Abstract
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death in the world. The multikinase inhibitor sorafenib only demonstrated marginal improvement in overall survival for advanced disease prompted the search for alternative treatment options. Human mesenchymal stem cells (MSCs) have the ability to home to tumor cells. However, its functional roles on the tumor microenvironment remain controversial. Herein, we showed that conditioned media derived from human fetal MSC (CM-hfMSCs) expressed high level of the insulin growth factor binding proteins IGFBPs and can sequester free insulin-like growth factors (IGFs) to inhibit HCC cell proliferation. The inhibitory effect of IGFBPs on IGF signaling was further evident from the reduction of activated IGF-1R and PI3K/Akt, leading eventually to the induction of cell cycle arrest. We also demonstrated that CM-hfMSCs could enhance the therapeutic efficacy of sorafenib and sunitinib. To the best of our knowledge, this is the first report to show that CM-hfMSCs has a tumor-specific, antiproliferative effect that is not observed with normal human hepatocyte cells and patient-derived matched normal tissues. Our results thus suggest that CM-hfMSCs can provide a useful tool to design alternative/adjuvant treatment strategies for HCC, especially in related function to potentiate the effects of chemotherapeutic drugs.
Introduction
Human hepatocellular carcinoma (HCC) is the fifth and the third leading cause of mortality in the world and Singapore respectively.1 Surgical resection and liver transplantation are the two curative treatments for HCC, but these are only applicable to a small proportion of patients with early tumors. Sorafenib, a multikinase inhibitor, is the only FDA-approved chemotherapy drug for systemic administration for advanced stage HCC.2 However, many solid tumors are rather resistant to chemotherapeutic drugs, and for those HCC patients who responded to sorafenib, the overall survival of HCC patients improved by only ~2 months.1 The observed marginal improvement prompted us to seek alternative, innovative therapies with greater clinical gains.
Mesenchymal stem cells (MSCs) are adult pluripotent progenitor cells of multiple mesenchymal lineages and have emerged as a potential option for regenerative medicine.3 Using a cocktail of various growth factors, MSCs can be induced to differentiate into hepatocyte-like phenotypes for functional liver replacement.4 In the field of cancer therapy, the most attractive feature of MSCs is its innate tumor homing properties. The ability of these MSCs to track microscopic tumors have significant clinical potential as these cells may potentially be employed for tracking or targeting metastasis and tumors, which are inaccessible for resection.5 Many research strategies have been developed to modify MSCs as an effective delivery vehicle for therapeutic genes. In HCC preclinical animal models, MSCs have been modified with antiangiogenic agent; pigment epithelium-derived factor,6 proapoptotic gene TRAIL7; cytokine such as interferon-β8; and oncolytic virus.9 The use of MSCs in clinical practice is hampered by the inability to monitor the transplanted cells in the patients, the lack of standardized clinical protocols and most important of all, its undefined role in tumorigenesis. Some reported that MSCs could promote tumor progression while others reported that MSCs exhibited an antitumor effect.10 MSCs are known to secrete, and respond to, various cytokines, chemokines, and growth factors.11 Thus, the precise effect of MSCs could be determined by the recipient cells that constitute the specific microenvironment. For example, conditioned media derived from MSC (CM-MSCs) was shown to increase survival, proliferation and migration of endothelial cells12; but inhibit cell cycle progression, migration, and contractibility of corneal fibroblast.13 Systemic infusion of MSCs or CM-MSCs has been demonstrated to protect against liver fibrosis in rodents14 and ameliorate fulminant liver failure in pigs.15 In the context of cancer, studies have reported that CM-MSCs derived from human Wharton's jelly stem cells promoted apoptosis and autophagy of cancer cells.16
We recently showed that MSCs exhibited an antitumor effect when cocultured with tumor cells.17 Because MSCs have been shown to gain or lose certain cell surface receptors during culture,18 we therefore hypothesized that the observed antitumor effect was mediated by the paracrine secretion of soluble factors from MSCs which affected the key signaling cascades via cell surface receptors expressed by the HCC tumors. The results showed that CM-MSCs secreted high levels of insulin binding proteins (IGFBPs), as well as, exerted an inhibitory effect on HCC proliferation. The antitumor effect was mediated through dysregulation of the insulin-like growth factor (IGF) signaling cascade; specifically, the activation of type-I IGF receptor (IGF-1R) on HCC was inhibited which disrupted the downstream phosphatidylinositol 3-kinase (PI3K)/Akt signaling events. Taken together, the results demonstrated that trophic factors secreted by MSCs possess antitumor effect in vitro and in vivo.
Results
Human fetal MSCs exerted a suppressive effect on the growth of HCC
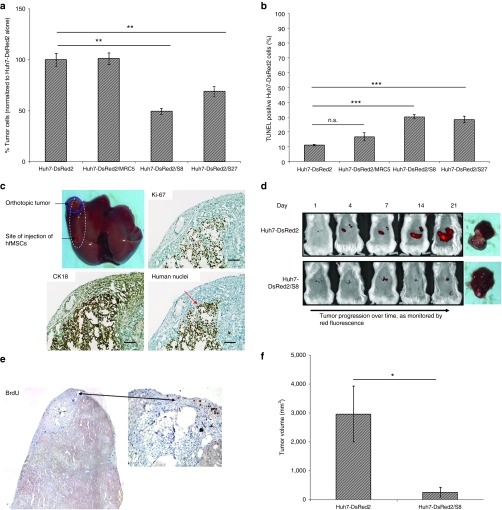
To characterize the biological effect of human fetal MSCs (hfMSCs) on the proliferation of HCC cells, Huh7-DsRed2 cells were cocultured in a 1:1 ratio with either immortalized human fetal lung fibroblast cells (MRC5) or hfMSCs (S8 and S27; Figure 1a). Cell viability assay showed that hfMSCs significantly inhibited the growth of Huh7-DsRed2 cells by ~50% and ~30%, respectively. An increase in the TUNEL-positive Huh7-DsRed2 cells in the presence of hfMSCs (either S8 or S27) could be detected (Figure 1b). A slight increase of ~5% was also observed when Huh7-DsRed2 was coculture with MRC5 but this increase was insignificant. Further, proliferation of Huh7-DsRed2 cells was inhibited when cocultured in hfMSCs as shown by the significant decrease in cell proliferation marker Ki-67. The percentage of Ki-67-positive Huh7-DsRed cells was 49% ± 0.77 in coculture of Huh7-DsRed2/S27 versus 92.6% ± 1.62 in coculture of Huh7-DsRed2/MRC5 (Supplementary Figure S1a). To evaluate whether migrating hfMSCs can suppress HCC growth in vivo, Huh7-DsRed2 cells were implanted into the livers of immunodeficient NOD/SCID mice. Tumor formation was confirmed by immunostaining of liver sections for human nuclei, cytokeratin 18 (CK18) and Ki-67 (Figure 1c, red circle), and monitored by DsRed2 imaging (Figure 1d). BrdU-labeled-S8 could be detected around and within the Huh7-DsRed2 tumor by BrdU staining, thus confirming that hfMSCs were capable of migrating to HCC (black arrowheads, Figure 1c). Compared to Huh7-DsRed2 tumor alone, the Huh7-DsRed2 tumor injected with S8 showed significant reduction in the DsRed2fluorescence intensity (Figure 1d) that correlated well with the reduction in tumor volume (Figure 1e; P < 0.05). Collectively, these results demonstrated that hfMSCs migrated towards HCC and inhibited tumor growth.
