Abstract
Antitransgene CD8+ T-cell responses are an important hurdle after recombinant adeno-associated virus (rAAV) vector-mediated gene transfer. Indeed, depending on the mutational genotype of the host, transgene amino-acid sequences of foreign origin can elicit deleterious cellular and humoral responses. We compared here two different major histocompatibility complex (MHC) class I epitopes of an engineered ovalbumin transgene delivered in muscle tissue by rAAV1 vector and found very different strength of CD8 responses, muscle destruction being correlated with the course of the immunodominant response. We further demonstrate that robust CD8+ T-cell priming can occur through the cross-presentation pathway but requires the presence of either a strong MHC class II epitope or antibodies to the transgene product. Finally, manipulating transgene subcellular localization, we found that provided we avoid transgene expression in antigen presenting cells, the poorly accessible cytosolic form of ovalbumin transgene lacking strong MHC II epitope, evades CD8+ T-cell priming and remains permanently expressed in muscle with no immune cell infiltration. Our results demonstrate that the intrinsic immunogenicity of transgenes delivered with rAAV vector in muscle can be manipulated in a rational manner to avoid adverse immune responses.
Introduction
Gene therapy of monogenic disorders relies on the replacement of a nonfunctional or a missing endogenous protein by a therapeutic gene product. Several clinical developments have been conducted using recombinant adeno-associated virus (rAAV) vectors as vehicles to express therapeutic transgenes in a target tissue, such as factor IX (FIX) in the liver of hemophilia B patients1 and lipoprotein lipase (LPL) in the muscle tissue of LPL deficient patients.2 As evidenced both in animal models and in humans, adverse immune responses against rAAV vector and the therapeutic transgenes themselves represent a major bottleneck, which limits the domain of application of these treatments.3,4 In addition to preexisting neutralizing antibodies and T-cell responses directed against vector capsids,1,5,6,7 immune responses directed towards the transgene have been observed for various transgenes delivered in mice8,9,10 and human.11 rAAV vector serotype, dose and route of administration, immunomodulatory properties and inflammatory status of the targeted tissue and pattern of transgene expression were all shown to influence immune responses directed against transgene of foreign origin.3,12 Indeed, the presence in the transgene product of amino acids sequences not encoded in the host genome and therefore not previously tolerated by the host immune system also represents a key factor in priming of antitransgene B- and T-cell responses, as shown in both murine and canine model of FIX gene therapy.13,14 Moreover, among the numerous potential peptides present within a protein, only a fraction of them are correctly processed by the antigen presentation machinery, possess adequate amino-acid sequence to bind given major histocompatibility complex (MHC) haplotype and are therefore presented to a dedicated T-cell repertoire, a general phenomenon known as immunodominance.15 Thus, few CD8 and CD4 epitopes of real therapeutic transgene have been discovered so far.8,16,17,18 In contrast, numerous model transgenes with known epitopes have been extensively studied, but much of them harbored only one known CD8 epitope in a given MHC background (Luciférase,19 Green Fluorescent Protein (GFP),20 β-galactosidase21), sometime associated with one known CD4 epitope (Ovalbumin (OVA),9 influenza virus hemagglutinin (HA)22), precluding the analysis of differential immunogenicity of multiple epitopes in a given transgene.
Due to the central role of dendritic cells (DC) during the initiation of adaptive immune responses,23,24 direct transduction of DC was suggested to be a key element driving cellular responses after gene transfer.25,26 Strategies aiming at preventing expression in antigen presenting cells (APC) through the use of tissue-specific promoters27,28,29 or miRNA-based regulation of transgene expression in the hematopoietic system30,31 have been used. In this later approach, target sequences of the hematopoietic-specific miRNA142.3p have been added to the transgene coding sequence to destabilize the transgene mRNA and prevent transgene product expression in APC, thus promoting effective immune tolerance for particular transgenes. While efficient at preventing direct presentation of transgene-derived antigens, these strategies cannot prevent the uptake and cross-presentation of transgene products by nontransduced APC patrolling in the target tissue long after gene transfer. In the context of rAAV-mediated gene transfer, cross-presentation of muscle-derived transgene products was shown to be sufficient to prime a functional antitransgene cytotoxic T lymphocyte (CTL) response.32 Moreover, studies performed in auto-immune disease models have highlighted the importance of helper CD4+ T-cells activity,33 antigen forms (soluble versus cell associated)34 and antigen-specific antibody responses35 as important determinants of the outcome of cross-presentation events of tissue-specific antigens. Indeed, higher immunogenicity of membrane-bound transgene products over soluble ones was reported in a gene therapy setting,10 but little attention was devoted to the role of MHC class II and B-cell epitopes in the initiation of adverse antitransgene CD8+ T-cell responses.
In this study, we explored how the core immunogenic properties of a transgene of foreign origin delivered in muscle with rAAV1 vector regulate CD8+ T-cell priming against multiple epitopes in a situation where its accessibility to the direct antigen-presentation pathways can be tightly restricted by the adjunction of miR-142-3p target sequences. Analyzing immune responses directed against different cell associated forms of OVA model transgene containing or not well-characterized MHC class I and II epitopes, we identified the immunodominance of individual MHC I epitope, the presence of a strong helper T-cell epitope and the presence of transgene-specific antibodies as three key determinants for the initiation of adverse CD8+ T-cell responses.
Results
Epitope-dependent CD8+ T-cell priming in the absence of transgene expression in APC
To explore if CD8+ T-cell priming is equally effective against multiple epitopes within a given transgene product, we engineered a recombinant rAAV serotype 1 (rAAV1) vector coding for the full-length membrane bound ovalbumin fused to UTY246 and DBY608 male HY antigen epitopes (rAAV1-mOVA-HY) (Figure 1a). In female C57Bl/6 mice (B6), mOVA-HY is a fully foreign antigen containing four well-characterized MHC class I (OVA257 and UTY246) and class II (OVA323 and DBY608) epitopes. Furthermore, to inhibit direct transgene expression in antigen presenting cells (APC), we additionally generated a second rAAV1 construct bearing four repeats of the miR-142-3p target (miR-142-3pT) sequence30 inserted in the 3′ untranslated region of the hPGK-driven mOVA-HY expression cassette.
