Abstract
Adoptive cell therapy with genetically modified T cells expressing a chimeric antigen receptor (CAR) is a promising therapy for patients with B-cell acute lymphoblastic leukemia. However, CAR-modified T cells (CAR T cells) have mostly failed in patients with solid tumors or low-grade B-cell malignancies including chronic lymphocytic leukemia with bulky lymph node involvement. Herein, we enhance the antitumor efficacy of CAR T cells through the constitutive expression of CD40 ligand (CD40L, CD154). T cells genetically modified to constitutively express CD40L (CD40L-modified T cells) demonstrated increased proliferation and secretion of proinflammatory TH1 cytokines. Further, CD40L-modified T cells augmented the immunogenicity of CD40+ tumor cells by the upregulated surface expression of costimulatory molecules (CD80 and CD86), adhesion molecules (CD54, CD58, and CD70), human leukocyte antigen (HLA) molecules (Class I and HLA-DR), and the Fas-death receptor (CD95). Additionally, CD40L-modified T cells induced maturation and secretion of the proinflammatory cytokine interleukin-12 by monocyte-derived dendritic cells. Finally, tumor-targeted CD19-specific CAR/CD40L T cells exhibited increased cytotoxicity against CD40+ tumors and extended the survival of tumor-bearing mice in a xenotransplant model of CD19+ systemic lymphoma. This preclinical data supports the clinical application of CAR T cells additionally modified to constitutively express CD40L with anticipated enhanced antitumor efficacy.
Introduction
Adoptive transfer of genetically modified tumor-specific T cells expressing a chimeric antigen receptor (CAR) is a novel therapeutic approach for cancer.1 CAR-modified T cells (CAR T cells) targeting the CD19 antigen have shown clinical benefit for some patients with B-cell malignancies.2,3,4,5 However, most patients with solid tumors or low-grade B-cell malignancies with bulky lymph node involvement have mostly failed to recapitulate these findings.1,3 Several possible limitations could explain the inability of CAR T cells to eradicate tumor cells. These include poor T-cell persistence/proliferation following adoptive transfer, inability of CAR T cells to counteract the local immunosuppressive tumor microenvironment, and/or loss of targeted antigen expression as demonstrated in a clinical case report of B-cell acute lymphoblastic leukemia.6,7
CD40 ligand (CD40L, CD154), a type II transmembrane protein belonging to the tumor necrosis factor gene superfamily, has the potential to enhance tumor-specific T-cell function. Initially identified on activated CD4+ T cells, expression of CD40L is inducible on a vast array of immune, hematopoietic, epithelial, endothelial, and smooth muscle cells.8,9 In activated T cells, CD40L is expressed within minutes, peaking within 6 hours, and then declining over the subsequent 12–24 hours.9 CD40L binds to its cognate receptor CD40 which is constitutively expressed on a variety of immune and nonimmune cells including B cells, macrophages, and dendritic cells (DCs).9 Significantly, CD40 is also expressed on several hematologic and nonhematologic malignancies including B-cell acute lymphoblastic leukemia, chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma, Hodgkin lymphoma, nasopharyngeal carcinoma, osteosarcoma, Ewing sarcoma, melanoma, breast, ovarian, and cervical carcinoma.10,11,12,13,14,15,16,17
