Salloum RM, Plumley DA, Flynn TC, Copeland EM. The effects of sepsis and endotoxemia on gut glutamine metabolism. Ann Surg. 1990;211:543–549; discussion 549-551;. doi: 10.1097/00000658-199005000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander JW. Nutrition and translocation. JPEN J Parenter Enteral Nutr. 1990;14:170S–174S. doi: 10.1177/014860719001400505. [DOI] [PubMed] [Google Scholar]
- 3.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 4.Lash LH, Hagen TM, Jones DP. Exogenous glutathione protects intestinal epithelial cells from oxidative injury. Proc Natl Acad Sci USA. 1986;83:4641–4645. doi: 10.1073/pnas.83.13.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao RK, Li L, Baker RD, Baker SS, Gupta A. Glutathione oxidation and PTPase inhibition by hydrogen peroxide in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol. 2000;279:G332–G340. doi: 10.1152/ajpgi.2000.279.2.G332. [DOI] [PubMed] [Google Scholar]
- 6.Kelly FJ. Glutathione content of the small intestine: regulation and function. Br J Nutr. 1993;69:589–596. doi: 10.1079/bjn19930058. [DOI] [PubMed] [Google Scholar]
- 7.Hum S, Koski KG, Hoffer LJ. Varied protein intake alters glutathione metabolism in rats. J Nutr. 1992;122:2010–2018. doi: 10.1093/jn/122.10.2010. [DOI] [PubMed] [Google Scholar]
- 8.Miralles-Barrachina O, Savoye G, Belmonte-Zalar L, Hochain P, Ducrotté P, Hecketsweiler B, Lerebours E, Déchelotte P. Low levels of glutathione in endoscopic biopsies of patients with Crohn's colitis: the role of malnutrition. Clin Nutr. 1999;18:313–317. doi: 10.1016/s0261-5614(98)80030-7. [DOI] [PubMed] [Google Scholar]
- 9.Sido B, Hack V, Hochlehnert A, Lipps H, Herfarth C, Dröge W. Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut. 1998;42:485–492. doi: 10.1136/gut.42.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molmenti EP, Ziambaras T, Perlmutter DH. Evidence for an acute phase response in human intestinal epithelial cells. J Biol Chem. 1993;268:14116–14124. [PubMed] [Google Scholar]
- 11.Jennings G, Bourgeois C, Elia M. The magnitude of the acute phase protein response is attenuated by protein deficiency in rats. J Nutr. 1992;122:1325–1331. doi: 10.1093/jn/122.6.1325. [DOI] [PubMed] [Google Scholar]
- 12.Lyoumi S, Tamion F, Petit J, Déchelotte P, Dauguet C, Scotté M, Hiron M, Leplingard A, Salier JP, Daveau M, et al. Induction and modulation of acute-phase response by protein malnutrition in rats: comparative effect of systemic and localized inflammation on interleukin-6 and acute-phase protein synthesis. J Nutr. 1998;128:166–174. doi: 10.1093/jn/128.2.166. [DOI] [PubMed] [Google Scholar]
- 13.Li JY, Lu Y, Hu S, Sun D, Yao YM. Preventive effect of glutamine on intestinal barrier dysfunction induced by severe trauma. World J Gastroenterol. 2002;8:168–171. doi: 10.3748/wjg.v8.i1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mårtensson J, Jain A, Meister A. Glutathione is required for intestinal function. Proc Natl Acad Sci USA. 1990;87:1715–1719. doi: 10.1073/pnas.87.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Hulst RR, van Kreel BK, von Meyenfeldt MF, Brummer RJ, Arends JW, Deutz NE, Soeters PB. Glutamine and the preservation of gut integrity. Lancet. 1993;341:1363–1365. doi: 10.1016/0140-6736(93)90939-e. [DOI] [PubMed] [Google Scholar]
- 16.Zhou YP, Jiang ZM, Sun YH, Wang XR, Ma EL, Wilmore D. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: a randomized, double-blind, controlled clinical trial. JPEN J Parenter Enteral Nutr. 2003;27:241–245. doi: 10.1177/0148607103027004241. [DOI] [PubMed] [Google Scholar]
- 17.Wusteman M, Tate H, Weaver L, Austin S, Neale G, Elia M. The effect of enteral glutamine deprivation and supplementation on the structure of rat small-intestine mucosa during a systemic injury response. JPEN J Parenter Enteral Nutr. 1995;19:22–27. doi: 10.1177/014860719501900122. [DOI] [PubMed] [Google Scholar]