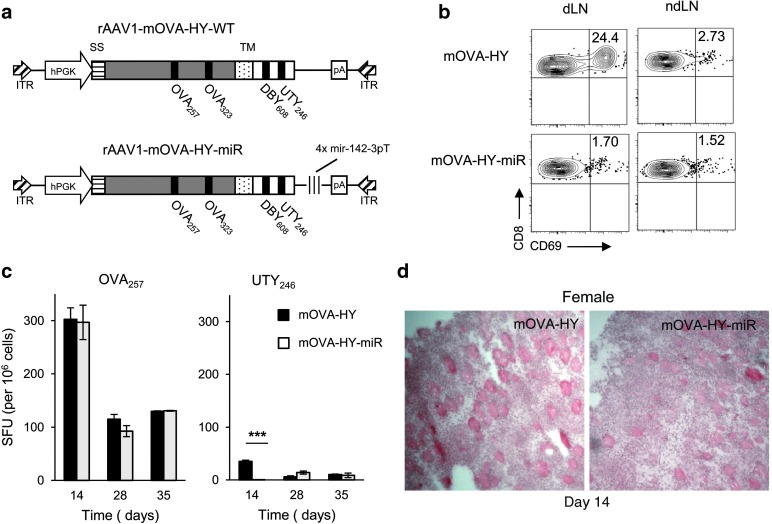
Figure 1.
Restriction of transgene expression to nonhematopoietic lineages has a minor influence on CD8+ T-cell priming. (a) The mOVA-HY construct comprises the leader peptide from the H-2Kb gene (SS), the full-length OVA cDNA, the H-2Db transmembrane sequence (TM) and, fused at the C-terminal, the DBY608 and UTY246 epitopes encompassed by 4–15 amino acids of their original protein sequence to ensure normal processing. In the mOVA-HY-miR construct, an additional sequence comprising four repeats of the miR142.3p target sequence were inserted in the 3′ untranslated region. (b) Rag2−/− female mice were injected i.m. at day 0 with rAAV1-mOVA-HY or rAAV1-mOVA-HYmiR. On day 3, mice were sacrificed and 2 × 105 cells from draining or nondraining lymph nodes were cocultured with 4.104 OT-1 cells. After 24 hours, CD69 activation was tested by flow cytometry. One representative experiment out of three is shown. (c–d) C57Bl/6 female mice were injected i.m. at day 0 with rAAV1-mOVA-HY (rAAV-WT) or rAAV1-mOVA-HYmir (rAAV-miR). (c) On day 14, 28, or 35 mice were sacrificed and their splenocytes were tested by IFN-γ ELISPOT assay against the MHC I–restricted OVA257 or UTY246 peptides. Data represent at least six to nine mice per group and per time point pooled from two independent experiments. (d) On day 14, tibialis anterior muscles from sacrificed mice were frozen and 7 µm thick transversal cryosections were stained with hematoxilin and eosin. Field size is 720 × 720 micron. One representative muscle out of three is shown.
To assess here whether miR-142-3pT sequence controls transgene expression and presentation by APC, Rag2−/− female mice were injected intramuscularly (i.m.) with either mOVA-HY or mOVA-HY-miR constructs. Their draining inguinal and popliteal lymph nodes or nondraining contralateral lymph nodes cells were isolated 3 days later and used in vitro as stimulating APC for anti-OVA257 OT-I TCR transgenic CD8+ T cells (OT-I). While draining lymph node cells from mOVA-HY immunized mice were able to present OVA257 epitope, as measured by overnight CD69 upregulation in tester OT-I cells (Figure 1b), no detectable OVA257 presentation was evidenced in mOVA-HY-miR immunized mice. This demonstrated that the miR-142-3pT sequence efficiently impaired early presentation of transgene-derived MHC class I epitopes in vivo, in line with its known regulation of transgene expression in cells of hematopoietic origin.30
To compare CD8+ T-cell priming against each MHC class I epitope, immunocompetent female B6 mice were injected i.m. with either mOVA-HY or mOVA-HY-miR constructs. Surprisingly, we observed similarly potent anti-OVA257 CD8+ T-cell responses after rAAV1-mediated gene transfer with both constructs (Figure 1c). In contrast, a tenfold lower CD8+ T-cell response was observed against anti-UTY246 epitope with mOVA-HY construct and this weak response was efficiently inhibited at day 14 by the adjunction of miR-142-3pT sequence (Figure 1c). Finally, local inflammation was further confirmed by histological inspection of muscle sections with both vectors (Figure 1d). As early as day 14, large areas of infiltration and muscle fibers destruction could be evidenced, in line with the strong anti-OVA257 CD8+ T-cell response detected by ELISPOT at that time point (Figure 1c). Altogether, these results indicate that strength of CD8+ T-cell priming is epitope-dependent and that miR-142-3pT–mediated destabilization of mOVA-HY mRNA in cells of hematopoietic origin affects CD8+ T-cell priming only against weakly immunogenic epitopes.
CD4+ T cell help controls CD8+ T-cell priming against tissue-restricted transgene
In addition to the core immunogenic properties of individual transgene-derived MHC I–restricted epitopes, the establishment of an antitransgene CD4+ helper T-cell response could influence the strength of CD8+ T-cell responses36,37, prompting us to analyze the CD4+ T-cell response induced by mOVA-HY and mOVA-HY-miR constructs. Similarly to the anti-OVA257 CD8+ T-cell response, the CD4+ T-cell response against the MHC class II–restricted DBY608 epitope derived from mOVA-HY-miR vector was not reduced nor delayed compared to the one triggered by mOVA-HY (Figure 2a). Surprisingly, neither of these two vectors was able to induce a detectable response against the MHC class II–restricted OVA323 epitope (data not shown). As the DBY608 epitope could behave as a dominant epitope masking the initiation of OVA323-specific immune response, we engineered a separate rAAV1 vector coding for the sole full-length membrane bound ovalbumin (mOVA) (Figure 2b). As compared with OVA protein emulsified in incomplete Freund's adjuvant (IFA), this later construct, however, elicited no detectable anti-OVA323 CD4+ T-cell responses, as measured by IFN-γ ELISPOT assay (Figure 2c). Of note, in female mice, the mOVA vector triggered a seven times weaker CD8+ T-cell response against the dominant OVA257 epitope compared to the one triggered with the mOVA-HY vector (Figure 2c). The impaired functional presentation of the OVA323 class II–restricted epitope in this setting was further confirmed by analyzing the in vivo activation of anti-OVA323 OT-II TCR transgenic CD4+ T cells (OT-II). While OT-II were readily expanded in recipient mice immunized with OVA protein emulsified in IFA, the rAAV1-mediated expression of the mOVA construct in muscle fibers failed to induce a detectable OT-II expansion (Figure 2d). This lack of anti-OVA323 response was observed at all time points and was also confirmed using a rAAV1 vector encoding for a secreted OVA protein (sOVA); in the same conditions, anti-DBY608 Marilyn T cells were expanded by mOVA-HY vector (Supplementary Figure S1). These results indicate that the DBY608 peptide is the major MHC class II–restricted epitope present in our mOVA-HY transgene.
Figure 2.
