Abstract
AIM: To evaluate the effects of combined radiofrequency ablation and transcatheter arterial embolization with iodized oil on ablation time, maximum output, coagulation diameter, and portal angiography in a porcine liver model.
METHODS: Radiofrequency ablation (RFA) was applied to in vivo livers of 10 normal pigs using a 17-gauge 3.0 cm expandable LeVeen RF needle electrode with or without transcatheter arterial embolization (TAE) with iodized oil (n = 5). In each animal, 2 areas in the liver were ablated. Direct portography was performed before and after RFA. Ablation was initiated at an output of 30 W, and continued with an increase of 10 W per minute until roll-off occurred. Ablation time and maximum output until roll-off, and coagulated tissue diameter were compared between the 2 groups. Angiographic changes on portography before and after ablation were also reviewed.
RESULTS: For groups with and without TAE with iodized oil, the ablation times until roll-off were 320.6 ± 30.9 seconds and 445.1 ± 35.9 seconds, respectively, maximum outputs were 69.0 ± 7.38 W and 87.0 ± 4.83 W and maximal diameters of coagulation were 41.7 ± 3.85 mm and 33.2 ± 2.28 mm. Significant reductions of ablation time and maximum output, and significantly larger coagulation diameter were obtained with RFA following TAE with iodized oil compared to RFA alone. Portography after RFA following TAE with iodized oil revealed more occlusion of the larger portal branches than with RFA alone.
CONCLUSION: RFA following TAE with iodized oil can increase the volume of coagulation necrosis with lower output and shorter ablation time than RFA alone in normal pig liver tissue.
Keywords: Liver, Radiofrequency ablation, Transcatheter arterial embolization, Iodized oil, Angiography, Hepatocellular carcinoma
INTRODUCTION
Percutaneous radiofrequency ablation (RFA) is widely used to treat focal malignant tumors such as hepatocellular carcinoma (HCC) and metastatic liver tumors[1-3]. It has been reported by many authors that this is a satisfactory therapeutic modality because it results in reliable local control and is minimally invasive[4,5]. The greatest disadvantage of RF ablation for treating liver tumors is difficulty of ablating large tumors due to the limited coagulation area, frequently observed in the treatment of large HCC. Inability to reliably creating adequate volumes of complete tumor coagulation limits the adoption of RF ablation for large hepatic tumors.
One characteristic of RF ablation is its susceptibility to the cooling effect of blood flow[6,7], a factor which has a considerable effect on RFA treatment. The reduced efficacy of RF ablation for large tumors reflects the in vivo biophysiological limitations imposed by perfusion-mediated vascular cooling, which limits heat-induced coagulation necrosis. HCCs, in particular, are supplied almost entirely by the hepatic arteries, and the abundant tumoral arterial blood flow has a cooling effect, which diminishes radiofrequency wave heating. Several investigators have been able to increase RF-induced coagulation necrosis by occluding blood flow to the liver during ablation procedures in animal models[7-10].
Transcatheter arterial embolization (TAE) using iodized oil mixed with an anticancer drug has been widely performed to treat HCC[11-13]. TAE can block hepatic arterial blood flow and attenuate the cooling effect of tumoral arterial blood flow. Recently, many clinical studies have shown that RF combined with TAE is effective on large HCC lesions[14,15].
In the present study, we evaluated whether TAE with iodized oil can increase RF-induced coagulation necrosis, and what modifications and effects are added in terms of ablation time, maximum output, and portal angiography by TAE with iodized oil.
MATERIALS AND METHODS
Animals and animal care
Approval of the institutional committee for the care of research animals was obtained before the study was initiated. This study was conducted in accordance with the guideline for the care and use of laboratory animals.
Ten healthy male pigs (weighing 52-59 kg; mean, 56.6 kg) were subjected to laparotomy under general anesthesia. The animals were sedated with an intramuscular injection of ketamine (5 mg/kg weight), xylazine (5 mg/kg weight), and atropine (0.02 mg/kg weight). General anesthesia was maintained with 1.5% fluothane containing a mixture of oxygen and nitrous oxide after tracheal intubation. Cardiac and respiratory parameters were monitored throughout the procedures. A longitudinal midline abdominal incision was made, and the peritoneum was opened. A 4 Fr. catheter (RC2; Clinical Supply, Gifu, Japan) was inserted into the proper hepatic artery via the femoral artery. The portal vein was exposed and punctured directly, and the 4 Fr. catheter was inserted. Hepatic arteriography and direct portography were performed before and after RFA. Hepatic arteriography was performed with an injection of contrast agent (Iomeprol 350 Ezai, Tokyo, Japan) at a rate of 3 mL/s for a total volume of 15 mL, and direct portography at a rate of 6 mL/s for a total volume of 24 mL. TAE was performed by selectively introducing a microcatheter (Sniper; Clinical Supply, Gifu, Japan) into the right and left hepatic arteries, and injecting iodized oil (10 mL per body ; lipiodol ultra-fluid: iodine addition products of the ethylesters of the fatty acid obtained from poppyseed oil, Nihon Schering, Osaka Japan) (LP) and 1 mm gelatin sponge particles in 5 animals. Animals were divided into 2 groups of 5 animals each: RFA-alone group and RFA following TAE with iodized oil group (LP-TAE group).