- 18.Quan ZF, Yang C, Li N, Li JS. Effect of glutamine on change in early postoperative intestinal permeability and its relation to systemic inflammatory response. World J Gastroenterol. 2004;10:1992–1994. doi: 10.3748/wjg.v10.i13.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alverdy JA, Aoys E, Weiss-Carrington P, Burke DA. The effect of glutamine-enriched TPN on gut immune cellularity. J Surg Res. 1992;52:34–38. doi: 10.1016/0022-4804(92)90275-5. [DOI] [PubMed] [Google Scholar]
- 20.van der Hulst RR, von Meyenfeldt MF, Tiebosch A, Buurman WA, Soeters PB. Glutamine and intestinal immune cells in humans. JPEN J Parenter Enteral Nutr. 1997;21:310–315. doi: 10.1177/0148607197021006310. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler TR, Bye RL, Persinger RL, Young LS, Antin JH, Wilmore DW. Effects of glutamine supplementation on circulating lymphocytes after bone marrow transplantation: a pilot study. Am J Med Sci. 1998;315:4–10. doi: 10.1097/00000441-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Newsholme P, Curi R, Pithon Curi TC, Murphy CJ, Garcia C, Pires de Melo M. Glutamine metabolism by lymphocytes, macrophages, and neutrophils: its importance in health and disease. J Nutr Biochem. 1999;10:316–324. doi: 10.1016/s0955-2863(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, Feng Z, Hoos A, Klimberg VS. Glutamine enhances gut glutathione production. JPEN J Parenter Enteral Nutr. 1998;22:224–227. doi: 10.1177/0148607198022004224. [DOI] [PubMed] [Google Scholar]
- 24.Ameho CK, Adjei AA, Harrison EK, Takeshita K, Morioka T, Arakaki Y, Ito E, Suzuki I, Kulkarni AD, Kawajiri A, et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41:487–493. doi: 10.1136/gut.41.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coëffier M, Marion R, Leplingard A, Lerebours E, Ducrotté P, Déchelotte P. Glutamine decreases interleukin-8 and interleukin-6 but not nitric oxide and prostaglandins e(2) production by human gut in-vitro. Cytokine. 2002;18:92–97. doi: 10.1006/cyto.2002.1027. [DOI] [PubMed] [Google Scholar]
- 26.Coëffier M, Miralles-Barrachina O, Le Pessot F, Lalaude O, Daveau M, Lavoinne A, Lerebours E, Déchelotte P. Influence of glutamine on cytokine production by human gut in vitro. Cytokine. 2001;13:148–154. doi: 10.1006/cyto.2000.0813. [DOI] [PubMed] [Google Scholar]
- 27.Jennings G, Elia M. Independent effects of protein and energy deficiency on acute-phase protein response in rats. Nutrition. 1991;7:430–434. [PubMed] [Google Scholar]
- 28.Fouin-Fortunet H, Besnier MO, Colin R, Wessely JY, Rosé F. Effects of ketoacids on liver glutathione and microsomal enzymes in malnourished rats. Kidney Int Suppl. 1989;27:S222–S226. [PubMed] [Google Scholar]
- 29.Grimble RF, Jackson AA, Persaud C, Wride MJ, Delers F, Engler R. Cysteine and glycine supplementation modulate the metabolic response to tumor necrosis factor alpha in rats fed a low protein diet. J Nutr. 1992;122:2066–2073. doi: 10.1093/jn/122.11.2066. [DOI] [PubMed] [Google Scholar]
- 30.Hunter EA, Grimble RF. Dietary sulphur amino acid adequacy influences glutathione synthesis and glutathione-dependent enzymes during the inflammatory response to endotoxin and tumour necrosis factor-alpha in rats. Clin Sci (Lond) 1997;92:297–305. doi: 10.1042/cs0920297. [DOI] [PubMed] [Google Scholar]
- 31.Jahoor F, Wykes LJ, Reeds PJ, Henry JF, del Rosario MP, Frazer ME. Protein-deficient pigs cannot maintain reduced glutathione homeostasis when subjected to the stress of inflammation. J Nutr. 1995;125:1462–1472. doi: 10.1093/jn/125.6.1462. [DOI] [PubMed] [Google Scholar]
- 32.Déchelotte P, Darmaun D, Rongier M, Hecketsweiler B, Rigal O, Desjeux JF. Absorption and metabolic effects of enterally administered glutamine in humans. Am J Physiol. 1991;260:G677–G682. doi: 10.1152/ajpgi.1991.260.5.G677. [DOI] [PubMed] [Google Scholar]
- 33.Inoue Y, Grant JP, Snyder PJ. Effect of glutamine-supplemented total parenteral nutrition on recovery of the small intestine after starvation atrophy. JPEN J Parenter Enteral Nutr. 1993;17:165–170. doi: 10.1177/0148607193017002165. [DOI] [PubMed] [Google Scholar]
