Abstract
[Purpose] To investigate the changes in body balance under ametropic conditions induced by spherical lenses in an upright position. [Subjects and Methods] Twenty subjects (10 males, 10 females) of average age 23.4±2.70 years participated and they were fully corrected by subjective refraction. To induce ametropic conditions (binocular myopia and hyperopia), lenses of ±0.50 D, ±1.00 D, ±1.50 D, ±2.00 D, ±3.00 D, ±4.00 D and ±5.00 D were used. General stability (ST), fall risk index (FI), and sway path (SP) were analyzed through changes in synchronization of left/right and toe/heel, as measured by the biofeedback system, TETRAX. Measurement was performed for 32 seconds for each condition. [Results] ST increased significantly from +0.50 D-induced myopia and from −1.00 D-induced hyperopia as compared with corrected emmetropia. FI increased significantly from +4.00 D-induced myopia and from −1.50 D-induced hyperopia as compared with corrected emmetropia. In SP, which means a change of body balance, toe/heel was significantly greater than left/right in all ametropic conditions. SP of right/left synchronization was not affected by the side of the dominant eye. [Conclusion] An uncorrected hyperope may cause subjects to have a higher risk of falling than an uncorrected myope. Therefore, clinical specialists should consider the refractive condition, especially hyperopia, when analyzing body balance.
Key words: Body balance, Ametropia, Fall risk index
INTRODUCTION
Human body balance can be defined as the ability to maintain the body’s center of gravity within the base of support with minimal sway1). For appropriate control of body balance, it is regulated by three different sensory systems: the visual, vestibular, and somatosensory systems2). Among these, the visual system, plays an important role in maintaining stable body balance by continuously providing information to the nervous system about the environment, body movement, and body position3).
The role of visual information in maintaining body balance has been studied for many years. For instance, comparative studies have demonstrated significant increases of postural sway under eyes closed conditions compared with eyes open, thus verifying the importance of the visual system for body balance4,5,6,7,8,9,10,11). Furthermore, several studies2, 12, 13) of the association of refractive error with postural ability have revealed that balance ability declines with an increase of refractive blur, and these studies have suggested that optimal refractive correction can reduce postural instability. However, changes of body balance under hyperopic conditions have rarely been studied and most previous studies have examined changes in postural sway under conditions of myopic refractive defocus.
The present study was performed in order to investigate changes in the general stability, the fall risk index, and the synchronization of right/left and toe/heel under conditions of hyperopia and also of myopia induced by spherical lenses for subjects with corrected emmetropia.
SUBJECTS AND METHODS
Twenty subjects (10 males, 10 females) of average age 23.4±2.70 years participated in this study. All subjects were healthy and had no neurological, otoneurological, or ophthalmological disease. Also, they were not taking any medication that might have interfered with balance control. The average spherical equivalent of the subjects was S-3.77±2.86 D. Those who were not corrected to 1.0 of VA and those having binocular dysfunctions such as poor stereopsis or amplitude of accommodation were excluded from the experiment. All the test subjects understood the purpose of this study and consented to participation therein. The study was conducted in conformity with the ethical principles of the Declaration of Helsinki.
To assess body balance, we used the TETRAX biofeedback system (Tetrax Portable Multiple System, Tetrax Ltd., Ranmat Gan, Israel). This system measures the postural sway on 4 force plates, one each for the toes and heels of each foot, and it is mainly used to evaluate the general stability (ST) of body balance and the fall risk index (FI)14). Increased values of these indices indicate that general instability and the chance of falling are proportionally elevated. The system is also capable of analyzing the sway path (SP) which is derived from the results of the synchronization of left/right and toe/heel.
First, the examiner corrected all subjects to emmetropia by subjective refraction with a phoropter (Ultramatic RX Master, Reichert, USA) and a 5 m visual chart. After this, the examiner asked the subjects to wear glasses with full correction and to stand in an upright position on the force plate. To induce ametropia (binocular hyperopia and myopia), spherical lenses of ±0.50 D, ±1.00 D, ±1.50 D, ±2.00 D, ±3.00 D, ±4.00 D, and ±5.00 D were used. Measurement was performed for 32 seconds for each condition. ST, FI, and SP were recorded as deviations from the values under the emmetropic condition. Each subject was instructed to keep looking at a fixed target at 30 meters distance during the measurements. After this, the subjects took a rest for one minute while the (±) lens was replaced, and for 10 minutes when the ametropic type was changed.
For data analysis, repeated-measures analysis of variance (ANOVA), the independent samples t-test, and frequency analysis were performed using SPSS for Windows (SPSS Inc., Chicago, USA).