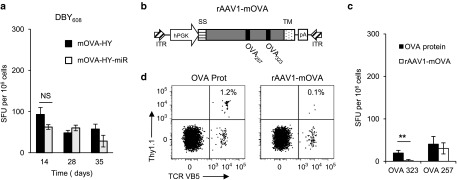
DBY608, but not OVA323, appears as a strong MHC II transgene-derived epitope. (a) C57Bl/6 female mice were injected i.m. at day 0 with rAAV1-mOVA-HY or rAAV1-mOVA-HYmir. On day 14, 28, or 35 mice were sacrificed and their splenocytes were tested by IFN-γ ELISPOT assay against the MHC II–restricted DBY608 peptide. Data represent six to nine mice per group and per time point pooled from two independent experiments. (b) The mOVA construct comprises the leader peptide from the H-2Kb gene (SS), the full-length OVA cDNA and the H-2Db transmembrane sequence (TM). (c–d) C57Bl/6 female mice were injected s.c. with 100 µg of OVA protein emulsified in IFA or i.m. with rAAV1-mOVA. (c) On day 14, mice were sacrificed and their splenocytes were tested by IFN-γ ELISPOT assay against the MHC II–restricted OVA323 or MHC I–restricted OVA257 peptides. Data represent three mice per group. (d) Alternatively, mice were transferred at day 0 with 106 congenic Thy1.1 TCR transgenic OT-II CD4+ T cells. On day 8, their PBL were stained to assess OT-II T cells expansion by flow cytometry analysis. Dot plots representative of three and gated on CD4+ 7-AAD− cells are shown.
This unique responsiveness to the DBY608 epitope provided us with an interesting model system to evaluate the impact of CD4+ T-cell help on the antitransgene CD8+ T-cell responses. Indeed, antitransgene CD4+ T-cell responses are not expected in male B6 mice since (i) male mice are naturally tolerant to male antigens such as DBY608 and UTY246 epitopes and (ii) the OVA323 epitope does not induce detectable CD4+ T cells. Comparing anti-OVA257 CD8+ T-cell responses in male and female B6 mice, we noted a reproducible fivefold reduction in the response against the mOVA-HY transgene in the absence of anti-DBY608 responses (Figure 3a). This lower anti-OVA257 T-cell response in male mice was similar to the one generated against the mOVA vector in female mice (Figure 2c), excluding a sex-related effect. More strikingly, in male recipients, the anti-OVA257 CD8+ T-cell response was delayed with the mOVA-HY-miR construct, with no detectable response by day 14 and a low albeit functional response by day 35 (Figure 3a). In line with the delayed CD8+ T-cell response, muscles from rAAV1-mOVA-HY-miR injected male mice presented no noticeable inflammation by day 14, while numerous muscle fibers from female mice appeared already surrounded by infiltrating cells (Figure 3b). To control for the presence of a potentially uncharacterized MHC II–restricted epitope in the mOVA-HY construct, we repeated these experiments in female mice after depletion of CD4+ T cells. Anti-CD4 GK1.5 antibody-mediated treatment was started 3 days before the injection of rAAV1 vectors and maintained until the end of the experiment, leading to profound and sustained depletion of CD4+ T cells (Figure 3c) and a lack of DBY608-specific immune responses as assessed by IFN-γ ELISPOT assay (data not shown). The anti-OVA257 immune response generated here in the absence CD4+ T cells (Figure 3d) was comparable to that obtained after injection of rAAV1-mOVA-HY into male mice or rAAV1-mOVA into female mice (Figures 2c and 3a), confirming the predominant role of the DBY608 epitope in the adaptive immune response directed against the mOVA-HY transgene. Interestingly, the emergence of a delayed anti-OVA257 CD8+ T-cell responses previously observed against the OVA-HY-miR transgene in male mice was also observed at day 28 in CD4-depleted female mice (Figure 3d).
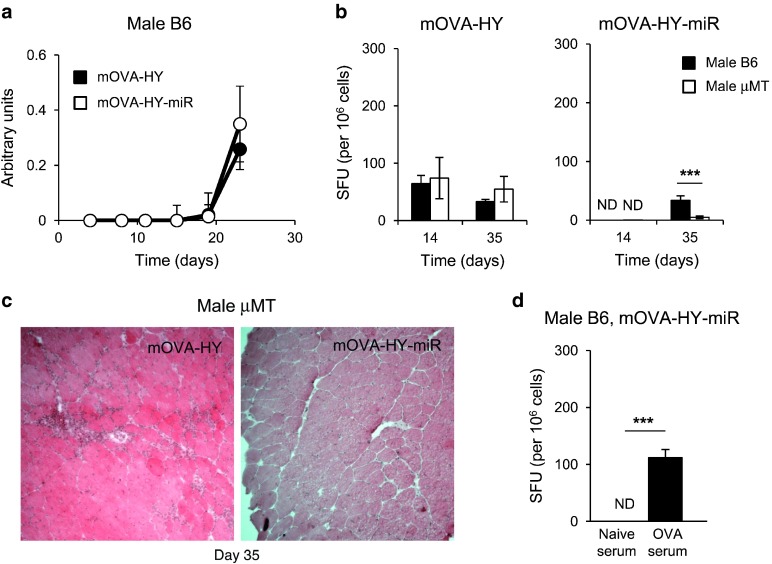
Figure 3.
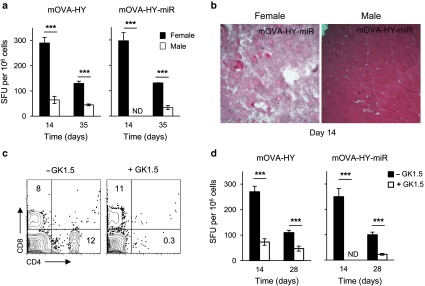
Antitransgene CD4+ helper T-cell response dictates the magnitude of antitransgene CD8+ T-cell responses and early efficacy of miR142-3pT–mediated regulation. (a–b) C57Bl/6 female or male mice were injected i.m. at day 0 with rAAV1-mOVA-HY or rAAV1-mOVA-HY-miR. (a) On day 14 or 35, mice were sacrificed and their splenocytes were tested by IFN-γ ELISPOT assay against OVA257 peptide. Data represent 6–10 mice per group and per time point pooled from two to four independent experiments. (b) On day 14, tibialis anterior muscles were frozen and 7 µm thick transversal cryosections were stained with hematoxilin and eosin. Field size is 720 × 720 micron. One representative muscle out of three is shown. (c–d) A group of C57Bl/6 female mice were injected by 60 μg of GK1.5 depleting antibody at day -3, 0, 7, 1, and 21. GK1.5-treated and control C57Bl/6 female mice were injected i.m. at day 0 with rAAV1-mOVA-HY or rAAV1-mOVA-HY-miR. (c) Their PBL were stained to assess the efficiency of CD4 depletion by flow cytometry. Representative dot plots for CD4 and CD8 expression on live PBL at day 14 are shown. (d) On day 14 or 28, mice were sacrificed and their splenocytes tested by IFN-γ ELISPOT assay against OVA257 peptide. Data represent 6–10 mice per group and per time point pooled from 2–4 independent experiments. ND, not detected.
