Abstract
AIM: To investigate the expression of ornithine decarboxylase (ODC) in precancerous and cancerous gastric lesions.
METHODS: We studied the expression of ODC in gastric mucosa from patients with chronic superficial gastritis (CSG, n = 32), chronic atrophic gastritis [CAG, n = 43; 15 with and 28 without intestinal metaplasia (IM)], gastric dysplasia (DYS, n = 11) and gastric cancer (GC, n = 48) tissues using immunohistochemical staining. All 134 biopsy specimens of gastric mucosa were collected by gastroscopy.
METHODS: The positive rate of ODC expression was 34.4%, 42.9%, 73.3%, 81.8% and 91.7% in cases with CSG, CAG without IM, CAG with IM, DYS and GC, respectively (P < 0.01), The positive rate of ODC expression increased in the order of CSG < CAG (without IM) < CAG (with IM) < DYS and finally, GC. In addition, ODC positive immunostaining rate was lower in well-differentiated GC than in poorly-differentiated GC (P < 0.05).
CONCLUSION: The expression of ODC is positively correlated with the degree of malignity of gastric mucosa and development of gastric lesions. This finding indicates that ODC may be used as a good biomarker in the screening and diagnosis of precancerous lesions.
Keywords: Ornithine decarboxylase, Gastric carcinoma, Precancerous lesions, Diagnosis, Immunohistochemistry
INTRODUCTION
Ornithine decarboxylase (ODC) is the first-rate limiting enzyme in the polyamine biosynthesis pathway[1]. ODC plays a critical role in cell proliferation[2], and it is implicated as an essential promoter in normal cell cycles. The activation of ODC is similarly related to tumor promotion and progression[3,4]. ODC activity appears to be directly coupled to the expression of ODC protein and increases with eukaryotic cell division in neoplasia and fetal development. Convincing evidence has confirmed that ODC plays an important role in the chemical carcinogenesis of mouse skin, and tumor formation can be blocked by the irreversible inhibitor of ODC, α-difluoromethylornithine[5-7]. ODC is overexpressed in a variety of cancers. Elevated levels of ODC have been found in gastric cancer[8], gliomas[9], breast cancer[10,11], colon cancer[12], pancreatic cancer[13], lung cancer[14], and prostate cancer[15]. In addition, increased expression of ODC has been found associated with gastric atrophy[16].
Although ODC overexpression is clearly associated with cancer development, a definitive causal role for ODC overexpression in carcinogenesis has only been shown in NIH/3T3 fibroblast cells[17]. Overexpression of ODC is sufficient to transform fibroblast cells in vitro, causing increased frequency of skin tumors in a transgenic mouse model[5,17].
Although several studies have evaluated ODC expre-ssion in gastric cancer, to date, no one has investigated its associations with precancerous gastric lesions, namely, in chronic atrophic gastritis (CAG), intestinal metaplasia (IM) and gastric dysplasia (DYS). There is no information presently available about the protein status of ODC in stomach mucosa collected by gastroscopy. A detailed study comparing the expression patterns of ODC in gastric carcinoma and precancerous tissues has not been conducted. Furthermore, whether overexpression of ODC is involved in the initiation or promotion of gastric cancer (GC) has not been established. Therefore, the aim of the present study was to investigate the expression of ornithine decarboxylase (ODC) in precancerous and cancerous gastric lesions.
