Abstract
The aims of the present study were to investigate the prevalence of temporomandibular disorder (TMD) in a group of patients with Parkinson’s disease (PD), and to analyze oral health according to the severity of the disease. [Methods] Signs and symptoms of TMD were evaluated using the Research Diagnostic Criteria for Temporomandibular Disorders, and oral health impact was measured using the Oral Health Impact Profile. The unpaired Student’s t-test was used to compare groups with and without TMD. Pearson’s correlation coefficients were calculated to determine correlations between the level of functional independence and oral health impact. Fisher’s exact test was used to test the association between TMD and the severity of symptoms of PD. [Results] Fifty-nine individuals with PD were analyzed. The prevalence of TMD was 20.33%. No statistically significant associations were found between TMD and the severity of PD. Oral health impact was considered weak, but a statistically significant difference between groups with and without TMD was found for psychological disability (p = 0.003). No significant correlation was found between the level of functional independence and oral health impact. [Conclusion] The prevalence of TMD among patients with Parkinson’s disease was 20.33%. A statistically significant difference between groups with and without TMD was found regarding the psychological disability domain.
Key words: Parkinson’s disease, Oral health, Temporomandibular joint disorder
INTRODUCTION
Parkinson’s disease (PD) is a chronic, progressive condition of the central nervous system characterized by the degeneration of dopaminergic neurons that leads to a reduction in dopamine and produces the major signs of the disease: trembling, especially in the upper limbs and extending to the neck and face; bradykinesia (slowness of voluntary motor actions), muscle stiffness resulting from the ineffective inhibition of antagonist muscles; and postural instability, which occurs due to the progressive loss of balance and postural reflexes1, 2). Axial impairment is considered one of the major indictors of disability in individuals with PD3). Motor symptoms are related to the development of postural abnormalities characterized by forward lean and flexion of the cervical spine, thoracic hyperkyphosis, protraction and abduction of the shoulders, and flexion of the arms1, 4, 5).
Studies have demonstrated that changes in neck posture can lead to alterations in the biomechanics of the temporomandibular joint, affecting both stomagnathic function and postural control6, 7). Deficient axial control and mandibular movements due to the progression of motor symptoms in individuals with PD8, 9) indicate that such individuals are subject to the development of temporomandibular disorder (TMD), which is defined as a set of clinical manifestations of mandibular dysfunction with or without pain caused by damage to the morphological or functional integrity of the temporomandibular system10). TMD has a multifactorial etiology and is related to myofunctional alterations, muscle and postural imbalances11), as well as parafunctional habits12), such as nail biting and clenching of the teeth, which cause muscle hyperactivity and microtraumas in the temporomandibular joint13). It is estimated that the prevalence of TMD in the elderly population is approximately 21%14).
Functional alterations related to the symptoms of TMD, such as orofacial pain affecting the temporomandibular joint and masticatory muscles, limited or deviated mandibular movements, and joint sounds15, 16), contribute to a perception of poor oral health. Indeed, the severity of the symptoms of TMD is reported to exert an impact on oral health17, 18), with a negative effect on the performance of activities of daily living. Moreover, the chronic, progressive nature of PD leads to impaired motor control, which has a negative impact on the maintenance of adequate oral hygiene3, 9, 19) and likely accounts for the greater impact on oral health among such individuals9).
Considering the evidence that characteristic clinical impairment in individuals with PD can lead to alterations in the stomatognathic system, the aims of the present study were to investigate the prevalence of TMD in a group of patients with PD at a rehabilitation center and analyze the oral health impact according to the severity of the disease.
SUBJECTS AND METHODS
A cross-sectional study was carried out involving patients at the Brazilian Parkinson’s Association in the city of Sao Paulo, Brazil. Male and female individuals were recruited from the physical therapy sector of the rehabilitation center. The following were the inclusion criteria: age 50 to 75 years, medical diagnosis of idiopathic PD, and adequate cognitive state based on the Brazilian version of the Mini Mental State Examination as assessed by, adopting the cutoff points proposed by Bertolucci et al.20): 13 for illiterate individuals, 18 for those with a low to medium level of schooling and 26 for those with a high level of schooling. Individuals with missing teeth, dentofacial deformities or signs and symptoms of TMD prior to the diagnosis of PD were excluded from the study.