Figure 1.
hfMSCs exhibit tumor homing and tumor suppressive properties in patient-derived orthotopic hepatocellular carcinoma (HCC) mouse model. (a) Huh7-DsRed2 cells were cocultured with MRC5, S8, or S27 for 72 hours in complete Dulbecco's modified Eagle's medium. Cell viability was performed by scoring the number of red-fluorescence cells in the various coculture systems, Huh7-DsRed2 cells in six random fields at 10× magnification in triplicate. (b) TUNEL-positive Huh7-DsRed2 cells were scored in eight random fields of view at 10× magnification in various coculture systems. All data shown were normalized to Huh7-DsRed2 alone and expressed as means ± standard error of the mean (SEM). (c) Bromodeoxyuridine (BrdU)-labeled S8 was injected subcapsularly at a distance of ~0.5 cm (indicated by red circle) adjacent from the orthotopically implanted Huh7-DsRed2 tumors as indicated by the blue circle. Photomicrographs showed the expression of BrdU, CK18, human nuclei, and Ki-67. Scale bar = 100 μm. (d) Noninvasive DsRed2 bioimaging was used to monitor tumor progression. Representative image from Huh7-DsRed2-bearing mice with or without S8 were shown. Red fluorescence indicates DsRed2 signal emitted by the tumor cells. (e) Corresponding tumor volumes were measured at the end of the experiment at day 21 after the entire liver were harvested. All data shown are means ± SEM, n ≤ 4. *P < 0.05; **P < 0.01. n.s., not significant.
The antitumor effect of hfMSCs was mediated via a paracrine signaling mechanism
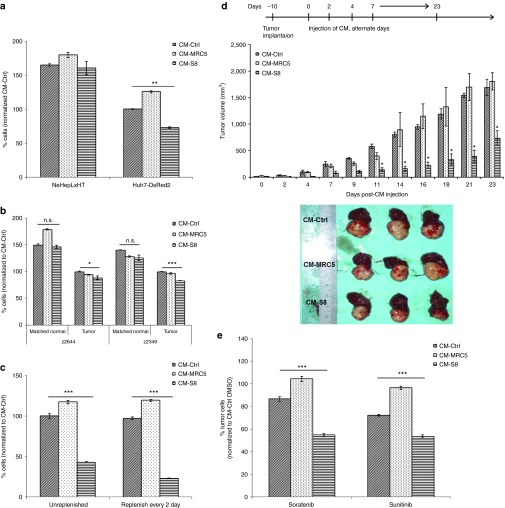
We postulated that the antitumor effect might be mediated through soluble factors secreted by hfMSCs in a paracrine manner. As shown in Figure 2a, treatment with CM-S8 decreased the percentage of HCC cells by 27% in comparison to control media collected concurrently (CM-Ctrl). The inhibitory effect was not observed in CM-MRC5. Similar antitumor effect was also observed in CM isolated from other donors (Supplementary Figure S1b) but amniotic membrane-derived CM-MSCs failed to suppress HCC growth in vitro (Supplementary Figure S1c). More importantly, CM-S8 did not suppress the proliferation of immortalized normal human neonatal hepatocytes NeHepLxHT cells (Figure 2a) and the matched histologically normal liver tissues derived from patients with primary HCC (z2644 and z2349; Figure 2b). Instead, patient-derived HCC cells treated with CM-S8 exhibited a significant reduction in cell viability compared to CM-MRC5 and CM-Ctrl-treated cells (Figure 2b). Together, these results demonstrated the tumor-specific suppressive effect of hfMSCs.
Figure 2.
CM-hfMSCs conferred hepatocellular carcinoma (HCC)-specific growth suppression in vitro and in vivo. (a) The effect of CM-Ctrl, CM-MRC5, and CM-S8 on Huh7-DsRed2 cells and immortalized normal human hepatocytes NeHepLxHT was assessed after 48 hours of incubation by cell counting kit (CCK)-8 assay. Data shown are presented as means ± SEM, n = 4, and normalized to CM-Ctrl-treated cells. (b) The effect of CM-Ctrl, CM-MRC5, and CM-S8 on short-term culture of HCC and its matched normal (z2644 and z2349) derived from two local patients was evaluated. Data shown are presented as means ± SEM, n = 3, and normalized to CM-Ctrl-treated cells. (c) Long-term effect of CM-hfMSCs on Huh7-DsRed2 cells was examined for a period of 7 days. CMs were either replenished every other day or unreplenished. All data are presented as means ± standard error of the mean (SEM) and normalized to CM-Ctrl-treated cells at the respective condition, n = 4. (d) Schematic diagram of the treatment regimen with CM-S8, CM-MRC5, and CM-Ctrl in mice bearing orthotopic patient-derived HCC tumor line, 26–1004. Intratumoral injection of CM was performed on alternate days from day 10 post-tumor implantation until day 18. Tumor volume was monitored until day 23. Tumors were then harvested at the end of the treatment. Bar chart depicts the mean tumor volumes of mice ± SEM, n = 3. Representative of whole liver images from each treatment group were shown. *P < 0.05; with respect to CM-Ctrl-treated cells. The combination effect of sunitinib/sorafenib and CM-S8 was tested in Huh7-DsRed2 cells. Cells were pretreated with CM-Ctrl, CM-MRC5, and CM-S8 for 48 hours followed by incubation in sunitinib or sorafenib-containing media for 24 hours. All data are presented as means ± SEM and normalized to dimethyl sulfoxide because this is the vehicle for sorafenib and sunitinib, n = 4; *P < 0.05; **P < 0.01; ***P < 0.001. n.s., not significant.
To show the potential clinical relevance of these results, we examined the anti-tumor effect of CM-hfMSCs over a period of 7 days. The tumor suppressive effect was consistently observed whether the CM was replenished every 2 days or unreplenished (Figure 2c). Nevertheless, the percentage of tumor cells treated with CM-hfMSCs that were replenished every 2 days reduced by an extra 20%. Treatment with CM-MRC5 also led to the increase in cell proliferation but this did not further increase upon the replenishment of CM. We next investigated the effect of CM-hfMSCs on the growth of patient-derived orthotopic HCC mouse xenograft. Intratumor injections of CM-hfMSCs, CM-MRC5, or CM-Ctrl were given on alternate day until day 18. As shown in Figure 2d, the tumor volume of mice injected with CM-hfMSCs were significantly reduced compared to those receiving either CM-MRC5 or CM-Ctrl. In contrast, tumors treated with CM-MRC5 or CM-Ctrl continued to grow. Collectively, these results demonstrated that CM-hfMSCs can confer tumor-specific proliferative inhibitory effect in vitro and in vivo.