- 34.Darmaun D, Déchelotte P. Role of leucine as a precursor of glutamine alpha-amino nitrogen in vivo in humans. Am J Physiol. 1991;260:E326–E329. doi: 10.1152/ajpendo.1991.260.2.E326. [DOI] [PubMed] [Google Scholar]
- 35.Scheppach W, Loges C, Bartram P, Christl SU, Richter F, Dusel G, Stehle P, Fuerst P, Kasper H. Effect of free glutamine and alanyl-glutamine dipeptide on mucosal proliferation of the human ileum and colon. Gastroenterology. 1994;107:429–434. doi: 10.1016/0016-5085(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 36.Papaconstantinou HT, Hwang KO, Rajaraman S, Hellmich MR, Townsend CM, Ko TC. Glutamine deprivation induces apoptosis in intestinal epithelial cells. Surgery. 1998;124:152–159; discussion 159-160;. [PubMed] [Google Scholar]
- 37.Evans ME, Jones DP, Ziegler TR. Glutamine prevents cytokine-induced apoptosis in human colonic epithelial cells. J Nutr. 2003;133:3065–3071. doi: 10.1093/jn/133.10.3065. [DOI] [PubMed] [Google Scholar]
- 38.Welbourne TC. Ammonia production and glutamine incorporation into glutathione in the functioning rat kidney. Can J Biochem. 1979;57:233–237. doi: 10.1139/o79-029. [DOI] [PubMed] [Google Scholar]
- 39.Gonzales S, Polizio AH, Erario MA, Tomaro ML. Glutamine is highly effective in preventing in vivo cobalt-induced oxidative stress in rat liver. World J Gastroenterol. 2005;11:3533–3538. doi: 10.3748/wjg.v11.i23.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basivireddy J, Jacob M, Balasubramanian KA. Oral glutamine attenuates indomethacin-induced small intestinal damage. Clin Sci (Lond) 2004;107:281–289. doi: 10.1042/CS20030390. [DOI] [PubMed] [Google Scholar]
- 41.Prabhu R, Thomas S, Balasubramanian KA. Oral glutamine attenuates surgical manipulation-induced alterations in the intestinal brush border membrane. J Surg Res. 2003;115:148–156. doi: 10.1016/s0022-4804(03)00212-9. [DOI] [PubMed] [Google Scholar]
- 42.Breuille D, Rose F, Arnal M, Melin C, Obled C. Sepsis modifies the contribution of different organs to whole-body protein synthesis in rats. Clin Sci (Lond) 1994;86:663–669. doi: 10.1042/cs0860663. [DOI] [PubMed] [Google Scholar]
- 43.Windmueller HG, Spaeth AE. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate. J Biol Chem. 1980;255:107–112. [PubMed] [Google Scholar]
- 44.Benard O, Madesh M, Anup R, Balasubramanian KA. Apoptotic process in the monkey small intestinal epithelium: I. Association with glutathione level and its efflux. Free Radic Biol Med. 1999;26:245–252. doi: 10.1016/s0891-5849(98)00164-6. [DOI] [PubMed] [Google Scholar]
- 45.Boudreau F, Yu SJ, Asselin C. CCAAT/enhancer binding proteins beta and delta regulate alpha1-acid glycoprotein gene expression in rat intestinal epithelial cells. DNA Cell Biol. 1998;17:669–677. doi: 10.1089/dna.1998.17.669. [DOI] [PubMed] [Google Scholar]
- 46.Tilg H, Vannier E, Vachino G, Dinarello CA, Mier JW. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J Exp Med. 1993;178:1629–1636. doi: 10.1084/jem.178.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Graaf TW, Van der Stelt ME, Anbergen MG, van Dijk W. Inflammation-induced expression of sialyl Lewis X-containing glycan structures on alpha 1-acid glycoprotein (orosomucoid) in human sera. J Exp Med. 1993;177:657–666. doi: 10.1084/jem.177.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams JP, Weiser MR, Pechet TT, Kobzik L, Moore FD, Hechtman HB. alpha 1-Acid glycoprotein reduces local and remote injuries after intestinal ischemia in the rat. Am J Physiol. 1997;273:G1031–G1035. doi: 10.1152/ajpgi.1997.273.5.G1031. [DOI] [PubMed] [Google Scholar]
- 49.Coëffier M, Marion R, Ducrotté P, Déchelotte P. Modulating effect of glutamine on IL-1beta-induced cytokine production by human gut. Clin Nutr. 2003;22:407–413. doi: 10.1016/s0261-5614(03)00040-2. [DOI] [PubMed] [Google Scholar]
- 50.Coëffier M, Claeyssens S, Hecketsweiler B, Lavoinne A, Ducrotté P, Déchelotte P. Enteral glutamine stimulates protein synthesis and decreases ubiquitin mRNA level in human gut mucosa. Am J Physiol Gastrointest Liver Physiol. 2003;285:G266–G273. doi: 10.1152/ajpgi.00385.2002. [DOI] [PubMed] [Google Scholar]