Considering daily variations in motor symptoms in individuals with PD due to the “on-off” phenomenon, the decision was made to perform the evaluations during the “on” period of medication. The evaluations were performed by a single examiner who had undergone a training exercise. Due to the clinical characteristics of the sample, the questionnaires were administered in interview format. The questions were always read in the same order and the response options for each question were presented.
Demographic data (age, sex, evolution of PD) were recorded on standardized charts. All individuals were evaluated for the effect of medications used to control the symptoms of PD. The modified Hoehn & Yahr21) scale was used for the classification of signs and symptoms of PD. This scale allows the classification of each individuals into seven stages of severity. Stages 1, 1.5 and 2 indicate mild disability; stages 2.5 and 3 indicate moderate disability and stages 4 and 5 indicate severe disability.
The Functional Independence Measure (FIM) was employed, which has been translated and validated for use on the Brazilian population was used to assess the subjects. The evaluation consists of the self-reported degree of assistance required from others for the performance of motor and cognitive tasks. Each activity is rated on a seven-point scale, for which 1 denotes complete dependence and 7 denotes complete independence22). As adequate cognitive capacity was one of the inclusion criteria, only the motor subscale was employed in the present study. Thus, the total score ranged from 13 to 91 points and the cutoff was 78 points, with lower scores indicating some degree of dependence and scores of 78 or higher indicating functional independence.
Signs and symptoms of TMD were evaluated using the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD), which is the gold standard for this type of evaluation. The RDC/TMD is made up of two axes. Axis I consists of an intraoral and extraoral clinical examination involving the analysis of mandibular movements and joint sounds as well as palpation of trigger points in the masticatory muscles. Axis II consists of a psychosocial questionnaire made up of 31 items. The diagnosis is determined with the aid of a correction key based on data from both axes23).
The Oral Health Impact Profile (OHIP-14)24) questionnaire was conducted. This measure is composed of 14 items distributed among seven subscales (functional limitation, physical pain, psychological discomfort, physical disability, psychological disability, social disability and handicap) addressing oral health status and its impact on social aspects. Each item has four response options: never (0 points), hardly ever (1 points), occasionally (2 points), fairly often (3 points) and very often (4 points). The total ranges from 0 to 56 points. Each item is attributed a weight. Oral health impact is considered weak when the score is between 0 and 9 points, moderate when the score is between 10 and 18 points and strong when the score is 19 points or higher25).
This study was according to the ethical principles of Declaration of Helsinki and the Regulating Guidelines and Norms for Research Involving Human Subjects stipulated in Resolution 196/96 of the Brazilian National Health Board. The study received approval from the Human Research Ethics Committee of University Nove de Julho, Brazil (process number: 437980). All participants signed a statement of informed consent.
Descriptive statistics (mean and standard deviation [SD]) were used for the characterization of the sample and distribution of the scores. The Kolmogorov-Smirnov test was used to determine the normality of the data distribution. The FIM results were dichotomized as “some degree of dependence” (< 78 points) and “independent” (≥ 78 points). The unpaired Student’s t-test was used to analyze differences in OHIP-14 scores between the groups with and without TMD. Pearson’s correlation coefficients were calculated to determine correlations between the FIM subscales and the OHIP-14. Fisher’s exact test was used to test the association between TMD and the severity of symptoms of PD. The Statistical Package for Social Sciences (SPSS) 15.0 for Windows was employed for all statistical tests, with a level of significance of to 5% (p < 0.05).