MATERIALS AND METHODS
Tissue samples
We collected gastric mucosal specimens from 134 patients including 32 with chronic superficial gastritis (CSG), 43 with CAG (15 with and 28 without IM), 11 with DYS and 48 with antral GC, during gastroscopy. These specimens were obtained from symptom-free subjects who volunteered to participate in gastroscopic screening for gastric cancer in the Department of Gastroenterology of the First Affiliated Hospital of Zhengzhou University. The 48 GC patients had not received any radiation therapy or chemotherapy. GC tissues could be further separated on the basis of differentiation grade. Histopathologically, all the 48 gastric specimens were confirmed as adenocarcinoma. All of the biopsies were taken from gastric antrum. The tumors were histologically graded as well-differentiated (17 patients, 35%), moderately-differentiated (10 patients, 21%) and poorly-differentiated (21 patients, 44%).The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Immunohistochemical staining for anti-ODC antibody
Tissues were fixed in 96% ethanol for 6 h at 4°C, embed-ded in paraffin, and cut into 5-mm thick sections. For Immunohistochemical (IHC) analysis, endogenous peroxidase activity was neutralized by treating the section in 1% hydrogen peroxide in phosphate-buffered saline for 20 min. For the microwave antigen retrieval procedure, slides were immersed in 10 mmol/L citrate buffer (pH 6.0) in a clean polyethylene chamber and placed inside a full-powered microwave, then heated for 10 min at 95°C to repair antigens, and subsequently cooled for 10-20 min. After being washed 3 times with phosphate-buffered saline, the sections were blocked in 10% mouse serum for 30-60 min to suppress nonspecific binding of IgG. Each tissue section was then incubated with primary mouse antihuman ODC monoclonal antibody (1:1000, Westbury, NY) for 2 h at room temperature. The slides were washed 3 times with phosphate-buffered saline and incubated with anti-mouse biotinylated secondary antibody (Vector, Burlingame, CA). The slides were colorized by DAB Reagent (Vectastain) and counterstained with hematoxylin. Negative controls were established by replacing the primary antibody with PBS supplemented with normal mouse or rabbit serum.
The staining results were evaluated according to the immunodetection of stain intensity and positive cells by two pathologists (Z Tan and L H Jiao), who discussed each case until they reached a consensus. Stain intensity is up to the standard of the relative stain intensity of most cells. The stain intensity could be from 0 to 3 (0, no staining; 1, shallow brown; 2, brown; 3, dark brown); and the positive cells in the observed stomach mucous cells ranged from 0 to 3 in percentage (0,no staining; 1, < 30%; 2, 30%-70%; and 3 > 70%). The samples were scored by their summation: 0-1 (-); 2-3 (+); 4 (++); 5-6 (+++).Any staining score ≥ 2 (+) was considered as positive expression.
Histopathological diagnosis for gastric epithelia was made according to the cellular morphological changes and tissue architecture using previously established criteria[18,19]. In brief, SCG, an inflammation manifested by mild lymphocyte and plasma-cell infiltration; CAG, glandular morphology disappeared partially or completely absent in the mucosa and replaced by connective tissues, inter-glandular space was infiltrated mainly by plasma cells and lymphocytes; IM, confirmed by the presence of goblet cells in gastric mucosa; and DYS, characterized by nuclear atypia with or without architectural abnormalities in the gastric epithelium without invasion. GC is characterized by invasion of neoplastic gastric cells through the basement membrane. Carcinomas were classified according to the histological classification of WHO and the Japanese Gastric Cancer Association[20,21].
Statistical analysis
The χ2 test was used for the percentage of samples with positive staining among lesions of different severities. SPSS 12.0 was used for statistical analyses. P < 0.05 was considered statistically significant.
RESULTS
Positive immunostaining for ODC was observed in the gastric epithelial cells and cancer cells with different rates in the lesions of CSG, CAG (without IM), CAG (with IM), DYS and GC. To estimate the difference, staining for ODC was carried out on whole sections. The positive immunostaining rate for ODC was 34.4% (11 of 32), 42.9% (12 of 28), 73.3% (11 of 15), 81.8% (9 of 11), and 91.7% (44 of 48), respectively (P < 0.001) (Table 1). ODC immunoreactivity was located mainly in the cytoplasm and cell membrane (Figure 1A-E).
Table 1.