RF procedure
The RF device used was a RF2000 radiofrequency ablation system (Boston Scientific Corporation, Natick, MA, USA) with an expansion-type electrode (LeVeen needle). A 17-gauge, 3.0-cm expandable 10-hook needle electrode was inserted directly into the liver with ultrasound guidance. After the needle position was confirmed with X ray and ultrasound, ablation was started. For each pig, 2 areas of the right and left lobes were ablated, yielding a total of 20 areas.
The RF 2000 generator monitors system impedance, the level of which determines the extent of tissue necrosis. In this apparatus, an increase of tissue impedance to current flow, which is caused by decreased conductivity of electrical current due to protein denaturation and loss of intracellular fluids, is measured in ohms. When tissue impedance rises above 200 Ω, the power output passively decreases to less than 10 W (roll-off). Roll-off indicates a precipitous drop of power output with a marked increase of tissue impedance because of tissue necrosis, which prohibits the passage of electrical current[16]. For all experiments, impedance and power output status were monitored on the display window of the RF2000 generator, and recorded on paper every 15 s.
A 2-phase application process was performed. The baseline power output was set at 30 W. Ablation was continued with an increase of 10 W per minute until roll-off occurred. After the first RO, ablation was resumed 30 seconds later at 70% of the maximum power achieved, and was completed when RO occurred a second time (Table 1). The total RF application time was calculated as the sum of the duration of the first and second phases of ablation.
Table 1.
Ablation protocol
| Baseline power output | Step up rate | Maximum output | Restart output | Application process |
| 30 W | 10 W/min | Until roll-off | 70% of the maximum output | Twice |
The baseline power output was set at 30 W. Ablation was continued with an increase of 10 W per minute until roll-off occurred. After the first RO, ablation was resumed 30 s later at 70% of the maximum power achieved, and was completed when RO occurred a second time.
Animals were sacrificed using an overdose of pento-barbital immediately after post-RFA angiography, and their livers were removed for gross pathologic analysis. Visible regions of coagulation necrosis were measured with calipers in fresh tissue prior to preservation. Measurements of the diameter of coagulation were based on the consensus of 2 observers. The 2 groups were compared with respect to ablation time and maximum output required for the occurrence of the second RO, as well as maximum coagulation diameter. Angiographic changes on portography before and after ablation were also compared. Data were analyzed using Student’s t-test with a significance level of 5%.
RESULTS
Both cardiac and respiratory parameters remained stable throughout the experiments. No thermal injuries to adjacent structures or organs occurred. Regions of RF ablation were easily distinguished from untreated tissue.
For the RFA-alone group and LP-TAE group, total ablation times were 445.1 ± 35.9 s and 320.6 ± 30.9 s, maximum outputs were 87.0 ± 4.83 W and 69.0 ± 7.38 W, and maximal diameters of coagulation were 33.2 ± 2.28 mm and 41.7 ± 3.85 mm, respectively (Table 2). Significant reductions of ablation time and maximum output and significantly larger coagulation diameter were obtained with RFA following LP-TAE compared to RFA alone.
Table 2.
Ablation time to roll-off, maximum output, and coagulation diameter
| Group with RFA alone | Group with RFA following LP-TAE | P value | |
| Ablation time to roll-off (s) | 445.1 ± 35.9 | 320.6 ± 30.9 | P < 0.05 |
| Maximum output (W) | 87.0 ± 4.83 | 69.0 ± 7.38 | P < 0.05 |
| Coagulation diameter (mm) | 33.2 ± 2.28 | 41.7 ± 3.85 | P < 0.05 |
Significant reductions in roll-off time and output and significantly greater coagulation diameter were obtained with RFA following LP-TAE compared with RFA alone. LP-TAE: Transcatheter arterial embolization with iodized oil (Lipiodol); RFA: radiofrequency ablation.