RESULTS
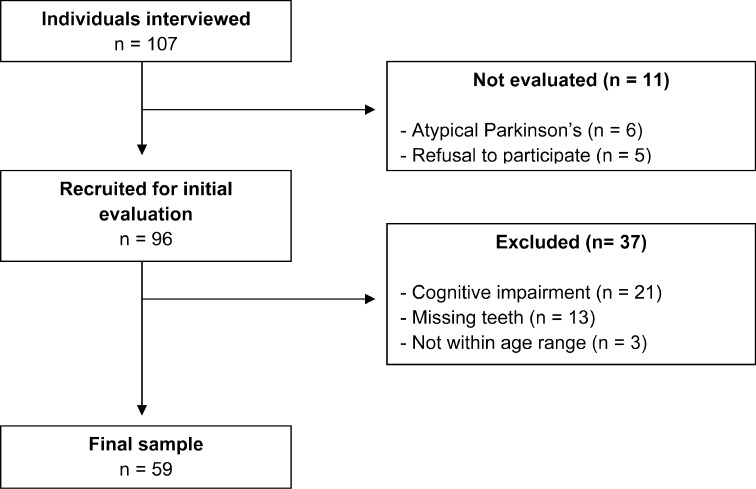
All individuals in the physical therapy sector of the Parkinson’s rehabilitation center were recruited. After the exclusion of those who did not meet the eligibility criteria, the final sample was made up of 59 individuals (Fig. 1).
Fig. 1.
Flowchart of sample selection procedure
Among the 59 participants evaluated, 50.84% were male and their mean age was 65.41 ± 8.77 years. The mean time elapsed since the diagnosis of PD was 7.11 ± 4.05 years. According to the Hoehn & Yahr scale, 83% of the sample had mild PD. Thirty-eight subjects were categorized as independent with regard to functional activities (Table 1).
Table 1. Characterization of sample.
| Variable | n | Mean ± SD |
|---|---|---|
| Age (years) | 65.4 ± 8.7 | |
| Time since diagnosis of PD | 7.1 ± 4.0 | |
| Gender (male/female) | (30/29) | |
| Motor impairment (Hoehn & Yahr) | ||
| Mild | 49 | |
| Moderate | 10 | |
| Total FIM score | 77.1 ± 5.8 | |
| Some dependence < 78 points (n) | 21 | |
| Independent ≥ 78 points (n) | 38 |
FIM: functional independence measure; PD: Parkinson’s disease; SD: standard deviation
The prevalence of TMD was 20.33% (n = 12) and this disorder was more frequent among the women (n = 7). Table 2 displays the distribution of the cases classified under diagnostic subtypes based on the RDC/TMD and distribution between sexes.
Table 2. Distribution of sample according to RDC/TMD and sex.
| Diagnosis | Male | Female | Total |
|---|---|---|---|
| RDC/TMD | |||
| Ib | 2 | - | 2 |
| IIa | 2 | 4 | 6 |
| IIb | - | 2 | 2 |
| IIIc | 1 | 1 | 2 |
Ib: myofascial pain with limited mouth opening; IIa: disc displacement with reduction; IIb: disc displacement without reduction; IIIc: osteoarthrosis; RDC: research diagnostic criteria
Fisher’s exact test revealed no significant association between TMD and PD severity (Table 3).
Table 3. Severity of PD according to presence or absence of TMD.
| Severity of PD (Hoehn & Yahr) | TMD | |
|---|---|---|
| Present | Absent | |
| Mild | 11 | 35 |
| Moderate | 1 | 12 |
| Total | 12 | 47 |
TMD: temporomandibular disorder; PD: Parkinson’s disease Fisher Exact Test.
Analyzing the entire sample (n = 59), oral health impact was weak for all OHIP-14 subscales. The greatest impacts were on the “physical disability” and “psychological discomfort” subscales (Table 4).