Immunohistochemical assay of ODC protein in gastric precancerous and cancerous lesions
| Histological grade |
ODC protein expression (n) |
Total | Positive rate (%)a | |||
| – | + | ++ | +++ | |||
| CSG | 21 | 9 | 2 | 0 | 32 | 34.4b |
| CAG (without IM) | 16 | 9 | 1 | 2 | 28 | 42.9bc |
| CAG (with IM) | 4 | 2 | 6 | 3 | 15 | 73.3 |
| DYS | 2 | 1 | 4 | 4 | 11 | 81.8 |
| GC | 4 | 4 | 23 | 17 | 48 | 91.7 |
P < 0.01, by treand;
P < 0.01, CSG vs CAG (with IM), CSG vs DYS, CSG vs GC, CAG (without IM) vs DYS and CAG (without IM) vs GC; and
P < 0.05, CAG (without IM) vs CAG (with IM). CSG: chronic superficial gastritis; CAG: chronic atrophic gastritis; IM: intestinal metaplasia; DYS: dysplasia; GC: gastric cancer.
Figure 1.
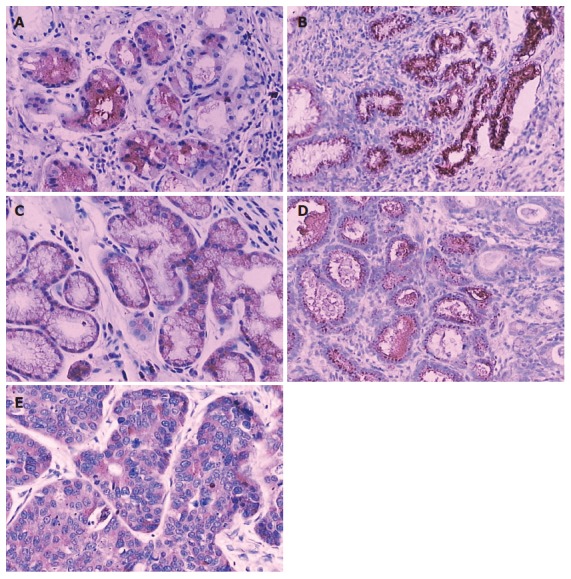
Immunoreactivity of ODC protein in gastric precancerous and cancerous lesions. A: Immunostaining of ODC in CSG, SP × 200; B: Immunostaining of ODC in CAG (Without IM). SP ×200; C: Immunostaining of ODC in CAG (with IM) SP × 200; D: Immunostaining of ODC in DYS, SP × 200; E: Immunostaining of ODC in GC, SP × 200. CSG: chronic superficial gastritis; CAG: chronic atrophic gastritis; IM: intestinal metaplasia; DYS: dysplasia; GC: gastric cancer.
The positive immunostaining rate for ODC was very low in CSG and CAG (without IM), and slightly increased in CAG (with IM) and DYS, and significantly increased in GC. ODC protein accumulation was higher in the GC than in CSG and CAG (without IM). In the cases of CAG (with IM) and DYS, it was also higher than in CSG and CAG (without IM). But it did not show significant difference among the groups with CAG (with IM), DYS and GC (Table 1). ODC positive immunostaining rate in the well-differentiated GC was lower than that in poorly-differentiated GC (Table 2).
Table 2.
IHC assay of ODC protein in gastric cancer
| Histological grade |
ODC protein expression (n) |
Total | Positive rate (%) | |||
| - | + | ++ | +++ | |||
| Well differentiated | 9 | 1 | 5 | 2 | 17 | 47.1a |
| Moderately differentiated | 1 | 3 | 2 | 4 | 10 | 90 |
| Poorly differentiated | 1 | 5 | 6 | 9 | 21 | 95.2 |
P < 0.05, well differentiated tissue vs either moderately or poorly differentiated tissues.
DISCUSSION
Gastric cancer has a high incidence in China and around the world. Gastric carcinogenesis is considered as a multistage, progressive process. It is of great importance to understand the biological processes of cancer initiation for early cancer detection. An early indicator for a patient predisposed to GC is abnormal hyperproliferation of gastric epithelial cells, such as in CAG, DYS and IM, which have all been considered as precancerous lesions for GC[22,23]. However, information about the mechanism of gastric carcinogenesis is very limited. Studies of ODC protein expression levels at different stages of gastric carcinogenesis may help answer why different stages of cancerous development occur.