CM-hfMSCs enhanced the antitumor effects of sorafenib and sunitinib
Currently, Sorafenib is the only FDA-approved drug available for treating HCC. Sunitinib, another molecular targeted drug, acts on b-RAF and which expression has been shown to correlate with the presence of multiple HCC nodules.19 Hence, we asked whether CM-hfMSCs was able to complement and potentiate the antitumor properties of sunitinib and sorafenib. Compared to single entity therapy, combination therapy of sorafenib or sunitinib with CM-S8, and not CM-Ctrl, further decreased the viability of tumor cells by ~32 and 19% respectively (Figure 2e). These results demonstrated that CM-hfMSCs can enhance the antitumor efficacy of sorafenib and sunitinib.
CM-hfMSCs inhibited HCC cell proliferation through induction of cell cycle arrest
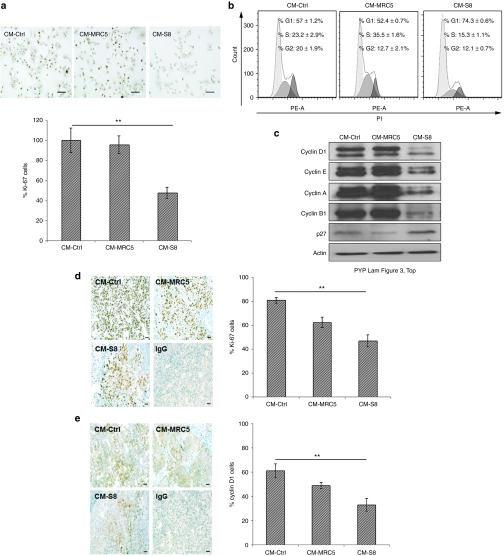
We next investigated whether the observed antitumor effect of CM-hfMSCs was due to the suppression of tumor growth or the induction of apoptosis. We did not observe significant TUNEL-positive cells or cleavage of procaspase-3 (Supplementary Figure S2a,b respectively). By contrast, the number of cells positively stained with the proliferation marker, Ki-67, was reduced in HCC treated with CM-hfMSCs but not CM-MRC5 compared to CM-Ctrl (Figure 3a). FACS analysis also showed a higher proportion of HCC cells in the G1 phase of the cell cycle (CM-hfMSCs, 74.3 ± 0.6%) comparing to the CM-Ctrl- and CM-MRC5-treated cells (57 ± 1.2% and 52.4 ± 0.7%, respectively; Figure 3b). Immunoblot analysis showed a reduction in cyclin D1, cyclin E, cyclin A, and cyclin B1 expression with the concomitant upregulation of p27Kip1 (Figure 3c). Consistent with the results observed in vitro, patient-derived HCC orthotopic tumors treated with CM-hfMSCs had a lower Ki-67 index (Figure 3d) and a corresponding reduction in cyclin D1-positive cells (Figure 3e) compared to HCC tumors treated with CM-MRC5 or CM-Ctrl. These results demonstrated that CM-hfMSCs inhibited the proliferation of HCC cells by the induction of cell cycle arrest at the G0/G1 phase.
Figure 3.
CM-hfMSCs induced cell cycle arrest of hepatocellular carcinoma (HCC) cells. (a) Immunocytochemistry staining of CM-treated-Huh7-DsRed2 cells against human Ki-67. Photomicrographs showed representative field images of Ki-67-stained cells. Scale bar = 100 µm. Bar graph represents percentage of Ki-67-positive cells (scored from 16 random field of view) normalized to CM-Ctrl-treated cells, n = 16. (b) Flow cytometry analysis of the cell cycle profile of CM-treated Huh7-DsRed2 cells. Representative diagrams were shown. (c) Immunoblot analysis of cell cycle–related proteins from CM-treated-Huh7-DsRed2 cell lysates. Protein loaded was normalized to actin. (d) Ki-67 and (e) cyclin D1 immunohistochemistry staining of cryosectioned tumor harvested from CM-Ctrl, CM-MRC5, and CM-S8-treated patient-derived tumor 26–1004. Photomicrographs showed representative images. Scale bar = 50 μm. Immunopositive cells were quantified (total 10 random field of view) and presented in the bar charts. Data are shown as means ± SEM, n = 10; **P < 0.01.
The tumor suppressive effect of hfMSCs was mediated through IGF receptor-1 signaling
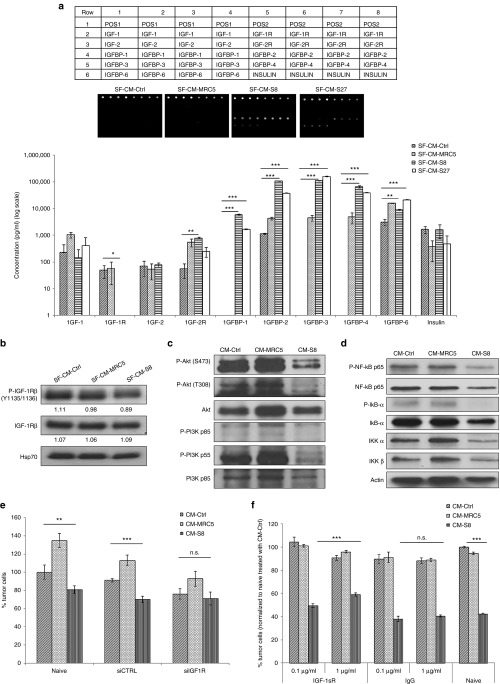
IGF signaling plays important roles in HCC tumorigenesis.20 During hepatocarcinogenesis, alterations in the expression of the molecules in the IGF pathways have been reported.21 To determine whether the tumor suppressive factors mediated by CM-hfMSCs may consist of molecules in the IGF pathways, an IGF-antibody array screen was performed. To minimize possible binding of nonspecific factors contained in foetal bovine serum to the array, the experiment was done using serum free conditioned media from S8; S27 and S33 (Supplementary Figure S3a,b). Similar tumor suppressive effect could be detected. Proteins that were differentially expressed in SF-CM-hfMSCs include IGFBP-1, -2, -3, -4, and -6; of which IGFBP1-3 were highly secreted by hfMSCs (Figure 4a). Conversely, no significant difference was observed for IGF-1, IGF-2, and insulin when compared to the controls (SF-CM-Ctrl and SF-CM-MRC5). Furthermore, HCC treated with CM-hfMSCs exhibited significantly reduced expression of phosphorylated IGF-1R (p-IGF-1R; Figure 4b). Tovar et al.22 have previously demonstrated the significant correlation between p-IGF-1R-related expression and the phosphatidylinositol gene signature. Therefore, we investigated whether the PI3K pathway was also affected. As shown in Figure 4c, HCC treated with CM-hfMSCs gave reduced levels of activated PI3K and Akt compared to the controls.
Figure 4.