In the RFA-alone group, disappearance of the small distal branches of the portal vein was observed on portography (Figure 1A), and a compensatory increase of hepatic arterial blood flow was noted on hepatic arteriography (Figure 1B). Subsegmental or distal portal branches disappeared in 8 ablated areas (80%), and segmental portal branches disappeared in 2 ablated areas (20%) on portography.
Figure 1.
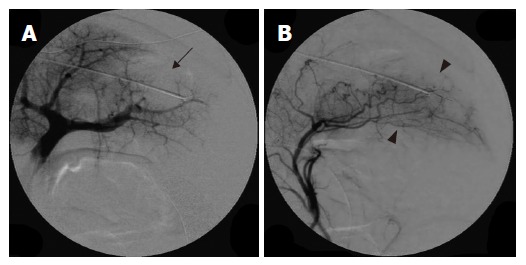
Portography revealing occlusion of distal small portal vein branches in the ablated area (arrow) (A) and hepatic arteriography showing a compensatory increase in hepatic arterial blood flow in response to occlusion of the portal vein branches (arrow head) (B) in group with RFA alone.
After LP-TAE, portal venous blood flow was decreased (Figure 2A) and the liver- enforced LP-TAE changed to dark red (Figure 2B). Disappearance of blood flow in large portal branches was recognized after RFA following LP-TAE (Figure 3). Segmental portal branches or larger branches such as the primary branch of the portal vein disappeared in 8 ablated areas (80%) on portography.
Figure 2.
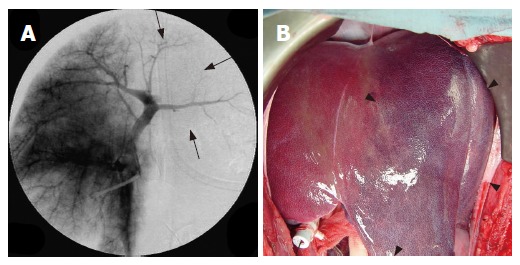
Portography revealing a decrease of portal venous blood flow in the left lobe after LP-TAE before RFA (arrow) (A) and photograph of liver showing the dark red left lobe after LP-TAE before RFA (arrow head) (B).
Figure 3.
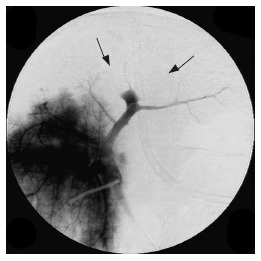
Portography showing large branches of portal veins have disa-ppeared (arrow) after RFA following LP-TAE.
Gross examination revealed a gray core of ablated tissue surrounded by a dark rim in all ablation areas. The RFA-alone group exhibited coagulation necrosis with sparing of large vessels (Figure 4A). In the LP-TAE group, the boundaries of coagulation necrosis were ill-defined, and the diameters of necrotic areas were increased (Figure 4B).
Figure 4.
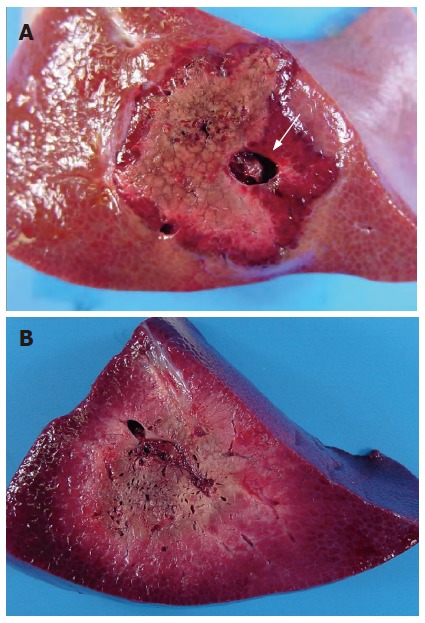
Region of necrosis avoiding large vessels (white arrow) in group with RFA alone (A), and showing ill-defined boundaries of coagulation necrosis and increase of the necrotic area in the group with RFA following LP-TAE (B).
DISCUSSION
The results of the present study demonstrate that RF ablation following TAE with iodized oil induce a larger coagulation necrosis with lower output and shorter ablation time than with RFA alone. To our knowledge, this is the first formal animal study evaluating the effects of combined RFA and TAE with iodized oil on ablation time, maximum power output, and portal angiographic changes.
