Abstract
Eye is a highly vascularised organ. There are chances that a foreign substance can enter the systemic circulation through the eye and cause oxidative stress and evoke immune response. Here the eyes of rabbits were exposed, for a period of 7 days, to 5 known ocular irritants: Cetyl pyridinium chloride (CPC), sodium salicylate (SS), imidazole (IMI), acetaminophen (ACT) and nicotinamide (NIC). The eyes were scored according to the draize scoring. Blood collected from the treated rabbit were analyzed for haematological and biochemical parameters. After sacrifice, histological analysis of the eye and analysis of pro-inflammatory biomarkers (IL-1α, IL-1β, IL-8 and TNF-α) in the cornea using ELISA was carried out. Spleen was collected and the proliferation capacities of spleenocytes were analyzed. Liver and brain were collected and assessed for oxidative stress. The eye irritation potential of the chemicals was evident from the redness and swelling of the conjunctiva and cornea. Histopathological analysis and ELISA assay showed signs of inflammation in the eye. However, the haematological and biochemical parameters showed no change. Spleenocyte proliferations showed only slight alterations which were not significant. Also oxidative stress in the brain and liver were negligible. In conclusion, chemicals which cause ocular irritation and inflammation did not show any systemic side-effects in the present scenario.
Keywords: Ocular irritation, Systemic effect, Oxidative stress, Antioxidants, Draize test, Spleenocytes
INTRODUCTION
The eye is a delicate sensory organ that captures visible light and converts it into sensory inputs which are processed in the retina and the central nervous system, giving rise to vision. Due to its external location, the eye is prone to damage by drying, harsh environmental condition, microbes as well as man-made injuries. Chemical/drug exposure to the eye can occur at the workplace/household, from the environment and also from chemicals intended to be used in the eye like cosmetics, opthamological drugs and so on. For this reason it is imperative to test chemicals for ocular irritation potential before commercialization. However, systemic absorption of the chemical/drug through ocular routes has not been well explored.
The eye is a highly vascularized organ. Any chemical on prolonged exposure can enter the blood stream via the eye and cause harm to other organs in the body. Studies have shown that a drug applied topically to the eye shows only minimal absorption (2~10%) due to the presence of natural barriers in the eye (1). This can be extrapolated to any chemical/drug that come in contact with the eye. The remainder of drug/chemical can enter the systemic circulation through the extensive network of conjunctival blood vessels and also via the nasolacrimal duct through the highly vascularized nasal mucosa (2,3). Exposure via this route also evades first pass metabolism and hence a large amount of chemical/drug will be present in the plasma. On top of that, reports suggest that systemic absorption is greater in case of inflamed eyes (4), which maybe due to the congestion of blood vessels and leaky vasculature of the compromised eye. Once these chemicals enter the eyes, they can damage various organs like liver, spleen, kidney, brain and so on. Hence ocular irritant chemicals pose additional threat of inducing dangerous and unforeseen systemic side-effects.
In this study, rabbit eyes were exposed to 5 known ocular irritants - 1) Cetyl pyridinium chloride (CPC), 2) Sodium Salicylate (SS), 3) Imidazole (IMI), 4) Acetaminophen (ACT) and Nicotinamide (NIC) repeatedly over a period of 7 days. Their ability to elicit inflammatory response in the eyes, modulates the blood parameters, affects spleenocyte proliferation and increases oxidative stress in liver and brain were assessed.
MATERIALS AND METHODS
Chemicals and reagents. CPC (Sigma), SS (Sigma), IMI (Merck), ACT (Sigma), NIC (Sigma), Rabbit IL-1β, IL-1α, IL-8 and TNF-α ELISA kits (Cusabio, China). Thiobarbituric acid (TBA), reduced glutathione (GSH), oxidized glutathione (GSSG), and dithio-bis-2-nitrobenzoic acid (DTNB) were purchased from Sigma Chemical Co., St. Louis, MO, USA. Pyrogallol (PG), Diethylene triamine penta acetic acid (DTPA), Trichloro acetic acid (TCA) and other chemicals and reagents used were of analytical grade.
Experimental animals. Studies were carried out using New Zealand white rabbits, procured from the Division of Laboratory Animal Sciences (DLAS), of Sree Chitra Tirunal Institute for Medical Sciences and Technology. Animals of both the sex were used for the study. They were housed in controlled environments (temperature - 22 ± 3℃; humidity -50 to 70%), fed with standard feed and free access to water and a 12 hr light and dark cycle was maintained. This study conformed to the guiding principles of Institutional Animal Ethics Committee (IAEC), Committee for the purpose of Control and Supervision of Experiments on Animals (CPC-SEA) and the Guide for the care and use of laboratory animals.
Exposure to ocular irritant chemicals. A total number of 15 animals, of both the sexes, were used for the study. Three animals per chemical were assigned. The right eye was exposed to known ocular irritant chemicals - CPC (15 mg/mL/day), SS (15 mg/mL/day), IMI (15 mg/mL/day), ACT (40 mg/mL/day) and NIC (40 mg/mL/day). The dose was determined based on in vitro tests (data not shown) where the dose at which cytotoxicity was evident in rabbit primary corneal cells was chosen. The chemicals were made up in physiological saline. The ocular irritant chemicals were applied to the right eye by administering them in the conjunctival sac. The left eye remained untreated and served as control (for scoring purpose and histopathology). For cytokine analysis, haematological and biochemical parameters and antioxidant assay, animals that were untreated were used as controls. The animals were exposed to the chemicals for a period of 7 days. At the end of the 7th day, the animals were kept for observation for 72 hr. A score from 0-3 (3-being the most severe) was given to eyes of each animal as per Draize test (5). Blood was collected from them at the end of the experimental period and analyzed for haematological and biochemical parameters. Then they were sacrificed by administering high dose of thiopentone through the marginal ear vein and eyes, spleen, liver and brain were collected for different experiments.