Overall, these results indicate that the presence of a strong helper epitope, as exemplified here with the male DBY608 epitope, can dictate both the intensity and the kinetic of antitransgene CTL responses. In the context of miR142-3pT–regulated vector to avoid direct transgene presentation by APCs, the absence of transgene-specific CD4+ T-cell responses was sufficient to delay for several weeks the induction of a functional antitransgene CD8+ T-cell response, albeit insufficient to prevent late CD8 priming.
Antitransgene antibodies requirement for CD8+ T-cell cross-priming
As antitransgene CD8+ T-cell responses preclude stable transgene expression and establishment of long-term transgene tolerance, we wished to delineate the cellular events leading to the delayed priming of a CD8+ T-cell response in the absence of both CD4+ T-cell help and direct presentation of transgene-derived epitopes. As auto-antibodies in an autoimmunity setting were shown to enhance cross-presentation of tissue specific antigens,35 we assessed whether anti-OVA antibodies would similarly be responsible for the priming of the delayed CD8+ T-cell response observed against the mOVA-HY-miR transgene in the absence of CD4+ T-cell help. In male mice, we found no differences in the kinetics and intensities of anti-OVA antibody responses against mOVA-HY or mOVA-HY-miR transgenes (Figure 4a). This indicates that restriction of antigen expression to nonhematopoietic lineages does not interfere with the initiation of antitransgene B-cell response. Interestingly, this also showed that antitransgene humoral response precedes anti-CD8+ T-cell response in rAAV1-mOVA-HY-miR injected mice by 7–10 days.
Figure 4.
Antitransgene B-cell response is required for CD8+ T-cell cross-priming in the absence of CD4+ T-cell responses. (a) C57Bl/6 male mice were injected i.m. at day 0 with rAAV1-mOVA-HY or rAAV1-mOVA-HY-miR. Every 4 days, blood sample were collected and sera were analyzed by ELISA assay for the presence of anti-OVA IgG. Data represent six mice per group pooled from two independent experiments. (b–c) C57Bl/6 or μMT male mice were injected i.m. at day 0 with rAAV1-mOVA-HY or rAAV1-mOVA-HY-miR. (b) On day 14 or 35, mice were sacrificed and their splenocytes tested by IFN-γ ELISPOT assay against OVA257 peptide. Data represent six mice per group per time point pooled from two independent experiments. (c) On day 35, tibialis anterior muscles were frozen and 7 µm thick transversal cryosections were stained with hematoxilin and eosin. Field size is 720 × 720 micron. One representative muscle out of three is shown. (d) C57Bl/6 male mice were injected i.m. with rAAV1-mOVA-HYmiR and coinjected i.v. with 100 μl of serum from naïve or OVA-immunized mice. On day 16, mice were sacrificed and their splenocytes tested by IFN-γ ELISPOT assay the OVA257 peptide. Data represent six mice per group pooled from two independent experiments. ND, not detected.
To evaluate the role of antitransgene B-cell responses in driving the delayed antitransgene CTL responses against the mOVA-HY-miR transgene in male mice, we injected B cells deficient male μMT mice with either rAAV1-mOVA-HY or rAAV1-mOVA-HY-miR vectors. While no differences in CD8+ T-cell responses against the mOVA-HY construct occurred between WT and μMT male mice, these responses were profoundly impaired in μMT male mice injected with rAAV1mOVA-HY-miR vector, both at early (day 14) and late (day 35) time points (Figure 4b). At this later time point, muscles of μMT male mice expressing the mOVA-HY-miR transgene appeared spared from cellular infiltration, in contrast with those expressing the mOVA-HY transgene (Figure 4c). It is also interesting to note that the impact of CD4+ T-cell help on the magnitude of antitransgene CTL response in this model was mostly independent from B-cell responses, as shown by the strong CD8+ T-cell responses observed in μMT female mice against both transgenes from day 14 (data not shown).
Next, to assess whether antitransgene antibodies could indeed enhance the priming of CD8+ T-cell against cross-presented epitopes in the absence of helper T-cell responses, we performed passive antitransgene antibodies transfer experiments. Male B6 mice were thus coinjected i.m. with the rAAV1-mOVA-HY-miR vector and i.v. with serum from either naïve (naïve serum) or OVA/IFA-immunized (OVA serum) mice. As expected, male mice injected with naïve serum did not elicit any detectable anti-OVA257 CD8+ T-cell response by day 16 (Figure 4d), whereas a strong anti-OVA257 CD8+ T-cell response was observed in OVA serum-injected male mice. Altogether, these data demonstrate that antitransgene B-cell responses trigger the initiation of delayed CD8+ T-cell responses whenever the transgene is not expressed in APC and lacks functional MHC II epitopes.
Impact of transgene subcellular localization on CD8+ T-cell response
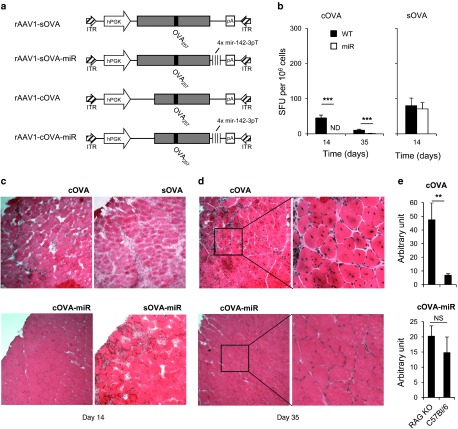
Overall, our results indicate that both CD4+ T-cell help and antitransgene antibodies are sufficient to trigger functional CD8+ T-cell responses against miR142-3pT–regulated transgenes. Thus, miR142-3pT–regulated transgene devoid of major MHC class II epitope and poorly accessible to antibodies should not trigger functional CD8+ T-cell responses in immunocompetent mice. To test this hypothesis, we produced four additional rAAV1 constructs encoding for cytoplasmic (cOVA) and secreted (sOVA) forms of the full-length ovalbumin, with or without miR142-3pT sequences (Figure 5a). Based on our above results with the membrane form of OVA (Figure 2), these two new OVA constructs are devoid of functional MHC class II epitope. Additionally, while the secreted form of OVA is readily accessible for antibody binding, the cytoplasmic OVA transgene should avoid antibody-mediated detection. Accordingly, we detected no OVA-specific antibodies in rAAV1-cOVA-miR injected mice by day 14, in contrast to rAAV1-cOVA, -sOVA, and -sOVA-miR injected mice (data not shown). Next, we analyzed anti-OVA257 CD8+ T-cell responses in these mice. While the cOVA construct generated a CD8+ T-cell response similar in strength and kinetics to that observed against mOVA transgene devoid of functional MHC II epitope, no response occurred after injection of rAAV1-cOVA-miR vector at all time points considered (Figure 5b). This was correlated with an absence of detectable infiltrates in transduced muscles at day 14 (Figure 5c) and, at day 35, muscles were still intact and spared from any infiltration whereas muscles injected with rAAV1-cOVA harbored numerous centro-nucleated muscle fibers indicative of an active reparation and regeneration process (Figure 5d). Importantly, quantifying OVA mRNA by Q-RT-PCR, we found that cOVA mRNA expression was identical at day 35 in immunocompetent and Rag2−/− mice injected with rAAV1-cOVA-miR vector in contrast to significant difference between immunocompetent and Rag2−/− B6 mice receiving rAAV1-cOVA (Figure 5e). Finally, rAAV1-sOVA-miR vector generated a rapid CD8+ T-cell response consistent, with the presence of anti-OVA antibodies (Figure 5b) leading to muscle destruction (Figure 5c).