Radiofrequency tumor ablation has been demonstrated to be a reliable method for creating heat-induced coagulation necrosis using a percutaneous approach[17]. A RF electrode is inserted into the tumor under ultrasound guidance. After attachment to the appropriate generator, a radiofrequency current is emitted from the electrode. RF energy can cause localized cell desiccation and necrosis. Radiofrequency current is emitted from the exposed, noninsulated portion of the electrode, and ion agitation is produced within the tissues surrounding the electrode. This agitation is converted by friction into heat, inducing cellular death via coagulation necrosis[18]. When the temperature within the tissue rises above 60°C, a region of necrosis surrounding the electrode begins to develop[19]. The extent of RF-induced coagulation necrosis depends on overall energy deposition, the duration of radiofrequency application, and radiofrequency electrode tip length[20]. A larger coagulation necrosis is formed with a 3.0 cm expandable electrode than with a 2.0 cm expandable electrode.
RF-induced heating is limited by perfusion-mediated vascular cooling[6,7]. A reduction of blood flow during RF application has been shown to increase coagulation necrosis in animal models[7-10]. Goldberg et al[7] applied RF to normal in vivo porcine liver with and without balloon occlusion of the portal vein and the hepatic artery, and found that greater coagulation could be achieved during portal venous occlusion. Chinn et al[9] compared 4 vascular occlusion groups: portal vein, hepatic artery, both hepatic artery and portal vein, and no occlusion, and reported that coagulation volume is the greatest with occlusion of both hepatic artery and portal vein. Sugimori et al[21] applied RF to normal pig livers using a 15-gauge LeVeen needle with a 2.0 cm expandable electrode at the maximum output of 60 w after transcatheter arterial infusion (TAI) of iodized oil, TAE with gelatin sponge, and TAE with iodized oil and gelatin sponge. They reported that RF ablation after TAE with iodized oil and gelatin sponge induces the largest coagulation necrosis, but there are no significant differences in RF ablation times with RFA alone and RFA combined TAE. In the present study, we applied RF to normal pig livers using a 17-gauge 3.0 cm expandable electrode until roll-off, and compared ablation time, maximum power output, and coagulation diameter between RFA alone and RFA following TAE with iodized oil and gelatin sponge, demonstrating that RF ablation following TAE with iodized oil and gelatin sponge could induce a larger coagulation necrosis with a lower output and a shorter ablation time than RFA alone with a significant difference. It is suggested that the cooling effects of blood flow are greater, so ablation range and expansion diameter of electrode are large.
Arterioportal communications occur through the sinusoids (transsinusoidal communications) and the peribiliary vascular plexus (transplexal communications)[22,23]. It is postulated that when more than a certain amount of iodized oil (lipiodol) is pooled in the sinusoids, a change occurs in the hepatic microcirculation and iodized oil flows into the portal vein through arterioportal communications after pooling in the sinusoids[24]. Therefore, injection of iodized oil into the hepatic artery results in a retrograde flow to the portal vein through the sinusoids and occlusion of portal vein branches[25]. Both hepatic artery and branches of the portal vein are simultaneously blocked after TAE with iodized oil, and perfusion-mediated vascular cooling effect limiting RF-induced coagulation necrosis is thus reduced. As a result, a larger coagulation necrosis is formed with lower output and shorter ablation time than with RFA alone. Furthermore, disappearance of blood flow in large portal branches was recognized after RFA following TAE with iodized oil in the present study, however, the necrosis achieved with RFA alone avoided the large vessels, probably due to the cooling effect of blood flow.
Because HCCs are hypervascular tumors and have abundant tumoral arterial blood flow, which diminishes radiofrequency wave heating, higher power output ablation compared to normal liver tissue is required until roll-off in opposition to the cooling effect. However, high-output RF ablation by RFA alone results in boiling and carbonization of the tissue near the electrode[7,19], leaving an incompletely ablated tumor, and increases the frequency of puncture and the risk of dissemination. It is presumed that scattered recurrence or rapid tumor growth after RFA is attributable to a rapid increase of local tissue temperature and intra-tumoral pressure due to high-output ablation, and bumping (explosion) in the early process of ablation might cause a spreading of incompletely ablated neoplastic cells via the portal vein[26-30]. Moreover, with RFA alone, a compensatory increase of hepatic arterial blood flow in response to occlusion of the portal vein branches after RFA might be related to a rapid growth of residual tumors. Because TAE with iodized oil can block hepatic arterial and portal blood flow and attenuate the vascular cooling effect, RF ablation with a low output for hypervascular hepatic tumors is enabled by combining TAE with iodized oil. Low output RF ablation combined with TAE with iodized oil can prevent a rapid increase of local tissue temperature and intra-tumoral pressure, and may prevent intraportal disseminations and intrahepatic scattered recurrence. Furthermore, TAE with iodized oil prior to RFA can cause ischemic necrosis of the tumors, thereby decreasing the number of neoplastic cells, which can reduce the risk of dissemination even if bumping (explosion) occurs. Our results strongly suggest that a combination of low output RFA and TAE with iodized oil might be efficacious for treating hypervascular large hepatic tumors without complications such as scattered recurrence or rapid tumor growth.