Histopathology. Eyes from the treated animals (test and control) were enucleated from each group and preserved in Davidson’s fluid. The preserved eyes were processed for histopathological analysis. The eyes were stained using Hematoxylin & Eosin staining and observed under the microscope to examine corneal thickening, macrophage infiltration, congestion of blood vessels etc, which are signs of inflammatory response.
Cytokine analysis.
Preparation of homogenate: The corneas were retrieved and 5 mL of phosphate buffered saline (pH-7.4) was added. They were homogenized under cold conditions using polytron homogenizer. The homogenate was then centrifuged at 12000 rpm for 30 min at 4℃. Supernatant was collected and used for ELISA assay. Corneas that were collected from untreated rabbits served as control.
Analysis of cytokines IL-1β, IL-1α, IL-8 and TNF-α using ELISA assay was carried out following the manufacturer’s instructions. Briefly, 100 μL of the sample was added to the wells and incubated for 2 hr at 37℃. After incubation, the liquid was removed and 100 μL of Biotin-Antibody (1X) was added to each well. It was incubated for 1 hr at 37℃. Supernatant was aspirated and was washed with wash buffer. After that, 100 μL HRP-Avidin (1X) was added to each well and incubated further for 1 hr at 37℃. Following this, supernatant was discarded and wells were washed 5 times in was buffer. 90 μL of TMB substrate was added and incubated in the dark for 15~30 min. The reaction was stopped by adding 50 μL stop solution and read at 450 nm spectrophotometrically (Labtech LT-4000 microplate reader). Triplicates of each samples were run. Concentration was estimated using standard curve of each cytokine.
Hematological and biochemical parameters. Blood was collected from the ear vein of rabbits (treated and control) into collecting tubes. The blood was collected in EDTA for analyzing hematological parameters such as hemoglobin concentration (HB), total erythrocyte count (RBC), white blood cell count (WBC), Platelet (PLT), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC). This was determined using hematology counter (Vet animal blood counter, USA).
Similarly, blood was collected in vacutainers without anticoagulant; serum was separated by centrifugation at 3000 rpm for biochemical estimation. Biochemical parameters such as Alkaline phosphatase (ALP), Aspartate transaminase (AST), Alanine trasnsaminase (AST), Gamma glutamyl transferase (GGT), uric acid, calcium, phosphorous, chlorides and creatinine were estimated using ERBA XL 300 Biochemical Fully automated analyzer (Erba Mannheim, Germany).
Spleenocyte proliferation assay. Proliferation of ocular irritant treated spleen was examined by estimating the incorporation of [3H]thymidine. Spleenocytes from the spleen of rabbits treated with the ocular irritant chemicals were isolated using histopaque (Sigma, USA) and cultured in RPMI 1640 (Himedia, India) supplemented with 10% FBS (Invitrogen, USA). Cells were seeded onto 96 well plates at a cell density of 2 × 105 cells per well and kept in 37℃ at 5% CO2. After 48 hr, 0.5 μCi of [3H]thymidine was added per well. Cells were harvested at 72 hr and radioactivity was measured in cpm using scintillation counter (Hidex, Finland). Data is reported as mean cpm of triplicate samples. Spleens from untreated rabbits were used as control.
Antioxidant enzyme assay of liver and brain.
Preparation of liver and brain homogenate: Liver and brain from the rabbits used for the sub acute ocular irritation study was collected. 10% liver and brain homogenate was prepared in phosphate buffered saline (PBS) and thereafter subjected to centrifugation at 3500 rpm for 10 min at 4℃. The resultant supernatants were maintained in an ice bath until used for the estimation of total protein, lipid peroxidation (LPO), glutathione reductase (GR), reduced glutathione (GSH), glutathione peroxidase (GPx) and superoxide dismutase (SOD) using standard protocols with slight modifications. Liver and brain from untreated rabbits served as control.
Total protein: Total proteins in the liver and brain homogenates of rabbits exposed to ocular irritant chemicals were estimated by the method of Lowry et al. (6) using bovine serum albumin as standard.
LPO: The extent of LPO in the above prepared liver and brain homogenate was determined by estimating the concentration of malondialdehyde (MDA), which is a secondary product of LPO. The protocol, as described by OkhawaMatsumoto and Fridovich (7), uses thiobarbituric acid (TBA) which reacts with thiobarbituric acid reactive substrates (TBARS) like MDA to form a pink coloured product which is measured at 532 nm. The concentration is expressed in nmol/mg protein.
GSH: The level of GSH in the 10% liver and brain homogenate was determined by the method of Moron et al. (8), with slight modifications, in which Ellman’s reagent or DTNB (5, 5'-dithiobis-(2-nitrobenzoic acid) reacts with GSH to form a spectrophotometrically detectable, yellow coloured product at 412 nm. The change in absorbance is a linear function of the GSH concentration in the reaction mixture and is based on the reaction of GSH with DTNB to give GSH-TNB, a compound that absorbs at 412 nm. The amount of GSH was expressed as nmol/mg protein.
GR: GR activity in liver and brain homogenate was determined by measuring the reduction of GSSG in the presence of NADPH as described by Mize and Langdon (9). Briefly, this assay measures the rate of NADPH oxidation to NADP+, which is accompanied by a decrease in absorbance at 340 nm. Thus, one GR unit is defined as the reduction of one μM of GSSG per minute at 25℃ and pH 7.6. Enzyme activity is measured in units/mg protein.
GPx: Activity of GPX in liver and brain homogenate was assayed by the method described by Rotruck (10). The remaining GSH after the enzyme catalyzed reaction complexes with DTNB, which absorbs at maximum wavelength of 412 nm. Enzyme activity was expressed as μg of GSH consumed/min/mg protein.
SOD: Assay of SOD was done in liver and brain tissue homogenate using modified pyrogallol autooxidation method (11) and is spectrophotometrically measured at 420 nm.
All measurements were carried out using Lambda 25, UV/Vis spectrophotometer, Perkin Elmer, USA.