RESULTS
The changes of ST and FI under each condition of ametropia are shown in Fig. 1. ST increased significantly (p<0.05) from +0.50 D-induced myopia and from −1.00 D-induced hyperopia as compared with the corresponding figures under corrected emmetropia. FI increased significantly (p<0.05) from +4.00 D-induced myopia and from −1.50 D-induced hyperopia as compared with the corresponding figures under corrected emmetropia.
Fig. 1.
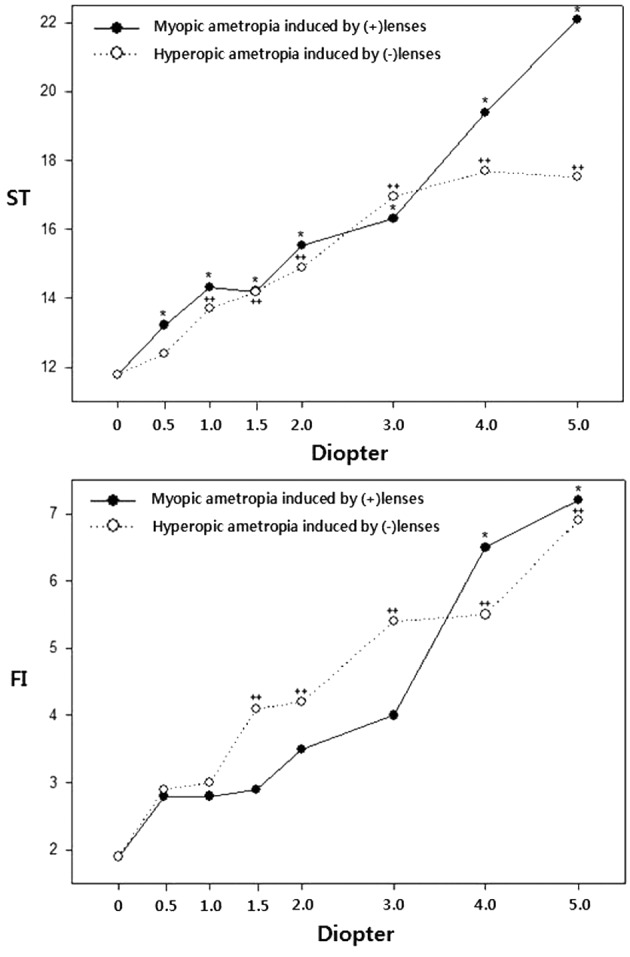
Changes in general stability (ST) and fall risk index (FI) in myopia and hyperopia induced by (±) spherical lenses. *p<0.05, ++p<0.05: significantly different from corrected emmetropia according to repeated measures ANOVA
The analysis results for left/right and toe/heel synchronization are given in Table 1. The values indicate there were differences in fluctuation regardless of sway path (slanted position) as compared with the corresponding figures under corrected emmetropia. In terms of the differences in fluctuation ranges, toe/heel was greater than left/right in all the ametropic conditions (p<0.05).
Table 1. Changes in sway path fluctuation regardless of the dominant eye in myopia and hyperopia induced by (±) spherical lenses and the significance of differences from corrected emmetropia.
| Defocus | Myopia | Hyperopia | Difference between myopia and hyperopia |
|||||
|---|---|---|---|---|---|---|---|---|
| L-R | T-H | L-R | T-H | L-R | T-H | |||
| ±0.50 | 2.20±1.10 | 5.74±4.78** | 2.52±1.79 | 4.93±4.31* | 0.493 | 0.575 | ||
| ±1.00 | 2.06±1.68 | 6.12±4.41** | 2.29±1.56 | 5.82±5.62* | 0.658 | 0.853 | ||
| ±1.50 | 2.08±1.86 | 6.84±5.37** | 1.71±1.27 | 8.01±6.55** | 0.461 | 0.539 | ||
| ±2.00 | 2.00±1.45 | 7.68±5.56** | 1.54±1.34 | 8.71±7.71** | 0.301 | 0.633 | ||
| ±3.00 | 2.01±1.73 | 6.33±5.69** | 2.28±1.56 | 8.14±7.24** | 0.607 | 0.384 | ||
| ±4.00 | 2.14±1.54 | 8.58±6.74** | 2.03±1.73 | 10.20±6.81** | 0.831 | 0.453 | ||
| ±5.00 | 2.47±2.04 | 8.84±8.58** | 1.96±1.65 | 9.55±8.69** | 0.388 | 0.796 | ||
Data are expressed by mean±SD. The number of subjects is twenty in all defocused group. L-R: Light-Left; T-H: Toe-Heel. *p<0.05, **p<0.01: significantly different from L-R of same ametropia. Difference between myopia and hyperopia is presented as the p-value.