Functionally, the CD40L/CD40 pathway mediates both humoral and cellular immunity through several mechanisms. B-cell activation/antigen presentation, immunoglobulin isotype switching, and germinal center development all rely upon the CD40L/CD40 pathway.9 DC antigen presentation, production of interleukin (IL)-12, and the generation of CD8+ T-cell immunity occur via the CD40L/CD40 pathway.18,19 T-cell proliferation, cytokine secretion, reversal of CD8+ T-cell exhaustion, and generation of memory phenotype are also mediated by the CD40L/CD40 pathway.20,21,22,23 The antitumor properties of the CD40L/CD40 pathway include direct tumor apoptosis (CD40 activation on the tumor) and licensing of DCs (via CD40) to generate an endogenous antitumor-specific T-cell response.24 Recombinant human CD40L or monoclonal agonistic antibodies to CD40 have been tested in phase 1 trials demonstrating objective tumor responses, and CLL tumor cells transduced with an adenovirus-encoding murine CD40L have been utilized as a tumor vaccine with promising clinical responses.25,26 In the latter, infusion of autologous Ad-CD40L-modified CLL in patients resulted in reduced leukemic burden, induction of leukemia-specific T cells, induction of CLL-specific antibodies (anti-ROR1 Ab), and an increase in serum cytokines (IL-12 and interferon-γ) demonstrating the capacity of CD40L expression to activate an endogenous antitumor response.26
Herein, we describe an approach to enhance CAR T cells through the constitutive expression of CD40 ligand. T cells modified to constitutively express CD40L (CD40L-modified T cells) demonstrated enhanced proliferation and secretion of proinflammatory cytokines in vitro, altered the phenotype/immunogenicity of CD40+ B-cell tumor cell lines or patient-derived CLL tumor cells, and induced DC maturation and secretion of the proinflammatory cytokine IL-12. T cells modified with a bicistronic retroviral vector encoding both a CD19-specific CAR (1928z) and the CD40L gene (1928z/CD40L T cells) exhibited enhanced in vitro cytotoxicity against a panel of CD19+ tumor cell lines and extended the survival of CD19+ tumor-bearing SCID/Beige mice when compared to mice treated with T cells expressing the CD19-targeted CAR alone. Collectively, these preclinical in vitro and in vivo data support the translation of the CAR/CD40L T cells approach to the clinical setting.
Results
Constitutive expression of CD40L by human T cells
We initially transduced T cells from healthy donors with the SFG-CD40L retroviral vector (Figure 1a). Retroviral transduction of T cells with the CD40L gene routinely resulted in ≥40% gene transfer with stable expression of CD40L in both CD4+ and CD8+ T-cell subsets (Figure 1b, Supplementary Figure S1). Proliferation of CD40L-modified T cells was significantly increased over the first 3 weeks of culture compared to mock-transduced T cells generated from the same three donors (Figure 1c, Supplementary Figure S2). Tissue culture media from CD40L-modified T cells was analyzed and shown to have significantly increased soluble CD40L (sCD40L) as expected, as well as significantly increased secretion of the proinflammatory cytokines interferon-γ and granulocyte–monocyte colony-stimulating factor when compared to the mock-transduced T cells (Figure 1d). Further characterization of CD40L-modified T cells demonstrated downregulation of PD1 expression without alteration of PDL1 or CD80 expression when compared to mock-tranduced T cells (Supplementary Figure S3).
Figure 1.
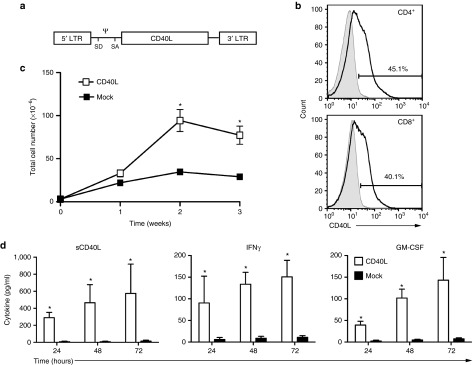
Constitutive expression of CD40L by human T cells. (a) Schematic of retroviral construct encoding human CD40L vector; LTR, long terminal repeat; SD, SA, splice donor and acceptor; Ψ, packaging element. (b) Flow cytometry of CD4+ and CD8+ CD40L-modified T cells following retroviral gene transfer; x-axis, APC-conjugated antihuman CD40L (CD154). Results shown are representative of at least three independent experiments. (c) Enhanced proliferation of CD40L-modified T cells compared to mock-transduced T cells. (d) Enhanced secretion of soluble CD40L (sCD40L), IFN-γ, and GM-CSF of CD40L-modified T cells compared to mock-transduced T cells. Results for proliferation and cytokine secretion are combined from three independent experiments. (*Denotes statistical significance where P < 0.001 using Mann–Whitney test). GM-CSF, granulocyte–monocyte colony-stimulating factor; IFN-γ, interferon-γ.