Tumor suppression is mediated through downregulation of insulin-like growth factor (IGF-1) signaling. (a) Protein array analysis detecting members of the IGF proteins family that are present in SF-CMs. Quantification of protein expression detected in SF-CM-Ctrl, SF-CM-MRC5, SF-CM-S8, and SF-CM-S27. Values shown are obtained from standard curve generated against the various proteins, n = 3. (b) Immunoblot analysis was performed on SF-CMs-treated Huh7-DsRed2 cell lysate using antibodies against P-IGF-1R-β and total IGF-1Rβ. Protein loaded was normalized to Hsp70. Immunoblot using antibodies against (c) P-Akt, total Akt, P-PI3K, total PI3K (d) P-NF-κB p65, total NF-κB p65, P-IκB-α, total IκB-α, IKK-α, and IKK-β was performed and normalized to actin. (e) Huh7-DsRed2 cells were transfected with Ctrl-RNAi or IGF-1R-RNAi for 24 hours. After which, the cells were treated with CM-Ctrl, CM-MRC5, or CM-S8 for 48 hours. Percentage of tumor cells was evaluated using cell counting kit (CCK-8) assay. Data shown are normalized to Ctrl-RNAi CM-Ctrl-treated cells and presented as means ± standard error of the mean (SEM), n = 4. (f) Huh7-DsRed2 cells were pretreated with monoclonal antibody against IGF-1R followed by incubation in CM-S8 and CM-MRC5 for 48 hours. Goat IgG and untreated cells served as control. Percentage of tumor cells was determined using CCK-8 assay after 48 hours incubation. Data shown are normalized to IgG, CM-Ctrl-treated cells and presented as means ± SEM, n = 3; *P < 0.05; **P < 0.01; ***P < 0.001. n.s., not significant.
IGF-1R signaling via the PI3K and Akt axis also involves NF-κB signaling which upon chronic activation can lead to fibrosis and cirrhosis, thus increasing the risk of HCC.23 We examined the effect of CM-hfMSCs on NF-κB signaling in HCC cells. In HCC cells treated with CM-S8, a reduced level of the activated NF-κB p65 subunit which correlated with reduced phosphorylation of IκB, was observed compared to the controls, suggesting that NF-κB was bound to IκB in the inactive form (Figure 4d). The expression level of IKK α and IKK β was also downregulated in the CM-S8-treated cells. These results therefore suggested that the tumor suppressive effect derived from CM-hfMSCs was modulated by elevated IGFBPs expression and the corresponding downregulation of the activated IGF-1R/PI3K/Akt and NF-κB signaling cascades.
The tumor suppressive effect of hfMSCs could be abolished by targeting knockdown of IGF-1R in HCC
The involvement of IGF-1R signaling in hfMSCs-mediated tumor suppression was further confirmed by the knockdown of IGF-1R in HCC cells. In IGF-1R-RNAi-treated HCC cells, the addition of CM-S8 did not exert an inhibitory effect compared to CM-Ctrl (Figure 4e). By contrast, naive cells or cells treated with either siCTRL or siIGF-1R consistently exhibited enhanced proliferation in the presence of CM-MRC5 (Figure 4e). Similarly, the inhibitory effect of CM-hfMSCs could be rescued by increasing the concentration of monoclonal antibodies against IGF-1R (Figure 4f). There was no significant difference observed in cells treated with CM-Ctrl or CM-MRC5. For both the IGF-1R-RNAi knockdown and IGF-1sR antibody treatment experiments, naive cells had been included as additional controls and the results obtained were similar to cells transfected with siCTRL (Figure 4e) or IgG (Figure 4f). Taken together, these results demonstrated that IGF-1R can be a critical pathway through which hfMSCs exerted its anti-HCC effect.
CM-hfMSCs suppressed growth of patient-derived cancer stem cells (CSC)
Emerging evidence indicates that IGF signaling is a central player in the induction and maintenance of epithelial mesenchymal transition and cell stemness which are processes involved in metastatic spread and resistance to cancer treatments.24 In head and neck squamous cell carcinoma (HNSCC), patient-derived tumor cells expressing high levels of aldehyde dehydrogenase (denoted as ALDhigh) have recently been shown to exhibit elevated IGF-1R phosphorylation.25 Aldehyde dehydrogenase (ALD) expression has been suggested to be a functional marker for HNSCC cancer stem cells.26 Thus, we investigated whether the antiproliferative activities of CM-hfMSCs could be extended to other cancer cells, particularly cells that display stem-like characteristics through the IGF-1R signaling pathway. As shown in Figure 5a, compared to CM-Ctrl, CM-hfMSCs treatment reduced the percentage of RPMI2650 tumor cells by 25%, similar to those observed in HCC tumor cells. CM-hfMSCs treatment on the various clones of ALD+ HNSCC cells demonstrated that the viability of these cells could similarly be reduced by ~20–30% (Figure 5b) with a concomitant decrease in the percentage of ALD+ cells (Figure 5c). Further analysis by immunoblotting showed a marked reduction of activated IGF-1R in SF-CM-hfMSCs-treated cells compared to SF-CM-MRC5-treated cells (Figure 5d), further confirming the role of IGF-1R signaling in CM-hfMSCs-mediated tumor suppression.
Figure 5.
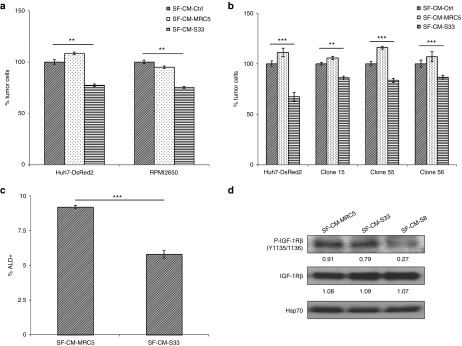
CM-hfMSCs inhibits aldehyde dehydrogenase-positive head and neck squamous cell carcinoma (HNSCC) cell proliferation which corresponded to reduced insulin-like growth factor (IGF)-1R expression levels. (a) The effect of SF-CM-hfMSCs on RPMI2650 HNSCC cells was evaluated by cell counting kit (CCK)-8 assay following 48 hours treatment. Data shown are normalized to SF-CM-Ctrl-treated cells and presented as means ± standard error of the mean (SEM), n = 3. (b) Different clones of HNSCC-CSC population derived from local head and neck cancer patient were subjected to SF-CMs treatment for 48 hours. Huh7-DsRed2 cells were included as positive reference to the activity of the various CMs. The effect was evaluated by CCK-8 assay. Data shown are normalized to respective SF-CM-Ctrl-treated cells and presented as means ± SEM, n = 4. (c) ALD-level of representative clone 56 was assessed following treatment with SF-CM-MRC5 and SF-CM-S33 by flow cytometry analysis. Data shown are means ± SEM, n = 3. (d) Endogenous protein expression of IGF-1R in SF-CMs-treated-HNSCC cells was examined using immunoblot analysis. **P < 0.01; ***P < 0.001.