Figure 5.
Inhibition of direct presentation prevents CD8+ T-cell priming to cytosolic transgene devoid of functional MHC class II epitope. (a) Secreted sOVA construct comprises the full-length OVA cDNA while cytoplasmic cOVA construct is deleted from 126 amino acids at the Cter OVA cDNA. For both constructs, additional constructs bearing four repeats of the miR142.3p target sequence in the 3′ untranslated region were generated. (b–d) C57Bl/6 female mice were injected i.m. at day 0 with rAAV1-cOVA, rAAV1-cOVA-miR, rAAV1-sOVA, or rAAV1-sOVA-miR. (b) At day 14 or 35, mice were sacrificed and their splenocytes tested by IFN-γ ELISPOT against the OVA257 peptide. Data represent six mice per group per time point pooled from two independent experiments. ND, not detected. At (c) day 14 or (d) day 35, tibialis anterior muscles from mice injected with rAAV1-cOVA or rAAV1-cOVA-miR were frozen and 7 µm thick transversal cryosections were stained with hematoxilin and eosin. Field size is 720 × 720 micron and inserts size is 300 × 300 micron. One representative muscle out of three is shown. (e) Rag2−/− or C57Bl/6 female mice were injected i.m. at day 0 with rAAV1-cOVA or rAAV1-cOVAmiR. On day 35, tibialis anterior muscles from sacrificed mice were removed and OVA mRNA was quantified by Q-RT-PCR. Data represent four mice per group. One of two different experiments is shown.
Discussion
Combining the use of different cell-associated forms of a model transgene encoding for multiple MHC class I and II epitopes and the careful dissection of transgene-specific CD8+ T-cell priming after rAAV-mediated gene transfer in muscle, we delineated three key features regulating the immunogenicity of a transgene: (i) the presence of immunodominant MHC I epitope, (ii) the presence of MHC II epitope and (iii) the accessibility of the transgene to antibody-mediated recognition. Moreover, we found that cross-priming depends on the intrinsic immunogenicity of the transgene and this can alter the efficiency of the miR142-3pT immunoregulation strategy used to avoid antitransgene response.
Indeed, the most surprising result in our study came from the initial observation that very efficient antitransgene CD8+ T-cell responses could be primed in the absence of direct presentation by APC. Early comparison of antitransgene immune responses triggered by rAd- and rAAV-mediated gene transfers suggested that transgene expression in APC was a necessary step for the induction of transgene-specific immune responses.25 On the other hand, suboptimal activation of CD8+ T cells directly on nonhematopoietic cells after rAAV2-OVA delivery in the liver,38 or cross-priming of CD8+ T cells after irradiation and bone-marrow reconstitution of mice injected in the muscle 9 weeks earlier32 have been shown. In line with the initial view, the use of tissue-specific promoters27,28,29 and more recently the addition of 3′ targets for endogenous microRNA30,39 confirmed the impact of antigen-expression in APC on antitransgene responses. Aside from the transgene itself, two other parameters could explain our results: the target tissue and the vector used. First, we choose the tibialis anterior muscle as a target tissue, an organ notably less tolerogenic than the liver13,40 used in the original miR142.3pT studies by Naldini and coll.30,39 The use of a rAAV1 vector instead of a lentiviral vector30,39 introduce a second potential variable, notably due to the known B-cell and CD8+ T-cell responses directed against the rAAV capsids.12 A prohibitive effect of either variable, however, is unlikely as miR-142-3p–mediated regulation was shown to promote tolerance to a muscle-targeted rAAV1-encoded transgene bearing little mutational differences with the host,31 a result that we reproduced in our study with the cytosolic OVA construct.
In agreement with our results, the sole use of microRNA target sequences was recently proven insufficient to promote long-term tolerance of two clinically relevant neo-antigens, muscle-targeted human α-sarcoglycan (SGCA) in sgca−/− dystrophic mice31 and liver-targeted α-L-iduronidase.41 Tissue-specific promoters have similarly demonstrated mixed results on the regulation of transgene-specific antibody42,43 and cellular responses,39 attributed at that time to the potential leakiness of such tissue-restricted promoters.44 These studies, as well as our present results, clearly suggest that efficient cross-presentation of transgene-derived epitopes acquired from transduced tissues is sufficient to prime a functional antitransgene CTL response, as previously observed for muscle-targeted β-galactosidase.32 The broad spectrum of impact of miR-142-3p–mediated regulation on antitransgene CTL responses described in this study, from no detectable impact in female immunized with a mOVA-HY construct, partial effect in male injected with a mOVA-HY construct, to lack of detectable immune response in mice injected with a cOVA construct, demonstrate that the immunogenicity of the transgene itself is a major parameter aside from the vector used and the choice of target tissue.
Our results demonstrated that the presence of a single foreign MHC class II epitope in the transgene itself, DBY608 here, controls the outcome of CD8+ T-cell priming following gene transfer even in the absence of direct APC transduction. It is well known that CD4+ T-cell help can greatly influence the outcome of the CD8+ T-cell priming, affecting both the intensity and quality of the CD8+ T-cell response being generated36,37,45 and the importance of this given MHC class II epitope for CD8+ T-cell priming has previously been discussed.46 Consistently, strong immune responses after rAAV-mediated gene-transfer were mostly detected against model transgene harboring identified MHC class II epitopes, such as OVA,9 HA,22 or FIX16 and not against transgene such as Luciferase, GFP, or β-galactosidase devoid of known MHC class II epitopes.