CD40L-modified T cells alter the phenotype of both CD40+ tumor cell lines and patient-derived CLL cells
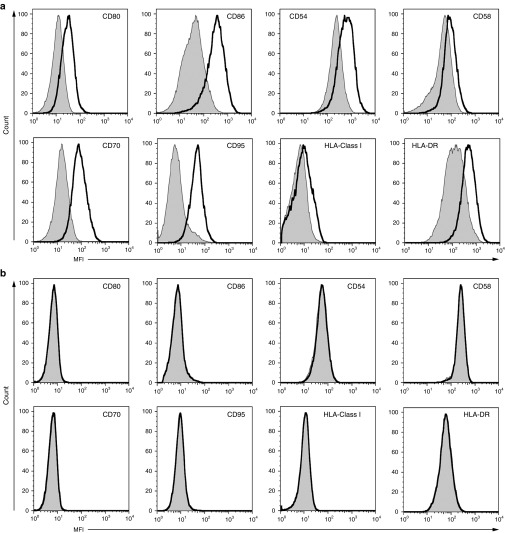
To investigate the ability of the CD40L/CD40 pathway to modify the phenotype of tumor cells, a coculture of CD40+ B-cell tumor cells and CD40L-modified T cells or mock-transduced T cells was performed. Cultures with CD40L-modified T cells, but not mock-transduced T cells, led to the upregulated expression of costimulatory molecules (CD80 and CD86), adhesion molecules (CD54, CD58, and CD70), HLA molecules (HLA Class I and HLA-DR), and the Fas-death receptor (CD95) on the surface of DOHH2 tumor cells (Figure 2a). Phenotypic changes were also evident when tumor cells were cultured in conditioned media (CD40L-modified T-cell media) containing elevated levels of sCD40L (Supplementary Figure S4). To determine if CD40 expression on the tumor cell is a requisite to alter tumor cell phenotype, coculture of the CD40− tumor cell line (NALM6) with CD40L-modified T cells and mock-transduced T cells was performed. These studies resulted in no alteration in the phenotype demonstrating the need for CD40 expression by the tumor to induce CD40L-mediated changes in tumor cell phenotype (Figure 2b).
Figure 2.
Augmented immunogenicity of CD40+ tumor cells by CD40L-modified T cells. (a) Flow cytometry showing upregulation of costimulatory molecules (CD80 and CD86), adhesion molecules (CD54, CD58, and CD70), HLA molecules (HLA Class I and HLA-DR), and the Fas-death receptor (CD95) on DOHH2 tumor cell line following coculture with CD40L-modified T cells (solid line) compared to culture with mock-transduced T cells from the same donor (gray line). (b) CD40− tumor (NALM6 shown) demonstrating no phenotypic changes following coculture with CD40L-modified T cells. All results are representative of at least three separate experiments. MFI, mean fluorescence intensity.
To further verify if this effect is clinically relevant, we cocultured CD40L-modified T cells derived from three separate CLL patients with autologous CLL tumor cells. Retroviral transduction of CLL patient-derived T cells routinely resulted in ≥40% gene transfer with stable expression of the CD40L gene (Figure 3a). In this setting, patient-derived CD40L-modified T cells, but not mock-transduced T cells, demonstrated the capacity to similarly upregulate costimulatory molecules, adhesion molecules, HLA molecules, and the Fas-death receptor on the surface of the autologous CLL cells (Figure 3b). Further characterization of CD40L-modified T cells in this culture demonstrated increased expression of IL-21R (Supplementary Figure S5) compared to mock-transduced T cells. Similar patterns of IL-21R expression on the B-cell CLL tumor cells and IL-21 within the supernate of these cocultures were noted.