Table 4. Impact of oral health based on each OHIP-14 subscale.
| Subscale | Mean ± SD |
|---|---|
| Functional limitation | 0.78 ± 0.62 |
| Physical pain | 0.99 ± 0.54 |
| Psychological discomfort | 1.07 ± 0.56 |
| Physical disability | 1.02 ± 0.59 |
| Psychological disability | 0.82 ± 0.47 |
| Social disability | 0.92 ± 0.61 |
| Handicap | 0.50 ± 0.27 |
| Total OHIP-14 | 6.14 ± 1.94 |
SD: standard deviation
A weak negative correlation was found between the severity of symptoms of PD and oral health impact (r = −0.167, p = 0.207). Comparing oral health impact between the groups with and without TMD, statistically significant differences were found regarding the “functional limitation”, “psychological discomfort”, “physical disability” and “psychological disability” subscales (Table 5).
Table 5. OHIP-14 subscale scores in groups with and without TMD.
| Dimension | Without TMD (n = 47) Mean ± SD |
With TMD (n = 12) Mean ± SD |
|
|---|---|---|---|
| Functional limitation | 0.71 ± 0.65 | 1.04 ± 0.45 | |
| Physical pain | 0.97 ± 0.55 | 1.11 ± 0.49 | |
| Psychological discomfort | 1.05 ± 0.61 | 1.16 ± 0.24 | |
| Physical disability | 1.00 ± 0.63 | 1.12 ± 0.38 | |
| Psychological disability | 0.77 ± 0.51 | 1.05 ± 0.17 | * |
| Social disability | 0.86 ± 0.64 | 1.15 ± 0.41 | |
| Handicap | 0.49 ± 0.28 | 0.55 ± 0.20 |
TMD: temporomandibular disorder; SD: standard deviation. *: statistical significance.
DISCUSSION
Despite evidence that individuals with PD exhibit deficits in axial control and mandibular function8, 9), to the best of our knowledge, there are no previous reports in the literature on the investigation of signs and symptoms of TMD in this population. The hypothesis of the present study was that common clinical manifestations in individuals with PD would be associated with TMD and the prevalence of this disorder would be greater than that found among elderly individuals with no neurological disease.
The prevalence of TMD in the present sample was 20.33%. Moreover, the disorder was more frequent among women (58.33%). This finding is in agreement with data reported in the literature that demonstrating a greater prevalence of TMD among females gender16).
The mean age of the present sample was 65.11 years. Abud et al.14) evaluated signs and symptoms of TMD in a sample of community-dwelling individuals aged 60 years and older with no neurological diseases and found a 21.9% prevalence rate of mild signs of TMD. Physiological changes in oral motor function stemming from the ageing process may be one of the factors linked to the occurrence of TMD in the elderly population26). Moreover, Bakke et al.9) found that orofacial functions of individuals with PD can be compromised due to the severity of the motor symptoms, which may also exert an influence on the occurrence of TMD in this population. However, no significant associations were found between motor impairment and a diagnosis of TMD in the present study. This may be partially explained by the fact that the sample was made up mostly of individuals in the mild stage of PD. A more in-depth evaluation of other factors, such as changes in posture and muscle tone, should be carried out for a better analysis of this relationship.
It is important to consider the impact of oral problems on quality of life and studies have shown that functional alterations associated with symptoms of TMD contribute to greater oral health impact, especially among individuals with orofacial pain17, 27, 28). The OHIP-14 has been used in recent studies to investigate the impact of TMD due to the satisfactory psychometric properties of this assessment tool17).
Oral health is influenced by a number of factors, including perceptions regarding general health. Brennan and Singh29) found an association between the perception of general health and oral health in a sample of elderly individuals, demonstrating that oral health is highly influenced by a poorer state of general health. In the present study, however, no significant correlation was found between motor impairment and oral health impact. This finding is in disagreement with data described by Bakke et al.9), who evaluated the impact of oral health in patients in moderate to advanced stages of PD. In the present sample, the majority of individuals were in less advanced stages of the disease, were only semi-dependent, and had good perceptions of their general health, with no impact on the performance of activities of daily living, as demonstrated by their high FIM scores. Moreover, participation in the social and preventive activities, to which individuals are submitted at Parkinson’s institutions perform, may have exerted influence on the findings.