Discussion
The IGF system comprises of circulating ligands such as IGF-1, IGF-2 and insulin, IGF binding proteins (IGFBP1-6) and their ligand binding type-I and type-II receptors.27 The IGFBPs function as transport proteins for IGF-1 and IGF-2 from the circulation to the peripheral tissues; thus, a reservoir of IGFs in the circulation can be maintained. IGFBPs also regulate the binding of IGFs to IGF-1 receptor, a transmembrane tyrosine kinase receptor that has high affinity for IGF-1 and -2, and is necessary for growth and metabolism in fetal development and normal tissues. Allelic losses and inactivating mutations of M6P/IGF2R,28 overexpressed levels of IGF-2 (ref. 29) and IGF-1R,22,30 and reduced levels of IGFBPs31,32 have all been reported in HCC.
Previously, we demonstrated that adult human bone marrow-derived MSCs could inhibit proliferation of human glioma cells through impaired endothelial progenitor cell recruitment and downregulation of proangiogenic factors, including platelet derived growth factor (PDGF)-BB, interleukin (IL)-1β, and IGF-1.17 In this study, we focused on characterizing the antitumor effect mediated by human fetal bone marrow–derived MSCs (hfMSCs) because in comparison to adult MSCs, these cells are more proliferative, easily expandable, and are less exposed to inflammatory and infectious microenvironment which will be of clinical relevance.33 Our findings are in agreement with Zhao et al.34 in that CM derived from adipose-derived MSCs could inhibit the proliferation of HCC in vitro. We did not observe significant level of cell death mediated by CM, similar to report by Giuffrida et al.35 where CM derived from human embryonic stem cells exerted a suppressive effect on proliferation but not cell death in ovarian, prostate, and breast adenocarcinoma. Having said this, the level of cell death seemed to be higher in a direct coculture system consisting of HCC and hfMSCs (Figure 1b). In a recent report, MSCs derived from Wharton jelly of the umbilical cord was shown to induce apoptosis in prostate cancer cells in coculture condition, via activation of JNK and downregulation of PI3K/Akt signaling.36 Even though in our studies, most of the suppressive effect of CM-hfMSCs on tumor cells is exerted through an inhibition on tumor cell proliferation (Figures 3 and 4c,d; Supplementary Figure S1a), the lack of significant apoptotic event may be attributed to suboptimal preparation of CM-hfMSCs. Nevertheless, findings from our studies have provided support that the suppressive effect of hfMSCs on tumor cell proliferation can occur through direct cell–cell contacts or in a paracrine fashion without direct cell contact in vitro and in vivo.
It is also worth noting that there are studies that reported the protumorigenic properties of CM-MSCs on HCC. In the study by Gong et al.,37 CM-MSCs harvested from the femur bone marrow of adult female Sprague-Dawley rats promoted the proliferation of HepG-2 cells. In another study, where the MSCs were derived from human HCC biopsy tissues, enhancement of Huh7 colony formation was observed in the presence of CM derived from these MSCs.38 The observed differences may be attributed to inherent biological differences in the source of MSCs, the heterogeneity of MSCs, the interaction of MSCs and its secreted factors with the surrounding microenvironment and other parameters, such as the dose and time of MSCs administration/analysis, as well as the optimal culture condition.39 Mamede et al.40 have demonstrated that the same amount of protein extracts derived from the human amniotic membrane exerted different extent of anti- or promitogenic effects on different tumor cells, suggesting that the effect of hfMSCs may be dependent on the type of dominating receptors that are expressed on specific tumor cells. We have independently observed that the extent of antiproliferative effect of CM-hfMSCs varied with different donors, suggesting that the intrinsic biological differences within the cells also contribute to the progression of tumorigenesis. We postulated that this is indeed the case since many growth factors are known to activate cell growth, motility, and survival pathways through binding to cell surface tyrosine kinase-bearing receptors. Interestingly, the results showed that the activation of IGF-1R was significantly reduced in HCC treated with CM-hfMSCs when compared to controls.
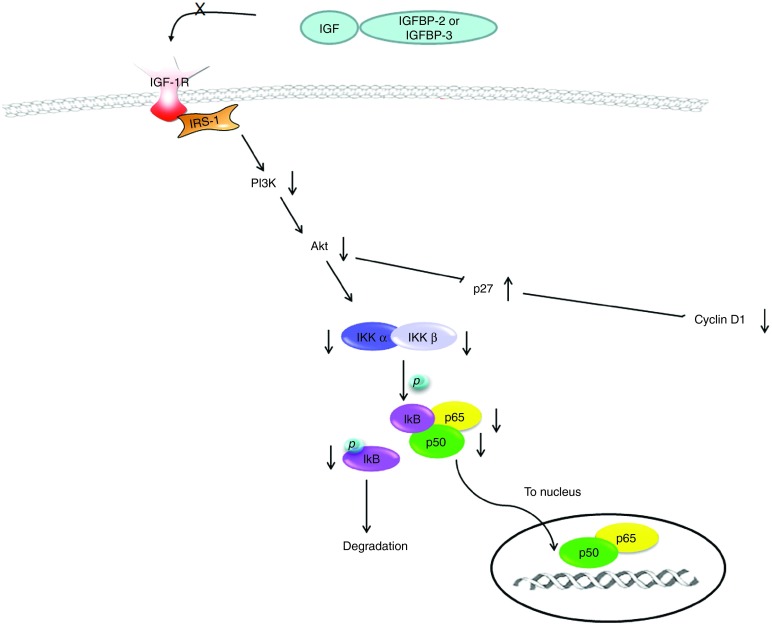
IGF-1R is highly activated in HCC tumors and the activation of IGF-1R, but not insulin receptor, is associated with the activation of ribosomal protein S6 (RPS6) and an increase in IGF-2 level,22 suggesting that IGF-2 may be responsible for IGF-1R activation. Subsequent signaling is transduced mainly through PI3K/Akt/mTOR pathway, which mediates cell survival and the RAS/RAF/MAP kinases that predominates cell proliferation.41 In our study, the results showed that the phosphorylation of IGF-1R was reduced in HCC treated with CM-hfMSCs compared to controls (Figures 4b and 5d). The expression levels of p85 subunit of PI3K and activated Akt were also reduced (Figure 4c) which could affect the activity of a number of downstream transcriptional regulators including NF-κβ via modulation of the inhibitor κB protein kinase (IKK) complex (Figure 4d). Furthermore, the results showed that p27Kip1, which acts as a negative regulator of cyclin and cdk activity,42 was significantly increased in Huh7 cells treated with CM-fhMSCs (Figure 3c). Cyclin D1 proteins, which have been associated with aggressive forms of human HCC,43 were downregulated along with various cyclins such as cyclin E, cyclin A, cyclin B1 and cell cycle regulator p27, all of which with known function in regulating cell proliferation, as illustrated in Figure 6. Overall, the repressed NF-κβ pathway through dysregulation of IGF signaling cascade impaired HCC proliferation by disrupting the cell cycle regulatory mechanisms in the cancer cells.
Figure 6.