Figure 3.
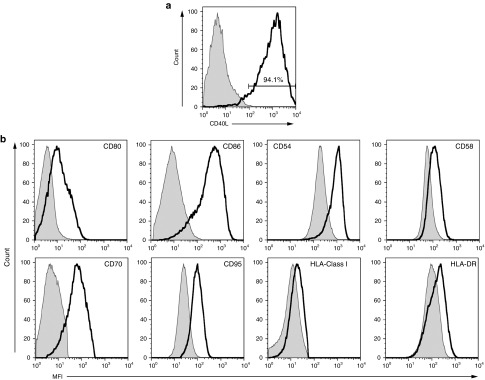
Augmented immunogenicity of CLL cells by autologous CD40L-modified T cells. (a) Flow cytometry of patient-derived CD40L-modified T cells following retroviral gene transfer with CD40L containing retroviral vector; x-axis, APC-conjugated antihuman CD40L (CD154). (b) Flow cytometry showing upregulation of costimulatory molecules (CD80 and CD86), adhesion molecules (CD54, CD58, and CD70), HLA molecules (HLA Class I and HLA-DR), and the Fas-death receptor (CD95) on CLL cells after coculturing with autologous CD40L-modified T cells (solid line) compared to cocultures with mock-transduced T cells from the same donor (gray line). All results are representative of at least three experiments using three separate donors. CLL, chronic lymphocytic leukemia; MFI, mean fluorescence intensity.
CD40L-modified T cells induce IL-12p70 secretion and mediate maturation of moDCs
Given the role of CD40L in DC maturation and secretion of the proinflammatory cytokine IL-12, we next investigated if CD40L-modified T cells could induce the same effect when cocultured with autologous monocyte-derived DCs (moDCs). Significantly, we found CD40L-modified T cell induced secretion of IL-12p70 in cocultures containing moDCs and autologous CD40L-modified T cells from three separate donors (Figure 4a). Range of CD40L-modified T cells transduction is provided in Supplementary Table S1. Maturation of moDCs, as determined by the surface expression of costimulatory molecules (HLA-DR, CD86, and CD83), was also demonstrated following coculture with CD40L-modified T cells but not following coculture with mock-transduced T cells (Figure 4b).
Figure 4.
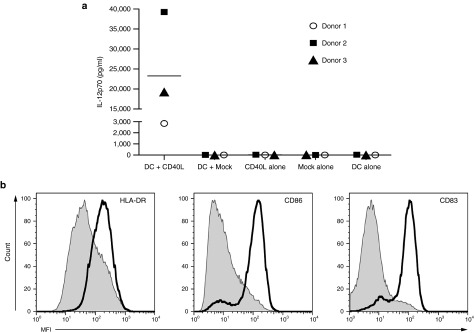
Secretion of IL-12 and maturation of monocyte-derived dendritic cells (moDCs) by CD40L-modified T cells. (a) Cytokine analysis of culture media for cocultures (24 hours) between moDCs and CD40L-modified T cells from three separate donors demonstrating elevated IL-12p70 secretion. (b) Flow cytometry of moDCs demonstrating maturation following coculture with CD40L-modified T cells (solid line). All results are representative of at least three separate experiments. IL-12, interleukin-12.