Another key result from our work is the demonstration that antitransgene antibodies can efficiently enhance CD8+ T-cell priming against membrane-bound transgene. This is reminiscent of the key role of autoantibodies in the regulation of self-antigen presentation35 and could result from a modified clearance of antigen-Ig complexes and altered targeting of DC subtypes in muscle-draining lymph nodes and/or a direct enhancement of antigen uptake by APCs through FcγR-mediated pathways. Unlike antitransgene CD4+ T-cell response, antitransgene antibodies formation was not as a prerequisite to mount an efficient immune antitransgene CTL response, as comparable CD8+ T-cell priming were observed with OVA-HY constructs in both μMT and WT C57Bl/6 female mice (data not shown). However, antitransgene B-cell response are of crucial importance in absence of strong CD4+ T-cell help, as evidenced by the absence of detectable CD8+ T-cell priming against the membrane-associated form of OVA encoded by the mOVA-HY-miR construct in B-cell deficient μMT male mice or against the cytosolic-associated form of OVA encoded by the cOVA-miR construct in WT mice. The idea that cytoplasmic transgene could evade such antibody-mediated cross-presentation is compatible with previous findings by Sarukhan et al. showing that β-gal, a protein normally cytoplasmic and nonimmunogenic after rAAV2-mediated intramuscular immunization, can trigger an functional CTL response when retargeted at the cell membrane.10
Finally, it is important to note that immune responses generated against each defined MHC class I epitope, and their sensitivity to miR-142-3p–mediated regulation, can be extremely different. The magnitude of the CD8+ T-cell response against the OVA257 epitope appeared ten times higher than the one against UTY246, a phenomenon referred to as immunodominance.15 This hierarchy was maintained independently of the nature of the antigen presentation pathway and miR-142-3p–mediated regulation only inhibited responses against the weaker of the two epitopes. In this latter case, we suggest that differences in MHC class I antigen processing and/or in the stability of subdominant epitope association with MHC class I molecules lead to a lower production of MHC-peptide complexes by the cross-presentation pathway. It is likely that the ordinarily weak response to the UTY246 epitope requires an above threshold amount of peptide-MHC complexes, which is not reached with miRNA142.3p-regulated AAV vector at day 14. The outcome in term of tolerance to the transgene itself serves as a reminder that lack of immunogenicity to a given epitope is not necessarily predictive of the global immune outcome. Given the currently limited knowledge on transgene-derived MHC I, but also MHC II, epitopes in current human clinical trials, this epitope-dependent immunogenicity suggests that T-cell assays to peptides banks are required to adequately follow antitransgene immune responses.1,2
Our study highlights a previously unappreciated importance of MHC class II epitopes and B-cell responses during the initiation of antitransgene CD8+ T-cell responses resulting either from the direct or the cross presentation pathways. Our results also delineate settings in which the addition of target sequences for miR142-3p mediates long-term expression of an immunogenic transgene. The miR142-3p sequence used here is fully conserved and highly expressed in hematopoietic cells from mice to humans,47 allowing translational applications in humans. All over, our study demonstrates that in addition to immune responses against rAAV vector capsids, the intrinsic immunogenicity of the transgene is worth considering for the design of rAAV gene therapy trials and may advocate for an appropriate immunomodulatory treatment.
Materials and Methods
Mice and animal experiments. Six to eight week old C57BL/6 mice were from Janvier (Le Genest Saint Isle, France). B6.129S2-Igh-6 tm1Cgn (μMT) and Rag2−/− Thy1.1 OT-II mice carrying a TCR specific for OVA323 bound to IAb were kind gift of Antonio Freitas and Olivier Lantz respectively. Rag1−/− OT-I mice carrying a TCR specific for OVA257 bound to Kb and CD45.1 Rag2−/− mice were bred in our animal facility. Mice were housed under specific pathogen-free conditions and handled in accordance with French and European directives. For intramuscular injection, mice were anesthetized and injected into the tibialis anterior with 25 μl of indicated rAAV vector (1.2 × 1010 vg per mice) diluted in phosphate-buffered saline (PBS). For OT-II cell transfer, 106 cells were injected into the retro-orbital venous sinus in a final volume of 200 μl of PBS 1×. For Ovalbumin immunization, mice were challenged subcutaneously at the base of the tail with 50 µg of Ovalbumin protein emulsified in IFA (BD-Difco, Le Pont de Claix, France).
Plasmid construction and recombinant AAV vector production. mOVA,48 cOVA,49 and sOVA50 cDNA were inserted by PCR in pSMD2 rAAV2 plasmid between the hPGK promoter and a polyA signal to create respectively pSMD2-mOVA, pSMD2-cOVA and pSMD2-sOVA. The pSMD2-mOVA-HY construct was generated by fusing the last coding codon of mOVA in pSMD2-mOVA to DBY608 and UTY246 epitopes encoding sequences encompassed respectively by 5 Nter and 15 Cter amino acids, and 4 Nter and 4 Cter amino acids of their original protein sequence to ensure normal processing. For pSMD2-mOVA-HY-mir, pSMD2-cOVA-mir, and pSMD2-sOVA-mir constructs, four repeats of the mir142.3p target sequence30 were inserted in the 3′ untranslated region of pSMD2-mOVA-HY, pSMD2-cOVA and pSMD2-sOVA constructs respectively. All expression cassettes were verified by sequencing.
For rAAV vector production, rAAV1-pseudotyped vectors were prepared by cotransfection in 293 cells of pSMD2 previous plasmids, pXX6 encoding adenovirus helper functions and pAAV9pITRCO2 that contains the rep and cap genes. Vector particles were purified on iodixanol gradients from cell lysates obtained 48 hours after transfection and titers were measured by quantitative real-time PCR.6
IFNγ-ELISPOT assay. Multiscreen nitrocellulose microplates (Millipore, Molsheim, France) were coated with antimouse IFNγ Ab (clone R4-6A2). Freshly isolated splenocytes (1.106/well and serial dilutions) were cultured in complete RPMI 1640 medium supplemented with 10% fetal calf serum (CM) with or without 1 µmol/l of indicated peptides. OVA257 (SIINFEKL), OVA323 (ISQAVHAAHAEINEAGR), UTY246 (WMHHNMDLI), and DBY608 (NAGFNSNRANSSRSS) peptides were synthesized by Proteogenix (Oberhausbergen, France). For each assay, Concanavalin A was added (5 µg/ml) as a positive control. After 20 hours, plates were incubated 2 hours with biotinylated antimouse IFNγ (clone XMG1.2) and 1.5 hours with alkaline phosphatase-conjugated streptavidin (Roche, Mannheim, Germany). Spots were developed by adding peroxidase substrates, 5-bromo-4,3-indolyl phosphate and nitroblue tetrazolium (Promega, Madison, WI) and counted using an AID reader (Autoimmun Diagnostika). Spot forming units (SFU) are represented after subtraction of background values obtained with unpulsed splenocytes (always below 10 spots/well).