Representation of the proposed mechanism of action for CM-hfMSCs on insulin-like growth factor (IGF)-expressing cells. Hepatocellular carcinoma (HCC) express high levels of IGF-2. IGFBPs present in CM-hfMSCs sequester IGF-expressed by HCC cells, thereby preventing IGF from binding to its receptor IGF-1R. Lack of ligand stimulation led to decreased phosphorylation of IGF-1R and downregulation of PI3K/Akt pathway. One of the downstream targets of Akt signaling, NF-κB signaling, was also downregulated. Reduced activation of Akt also led to increased accumulation of the cdk p27Kip1, resulting in cell cycle arrest.
The role of IGFBPs has been suggested as tumor suppressors, as reduced mRNA levels of IGFBP-1,-2; -3, -4, -7 have been detected in surgical specimens in human HCC tissues31,32,44,45 respectively. The findings of IGFBP-3 downregulation in clinically early HCC specimens and HCC with cirrhosis suggests that the downregulation of IGFBP-3 may contribute to initial deregulation of the IGF signaling axis in HCC.22 Downregulation of IGFBP-3 could also be mediated through promoter hypermethylation.46 In our study, all IGFBPs available in the cytokine arrays were highly elevated in CM-hfMSCs, particularly those from IGFBP-1, -2, and -3 (Figure 4b). IGFBP-7 was not provided in the array and it is therefore undetermined whether IGFBP-7 proteins are also secreted by hfMSCs at high levels. It is interesting to note that high levels of IGFBPs are produced by these undifferentiated stem cells. Igfbp-3 gene is identified as one of the top three upregulated genes in a study that compares bone marrow stromal cells with the mature bone marrow–derived osteoblasts.47 The antiproliferative effect of CM-hfMSCs may be a direct effect of proteolysis of IGFBP-3 as some of these fragments have been reported to inhibit cell proliferation.48 Recently, the C-terminal of IGFBP-4 was also demonstrated to inhibit the capillary-like tube formation of human brain endothelial cells and possess antitumor effects.49 Other possible anti-tumor mechanism of IGFBPs includes the binding of IGFBPs to IGF-1, thereby depriving the ligand's ability to bind and activate IGF-1R-dependent signaling cascade. IGF-1 could also downregulate IGF-1R by increasing the receptor ubiquitin degradation.50 The precise mode of action through which the high levels of IGFBPs exert their inhibitor effect via IGF-1R will require further investigations.
Unlike conventional IGF inhibitors that target the receptor tyrosine kinases, CM from MSCs contain a cocktail of various factors that confers protection to injured tissues,14 and yet, could induce tumor cell death as presented in the current study and by others.16,34,35,36 Furthermore, the antiproliferative effect could be enhanced in combination with molecular targeting agents, such as sorafenib and sunitinib, to overcome drug resistance. To address the potential use of MSCs in cancer therapy will require rigorous studies to delineate the various factors produced under specific disease condition.
In conclusion, the uniqueness of our study is that we have demonstrated the anti-proliferative activities of CM-hfMSCs using patient-derived orthotopic HCC mouse model rather than the implantation of HCC cells subcutaneously, and with primary fresh cultures derived from HCC and its corresponding matched normal tissues as control. This observation is of critical importance for future clinical application. Furthermore, we showed that human fetal MSCs secreted high levels of IGFBPs which sequestered free IGFs, thereby inhibited HCC progression. The inhibitory effect of IGFBPs on IGF signaling is evident from the decreased level of activated IGF-1R and PI3K/Akt, and affected HCC proliferation. The results suggested that the administration of CM-hfMSCs may provide an alternative/adjuvant treatment for HCC and potentiate the effects of chemotherapeutic drugs.
Materials and Methods
Refer to Supplementary Information for detailed information.
Cell culture and ethics. Fetal tissue collection was approved by the Domain Specific Review Board of National University Hospital, Singapore in compliance with international guidelines regarding the use of fetal tissue for research. Human fetal MSCs (hfMSCs) S8, S27, S33, and S64 were isolated from the fetal femur collected after clinically indicated termination of pregnancy as previously described.33 In this study, Huh7-DsRed2 and Huh7 parental cells is denoted as HCC cells. The use of patient-derived HCC, denoted as primary HCC cells, its adjacent match normal liver tissues, and patient-derived HNSCC were approved by SingHealth Central Institutional Review Board, Singapore (Refer Supplementary Information). Additional information regarding culturing of cells can be found under Supplementary Information.
Collection of CM and tumor viability assay. For collection of CM-hfMSCs and CM-MRC5, cells were grown in complete DMEM media; CM-Ctrl was also incubated in parallel. CM and CM-Ctrl were collected after 48 hours and centrifuged at 3,000 rpm for 5 minutes at 4 °C to ensure complete removal of cellular debris. CM were then concentrated 30-fold using Vivaspin-20 centrifugal concentrators (Sartorium Stedim UK, Epsom, UK).
To determine the effect of CM, HCC cells were incubated with CMs for 48 hours and the percentage of viable cells was determined by cell counting kit (CCK)-8 assay (Dojindo Laboratories, Kumamoto, Japan) at optical density 450 nm using the Victor spectrophotometer (PerkinElmer Life Sciences, Waltham, MA).
To check the effect of IGF-receptor knockdown, HCC cells were first tranfected with siRNA for 48 hours, followed by treatment with CM-hfMSCs for additional 48 hours.
For combination treatment with sorafenib or sunitinib, HCC cells were first treated with CM-hfMSCs for 48 hours followed by 24 hours incubation with either sunitinib (9 μmol/l in dimethyl sulfoxide; Pfizer, New York, NY) or sorafenib (10 μmol/l; Bayer Pharmaceuticals, West Haven, CT).
For investigating the effect of IGF-1R neutralization, HCC cells were preincubated with 0.1 and 1 μg/ml of neutralization antibody against IGF-1R (Sigma-Aldrich, Saint Louis, MO) for 3 hours prior to the addition of CM-hfMSCs.
Animal work. This study was approved by SingHealth Central Institutional Review Board, Singapore and was performed according to the guidelines and protocols approved by the Institutional Animal Care and Use Committee at the Singapore General Hospital.
To evaluate the antitumor effect of CM-hfMSCs in vivo, CMs (3,000 µg/10 µl) was administered on alternate days into orthotopic pre-established patient-derived HCC tumors on immunodeficient mice. Tumor volumes were recorded every 2 days. At the end of the treatment period, livers were harvested and cryopreserved.
Immunohistochemistry. The following antibodies were used: mouse anti-human Ki-67 (DakoCytomation, Glostrup, Denmark), rabbit anti-human cyclin D1 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-human nuclei (Merck Millipore, Billerica, MA), mouse anti-BrdU (Becton Dickinson, Franklin Lake, NJ). Refer Supplementary Information for detailed information.
Immunoblotting. The following primary antibodies were used: cyclin D1, cyclin A, cyclin E, cyclin B1 (Santa Cruz Biotechnology), p27, P-IκB-α, IκB-α, P-NF-κB p65, NF-κB p65, IKK α, IKK β, P-PI3K p55/p85, PI3K, P-Akt (T308), P-Akt (S473), Akt, P-IGF-1Rβ (Y1135/1136), IGF-1Rβ, caspase-3, Hsp70 (Cell Signaling Technology), and Actin (NeoMarkers, Fremont, CA). Refer Supplementary Information for detailed information.