Expression of both CAR and CD40L by T cells results in enhanced in vitro cytotoxicity
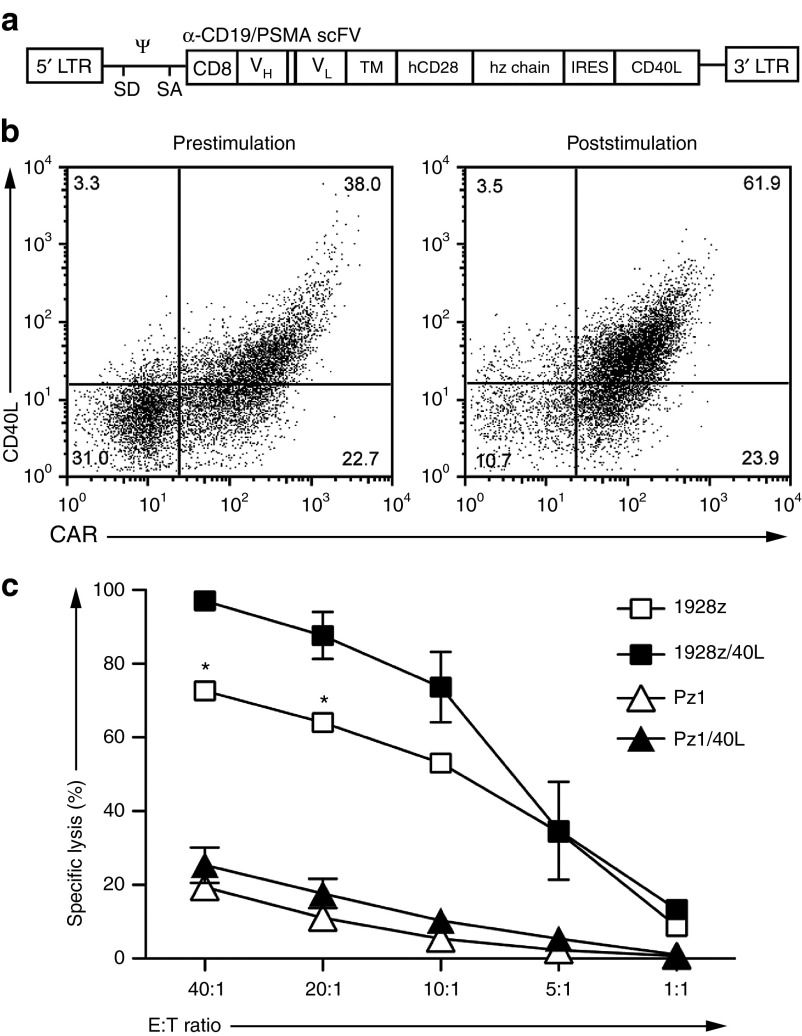
We next assessed the ability of T cells to express both the anti-CD19 CAR (1928z) and CD40L using a bicistronic retroviral vector (1928z/CD40L) (Figure 5a). Transduction of T cells routinely resulted in ≥40% expression of both 1928z and CD40L on 1928z/CD40L T cells (Figure 5b). Control retroviral vectors were also generated including the anti-CD19 CAR (1928z) and anti-PSMA CAR (Pz1 and Pz1/CD40L) (Figure 5b). Proliferation of 1928z T cells and 1928z/CD40L T cells following stimulation on artificial antigen-presenting cells (AAPCs) was measured and found to be similar. We speculate the lack of enhanced expansion of 1928z/40L CAR T cells compared to 1928z CAR T cells may be due in part to the overriding strength of the CD28 costimulatory signals in the second generation CAR construct. To test this we utilized our previously published first generation CAR (19z1) and generated a 19z1/40L vector.27 Following stimulation on AAPCs we noted enhanced proliferation of 19z1/CD40L T cells compared to 19z1 T cells alone (Supplementary Figure S7). To assess in vitro antitumor activity of 1928z/CD40L T cells, a standard 4-hour 51Cr release assay was performed. Constitutive expression of CD40L statistically enhanced the lytic capacity of 1928z T cells against CD19+ tumor cells when compared to a panel of control T cells including T cells modified to express the 1928z CAR alone (Figure 5c, Supplementary Figure S6). Furthermore, 1928z/40L T cells induced secretion of IL-12p70 in cocultures containing autologous moDCs (Supplementary Figure S8).
Figure 5.