Statistical analysis. All experiments were repeated thrice and all data are presented as the mean with the standard deviation (mean ± SD). Significance has been calculated using Student’s t-test. * denotes a statistical significance (p value * ≤ 0.05) with respect to control.
RESULTS
Scoring. The treated right eye and the control left eye were scored based on severity from 0-3, where 3 were assigned most severe. It can be seen that prolonged treatment caused swelling in the cornea. Redness, chemosis and discharge from the conjunctiva was evident. This is shown in Table 1.
Table 1. In vivo scoring of rabbit eyes, after exposure to ocular irritant chemicals (sub acute).
| Control | CPC | SS | IMI | ACT | NIC | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cornea | Degree of opacity | 0 | 0 | 1 | 0 | 0 | 0 |
| Area of cornea | 0 | 3 | 3 | 2 | 2 | 2 | |
| Iris | 0 | 2 | 2 | 1 | 2 | 2 | |
| Conjuctiva | Redness | 0 | 3 | 3 | 2 | 2 | 2 |
| Chemosis | 0 | 2 | 2 | 2 | 2 | 2 | |
| Discharge | 0 | 3 | 2 | 2 | 1 | 2 | |
CPC: Cetyl pyridinium chloride, SS: Sodium Salicylate, IMI: Imidazole ACT: Acetaminophen, NIC: Nicotinamide. All values are Mean ± SD, n = 3.
0: normal, 1: slight, 2: moderate, 3: severe.
Histopathology. Histopathological analysis of eyes of rabbits treated with CPC showed an oedematous cornea with macrophage and neutrophil infiltration (Fig. 1B). There was also acute inflammation and congestion of blood vessels in the ciliary body of the eye, with respect to control. From Fig. 1C, it was seen that the cornea had increased in size in comparison to control, due to oedema, in SS treated animals. Mild acute inflammation of the ciliary body was also noticed.The eyes of rabbits treated with IMI did not show any significant signs of inflammation in the cornea (Fig. 1D). But ciliary body showed congested blood vessels, which can be an indication of inflammation. Rabbits treated with ACT showed an odematous cornea when compared with control, as evident from Fig. 1E. However, mild acute inflammation was noticed in the ciliary body of the exposed animals. NIC treated rabbits showed oedema in the cornea, when compared to control, as seen in Fig. 1F. There was acute inflammation in the ciliary body, which was clear from the infiltration of macrophages and neutrophils.
Fig. 1. Histopathology of cornea using H&E staining. (A) Control (B) Cetyl Pyridinum Chloride (CPC), arrow indicates macrophage infiltration (C) Sodium Salicylate (SS) (D) Imidazole (IMI) (E) Acetaminophen (ACT) (F) Nicotinamide (NIC), arrow indicates macrophage infiltration.
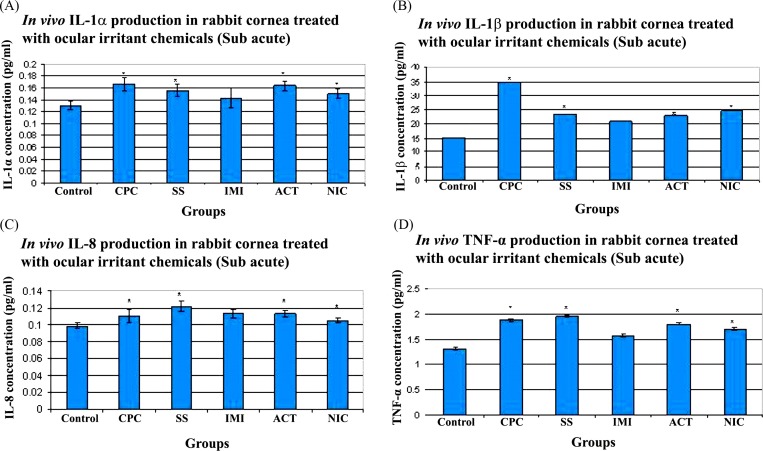
Cytokine analysis. The production of IL-1α, IL-1β, IL-8 and TNF-α, in rabbit cornea, after treatment for 7 days with CPC, SS, IMI, ACT and NIC are shown in Fig. 2. In Fig. 2A, it can be clearly seen that CPC (0.166 ± 0.01 pg/mL), SS (0.156 ± 0.01 pg/mL), ACT (0.163 ± 0.007 pg/mL) and NIC (0.149 ± 0.01 pg/mL) induced a significant increase in the production of IL-1α in comparison with control (0.130 ± 0.01 pg/mL). IMI (0.143 ± 0.01 pg/mL) also showed a minor increase in IL-1α when compared to control, but it was statistically insignificant. It was seen that IL-1β production was significantly stimulated in the cornea of rabbits treated with ocular irritant chemicals, when evaluated with control (Fig. 2B). CPC (34.77 ± 0.02 pg/mL) showed the most increased production with respect to control (15.02 ± 0.002 pg/mL). IMI (20.73 ± 0.007 pg/mL) treated corneas, even though showed slight alteration with that of control, was found to be statistically insignificant. There was a marked increase in IL-8, a chemotactic factor, in the cornea of rabbits treated with the different ocular irritant chemicals, with respect to control (Control: 0.098 ± 0.003; CPC: 0.11 ± 0.007; SS: 0.121 ± 0.005; IMI: 0.112 ± 0.005; ACT: 0.113 ± 0.003 and NIC: 0.105 ± 0.002). This was found to be statistically significant when compared with control, except for IMI treated corneas. This is illustrated in Fig. 2C. TNF-α, showed a noticeable increase in production, as evident in Fig. 2D, in the rabbit cornea that were treated with CPC (1.88 ± 0.02 pg/mL), SS (1.96 ± 0.01 pg/mL), NIC (1.70 ± 0.01 pg/mL) and ACT (1.80 ± 0.02 pg/mL) with regards to control (1.31 ± 0.01 pg/mL). However, IMI (1.57 ± 0.01 pg/mL) treated animals showed statistically insignificant variation in comparison to control.