In conclusion, reduction of arterial and portal blood flow due to TAE with iodized oil before RF increases coagulation necrosis with lower output and shorter ablation time than with RFA alone. Strategies for combining RFA with TAE with iodized oil to reduce tumoral blood flow might improve treatment effect on hypervascular large hepatic tumors.
Footnotes
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
References
- 1.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 2.Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, Paties CT, Silverman DE, Buscarini L. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759–768. doi: 10.2214/ajr.167.3.8751696. [DOI] [PubMed] [Google Scholar]
- 3.Lencioni R, Cioni D, Bartolozzi C. Percutaneous radiofrequency thermal ablation of liver malignancies: techniques, indications, imaging findings, and clinical results. Abdom Imaging. 2001;26:345–360. doi: 10.1007/s002610000194. [DOI] [PubMed] [Google Scholar]
- 4.Rossi S, Buscarini E, Garbagnati F, Di Stasi M, Quaretti P, Rago M, Zangrandi A, Andreola S, Silverman D, Buscarini L. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998;170:1015–1022. doi: 10.2214/ajr.170.4.9530052. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg SN, Gazelle GS, Solbiati L, Livraghi T, Tanabe KK, Hahn PF, Mueller PR. Ablation of liver tumors using percutaneous RF therapy. AJR Am J Roentgenol. 1998;170:1023–1028. doi: 10.2214/ajr.170.4.9530053. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg SN, Gazelle GS. Radiofrequency tissue ablation: physical principles and techniques for increasing coagulation necrosis. Hepatogastroenterology. 2001;48:359–367. [PubMed] [Google Scholar]
- 7.Goldberg SN, Hahn PF, Tanabe KK, Mueller PR, Schima W, Athanasoulis CA, Compton CC, Solbiati L, Gazelle GS. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9:101–111. doi: 10.1016/s1051-0443(98)70491-9. [DOI] [PubMed] [Google Scholar]
- 8.Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227:559–565. doi: 10.1097/00000658-199804000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinn SB, Lee FT, Kennedy GD, Chinn C, Johnson CD, Winter TC, Warner TF, Mahvi DM. Effect of vascular occlusion on radiofrequency ablation of the liver: results in a porcine model. AJR Am J Roentgenol. 2001;176:789–795. doi: 10.2214/ajr.176.3.1760789. [DOI] [PubMed] [Google Scholar]
- 10.Sugimori K, Morimoto M, Shirato K, Kokawa A, Tomita N, Saito T, Nozawa A, Hara M, Sekihara H, Tanaka K. Radiofrequency ablation in a pig liver model: effect of transcatheter arterial embolization on coagulation diameter and histologic characteristics. Hepatol Res. 2002;24:164. doi: 10.1016/s1386-6346(02)00030-x. [DOI] [PubMed] [Google Scholar]
- 11.Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397–401. doi: 10.1148/radiology.148.2.6306721. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1989;170:783–786. doi: 10.1148/radiology.170.3.2536946. [DOI] [PubMed] [Google Scholar]
- 13.Uchida H, Ohishi H, Matsuo N, Nishimine K, Ohue S, Nishimura Y, Maeda M, Yoshioka T. Transcatheter hepatic segmental arterial embolization using lipiodol mixed with an anticancer drug and Gelfoam particles for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1990;13:140–145. doi: 10.1007/BF02575465. [DOI] [PubMed] [Google Scholar]
- 14.Buscarini L, Buscarini E, Di Stasi M, Quaretti P, Zangrandi A. Percutaneous radiofrequency thermal ablation combined with transcatheter arterial embolization in the treatment of large hepatocellular carcinoma. Ultraschall Med. 1999;20:47–53. doi: 10.1055/s-1999-14233. [DOI] [PubMed] [Google Scholar]
- 15.Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchianò A, Fornari F, Quaretti P, Tolla GD, Ambrosi C, Mazzaferro V, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119–126. doi: 10.1148/radiology.217.1.r00se02119. [DOI] [PubMed] [Google Scholar]