Polyamines, such as putrescine, spermidine and spermine, play important roles in cell proliferation and differentiation. ODC is a rate-limiting enzyme in the biosynthesis of polyamines, and the ODC gene is considered as an immediate early gene as well as an oncogene[2,23,24]. The expression rate of ODC is invariably associated with a cell’s proliferate activity. ODC protein is 50 kDa monomer and about 100 kDa as the active homodimer is formed. It has a rapid turnover rate with a half-life at 15 min[25]. While ODC activity may be correlated with the oncogenesis and progression of gastric cancer, the expression pattern of ODC in precancerous gastric lesions have not yet been elucidated.
In the present study, we conducted a detailed IHC comparison between precancerous and cancerous gastric lesions. The expression of ODC is positively correlated with the degree of malignity of gastric mucosa and the development of gastric lesions. With the likelihood of malignant lesions progressed from normal to CSG < CAG < DYS < GC, the positive immunostaining rates for ODC similarly increased, showing a good linear correlation between ODC expression and lesion progression. We found that the positive immunostaining rate of ODC is abnormally high in CAG (with IM), DYS and GC, indicative of abnormally high cell proliferation activity. This indicates that ODC expression may be related to the proliferative status of gastric mucosa epithelial cells. These data are consistent with the views of Patchett and others who have suggested that the presence of atrophy and intestinal metaplasia are strongly associated with increased levels of ODC activity[26]. The present results indicate that increased expression of ODC may be an important molecular event, involved in the early stages of gastric carcinogenesis. The high coincidental expression of ODC protein accumulation may be an important event to enhance GC and a useful biomarker to assess risk for the development of GC[27]. This conclusion differs from Patchett’s, which suggested that measurement of mucosal ODC activity may not be a valuable clinical marker of increased cancer risk [28]. We believe that variations in technique, materials and methods may partly explain these distinctions.
Another interesting observation is that the expression of ODC directly correlates with the differentiable condition of GC. The ODC positive immunostaining rate in well-differentiated GC was lower than in both the poorly-differentiated and moderately-differentiated GC. This result is consistent with findings in human colon carcinomas[29]. The present results indicate that increased expression of ODC may reflect the differentiated condition of stomach mucosa.
The regulation of ODC expression can occur at multiple levels including transcription, translation and protein degradation[30-32]. The ODC antizyme is a major factor in the regulation of ODC[33,34]. ODC antizyme binds to monomeric ODC, stimulating the degradation of ODC, and therefore, decreasing the exogenous level of ODC protein[35]. We presume that gastric mucosa epithelial cells may have different intrinsic ODC antizyme levels. ODC antizyme levels may decrease significantly while the degree of malignity of the gastric mucosa increases. This hypothesis requires careful investigation in follow-up studies.
In conclusion, the expression of ODC is positively correlated with the degree of malignity of gastric mucosa and development of gastric lesions. This finding indicates that ODC may be used as a good biomarker in the screening and diagnosis of precancerous lesions.
ACKNOWLEDGMENTS
The authors wish to thank Professor Ouyang Q of Huaxi Hospital of Sichuan University and Professor Zhen Tan of the Department of Pathology, the Affiliated Hospital of Hainan Medical College for revisions of this manuscript.
COMMENTS
Background
Ornithine decarboxylase (ODC) catalyzes the first step in the polyamine biosynthetic pathway forming putrescine, which is then converted into the polyamines spermidine and spermine. Polyamine content plays an important role in both normal and neoplastic growth and alterations of polyamine synthesis via changes in ODC content occur in response to tumor promoters and carcinogens. The amount of ODC is altered in response to many growth factors, oncogenes, and tumor promoters and to changes in polyamine levels. ODC is overexpressed in a variety of cancers. Gastric cancer has a high incidence in China and around the world. Gastric carcinogenesis is considered as a multistage, progressive process. It is of great importance to understand the biological processes of cancer initiation for early cancer detection. An early indicator for a patient predisposed to GC is abnormal hyperproliferation of gastric epithelial cells, such as in CAG, DYS and IM, which have all been considered as precancerous lesions for GC. Studies of ODC protein expression levels at different stages of gastric carcinogenesis may help answer why different stages of cancerous development occur.