Fig. 2. Cytokine production in rabbit cornea treated with ocular irritant chemicals (Sub acute). CPC- Cetyl Pyridinum Chloride, SS-Sodium Salicylate, IMI- Imidazole, ACT- Acetaminophen and NIC- Nicotinamide. (A) IL-1α production (B) IL-1β production (C) IL-8 production (D) TNF-α production. * Denotes p ≤ 0.05. n = 3.
Hematological and Biochemical parameter. Blood collected from the rabbits after sub acute exposure to ocular irritant chemicals was subjected to hematological and biochemical parameters. HB showed a slight decrease when compared to control. However, they are within the normal range. From the rest of the hematological parameters (WBC, RBC, PLT, MCV, MCH and MCHC) shown in Table 2, it can be seen that there are slight variations in the values when compared with control but was within the normal range. They were also statistically not insignificant.
Table 2. Hematological parameter of rabbits treated with ocular irritant chemicals (sub acute).
| Parameters | Control | CPC | SS | IMI | ACT | NIC |
|---|---|---|---|---|---|---|
|
| ||||||
| HB (g/dL) | 15.05 ± 2.62 | 12.00 ± 0.87 | 13.13 ± 0.59 | 12.90 ± 0.57 | 13.47 ± 1.19 | 13.27 ± 1.27 |
| WBC × 103/mm3 | 5.70 ± 1.41 | 6.27 ± 1.57 | 5.73 ± 2.52 | 8.05 ± 2.19 | 4.17 ± 1.93 | 6.07 ± 2.99 |
| RBC × 106/mm3 | 7.08 ± 1.12 | 5.78 ± 0.25 | 5.97 ± 0.48 | 5.97 ± 0.36 | 6.21 ± 0.68 | 6.40 ± 0.79 |
| PLT | 229.00 ± 26.87 | 552.00 ± 378.71 | 339.46 ± 289.35 | 495.5 ± 238.29 | 316.33 ± 65.58 | 335.59 ± 291.77 |
| MCV (μm3) | 69.50 ± 0.71 | 65.87 ± 1.83 | 69.60 ± 3.12 | 68.20 ± 0.84 | 68.00 ± 1.35 | 66.67 ± 2.87 |
| MCH (pg) | 21.70 ± 0.28 | 20.73 ± 0.55 | 22.07 ± 1.17 | 21.55 ± 0.35 | 21.70 ± 0.61 | 20.77 ± 0.76 |
| MCHC (g/dL) | 31.10 ± 0.14 | 31.50 ± 0.10 | 31.70 ± 0.44 | 31.60 ± 0.01 | 31.87 ± 0.50 | 31.20 ± 0.17 |
CPC: Cetyl pyridinium chloride, SS: Sodium Salicylate, IMI: Imidazole ACT: Acetaminophen, NIC: Nicotinamide.
All values are Mean ± SD, n = 3.
Biochemical parameters (SGPT, SGOT, ALP, GGT, uric acid, bilirubin, calcium, phosphorous, chlorides and creatinine) are shown in Table 3. There was a slight decrease in uric acid levels when compared with that of control. SGPT levels were also slightly elevated than control. However, both uric acid and SGPT alteration was not statistically significant. There are variations seen in the rest of the parameters of the treated group when compared to control, but are not significant and fall within the normal range.
Table 3. Biochemical parameter of rabbits treated with ocular irritant chemicals (sub acute).
| Parameters | Control | CPC | SS | IMI | ACT | NIC |
|---|---|---|---|---|---|---|
|
| ||||||
| AST (IU/L) | 44.00 ± 3.53 | 74.43 ± 21.60 | 99.16 ± 16.46 | 106.03 ± 18.33 | 103.93 ± 20.11 | 111.96 ± 13.47 |
| ALT (IU/L) | 21.30 ± 1.41 | 18.20 ± 3.47 | 18.06 ± 4.82 | 17.70 ± 0.95 | 23.53 ± 5.11 | 16.66 ± 3.84 |
| ALP (IU/L) | 61.00 ± 2.82 | 108.66 ± 37.52 | 72.33 ± 50.33 | 102.00 ± 56.78 | 64.33 ± 9.71 | 89.66 ± 39.31 |
| GGT (IU/L) | 10.85 ± 0.77 | 6.13 ± 5.82 | 11.83 ± 2.97 | 11.26 ± 1.71 | 5.36 ± 1.12 | 8.20 ± 3.11 |
| Uric acid (mg/dL) | 2.53 ± 3.06 | 0.50 ± 0.54 | 0.11 ± 0.16 | 0.05 ± 0.09 | 0.68 ± 0.72 | 0.72 ± 0.01 |
| Calcium (mg/dL) | 13.40 ± 0.14 | 12.76 ± 0.50 | 13.86 ± 2.04 | 13.00 ± 0.78 | 13.20 ± 0.61 | 13.13 ± 0.11 |
| Phosphorous (mg/dL) | 3.43 ± 0.17 | 4.72 ± 0.78 | 4.96 ± 0.38 | 4.87 ± 0.69 | 4.92 ± 0.07 | 4.76 ± 0.27 |
| Chlorides (mmol/L) | 104.3 ± 9.33 | 112.3 ± 3.60 | 108.7 ± 3.43 | 115.4 ± 3.72 | 112.36 ± 2.35 | 110.7 ± 1.58 |
| Creatinine (mg/dL) | 1.22 ± 0.24 | 0.97 ± 0.12 | 1.35 ± 0.70 | 1.146 ± 0.08 | 1.12 ± 0.13 | 1.04 ± 0.07 |
CPC: Cetyl pyridinium chloride, SS: Sodium Salicylate, IMI: Imidazole ACT: Acetaminophen, NIC: Nicotinamide.
All values are Mean ± SD, n = 3.