Quantification of OVA mRNA in transduced muscles. For quantification of OVA mRNA, 100 ng of total RNA were reverse transcribed using iScript DNA Synthesis Kit (Biorad, Marnes-la-Coquette, France). Then, 2 μl of cDNA were subjected to real-time PCR amplification using Ova-F (5′-AAGCAGGCAGAGAGGTGGTA-3′), Ova-R (5′-GAATGGATGGTCAGCCCTAA-3′), β-actin-F (5′-AAGATCTGGCACCACACCTTCT-3′), and β-actin-R (5′-TTTTCACGGTTGGCCTTAGG-3′) primers. For all reaction mixtures, 10 μl of Fast Start Universal SYBR green mastermix (Roche, Boulogne-Billancourt, France) was used in a final volume of 20 μl, OVA primers were used at 500 nmol/l and β-actin primers at 400 nmol/l. The absolute amount of OVA mRNA for each sample was then normalized against the β-actin mRNA amount (arbitrary units) and determined using serial dilutions of pAAV–CMV–OVA plasmid and β-actin purified PCR product.
Fluorescence-activated cell sorting analysis. All reagents used for flow cytometry were purchased from BD-Biosciences (Le Pont de Claix, France). For PBL staining, erythrocytes were eliminated by hypotonic shock with PharMLysis buffer. Cell suspensions were first incubated with anti-FcγRIII/II (2.4G2) mAb for 15 minutes at 4 °C and then stained for 30 minutes at 4 °C in PBS 1× BSA 0.1% with saturating amounts of combinations of the following mAbs: FITC anti-CD4, PE anti-CD69, Pacific Blue anti-CD8, and APC-Cy7 anti-CD45.2. Dead cells were excluded using 7-actinomycine D (Sigma-Aldrich, Lyon, France) or LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit (Invitrogen, Saint-Aubin, France). Flow cytometric analysis was performed on a BD FACScalibur or FACSCanto II flow cytometer (BD Biosciences). Data were analyzed using FlowJo (Tree Star) software.
Anti-OVA IgG ELISA. ELISA microtiter plates (Nunc.) were coated overnight with 50 μl per well of a 10 μg/ml dilution of OVA protein (Sigma-Aldrich) in carbonate buffer pH 9.5. Plates were then extensively washed and then blocked for 2 hours with PBS 1×-BSA 1%-milk 5%. Serial dilutions of experimental sera as well as of a reference serum from an OVA protein-immunized mice, were diluted in PBS 1×-BSA 1% and incubated for 2 hours at room temperature. Bound anti-OVA IgG were detected with HRP-conjugated goat antimouse IgG (Vector Laboratories, Eurobio, Les Ulis, France) and revealed with TMB substrate reagent set (BD Biosciences). The reaction was stopped after 3–5 minutes with 2 mol/l H2SO4 and the absorbance at 450 nm was determined. All sera were tested in duplicate and antibodies levels are represented as a ratio of the reference serum (arbitrary units).
Statistical analysis. All data are shown as mean ± SEM. For all statistical analyses, an unpaired t-test was performed. Data were considered significant when P values were <0.05. (NS) nonsignificant, *P < 0.05 and ***P < 0.001.
SUPPLEMENTARY MATERIAL Figure S1. Response of OT-ll and Marilyn transgenic T cells to mOVA-HY transgene.
Acknowledgments
We thank Guillaume Précigout (INSERM U974), Françoise Grela, and Célia Casset (INSERM U1151) for their technical help. This work was supported by the Association Française contre les Myopathies (AFM) and the Agence Nationale de la Recherche (ANR-11-JSV3). M.C. was supported by the French Ministry of Research and by AFM. The authors declare no conflict of interest.
Supplementary Material
Response of OT-ll and Marilyn transgenic T cells to mOVA-HY transgene.
References
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ.et al. (2006Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response Nat Med 12342–347. [DOI] [PubMed] [Google Scholar]
- Gaudet D, Méthot J, Déry S, Brisson D, Essiembre C, Tremblay G.et al. (2013Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial Gene Ther 20361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays LE, Wilson JM. The complex and evolving story of T cell activation to AAV vector-encoded transgene products. Mol Ther. 2011;19:16–27. doi: 10.1038/mt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2011;11:321–330. doi: 10.2174/156652311796150354. [DOI] [PubMed] [Google Scholar]
- Brantly ML, Spencer LT, Humphries M, Conlon TJ, Spencer CT, Poirier A.et al. (2006Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults Hum Gene Ther 171177–1186. [DOI] [PubMed] [Google Scholar]
- Lorain S, Gross DA, Goyenvalle A, Danos O, Davoust J, Garcia L. Transient immunomodulation allows repeated injections of AAV1 and correction of muscular dystrophy in multiple muscles. Mol Ther. 2008;16:541–547. doi: 10.1038/sj.mt.6300377. [DOI] [PubMed] [Google Scholar]
- Veron P, Leborgne C, Monteilhet V, Boutin S, Martin S, Moullier P.et al. (2012Humoral and cellular capsid-specific immune responses to adeno-associated virus type 1 in randomized healthy donors J Immunol 1886418–6424. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Doucet C, Leboeuf M, Lemonnier FA, Danos O, Davoust J.et al. (2003Identification of an HLA-A*0201-restricted epitopic peptide from human dystrophin: application in duchenne muscular dystrophy gene therapy Mol Ther 8274–283. [DOI] [PubMed] [Google Scholar]
- Brockstedt DG, Podsakoff GM, Fong L, Kurtzman G, Mueller-Ruchholtz W, Engleman EG. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin Immunol. 1999;92:67–75. doi: 10.1006/clim.1999.4724. [DOI] [PubMed] [Google Scholar]
- Sarukhan A, Soudais C, Danos O, Jooss K. Factors influencing cross-presentation of non-self antigens expressed from recombinant adeno-associated virus vectors. J Gene Med. 2001;3:260–270. doi: 10.1002/jgm.175. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S.et al. (2010Dystrophin immunity in Duchenne's muscular dystrophy N Engl J Med 3631429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss AK, Muruve DA. Immunity to adeno-associated virus vectors in animals and humans: a continued challenge. Gene Ther. 2008;15:808–816. doi: 10.1038/gt.2008.54. [DOI] [PubMed] [Google Scholar]
- Cao O, Hoffman BE, Moghimi B, Nayak S, Cooper M, Zhou S.et al. (2009Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B Mol Ther 171733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD. Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4:192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu Q, Yang P, Hsu HC, Mountz JD. Determination of specific CD4 and CD8 T cell epitopes after AAV2- and AAV8-hF.IX gene therapy. Mol Ther. 2006;13:260–269. doi: 10.1016/j.ymthe.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Tangri S, Mothé BR, Eisenbraun J, Sidney J, Southwood S, Briggs K.et al. (2005Rationally engineered therapeutic proteins with reduced immunogenicity J Immunol 1743187–3196. [DOI] [PubMed] [Google Scholar]