Research frontiers
It can be seen in the following four aspects: (1) expression of ODC in cancers; (2)regulation of ornithine decarboxylase; (3) inhibitor of ODC-antizyme; and (4)role of ODC and antizyme in carcinogenesis
Innovations and breakthroughs
Although several studies have evaluated ODC expression in gastric cancer, to date, no one has investigated its associations with precancerous gastric lesions, namely, in chronic atrophic gastritis (CAG), intestinal metaplasia (IM) and gastric dysplasia (DYS). The authors found the expression of ODC is positively correlated with the degree of malignity of gastric mucosa and the development of gastric lesions. The positive immunostaining rates for ODC similarly increased, showing a good linear correlation between ODC expression and lesion progression. This finding indicates that ODC may be used as a good biomarker in the screening and diagnosis of precancerous lesions.
Applications
The authors found the expression of ODC is positively correlated with the degree of malignity of gastric mucosa and the development of gastric lesions. With the likelihood of malignant lesions progressed from normal to CSG < CAG < DYS < GC, the positive immunostaining rates for ODC similarly increased, showing a good linear correlation between ODC expression and lesion progression. Therefore, ODC may be used as a good biomarker in future in screening and diagnosing precancerous and cancerous gastric lesions
Terminology
ODC: ornithine decarboxylase; CAG: chronic atrophic gastritis; IM: intestinal metaplasia; DYS: gastric dysplasia; GC: gastric cancer.
Peer review
This paper investigates the expression and diagnostic value of ODC, a key rate-limiting enzyme in polyamine biosynthesis, in gastric precursor and cancer. The expression of ODC is positively correlated with the degree of malignancy of gastric mucosa, in the order of chronic superficial gastritis, chronic atrophic gastritis (without intestinal metaplasia), chronic atrophic gastritis (with intestinal metaplasia), gastric dysplasia and gastric cancer. They concluded that ODC can be a good indicator in the screening and diagnosis of gastric precursor and cancerous lesions.
Footnotes
Supported by Miao Pu Foundation of Hainan Medical College, No. 2004108 and Natural Science Foundation of Hainan Province, No. 80582
S- Editor Zhu LH L- Editor Ma JY E- Editor Ma WH
References
- 1.Thomas T, Thomas TJ. Polyamine metabolism and cancer. J Cell Mol Med. 2003;7:113–126. doi: 10.1111/j.1582-4934.2003.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auvinen M, Paasinen A, Andersson LC, Hölttä E. Ornithine decarboxylase activity is critical for cell transformation. Nature. 1992;360:355–358. doi: 10.1038/360355a0. [DOI] [PubMed] [Google Scholar]
- 3.Pegg AE. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988;48:759–774. [PubMed] [Google Scholar]
- 4.Tabib A, Bachrach U. Role of polyamines in mediating malignant transformation and oncogene expression. Int J Biochem Cell Biol. 1999;31:1289–1295. doi: 10.1016/s1357-2725(99)00098-9. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien TG, Megosh LC, Gilliard G, Soler AP. Ornithine decarboxylase overexpression is a sufficient condition for tumor promotion in mouse skin. Cancer Res. 1997;57:2630–2637. [PubMed] [Google Scholar]
- 6.Soler PA, Gilliard G, Megosh L, George K, O'Brien TG. Polyamine regulates expression of the neoplastic phenotype in mouse skin. Cancer Res. 1988;58:1654–1659. [PubMed] [Google Scholar]