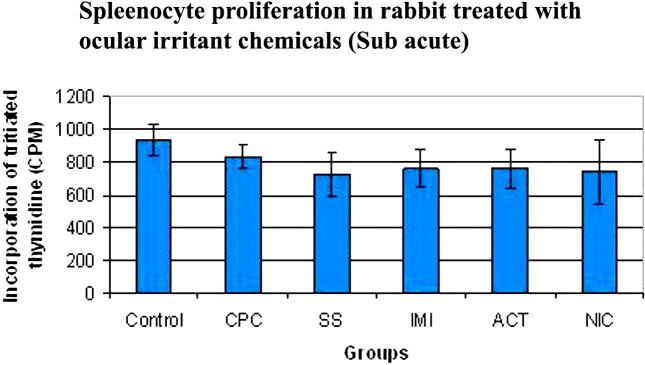
Spleenocyte proliferation assay. It is seen from Fig. 3, that the spleenocyte proliferation has some variation in the animals that were treated with the ocular irritant chemicals, when compared to control. However, since it is statistically in significant, it is safe to assume that the ocular irritant chemicals did not affect the spleenocyte proliferation capacity.
Fig. 3. Spleenocyte proliferation in rabbits treated with ocular irritant chemicals (Sub acute). CPC- Cetyl Pyridinum Chloride, SS-Sodium Salicylate, IMI- Imidazole, ACT- Acetaminophen and NIC- Nicotinamide. n = 3.
Antioxidant enzyme assay.
LPO: The liver of rabbits treated for 7 days with ocular irritant chemicals did not show any significant increase in LPO in comparison to control, as seen in Fig. 4A. MDA concentration in control was 2.98 ± 0.84 nmol/mg protein, whereas values of CPC, SS, IMI, ACT and NIC were as follows: 3.77 ± 0.38, 3.96 ± 0.51, 4.03 ± 0.12, 4.55 ± 0.14 and 4.01 ± 0.65 nmol/mg protein respectively.
Fig. 4. Lipid peroxidation (LPO) in rabbits treated with ocular irrirant chemicals (Sub acute). CPC- Cetyl Pyridinum Chloride, SS- Sodium Salicylate, IMI- Imidazole, ACT- Acetaminophen and NIC- Nicotinamide. (A) LPO in liver and (B) LPO in brain. n = 3.

Rabbit exposed to ocular irritant chemicals in the eyes showed slight increase in LPO in the brain (CPC: 10.22 ± 2.02, SS: 15.53 ± 5.10, IMI: 8.98 ± 2.22, ACT:10.10 ± 0.71 and NIC: 12.61 ± 2.74 nmol/mg protein), which was insignificant, when compared to control (8.89 ± 0.45 nmol/mg protein). This is depicted in Fig. 4B.
GSH: GSH concentration in the liver of rabbits treated with CPC (1.53 ± 0.44 nmol/mg protein), SS (1.57 ± 0.66 nmo/mg protein), IMI (1.40 ± 0.19 nmol/mg protein), ACT (1.25 ± 0.41 nmol/mg protein) and NIC (1.22 ± 0.43 nmol/mg protein) were statistically insignificant, when compared with control (1.61 ± 0.15 nmol/mg protein). This is shown in Fig. 5A.
Fig. 5. Concentration of reduced Glutathione (GSH) in rabbits treated with ocular irritant chemicals (Sub acute). CPC- Cetyl Pyridinum Chloride, SS- Sodium Salicylate, IMI- Imidazole, ACT- Acetaminophen and NIC- Nicotinamide. (A) GSH in liver (B) GSH in brain. n = 3.
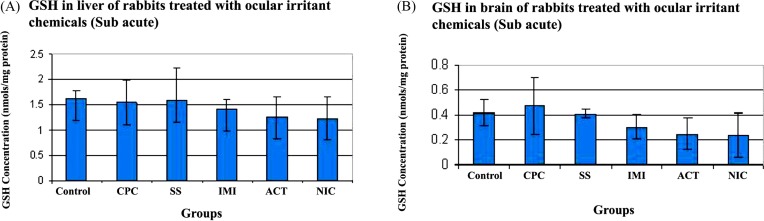
Concentration of GSH in the brain homogenate of treated rabbits is shown in Fig. 5B. Though there is a slight decrease in GSH levels with respect to control (Control - 0.42 ± 0.1, CPC - 0.47 ± 0.22, SS - 0.41 ± 0.03, IMI - 0.3 ± 0.10, ACT - 0.25 ± 0.12 and NIC - 0.23 ± 0.18 nmol/mg protein) it still remains statistically insignificant.
GR: The activity of GR in the liver (Fig. 6A) and brain (Fig. 6B) of animals exposed to ocular irritant chemicals showed slight variations with regards to control. However, both in liver and brain, it was statistically insignificant. Control (liver - 0.26 ± 0.11; brain - 0.49 ± 0.08), CPC (liver - 0.21 ± 0.05; brain - 0.63 ± 0.05 units/mg protein), SS (liver - 0.17 ± 0.03; brain - 0.45 ± 0.08 units/mg protein), IMI (liver - 0.16 ± 0.004; brain - 0.46 ± 0.08 units/mg protein), ACT (liver - 0.28 ± 0.05; brain - 0.41 ± 0.8 units/mg protein) and NIC (liver - 0.26 ± 0.03; brain - 0.26 ± 0.09 units/mg protein).
Fig. 6. Activity of Glutathione reductase (GR) in rabbits treated with ocular irritant chemicals (Sub acute). CPC- Cetyl Pyridinum Chloride, SS- Sodium Salicylate, IMI- Imidazole, ACT- Acetaminophen and NIC- Nicotinamide. (A) GR activity in liver (B) GR activity in brain. n = 3.

GPx: Liver homogenates treated with CPC (0.15 ± 0.04), SS (0.13 ± 0.005), IMI (0.16 ± 0.01), ACT (0.15 ± 0.01) and NIC (0.13 ± 0.004) was almost similar to that of control (0.14 ± 0.002) value. This is shown in Fig. 7A. All the values are expressed in units/mg protein.