- 16.Cabassa P, Donato F, Simeone F, Grazioli L, Romanini L. Radiofrequency ablation of hepatocellular carcinoma: long-term experience with expandable needle electrodes. AJR Am J Roentgenol. 2006;186:S316–S321. doi: 10.2214/AJR.05.0243. [DOI] [PubMed] [Google Scholar]
- 17.McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990;25:267–270. doi: 10.1097/00004424-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Cosman ER, Nashold BS, Ovelman-Levitt J. Theoretical aspects of radiofrequency lesions in the dorsal root entry zone. Neurosurgery. 1984;15:945–950. [PubMed] [Google Scholar]
- 19.Goldberg SN, Gazelle GS, Halpern EF, Rittman WJ, Mueller PR, Rosenthal DI. Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad Radiol. 1996;3:212–218. doi: 10.1016/s1076-6332(96)80443-0. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg SN, Gazelle GS, Dawson SL, Rittman WJ, Mueller PR, Rosenthal DI. Tissue ablation with radiofrequency: effect of probe size, gauge, duration, and temperature on lesion volume. Acad Radiol. 1995;2:399–404. doi: 10.1016/s1076-6332(05)80342-3. [DOI] [PubMed] [Google Scholar]
- 21.Sugimori K, Nozawa A, Morimoto M, Shirato K, Kokawa A, Saito T, Numata K, Tanaka K. Extension of radiofrequency ablation of the liver by transcatheter arterial embolization with iodized oil and gelatin sponge: results in a pig model. J Vasc Interv Radiol. 2005;16:849–856. doi: 10.1097/01.RVI.0000157780.44868.78. [DOI] [PubMed] [Google Scholar]
- 22.Cho KJ, Lunderquist A. The peribiliary vascular plexus: the microvascular architecture of the bile duct in the rabbit and in clinical cases. Radiology. 1983;147:357–364. doi: 10.1148/radiology.147.2.6836115. [DOI] [PubMed] [Google Scholar]
- 23.Itai Y, Matsui O. Blood flow and liver imaging. Radiology. 1997;202:306–314. doi: 10.1148/radiology.202.2.9015047. [DOI] [PubMed] [Google Scholar]
- 24.Miller DL, O'Leary TJ, Girton M. Distribution of iodized oil within the liver after hepatic arterial injection. Radiology. 1987;162:849–852. doi: 10.1148/radiology.162.3.3027747. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura H, Hashimoto T, Oi H, Sawada S. Iodized oil in the portal vein after arterial embolization. Radiology. 1988;167:415–417. doi: 10.1148/radiology.167.2.2833765. [DOI] [PubMed] [Google Scholar]
- 26.Nicoli N, Casaril A, Abu Hilal M, Mangiante G, Marchiori L, Ciola M, Invernizzi L, Campagnaro T, Mansueto G. A case of rapid intrahepatic dissemination of hepatocellular carcinoma after radiofrequency thermal ablation. Am J Surg. 2004;188:165–167. doi: 10.1016/j.amjsurg.2003.12.061. [DOI] [PubMed] [Google Scholar]
- 27.Ruzzenente A, Manzoni GD, Molfetta M, Pachera S, Genco B, Donataccio M, Guglielmi A. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol. 2004;10:1137–1140. doi: 10.3748/wjg.v10.i8.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotoh K, Enjoji M, Arimura E, Morizono S, Kohjima M, Sakai H, Nakamuta M. Scattered and rapid intrahepatic recurrences after radio frequency ablation for hepatocellular carcinoma. World J Gastroenterol. 2005;11:6828–6832. doi: 10.3748/wjg.v11.i43.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotoh K, Morizono S, Kohjima M, Enjoji M, Sakai H, Nakamuta M. Evaluation of liver parenchymal pressure and portal endothelium damage during radio frequency ablation in an in vivo porcine model. Liver Int. 2005;25:1217–1223. doi: 10.1111/j.1478-3231.2005.01167.x. [DOI] [PubMed] [Google Scholar]
- 30.Nakai M, Shiraki T, Higashi K, Maeda M, Sahara S, Takeuchi N, Kimura M, Terada M, Sato M. Low-output radiofrequency ablation combined with transcatheter arterial oily-chemoembolization for hepatocellular carcinoma. Nihon Igaku Hoshasen Gakkai Zasshi. 2005;65:124–126. [PubMed] [Google Scholar]