In the comparison of oral health impact between individuals with and without TMD, higher OHIP-14 scores were found among those with TMD, despite the weak impact indicated by the different subscales. This difference was significant with regard to psychological disability. It has been demonstrated that all diagnoses resulting from the RDC/TMD have a significant impact on oral health30). Moreover, orofacial pain is reported to be the main factor related to a greater negative oral health impact17, 27, 28, 30). This may explain the present findings, as only two individuals were classified with myofascial pain.
Although we did not find demonstrate significant differences in the present data, it should be stressed that evaluations and interventions involving individuals with PD mainly address motor aspects (such as gait)31) and cognitive aspects3), which may sometimes make such individuals overlook symptoms of equal importance to their health and quality of life. This may influence the measurement of symptoms of TMD and the perception of oral health, as these aspects are generally analyzed based on self-reports.
The present study had the inherent limitations of a cross-sectional design, which only allows the establishment of associations and does not permit conclusions regarding causality. Thus, longitudinal studies should be carried out to determine the cause-and-effect relationships of the variables analyzed. Studies should also be carried out to investigate other factors with a more global therapeutic approach for individuals with PD. Such investigations could offer valuable information on the efficacy of therapeutic and prevention strategies for this population.
Acknowledgments
The authors are grateful to the Brazilian fostering agency Fundação de Amparo à Pesquisa de São Paulo (FAPESP; Process 2012/04158-0 and 2012/03643-7) and the Brasil Parkinson Association in the city of São Paulo.
REFERENCES
- 1.Jankovic J: Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry, 2008, 79: 368–376. [DOI] [PubMed] [Google Scholar]
- 2.de Lau LM, Breteler MM: Epidemiology of Parkinson’s disease. Lancet Neurol, 2006, 5: 525–535. [DOI] [PubMed] [Google Scholar]
- 3.Muslimovic D, Post B, Speelman JD, et al. CARPA Study Group: Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology, 2008, 70: 2241–2247. [DOI] [PubMed] [Google Scholar]
- 4.Doherty KM, van de Warrenburg BP, Peralta MC, et al. : Postural deformities in Parkinson’s disease. Lancet Neurol, 2011, 10: 538–549. [DOI] [PubMed] [Google Scholar]
- 5.Benatru I, Vaugoyeau M, Azulay JP: Postural disorders in Parkinson’s disease. Neurophysiol Clin, 2008, 38: 459–465. [DOI] [PubMed] [Google Scholar]
- 6.Olmos SR, Kritz-Silverstein D, Halligan W, et al. : The effect of condyle fossa relationships on head posture. Cranio, 2005, 23: 48–52. [DOI] [PubMed] [Google Scholar]
- 7.Tingey EM, Buschang PH, Throckmorton GS: Mandibular rest position: a reliable position influenced by head support and body posture. Am J Orthod Dentofacial Orthop, 2001, 120: 614–622. [DOI] [PubMed] [Google Scholar]
- 8.Robertson LT, Hammerstad JP: Jaw movement dysfunction related to Parkinson’s disease and partially modified by levodopa. J Neurol Neurosurg Psychiatry, 1996, 60: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakke M, Larsen SL, Lautrup C, et al. : Orofacial function and oral health in patients with Parkinson’s disease. Eur J Oral Sci, 2011, 119: 27–32. [DOI] [PubMed] [Google Scholar]
- 10.Ingawalé S, Goswami T: Temporomandibular joint: disorders, treatments, and biomechanics. Ann Biomed Eng, 2009, 37: 976–996. [DOI] [PubMed] [Google Scholar]