Fig. 7. Activity of Glutathione peroxidase (GPx) in rabbits treated with ocular irritant chemicals (Sub acute). CPC- Cetyl Pyridinum Chloride, SS- Sodium Salicylate, IMI- Imidazole, ACT- Acetaminophen and NIC- Nicotinamide. (A) GPx activity in liver (B) GPx activity in brain. n = 3.

It is seen in Fig. 7B that in brain homogenates as well, the GPx activity was not significant in the treated group (CPC - 0.15 ± 0.04, SS - 0.14 ± 0.01, IMI - 0.12 ± 0.01, ACT - 0.13 ± 0.02 and NIC - 0.12 ± 0.04 units/mg protein) even though there were slight alteration when compared to control (0.17 ± 0.002 units/mg protein).
SOD: From Fig. 8A, it can be seen that the SOD activity in the liver homogenate of ocular irritant exposed animals (CPC - 0.08 ± 0.005, SS - 0.08 ± 0.004, IMI - 0.06 ± 0.003, ACT - 0.08 ± 0.007 and NIC - 0.08 ± 0.01 units/mg protein) were similar to that of control (0.11 ± 0.008 units/mg protein).
Fig. 8. Activity of Superoxide dismutase (SOD) in rabbits treated with ocular irritant chemicals (Sub acute). CPC- Cetyl Pyridinum Chloride, SS- Sodium Salicylate, IMI- Imidazole, ACT- Acetaminophen and NIC- Nicotinamide. (A) SOD activity in liver (B) SOD activity in brain. n = 3.
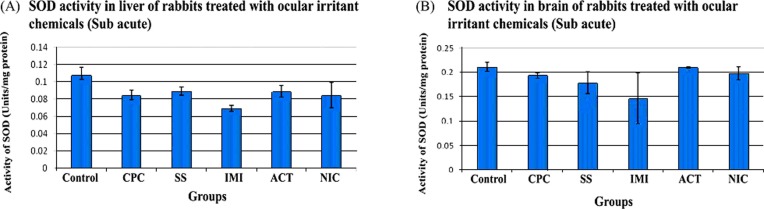
SOD activity in the brain of treated animals (CPC -0.19 ± 0.01, SS - 0.17 ± 0.02, IMI - 0.14 ± 0.07, ACT - 0.21 ± 0.001 and NIC - 0.19 ± 0.01 units/mg protein) was comparable with that of control (0.21 ± 0.01 units/mg protein) as shown in Fig. 8B.
DISCUSSION
Due to the external location of the eye, it encounters various harsh environmental abuses, microbes as well as manmade injuries. It is well protected by barriers like conjuctiva, tear, cornea, which keep the systemic circulation separate from the eye. So they can prevent any substance, which comes in contact with it, from entering the systemic circulation.However the conjuctiva consists of large intercellular spaces and is highly vascularized and can allow the entry of certain chemicals and substances (12). Moreover, these substances can enter via the nasolacrimal duct through the highly vascularized nasal mucosa and on to the systemic circulation (3). Drugs which enter the systemic circulation through these paths evade the first pass metabolism and hence their concentration will be high in the plasma (13). It has been reported that about 2% of the drug that is administered ocularly can be retained in the eye and the rest of the drug enters the systemic circulation through the above mentioned routes. Also, the cornea which is highly impenetrable to macromolecules due to the presence of tight junction can be breached under certain conditions like inflammation (14). Hence when an eye is exposed to an eye irritant chemical, it can potentially cause inflammation and make the cornea leaky. This permits paracellular transport and entry into the systemic circulation. Hence this study focuses on the systemic effect of ocular irritant chemicals on prolonged or sub acute exposure to the eye.
For this study five known ocular irritant chemicals were chosen - CPC, SS, IMI, ACT and NIC. Numerous studies have shown the eye irritation potential of these chemicals (15-18).These chemicals were chosen so as to slightly infringe the corneal barriers and make it more permeable for the entry into systemic circulation. In the present study, the ability of these chemicals to induce inflammation in the eye, alter the haematological and biochemical parameters, affect the spleenocyte proliferation capacity and cause oxidative damage to the liver and brain was assessed.
All the chemicals showed typical draize score which categorized them from moderate to severe irritant. It was seen that CPC showed redness, odema and discharge on the second day of exposure. This is because CPC is a more potent irritant when compared to the other chemicals.All the other chemicals showed prominent signs of irritation from the 4th day onwards. To understand whether this trauma resulted in inflammation, histopathological analysis and pro-inflammatory cytokine analysis was carried out.
When inflammation occurs, the blood vessels become congested to supply more blood to the affected areas. This cause the redness and migration of the macrophages and neutrophils and fluid accumulation. From the histopathological analysis of the eye, it was clear that the chemicals could evoke inflammatory response. All these were evidenced by the macrophage/neutrophil infiltration, oedematous cornea and congested blood vessels. IMI, however, did not show significant signs of inflammation, when compared with the rest of the chemicals. This could be because of the anti-inflammatory properties of IMI (18). CPC treated animals showed inflammation in the cornea and ciliary body whereas inflammation was restricted to the ciliary bodies in the other entire chemical (SS, ACT and NIC) treated eyes. Ciliary body is rich in blood vessels and any local inflammatory reaction can induce lymphocyte migration to the ciliary body. It has been reported that sensitized lymphocytes can reach and attack corneal endothelial cells through the ciliary body, iris and aqueous humor, and eventually lead to immune rejection of graft tissue. Five days after corneal transplantation, lymphocytes were detected in the limbus and pars plana and they gradually migrated to the cornea, iris and anterior chamber region (19). Since the visual signs of inflammation (from draize score) suggest the late onset of inflammation in SS, ACT and NIC treated animals, this can be the reason for the presence of infiltrating cells in the ciliary body rather than cornea. In contrast, macrophage and neutrophil infiltration was evident in cornea as well as ciliary body of CPC treated animals. Signs of ocular irritation were seen in the initial days of exposure to CPC, giving time for the immune cells to migrate to the cornea too.