The distribution of the tendency of SP in left/right synchronization dependent on the side of the dominant eye is shown in Table 2. Among the 11 subjects with a right-dominant eye in induced myopia, 27.3% of the subjects slanted constantly toward the left side and 18.2% of the subjects slanted constantly toward the right side, and there was no consistent SP in 54.5% of the subjects. In the 9 subjects with a left-dominant eye in induced myopia, 22.2% of the subjects slanted constantly toward the left side and 22.2% of the subjects slanted constantly the toward right side, and 55.6% of the subjects did not show any particular tendency.
Table 2. Distribution of the sway path tendency according to the side of the dominant eye in myopia and hyperopia induced by (±) spherical lenses.
| Dominant eye | Sway path | Myopia | Hyperopia |
|---|---|---|---|
| Right | Toward left | 3 (27.3%) | 1 (9.1%) |
| Toward right | 2 (18.2%) | 2 (18.2%) | |
| No tendency | 6 (54.5%) | 8 (72.2%) | |
| 11 (100%) | |||
| Left | Toward left | 2 (22.2%) | 1 (11.1%) |
| Toward right | 2 (22.2%) | 1 (11.1%) | |
| No tendency | 5 (55.6%) | 7 (77.8%) | |
| 9 (100%) | |||
In terms of constant tendency of SP under a hyperopic condition, the slant proportion was 9.1% toward the left side and 18.2% toward the right side in the 11 subjects with a right-dominant eye, and 11.1% toward the left side and 11.1% toward the right side in the 9 subjects with a left-dominant eye.
DISCUSSION
The visual contribution to balance control strongly depends on the performance of the visual system. Edwards11) reported an increase in body instability of about 28% with the addition of a +5 D lens and Paulus et al.8) also demonstrated a result showing an approximate 25% increase in postural instability due to retinal blur induced by +4 D and +6 D lenses. In addition, they found a similar increase in postural instability, when subjects with myopic refractive error between 3 and 5 D removed their corrected spectacles. Furthermore, Anand et al.2, 12) analyzed the standing stability in younger and older subjects with added lenses of +1 D, +2 D, +4 D and +8 D under more challenging conditions, when the normal information from the vestibular and somatosensory systems was disrupted. As stated above, poor vision due to myopic defocus can reduce postural stability and can significantly increase the risk of falls and fractures in older people15). However, so far, studies on the connection between the hyperopic refractive state and body balance have been insufficient. Since many researchers have reported that increasing interference with the decrease of visual acuity is accompanied by a relatively increased amount of sway of the human body, we focused on the changes of body balance under conditions of hyperopia and also of myopia.
Based on our results, the increase of ST implies that even retinal blur caused by uncorrected myopia as small as −0.50 D can affect the general stability of body balance. Moreover, even if there is no retinal blur because of accommodative function, uncorrected hyperopia such as +1.00 D can also affect the general stability of body balance. A significant increase of FI occurred from −1.50 D-induced hyperopia and from +4.00 D-induced myopia. These changes indicate that the hyperopic condition has a higher fall risk than the myopic condition. Under the hyperopic condition, strain caused by excessive accommodation for focusing becomes the cause of physiologic dizziness which tends to adversely influence body balance. Physiologic dizziness that occurs in normal persons as a result of physiologic stimulation of the three sensory systems leads to visual disturbances causing distortion of images16) and typically dizziness may result in loss of balance and falls17). Accordingly, dizziness should be considered as an influencing factor in conjunction with mechanical contributions from the musculoskeletal and joint systems, vestibular proprioceptive information, haptic cognitive status, and even brain structure, all of which affect body balance in normal healthy adults18,19,20,21,22).
Anteroposterior stability is controlled by the ankle, whereas mediolateral stability is controlled by the hips23). Refractive blur may have a greater effect on the visual stimuli that provide information for control of anteroposterior stability than on the stimuli providing information for mediolateral stability2). Our results show that the fluctuation of toe/heel synchronization was significantly greater than that of left/right synchronization under both conditions of hyperopia and myopia. However, a difference of fluctuation between hyperopia and myopia was not found in every synchronization of left/right or toe/heel. Whether under hyperopia or myopia, the type of ametropia does not significantly affect synchronization. Gentaz24) suggested that there is a preferred eye (called the “postural eye”) that allows better postural stability than the non-preferred eye. Additionally, the tendency in SP of left/right followed three patterns: toward the left, toward the right, and no tendency. We investigated the relationship between these tendencies and the side of the dominant eye and our results revealed that more than half of the subjects showed no tendency and that a noticeable relationship was not shown in the rest of the subjects, despite increase in uncorrected refraction power. Consequently, the postural eye may not necessarily be the dominant eye.
In conclusion, we found that uncorrected hyperopia might be the cause of a higher fall risk than uncorrected myopia. In addition, though this study was limited by the age range of the subjects, refractive errors should be appropriately corrected both in children and older patients, who tend to exhibit a high prevalence rate of hyperopia.