Efficient transduction of human T cells with a CAR/CD40L vector demonstrates enhanced cytotoxicity. (a) Schematic of retroviral construct containing 1928z-IRES-CD40L and Pz1-IRES-CD40L genes; LTR, long terminal repeat; SD, SA, splice donor and acceptor; Ψ, packaging element; CD8 indicates CD8 leader sequence; scFv, single chain variable fragment; VH and VL, variable heavy and light chains; TM, transmembrane domain. (b) FACS analysis of human T cells transduced to express 19-28z/CD40L vector (prestimulation) with subsequent enhanced expression of CAR/CD40L following coculture on AAPCs (NIH 3T3 fibroblasts expressing CD19 and CD80; 1928z/CD40LT cells shown) used for in vivo experiments. x-axis, PE-conjugated 1928z CAR-specific antibody (19e3); y-axis, APC-conjugated antihuman CD40L (CD154). Results are representative of at least three independent experiments. (c) As determined by standard 51Cr release assay 19-28z/40L T cells have significant increased ability to lyse DOHH2 tumor cells compared to 19-28z T cells. Results of cytotoxicity assay are combined from three independent experiments and effectors have not undergone prestimulation on AAPCs. (*Denotes statistical significance where P < 0.05 using Mann–Whitney test). AAPC, artificial antigen-presenting cell; FACS, fluorescence-activated cell sorting.
Expression of 1928z/40L by T cells results in enhanced in vivo cytotoxicity in a systemic model of follicular lymphoma
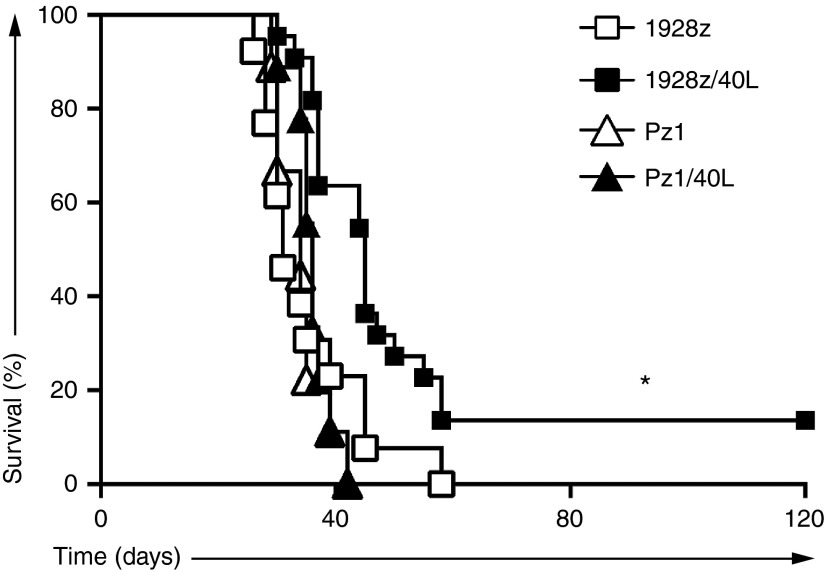
To investigate the in vivo antitumor activity of 1928z/CD40L T cells, we utilized a xenotransplant model of systemic DOHH2 lymphoma. We have previously observed that systemic DOHH2 tumor cells are markedly refractory to CD19-targeted CAR T-cell therapy in SCID/Beige mice (unpublished data, Figure 6). To assess whether further modification of CAR T cells to constitutively express CD40L could enhance the antitumor efficacy in this model, we inoculated and treated SCID/Beige mice bearing systemic DOHH2 tumor with CAR/CD40L T cells. Significantly, treatment with 1928z/CD40L T cells compared to treatment with 1928z T cells or control T cells (Pz1 and Pz1/CD40L T cells) demonstrated enhanced survival and resulted in long-term survival in mice treated with 1928z/40L T cells (Figure 6).
Figure 6.