To confirm inflammatory response, ELISA assay for various pro-inflammatory cytokines (IL-1α, IL-1β, IL-8 and TNF-α) was carried out. IL-1α and IL-1β are constitutively produced by the keratocytes and corneal fibroblasts (20). TNF-α is another pro-inflammatory cytokine. IL-1 and TNF-α act synergistically and govern the expression of cytokines like IL-6, IL-8 and so on (21). IL-8 activates polymorphonuclear neutrophil and macrophages and causes their chemotaxis in the affected region (22,23). All the chemicals except IMI showed statistically significant increase in cytokine level as compared to control. Interestingly, reports suggest that IMI and their derivatives show antiinflammatory activity and cytokine (IL-1, TNF-α) suppressive activity (24). Results from the current study reflect the same observation as well. Hence this might be the reason that no significant inflammatory response was evoked in IMI treated rabbits. These results suggest that all the chemicals except IMI were able to induce an inflammatory response in the eye.
Since these chemicals (except IMI) could cause inflammation, there is a greater chance for them to enter the systemic circulation through the leaky vasculature. The hematological parameters of blood were analyzed. There was no significant alteration when compared to control and did not show any abnormal WBC count or any variable platelet count. However there was a slight decrease in HB levels, but since it was within the normal range (9.4~17.4 g/dL), it cannot be concluded that the animals became anemic after treatment. This discrepancy can be attributed to animal to animal variation. The biochemical parameters showed slight variation in AST levels and uric acid levels. Yet the slight increase in the AST levels was within normal range (55~260 IU/L). The decrease in uric acid levels in the test when compared to the control indicates hypourecemia, which can be due to drug toxicity, dehydration and starvation or genetic disorder. However, the rest of the biochemical parameters (ALT, ALP, GGT, bilirubin, calcium, phosphorous, chlorides and creatinine) fell within the normal range suggesting normal liver and kidney functionality. Hence we cannot conclusively say that decrease in uric acid levels is an indication of toxicity. Further investigation is required for that.
Increase in DNA synthesis indicates the proliferating capacity of cells. Since spleen is a lymphoid organ, any foreign material that comes through the blood will come in contact with the spleen and cause immuno-modulatory effects. To study this, DNA synthesis was measured by uptake of tritiated thymidine. No stimulatory or inhibitory effect was observed as the thymidine level was comparable with control. This indicates that no particular immune response was evoked by the ocular irritant chemicals.
Liver and brain are two of the most vital organs. Liver is responsible for the detoxification of foreign substances and is susceptible to oxidative damage. Brain is the centre of the nervous system and is highly protected by the blood brain barrier (BBB). However, some chemicals can pass through the BBB and cause oxidative stress. Oxidative stress is believed to be involved in the pathogenesis of neuro degenerative diseases (25). Hence it is important to understand if the ocular irritant chemicals are capable of causing oxidative stress. Assessing the status of various antioxidant enzyme and oxidative stress markers gives an idea of the extent of damage caused by the ocular irritant chemicals that can enter the systemic circulation. Oxidative stress is caused by the imbalance between production of reactive oxygen species (ROS) and their elimination by the antioxidant defense mechanism, which damages the important biomolecules and cells (26). LPO is a common consequence of oxidative stress. The free radicals attack lipids and generate peroxyl radical and trigger a chain of reaction (27). In this study, there was no marked difference in LPO when compared to control, suggesting that there was no oxidative damage to the lipid bi-layer membranes. To further confirm that there was no oxidative stress in liver and brain, concentration of GSH and activity of GR and GPx enzymes were estimated. GSH plays a central role in the cellular redox homeostasis.GSH is a tripeptide which gets oxidized to glutathione disulfide (GSSG) during oxidative stress by donating and electron to GPx (28). GR is responsible for the recyling of GSSG back to GSH to maintain the balance (29,30). In the current study, there was a slight decrease in GSH concentration in the liver and brain, which was not significant. The activity of GR and GPx were also showing slight alterations, which was not relevant. This indicates further that the ocular irritant chemicals were unable to generate free radicals and cause excessive oxidative damage. SOD is another enzyme that aids is the dismutation of oxygen anions to H2O2 and molecular oxygen (26). In the present study, it can be seen that the ocular irritant chemicals were not able to produce significant amount of super oxide free radicals in the liver and brain, as the activity of SOD enzyme did not show much variability when compared to control.
To sum it up, in this present study, known ocular irritant chemicals were exposed to rabbit cornea in order to assess the systemic side-effects of these chemicals. Inflammatory response in the eye was evident from histopathology and cytokine release profile. However, the hematological and biochemical parameters did not show any marked change. The replication capacities of spleenocytes were not compromised. Also, the ocular irritant chemicals, which entered the systemic circulation was incapable of doing much oxidative damage to the liver and brain. In conclusion, even though there are chances that a foreign chemical can enter the systemic circulation though the naso-lacirmal system or conjunctival blood vessels, it was not able to cause any significant side-effects in the present scenario. It was also found that the ability of the chemicals to induce inflammation did not aid further in causing systemic problems. However, further studies are required using a variety of other chemicals and higher doses to estimate the systemic problems caused when exposed to the eye. Also, the mechanism of eye irritation caused by IMI needs to be looked into further.
Acknowledgments
The authors wish to express their sincere thanks to ICMR (Project No. 5/20/4(Bio)/10-NCD-1), for granting the funds to carry out the work. The authors are also grateful to Director and Head, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram for their encouragements and support for this study. The technical support of Dr Sabareeshwaran (Histopathology analysis), Mr. Shaji. S and Mr. Hari Kumar are kindly acknowledged.