- 11.Park Y, Bae Y: Change of range of motion of the temporomandibular joint after correction of mild scoliosis. J Phys Ther Sci, 2014, 26: 1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauriti L, Motta LJ, Silva PF, et al. : Are occlusal characteristics, headache, parafunctional habits and clicking sounds associated with the signs and symptoms of temporomandibular disorder in adolescents? J Phys Ther Sci, 2013, 25: 1331–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim PF, Smith S, Bhalang K, et al. : Development of temporomandibular disorders is associated with greater bodily pain experience. Clin J Pain, 2010, 26: 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abud MC, dos Santos JF, da Cunha VP, et al. : TMD and GOHAI indices of Brazilian institutionalised and community-dwelling elderly. Gerodontology, 2009, 26: 34–39. [DOI] [PubMed] [Google Scholar]
- 15.Dworkin SF, Huggins KH, LeResche L, et al. : Epidemiology of signs and symptoms in temporomandibular disorders: clinical signs in cases and controls. J Am Dent Assoc, 1990, 120: 273–281. [DOI] [PubMed] [Google Scholar]
- 16.Magnusson T, Egermark I, Carlsson GE: A longitudinal epidemiologic study of signs and symptoms of temporomandibular disorders from 15 to 35 years of age. J Orofac Pain, 2000, 14: 310–319. [PubMed] [Google Scholar]
- 17.Dahlström L, Carlsson GE: Temporomandibular disorders and oral health-related quality of life. A systematic review. Acta Odontol Scand, 2010, 68: 80–85. [DOI] [PubMed] [Google Scholar]
- 18.Barros VM, Seraidarian PI, Côrtes MI, et al. : The impact of orofacial pain on the quality of life of patients with temporomandibular disorder. J Orofac Pain, 2009, 23: 28–37. [PubMed] [Google Scholar]
- 19.Terriff DL, Williams JV, Patten SB, et al. : Patterns of disability, care needs, and quality of life of people with Parkinson’s disease in a general population sample. Parkinsonism Relat Disord, 2012, 18: 828–832. [DOI] [PubMed] [Google Scholar]
- 20.Bertolucci PH, Brucki SM, Campacci SR, et al. : [The Mini-Mental State Examination in a general population: impact of educational status]. Arq Neuropsiquiatr, 1994, 52: 1–7. [PubMed] [Google Scholar]
- 21.Hoehn MM, Yahr MD: Parkinsonism: onset, progression and mortality. Neurology, 1967, 17: 427–442. [DOI] [PubMed] [Google Scholar]
- 22.Linacre JM, Heinemann AW, Wright BD, et al. : The structure and stability of the functional independence measure. Arch Phys Med Rehabil, 1994, 75: 127–132. [PubMed] [Google Scholar]
- 23.Dworkin SF, LeResche L: Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord, 1992, 6: 301–355. [PubMed] [Google Scholar]
- 24.Slade GD: Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol, 1997, 25: 284–290. [DOI] [PubMed] [Google Scholar]
- 25.Allen PF, Locker D: Do item weights matter? An assessment using the oral health impact profile. Community Dent Health, 1997, 14: 133–138. [PubMed] [Google Scholar]
- 26.Kafas P, Leeson R: Assessment of pain in temporomandibular disorders: the bio-psychosocial complexity. Int J Oral Maxillofac Surg, 2006, 35: 145–149. [DOI] [PubMed] [Google Scholar]
- 27.Rener-Sitar K, Celebić A, Mehulić K, et al. : Factors related to oral health related quality of life in TMD patients. Coll Antropol, 2013, 37: 407–413. [PubMed] [Google Scholar]
- 28.John MT, Reissmann DR, Schierz O, et al. : Oral health-related quality of life in patients with temporomandibular disorders. J Orofac Pain, 2007, 21: 46–54. [PubMed] [Google Scholar]
- 29.Brennan DS, Singh KA: General health and oral health self-ratings, and impact of oral problems among older adults. Eur J Oral Sci, 2011, 119: 469–473. [DOI] [PubMed] [Google Scholar]
- 30.Reissmann DR, John MT, Schierz O, et al. : Functional and psychosocial impact related to specific temporomandibular disorder diagnoses. J Dent, 2007, 35: 643–650. [DOI] [PubMed] [Google Scholar]
- 31.Cholewa J, Gorzkowska A, Szepelawy M, et al. : Influence of functional movement rehabilitation on quality of life in people with Parkinson’s disease. J Phys Ther Sci, 2014, 26: 1329–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]