Cytokine array analysis. RayBio Quantikine Human IGF Signaling Array I (RayBiotech, Norcross, GA) that contains antibodies targeting to 10 proteins of the IGF family was used. Eighteen micrograms of SF-CMs were used according to manufacturer's instructions. Signals were visualized through Axon GenePix 4000B laser scanner equipped with Cy3 wavelength and quantified using Gene Pix data analysis software (Molecular Devices, Sunnyvale, CA).
Statistical analysis. Statistical analyses were performed using Graphpad Prism 3.0 (Graphpad Software, San Diego, CA). Comparison between more than two treatment groups was made using one-way analysis of variance with the Tukey post hoc test. All reported P values are two-sides, nonpaired and values of < 0.05 are considered as statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Tumor suppressive effect was also observed in CM-hfMSCs derived from other donors. Figure S2. CM-hfMSCs did not induce apoptosis of HCC cells. Figure S3. Tumor suppressive effect was retained in serum free CM-hfMSCs.
Acknowledgments
We would like to thank Alan TL Lam (Bioprocessing Technology Institute, Singapore), Caroline Lee and Edita Aliwarga (National Cancer Centre), Patrizia Danielli (University of Pavia Italy), for providing technical assistance. Special thanks to Mac Ho (National Cancer Centre) for useful discussion. This project is funded by grants from the National Medical Research Council (1201/2009) and the National Cancer Centre Research Foundation. Jerry KY Chan received salary support from the National Medical Research Council (CSA/012/2009 and CSA/043/2012). The authors declare no potential conflicts of interest.
Supplementary Material
References
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- Cabibbo G, Rolle E, De Giorgio M, Genco C, Pressiani T, Spada F, et al. HCC Working Group Management of cirrhotic patients with hepatocellular carcinoma treated with sorafenib. Expert Rev Anticancer Ther. 2011;11:1807–1816. doi: 10.1586/era.11.139. [DOI] [PubMed] [Google Scholar]
- Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- Sun B, Roh KH, Park JR, Lee SR, Park SB, Jung JW, et al. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 2009;11:289–98, 1 p following 298. doi: 10.1080/14653240902807026. [DOI] [PubMed] [Google Scholar]
- Gao Y, Yao A, Zhang W, Lu S, Yu Y, Deng L, et al. Human mesenchymal stem cells overexpressing pigment epithelium-derived factor inhibit hepatocellular carcinoma in nude mice. Oncogene. 2010;29:2784–2794. doi: 10.1038/onc.2010.38. [DOI] [PubMed] [Google Scholar]
- Yulyana Y, Endaya BB, Ng WH, Guo CM, Hui KM, Lam PY, et al. Carbenoxolone enhances TRAIL-induced apoptosis through the upregulation of death receptor 5 and inhibition of gap junction intercellular communication in human glioma. Stem Cells Dev. 2013;22:1870–1882. doi: 10.1089/scd.2012.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Xie DY, Lin BL, Zhang GL, Wang PP, Peng L, et al. Interferon-β gene-modified human bone marrow mesenchymal stem cells attenuate hepatocellular carcinoma through inhibiting AKT/FOXO3a pathway. Br J Cancer. 2013;109:1198–1205. doi: 10.1038/bjc.2013.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong HT, Federspiel MJ, Guo CM, Ooi LL, Russell SJ, Peng KW, et al. Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. J Hepatol. 2013;59:999–1006. doi: 10.1016/j.jhep.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F., 3rd Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth. Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapova IA, Gaudette GR, Brink PR, Robinson RB, Rosen MR, Cohen IS, et al. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells. 2007;25:1761–1768. doi: 10.1634/stemcells.2007-0022. [DOI] [PubMed] [Google Scholar]
- Watson SL, Marcal H, Sarris M, Di Girolamo N, Coroneo MT, Wakefield D. The effect of mesenchymal stem cell conditioned media on corneal stromal fibroblast wound healing activities. Br J Ophthalmol. 2010;94:1067–1073. doi: 10.1136/bjo.2009.165837. [DOI] [PubMed] [Google Scholar]
- Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitraruch S, Mitry RR, Dhawan A. Intraportal transplantation of human bone marrow mesenchymal stem cells in pigs with fulminant hepatic failure. Hepatology. 2013;57:2088. doi: 10.1002/hep.25971. [DOI] [PubMed] [Google Scholar]
- Gauthaman K, Yee FC, Cheyyatraivendran S, Biswas A, Choolani M, Bongso A. Human umbilical cord Wharton's jelly stem cell (hWJSC) extracts inhibit cancer cell growth in vitro. J Cell Biochem. 2012;113:2027–2039. doi: 10.1002/jcb.24073. [DOI] [PubMed] [Google Scholar]
- Ho IA, Toh HC, Ng WH, Teo YL, Guo CM, Hui KM, et al. Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells. 2013;31:146–155. doi: 10.1002/stem.1247. [DOI] [PubMed] [Google Scholar]
- Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Colombino M, Sperlongano P, Izzo F, Tatangelo F, Botti G, Lombardi A, et al. BRAF and PIK3CA genes are somatically mutated in hepatocellular carcinoma among patients from South Italy. Cell Death Dis. 2012;3:e259. doi: 10.1038/cddis.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuhahn K, Schirmacher P. Reactivation of the insulin-like growth factor-II signaling pathway in human hepatocellular carcinoma. World J Gastroenterol. 2008;14:1690–1698. doi: 10.3748/wjg.14.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- Tovar V, Alsinet C, Villanueva A, Hoshida Y, Chiang DY, Solé M, et al. IGF activation in a molecular subclass of hepatocellular carcinoma and pre-clinical efficacy of IGF-1R blockage. J Hepatol. 2010;52:550–559. doi: 10.1016/j.jhep.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Karin M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene. 2008;27:6228–6244. doi: 10.1038/onc.2008.300. [DOI] [PubMed] [Google Scholar]
- Malaguarnera R, Belfiore A. The emerging role of insulin and insulin-like growth factor signaling in cancer stem cells. Front Endocrinol (Lausanne) 2014;5:10. doi: 10.3389/fendo.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong HS, Chong FT, Sew PH, Lau DP, Wong BH, Teh BT, et al. Targeting cancer stem cell plasticity through modulation of epidermal growth factor and insulin-like growth factor receptor signaling in head and neck squamous cell cancer. Stem Cells Transl Med. 2014;3:1055–1065. doi: 10.5966/sctm.2013-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, et al. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32:1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulstone E, Prince S, Zaccheo O, Burns JL, Harper J, Jacobs C, et al. Insulin-like growth factor ligands, receptors, and binding proteins in cancer. J Pathol. 2005;205:145–153. doi: 10.1002/path.1712. [DOI] [PubMed] [Google Scholar]