REFERENCES
- 1.Shumway-Cook A, Anson D, Haller S: Postural sway biofeedback: its effect on reestablishing stance stability in hemiplegic patients. Arch Phys Med Rehabil, 1988, 69: 395–400. [PubMed] [Google Scholar]
- 2.Anand V, Buckley J, Scally A, et al. : The effect of refractive blur on postural stability. Ophthalmic Physiol Opt, 2002, 22: 528–534. [DOI] [PubMed] [Google Scholar]
- 3.Lord SR: Visual risk factors for falls in older people. Age Ageing, 2006, 35: ii42–ii45. [DOI] [PubMed] [Google Scholar]
- 4.Isotalo E, Kapoula Z, Feret PH, et al. : Monocular versus binocular vision in postural control. Auris Nasus Larynx, 2004, 31: 11–17. [DOI] [PubMed] [Google Scholar]
- 5.Henriksson NG, Johansson G, Olsson LG, et al. : Electric analysis of the Romberg test. Acta Otolaryngol, 1966, 224: 224–, 272.. [DOI] [PubMed] [Google Scholar]
- 6.Travis RC: An experimental analysis of dynamic and static equilibrium. J Exp Psychol, 1945, 35: 216–234. [Google Scholar]
- 7.Magnusson M, Enbom H, Johansson R, et al. : Significance of pressor input from the human feet in anterior-posterior postural control. The effect of hypothermia on vibration-induced body-sway. Acta Otolaryngol, 1990, 110: 182–188. [DOI] [PubMed] [Google Scholar]
- 8.Paulus WM, Straube A, Brandt T: Visual stabilization of posture. Physiological stimulus characteristics and clinical aspects. Brain, 1984, 107: 1143–1163. [DOI] [PubMed] [Google Scholar]
- 9.Lord SR, Clark RD, Webster IW: Postural stability and associated physiological factors in a population of aged persons. J Gerontol, 1991, 46: M69–M76. [DOI] [PubMed] [Google Scholar]
- 10.Lord SR, Ward JA: Age-associated differences in sensori-motor function and balance in community dwelling women. Age Ageing, 1994, 23: 452–460. [PubMed] [Google Scholar]
- 11.Edwards AS: Body sway and vision. J Exp Psychol, 1946, 36: 526–535. [DOI] [PubMed] [Google Scholar]
- 12.Anand V, Buckley JG, Scally A, et al. : Postural stability in the elderly during sensory perturbations and dual tasking: the influence of refractive blur. Invest Ophthalmol Vis Sci, 2003, 44: 2885–2891. [DOI] [PubMed] [Google Scholar]
- 13.Straube A, Paulus W, Brandt T: Influence of visual blur on object-motion detection, self-motion detection and postural balance. Behav Brain Res, 1990, 40: 1–6. [DOI] [PubMed] [Google Scholar]
- 14.Jeon BJ, Cha TH: The effects of balance of low vision patients on activities of daily living. J Phys Ther Sci, 2013, 25: 693–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lord SR, Dayhew J: Visual risk factors for falls in older people. J Am Geriatr Soc, 2001, 49: 508–515. [DOI] [PubMed] [Google Scholar]
- 16.Maria BL: Current management in child neurology. In: Management of dizziness in children, 3rd ed. Hemilton: BC Decker Inc., 2005, pp 370–376. [Google Scholar]
- 17.Huijbregts P, Vidal P: Dizziness in orthopaedic physical therapy practice: classification and pathophysiology. J Manual Manip Ther, 2004, 12: 199–214. [Google Scholar]
- 18.Horak FB: Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing, 2006, 35: ii7–ii11. [DOI] [PubMed] [Google Scholar]
- 19.Jeka JJ, Lackner JR: Fingertip contact influences human postural control. Exp Brain Res, 1994, 100: 495–502. [DOI] [PubMed] [Google Scholar]
- 20.Lackner JR, DiZio P: Vestibular, proprioceptive, and haptic contributions to spatial orientation. Annu Rev Psychol, 2005, 56: 115–147. [DOI] [PubMed] [Google Scholar]
- 21.Winter DA, Patla AE, Ishac M, et al. : Motor mechanisms of balance during quiet standing. J Electromyogr Kinesiol, 2003, 13: 49–56. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan EV, Rose J, Rohlfing T, et al. : Postural sway reduction in aging men and women: relation to brain structure, cognitive status, and stabilizing factors. Neurobiol Aging, 2009, 30: 793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter DA, Patla AE, Frank JS: Assessment of balance control in humans. Med Prog Technol, 1990, 16: 31–51. [PubMed] [Google Scholar]
- 24.Gentaz R: L’oeil postural. Agressologie, 1988, 29: 685–686. [PubMed] [Google Scholar]