- Lin SW, Hensley SE, Tatsis N, Lasaro MO, Ertl HC. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J Clin Invest. 2007;117:3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberis MP, Bell CL, Wilson JM. Identification of the murine firefly luciferase-specific CD8 T-cell epitopes. Gene Ther. 2009;16:441–447. doi: 10.1038/gt.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han WG, Unger WW, Wauben MH. Identification of the immunodominant CTL epitope of EGFP in C57BL/6 mice. Gene Ther. 2008;15:700–701. doi: 10.1038/sj.gt.3303104. [DOI] [PubMed] [Google Scholar]
- Oukka M, Colucci-Guyon E, Tran PL, Cohen-Tannoudji M, Babinet C, Lotteau V.et al. (1996CD4 T cell tolerance to nuclear proteins induced by medullary thymic epithelium Immunity 4545–553. [DOI] [PubMed] [Google Scholar]
- Gross DA, Leboeuf M, Gjata B, Danos O, Davoust J. CD4+CD25+ regulatory T cells inhibit immune-mediated transgene rejection. Blood. 2003;102:4326–4328. doi: 10.1182/blood-2003-05-1454. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ.et al. (2000Immunobiology of dendritic cells Annu Rev Immunol 18767–811. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Jooss K, Yang Y, Fisher KJ, Wilson JM. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geest BR, Van Linthout SA, Collen D. Humoral immune response in mice against a circulating antigen induced by adenoviral transfer is strictly dependent on expression in antigen-presenting cells. Blood. 2003;101:2551–2556. doi: 10.1182/blood-2002-07-2146. [DOI] [PubMed] [Google Scholar]
- Cordier L, Gao GP, Hack AA, McNally EM, Wilson JM, Chirmule N.et al. (2001Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies Hum Gene Ther 12205–215. [DOI] [PubMed] [Google Scholar]
- Ziegler RJ, Lonning SM, Armentano D, Li C, Souza DW, Cherry M.et al. (2004AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of alpha-galactosidase A and the induction of immune tolerance in Fabry mice Mol Ther 9231–240. [DOI] [PubMed] [Google Scholar]
- Fougerousse F, Bartoli M, Poupiot J, Arandel L, Durand M, Guerchet N.et al. (2007Phenotypic correction of alpha-sarcoglycan deficiency by intra-arterial injection of a muscle-specific serotype 1 rAAV vector Mol Ther 1553–61. [DOI] [PubMed] [Google Scholar]
- Brown BD, Venneri MA, Zingale A, Sergi Sergi L, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- Boisgerault F, Gross DA, Ferrand M, Poupiot J, Darocha S, Richard I.et al. (2013Prolonged gene expression in muscle is achieved without active immune tolerance using microrRNA 142.3p-regulated rAAV gene transfer Hum Gene Ther 24393–405. [DOI] [PubMed] [Google Scholar]
- Xu D, Walker CM. Continuous CD8? T-cell priming by dendritic cell cross-presentation of persistent antigen following adeno-associated virus-mediated gene delivery. J Virol. 2011;85:12083–12086. doi: 10.1128/JVI.05375-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Davey GM, Sutherland RM, Kurts C, Lew AM, Hirst C.et al. (2001Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo J Immunol 1666099–6103. [DOI] [PubMed] [Google Scholar]
- Harbers SO, Crocker A, Catalano G, D'Agati V, Jung S, Desai DD.et al. (2007Antibody-enhanced cross-presentation of self antigen breaks T cell tolerance J Clin Invest 1171361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha B, Tanchot C. Towards a cellular definition of CD8+ T-cell memory: the role of CD4+ T-cell help in CD8+ T-cell responses. Curr Opin Immunol. 2004;16:259–263. doi: 10.1016/j.coi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- Wuensch SA, Spahn J, Crispe IN. Direct, help-independent priming of CD8+ T cells by adeno-associated virus-transduced hepatocytes. Hepatology. 2010;52:1068–1077. doi: 10.1002/hep.23745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BD, Cantore A, Annoni A, Sergi LS, Lombardo A, Della Valle P.et al. (2007A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice Blood 1104144–4152. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y.et al. (2003Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer J Clin Invest 1111347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn MJ, McElmurry RT, Lees CJ, DeFeo AP, Chen ZY, Kay MA.et al. (2011Minicircle DNA-based gene therapy coupled with immune modulation permits long-term expression of a-L-iduronidase in mice with mucopolysaccharidosis type I Mol Ther 19450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Mingozzi F, Rodriguéz-Colôn SM, Joseph S, Dobrzynski E, Suzuki T.et al. (2004Therapeutic levels of factor IX expression using a muscle-specific promoter and adeno-associated virus serotype 1 vector Hum Gene Ther 15783–792. [DOI] [PubMed] [Google Scholar]
- Sun B, Zhang H, Franco LM, Brown T, Bird A, Schneider A.et al. (2005Correction of glycogen storage disease type II by an adeno-associated virus vector containing a muscle-specific promoter Mol Ther 11889–898. [DOI] [PubMed] [Google Scholar]
- Veron P, Boutin S, Martin S, Chaperot L, Plumas J, Davoust J.et al. (2009Highly efficient transduction of human plasmacytoid dendritic cells without phenotypic and functional maturation J Transl Med 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- Tyznik AJ, Bevan MJ. The surprising kinetics of the T cell response to live antigenic cells. J Immunol. 2007;179:4988–4995. doi: 10.4049/jimmunol.179.8.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Varambally S, Maher CA, Cao Q, Chockley P, Toubai T.et al. (2011Targeting of microRNA-142-3p in dendritic cells regulates endotoxin-induced mortality Blood 1176172–6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbo S, Delagrèverie H, Arnoult C, Authier FJ, Tron F, Boyer O. Functional tolerance of CD8+ T cells induced by muscle-specific antigen expression. J Immunol. 2008;181:408–417. doi: 10.4049/jimmunol.181.1.408. [DOI] [PubMed] [Google Scholar]
- Yang J, Sanderson NS, Wawrowsky K, Puntel M, Castro MG, Lowenstein PR. Kupfer-type immunological synapse characteristics do not predict anti-brain tumor cytolytic T-cell function in vivo. Proc Natl Acad Sci USA. 2010;107:4716–4721. doi: 10.1073/pnas.0911587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzynski E, Mingozzi F, Liu YL, Bendo E, Cao O, Wang L.et al. (2004Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer Blood 104969–977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Response of OT-ll and Marilyn transgenic T cells to mOVA-HY transgene.