- Oka Y, Waterland RA, Killian JK, Nolan CM, Jang HS, Tohara K, et al. M6P/IGF2R tumor suppressor gene mutated in hepatocellular carcinomas in Japan. Hepatology. 2002;35:1153–1163. doi: 10.1053/jhep.2002.32669. [DOI] [PubMed] [Google Scholar]
- Breuhahn K, Vreden S, Haddad R, Beckebaum S, Stippel D, Flemming P, et al. Molecular profiling of human hepatocellular carcinoma defines mutually exclusive interferon regulation and insulin-like growth factor II overexpression. Cancer Res. 2004;64:6058–6064. doi: 10.1158/0008-5472.CAN-04-0292. [DOI] [PubMed] [Google Scholar]
- Desbois-Mouthon C, Baron A, Blivet-Van Eggelpoël MJ, Fartoux L, Venot C, Bladt F, et al. Insulin-like growth factor-1 receptor inhibition induces a resistance mechanism via the epidermal growth factor receptor/HER3/AKT signaling pathway: rational basis for cotargeting insulin-like growth factor-1 receptor and epidermal growth factor receptor in hepatocellular carcinoma. Clin Cancer Res. 2009;15:5445–5456. doi: 10.1158/1078-0432.CCR-08-2980. [DOI] [PubMed] [Google Scholar]
- Huynh H, Chow PK, Ooi LL, Soo KC. A possible role for insulin-like growth factor-binding protein-3 autocrine/paracrine loops in controlling hepatocellular carcinoma cell proliferation. Cell Growth Differ. 2002;13:115–122. [PubMed] [Google Scholar]
- Gong Y, Cui L, Minuk GY. The expression of insulin-like growth factor binding proteins in human hepatocellular carcinoma. Mol Cell Biochem. 2000;207:101–104. doi: 10.1023/a:1007010818094. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Teoh SH, Chong MS, Lee ES, Tan LG, Mattar CN, et al. Neo-vascularization and bone formation mediated by fetal mesenchymal stem cell tissue-engineered bone grafts in critical-size femoral defects. Biomaterials. 2010;31:608–620. doi: 10.1016/j.biomaterials.2009.09.078. [DOI] [PubMed] [Google Scholar]
- Zhao W, Ren G, Zhang L, Zhang Z, Liu J, Kuang P, et al. Efficacy of mesenchymal stem cells derived from human adipose tissue in inhibition of hepatocellular carcinoma cells in vitro. Cancer Biother Radiopharm. 2012;27:606–613. doi: 10.1089/cbr.2011.1150. [DOI] [PubMed] [Google Scholar]
- Giuffrida D, Rogers IM, Nagy A, Calogero AE, Brown TJ, Casper RF. Human embryonic stem cells secrete soluble factors that inhibit cancer cell growth. Cell Prolif. 2009;42:788–798. doi: 10.1111/j.1365-2184.2009.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han I, Yun M, Kim EO, Kim B, Jung MH, Kim SH. Umbilical cord tissue-derived mesenchymal stem cells induce apoptosis in PC-3 prostate cancer cells through activation of JNK and downregulation of PI3K/AKT signaling. Stem Cell Res Ther. 2014;5:54. doi: 10.1186/scrt443. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gong P, Wang Y, Wang Y, Jin S, Luo H, Zhang J, et al. Effect of bone marrow mesenchymal stem cells on hepatocellular carcinoma in microcirculation. Tumour Biol. 2013;34:2161–2168. doi: 10.1007/s13277-013-0749-4. [DOI] [PubMed] [Google Scholar]
- Hernanda PY, Pedroza-Gonzalez A, van der Laan LJ, Bröker ME, Hoogduijn MJ, Ijzermans JN, et al. Tumor promotion through the mesenchymal stem cell compartment in human hepatocellular carcinoma. Carcinogenesis. 2013;34:2330–2340. doi: 10.1093/carcin/bgt210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H. Stricter standards sought to curb stem-cell confusion. Nature. 2013;499:389. doi: 10.1038/499389a. [DOI] [PubMed] [Google Scholar]
- Mamede AC, Laranjo M, Carvalho MJ, Abrantes AM, Pires AS, Brito AF, et al. Effect of amniotic membrane proteins in human cancer cell lines: an exploratory study. J Membr Biol. 2014;247:357–360. doi: 10.1007/s00232-014-9642-3. [DOI] [PubMed] [Google Scholar]
- Scagliotti GV, Novello S. The role of the insulin-like growth factor signaling pathway in non-small cell lung cancer and other solid tumors. Cancer Treat Rev. 2012;38:292–302. doi: 10.1016/j.ctrv.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Nishida N, Fukuda Y, Komeda T, Kita R, Sando T, Furukawa M, et al. Amplification and overexpression of the cyclin D1 gene in aggressive human hepatocellular carcinoma. Cancer Res. 1994;54:3107–3110. [PubMed] [Google Scholar]
- Ranke MB, Maier KP, Schweizer R, Stadler B, Schleicher S, Elmlinger MW, et al. Pilot study of elevated levels of insulin-like growth factor-binding protein-2 as indicators of hepatocellular carcinoma. Horm Res. 2003;60:174–180. doi: 10.1159/000073229. [DOI] [PubMed] [Google Scholar]
- Chen D, Yoo BK, Santhekadur PK, Gredler R, Bhutia SK, Das SK, et al. Insulin-like growth factor-binding protein-7 functions as a potential tumor suppressor in hepatocellular carcinoma. Clin Cancer Res. 2011;17:6693–6701. doi: 10.1158/1078-0432.CCR-10-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T, Yumoto Y, Nouso K, Nakatsukasa H, Onishi T, Fujikawa T, et al. Reduced expression of insulin-like growth factor binding protein-3 and its promoter hypermethylation in human hepatocellular carcinoma. Cancer Lett. 2002;176:149–158. doi: 10.1016/s0304-3835(01)00736-4. [DOI] [PubMed] [Google Scholar]
- Monticone M, Liu Y, Tonachini L, Mastrogiacomo M, Parodi S, Quarto R, et al. Gene expression profile of human bone marrow stromal cells determined by restriction fragment differential display analysis. J Cell Biochem. 2004;92:733–744. doi: 10.1002/jcb.20120. [DOI] [PubMed] [Google Scholar]
- Lalou C, Lassarre C, Binoux M. A proteolytic fragment of insulin-like growth factor (IGF) binding protein-3 that fails to bind IGFs inhibits the mitogenic effects of IGF-I and insulin. Endocrinology. 1996;137:3206–3212. doi: 10.1210/endo.137.8.8754741. [DOI] [PubMed] [Google Scholar]
- Moreno MJ, Ball M, Rukhlova M, Slinn J, L'abbe D, Iqbal U, et al. IGFBP-4 anti-angiogenic and anti-tumorigenic effects are associated with anti-cathepsin B activity. Neoplasia. 2013;15:554–567. doi: 10.1593/neo.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Fang Y, Dong W, Mu X, Liu Q, Du J. IGF-1 receptor is down-regulated by sunitinib induces MDM2-dependent ubiquitination. FEBS Open Bio. 2012;2:1–5. doi: 10.1016/j.fob.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.