- 7.Megosh LC, Hu J, George K, O'Brien TG. Genetic control of polyamine-dependent susceptibility to skin tumorigenesis. Genomics. 2002;79:505–512. doi: 10.1006/geno.2002.6736. [DOI] [PubMed] [Google Scholar]
- 8.Mori M, Honda M, Shibuta K, Baba K, Nakashima H, Haraguchi M, Koba F, Ueo H, Sugimachi K, Akiyoshi T. Expression of ornithine decarboxylase mRNA in gastric carcinoma. Cancer. 1996;77:1634–1638. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1634::AID-CNCR32>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 9.Ernestus RI, Röhn G, Schröder R, Els T, Klekner A W, Klug N. Polyamine metabolism in brain tumours: diagnostic relevance of quantitative biochemistry. J Neurol Neurosurg Psychiatry. 2001;71:88–92. doi: 10.1136/jnnp.71.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mimori K, Mori M, Shiraishi T, Tanaka S, Haraguchi M, Ueo H, Shirasaka C, Akiyoshi T. Expression of ornithine decarboxylase mRNA and c-myc mRNA in breast tumours. Int J Oncol. 1998;12:597–601. doi: 10.3892/ijo.12.3.597. [DOI] [PubMed] [Google Scholar]
- 11.Cañizares F, Salinas J, de las Heras M, Diaz J, Tovar I, Martinez P, Peñafiel R. Prognostic value of ornithine decarboxylase and polyamines in human breast cancer: correlation with clinicopathologic parameters. Clin Cancer Res. 1999;5:2035–2041. [PubMed] [Google Scholar]
- 12.Berdinskikh NK, Ignatenko NA, Zaletok SP, Ganina KP, Chorniy VA. Ornithine decarboxylase activity and polyamine content in adenocarcinomas of human stomach and large intestine. Int J Cancer. 1991;47:496–498. doi: 10.1002/ijc.2910470404. [DOI] [PubMed] [Google Scholar]
- 13.Subhi AL, Tang B, Balsara BR, Altomare DA, Testa JR, Cooper HS, Hoffman JP, Meropol NJ, Kruger WD. Loss of methylthioadenosine phosphorylase and elevated ornithine decarboxylase is common in pancreatic cancer. Clin Cancer Res. 2004;10:7290–7296. doi: 10.1158/1078-0432.CCR-04-0972. [DOI] [PubMed] [Google Scholar]
- 14.Tian H, Huang Q, Li L, Liu XX, Zhang Y. Gene expression of ornithine decarboxylase in lung cancers and its clinical significance. Acta Biochim Biophys Sin (Shanghai) 2006;38:639–645. doi: 10.1111/j.1745-7270.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- 15.Young L, Salomon R, Au W, Allan C, Russell P, Dong Q. Ornithine decarboxylase (ODC) expression pattern in human prostate tissues and ODC transgenic mice. J Histochem Cytochem. 2006;54:223–229. doi: 10.1369/jhc.5A6672.2005. [DOI] [PubMed] [Google Scholar]
- 16.Konturek PC, Rembiasz K, Konturek SJ, Stachura J, Bielanski W, Galuschka K, Karcz D, Hahn EG. Gene expression of ornithine decarboxylase, cyclooxygenase-2, and gastrin in atrophic gastric mucosa infected with Helicobacter pylori before and after eradication therapy. Dig Dis Sci. 2003;48:36–46. doi: 10.1023/a:1021774029089. [DOI] [PubMed] [Google Scholar]
- 17.Moshier JA, Dosescu J, Skunca M, Luk GD. Transformation of NIH/3T3 cells by ornithine decarboxylase overexpression. Cancer Res. 1993;53:2618–2622. [PubMed] [Google Scholar]
- 18.Wang LD, Shi ST, Zhou Q, Goldstein S, Hong JY, Shao P, Qiu SL, Yang CS. Changes in p53 and cyclin D1 protein levels and cell proliferation in different stages of human esophageal and gastric-cardia carcinogenesis. Int J Cancer. 1994;59:514–519. doi: 10.1002/ijc.2910590414. [DOI] [PubMed] [Google Scholar]