References
- 1.Hopkins G.A., Lyle W.M. Potential systemic effects of six common ophthalmic drugs. J. Am. Optom. Assoc. (1977);48:1241–1245. [PubMed] [Google Scholar]
- 2.Leader B.J., Calkwood J.C. Peril to the nerveglaucoma and clinical neuro-opthalmology. Kugler publications; New Orleans: (1996). pp. 65–70. [Google Scholar]
- 3.Gray C. Systemic toxicity with topical ophthalmic medications in children. Paediatr. Perinat. Drug Ther. (2006);7:23–29. doi: 10.1185/146300905X75334. [DOI] [Google Scholar]
- 4.Salminen L. Review: systemic absorption of topically applied ocular drugs in humans. J. Ocul. Pharmacol. Ther. (1990);6:243–249. doi: 10.1089/jop.1990.6.243. [DOI] [PubMed] [Google Scholar]
- 5.Draize J.H., Woodard G., Calvery H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. (1944);82:377–390. [Google Scholar]
- 6.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. (1951);193:265–275. [PubMed] [Google Scholar]
- 7.Okhawa-Matsumoto A., Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu-, Zn-SOD in mitochondria. J. Biol. Chem. (2001);276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 8.Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione Stransferase activities in rat lung and liver. Biochim. Biophys. Acta. (1979);582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 9.Mize C.E., Langdon R.G. Hepatic glutathione reductase: purification and general kinetic properties. J. Biol. Chem. (1962);237:1589–1595. [PubMed] [Google Scholar]
- 10.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: biochemical role as a component of glutathione peroxidase. Science. (1973);179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 11.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. (1974);47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y.C., Chiang B., Wu X., Prausnitz M.R. Ocular delivery of macromolecules. J. Controlled Release. (2014);190:172–181. doi: 10.1016/j.jconrel.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Delivery Rev. (2006);58:1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Yi X., Wang Y., Yu F.S. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Invest. Ophthalmol. Visual Sci. (2000);41:4093–4100. [PubMed] [Google Scholar]
- 15.Green K., Bowman K.A., Elijah R.D., Mermelstein R., Kilpper R.W. Dose-effect response of the rabbit eye to cetylpyridinium chloride. Cutaneous Ocul. Toxicol. (1985);4:13–26. doi: 10.3109/15569528509068355. [DOI] [Google Scholar]
- 16.Takahashi Y., Koike M., Honda H., Ito Y., Sakaguchi H., Suzuki H., Nishiyama N. Development of the short time exposure (STE) test: An in vitro eye irritation test using SIRC cells. Toxicol. In Vitro. (2008);22:760–770. doi: 10.1016/j.tiv.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Spielmann H., Kalweit S., Liebsch M., Wirnsberger T., Gerner I., Bertram-Neis E., Krauser K., Kreiling R., Miltenburger H.G., Pape W., Steiling W. Validation study of alternatives to the Draize eye irritation test in Germany: Cytotoxicity testing and HET-CAM test with 136 industrial chemicals. Toxicol. In Vitro. (1993);7:505–510. doi: 10.1016/0887-2333(93)90055-A. [DOI] [PubMed] [Google Scholar]
- 18.Bagley D.M., Gardner J.R., Holland G., Lewis R.W., Vrijhof H., Walker A.P. Eye Irritation: Updated reference chemicals data bank. Toxicol. In Vitro. (1999);13:505–510. doi: 10.1016/S0887-2333(99)00015-6. [DOI] [PubMed] [Google Scholar]
- 19.Wang F.H., Chen M., Liu T., Zang X.J., Gong H.Q., Shi W.Y. Lymphocyte infiltration and activation in iris-ciliary body and anterior chamber of mice in corneal allograft rejection. Int. J. Ophthalmol. (2012);5:681–686. doi: 10.3980/j.issn.2222-3959.2012.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weng J., Mohan R.R., Li Q., Wilson S.E. IL-1 upregulates keratinocyte growth factor and hepatocyte growth factor mRNA and protein production by cultured stromal fibroblast cells: Interleukin-1 beta expression in the cornea. Cornea. (1997);16:465–471. doi: 10.1097/00003226-199707000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Wilson S.E., Mohan R.R., Mohan R.R., Ambrósio R., Jr., Hong J., Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retinal Eye Res. (2001);20:625–637. doi: 10.1016/S1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 22.Ferrick M.R., Thurau S.R., Oppenheim M.H., Herborf C.P., Ni M., Zachariae C.O., Marsushima K., Chan C.C. Ocular inflammation stimulated by intravitreal interleukin-8 and interleukin-1. Invest. Ophthalmol. Visual Sci. (1991);32:1534–1539. [PubMed] [Google Scholar]
- 23.Miller M.D., Krangel M.S. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit. Rev. Immunol. (1992);12:17–46. [PubMed] [Google Scholar]
- 24.Reddy M.P., Webb E.F., Cassatt D., Maley D., Lee J.C., Griswold D.E., Truneh A. Pyridinyl imidazoles inhibit the inflammatory phase of delayed type hypersensitivity reactions without affecting T-dependent immune responses. Int. J. Immunopharmacol. (1994);16:795–804. doi: 10.1016/0192-0561(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 25.Melo A., Monteiro L., Lima R.M., Oliveira D.M., Cerqueira M.D., El-Bachá R.S. Oxidative stress in neurodegenerative diseases: mechanisms and therapeutic perspectives. Oxid. Med. Cell. Longevity. (2011);2011:467180. doi: 10.1155/2011/467180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biol. Med. (2010);49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin H., Xu L., Porter N.A. Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. (2011);111:5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 28.Circu M.L., Aw T.Y. Reactive oxygen species, cellular redox systems and apoptosis. Free Radical Biol. Med. (2010);48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rall T.W., Lehninger A.L. Glutathione reductase of animal tissues. J. Biol. Chem. (1952);194:119–130. [PubMed] [Google Scholar]
- 30.Sastre J., Palladro F.V., Viña J. Glutathione. Handb. Environ. Chem. (2005);20:91–108. [Google Scholar]