- 19.Rugge M, Correa P, Dixon MF, Hattori T, Leandro G, Lewin K, Riddell RH, Sipponen P, Watanabe H. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000;24:167–176. doi: 10.1097/00000478-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Fenoglio-Preiser C, Mu-oz N, Carneiro F. Tumors of the stomach. In: Hamilton SR, Aaltonen LA, editors. World Health Organization classification of tumours, Pathology and genetics of tumours of the digestive system. 1st ed. Lyon: International Agency for Research on Cancer; 2000. pp. 37–38. [Google Scholar]
- 21.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 2nd ed. Tokyo: Kanehara & Co. Ltd Pub; 1998. pp. 1317–1321. [DOI] [PubMed] [Google Scholar]
- 22.You WC, Blot WJ, Li JY, Chang YS, Jin ML, Kneller R, Zhang L, Han ZX, Zeng XR, Liu WD. Precancerous gastric lesions in a population at high risk of stomach cancer. Cancer Res. 1993;53:1317–1321. [PubMed] [Google Scholar]
- 23.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 24.Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci USA. 1996;93:1065–1070. doi: 10.1073/pnas.93.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pendeville H, Carpino N, Marine JC, Takahashi Y, Muller M, Martial JA, Cleveland JL. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol Cell Biol. 2001;21:6549–6558. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patchett SE, Katelaris PH, Zhang ZW, Alstead EM, Domizio P, Farthing MJ. Ornithine decarboxylase activity is a marker of premalignancy in longstanding Helicobacter pylori infection. Gut. 1996;39:807–810. doi: 10.1136/gut.39.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okuzumi J, Yamane T, Kitao Y, Tokiwa K, Yamaguchi T, Fujita Y, Nishino H, Iwashima A, Takahashi T. Increased mucosal ornithine decarboxylase activity in human gastric cancer. Cancer Res. 1991;51:1448–1451. [PubMed] [Google Scholar]
- 28.Patchett SE, Alstead EM, Butruk L, Przytulski K, Farthing MJ. Ornithine decarboxylase as a marker for premalignancy in the stomach. Gut. 1995;37:13–16. doi: 10.1136/gut.37.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pegg AE. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986;234:249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu HY, Liu XX, Jiang CY, Zhang Y, Bian JF, Lu Y, Geng Z, Liu SL, Liu CH, Wang XM, et al. Cloning and expression of ornithine decarboxylase gene from human colorectal carcinoma. World J Gastroenterol. 2003;9:714–716. doi: 10.3748/wjg.v9.i4.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pegg AE, Shantz LM, Coleman CS. Ornithine decarboxylase: structure, function and translational regulation. Biochem Soc Trans. 1994;22:846–852. doi: 10.1042/bst0220846. [DOI] [PubMed] [Google Scholar]
- 32.Shantz LM, Pegg AE. Translational regulation of ornithine decarboxylase and other enzymes of the polyamine pathway. Int J Biochem Cell Biol. 1999;31:107–122. doi: 10.1016/s1357-2725(98)00135-6. [DOI] [PubMed] [Google Scholar]
- 33.Ivanov IP, Matsufuji S, Murakami Y, Gesteland RF, Atkins JF. Conservation of polyamine regulation by translational frameshifting from yeast to mammals. EMBO J. 2000;19:1907–1917. doi: 10.1093/emboj/19.8.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coffino P. Regulation of cellular polyamines by antizyme. Nat Rev Mol Cell Biol. 2001;2:188–194. doi: 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov IP, Rohrwasser A, Terreros DA, Gesteland RF, Atkins JF. Discovery of a spermatogenesis stage-specific ornithine decarboxylase antizyme: antizyme 3. Proc Natl Acad Sci USA. 2000;97:4808–4813. doi: 10.1073/pnas.070055897. [DOI] [PMC free article] [PubMed] [Google Scholar]