Tumor eradication and long-term survival following 1928z/CD40L T-cell infusion. Survival curve of SCID-Beige mice inoculated with DOHH2 tumor cells by intravenous (i.v.) injection 2 days before a single i.v. dose of CAR-modified T cells. Enhanced long-term survival was demonstrated in mice treated with 1928z/CD40L T cells (n = 22) as compared to a panel of control T cells (1928z group n = 13; Pz1 and Pz1/40L group n = 9). Results are combined from two independent experiments. (*Denotes statistical significance between the 1928z/40L group versus 1928z group where P < 0.005). CAR, chimeric antigen receptor.
Discussion
Adoptive therapy utilizing CAR T cells has shown promising clinical responses in patients with B-cell malignancies.2,3,4,5 However, CAR T cells have limited success against most other tumors most specifically solid tumors or low-grade B-cell malignancies.1,3 In their current form, CAR T cells have not demonstrated the ability to overcome the immunosuppressive tumor microenvironment or respond to tumor escape following target antigen loss.6,7 One possible method for CAR T cells to overcome these limitations is through the recruitment of an endogenous antitumor immune response. Constitutive expression of CD40L may facilitate this by improving CAR T-cell cytolytic capacity/proliferation, augment tumor immunogenicity, and improve DC antigen presentation/function.
To assess the effect of constitutive expression of CD40L in T cells we developed a retroviral vector containing the CD40L gene. When transduced in T cells both CD4+ and CD8+ T-cell subsets demonstrated constitutive expression of CD40L (Figure 1b). While more commonly associated with CD4+ T cells, CD40L expression and function (development of memory CD8+ T cells) have recently been reported.28 CD40L expression is also known to enhance T-cell proliferation and secretion of proinflammatory TH1 cytokines (interferon-γ, granulocyte–monocyte colony-stimulating factor).20,21 As expected, CD40L-modified T cells secrete proinflammatory cytokines and demonstrate enhanced proliferation when compared to similarly activated but mock-transduced T cells from the same donor. Expansion, persistence, and generation of homeostatic cytokines have been correlated with successful antitumor results in patients with B-cell malignancies treated with CAR T cells.29 To this end, arming T cells through the constitutive expression of CD40L has at least the potential to enhance CAR T-cell antitumor function. We also noted CD40L-modified T cells have decreased expression of PD1 which has been noted to be increased on the surface of CD19-specific CAR T cells following adoptive transfer in patients with relapsed/refractory non-Hodgkin lymphoma possibly contributing to the limited persistence of T cell in this study.30 Furthermore, CAR T cells have demonstrated enhanced function through an antigen-independent auto-/trans-costimulation following expression of 4-1BBL and CD80 which could explain the effects seen following constitutive expression of CD40L by T cells.31
Avoiding immunosurveillance through the downregulation of cell surface proteins (HLA Class I, costimulatory molecules, and/or adhesion molecules) is often employed by tumors to avoid elimination.6,32,33 Apoptotic resistance can also occur with the loss of the Fas-death receptor on the surface of malignant cells.34 To counteract this, CD40L mediates the upregulation of costimulatory molecules (CD80 and CD86), adhesion molecules (CD54, CD58, and CD70), HLA molecules (HLA Class I and HLA-DR) and facilitate apoptosis through the Fas/FasL pathway on malignant B-cell tumors.35,36 CD40L-modified T cells predictably modified the phenotype of CD40+ tumor cells resulting in the upregulated expression of these critical surface proteins thereby counteracting the tumor cells' ability for immune evasion. This effect was dependent on the expression of CD40 by the tumor cells as the phenotypic changes were absent when CD40− tumor cells were cocultured with CD40L-modified T cells. This effect was also seen in a more clinically relevant setting wherein cocultured CD40L-modified T cells derived from CLL patients augmented the immunogenicity of autologous CLL cells. This finding demonstrates the retained ability of T cells to augment the immunogenicity of autologous malignant cells through constitutive CD40L expression. Importantly, cell to cell contact is not a requisite for this effect as media containing elevated levels of sCD40L from CD40L-modified T cells led to similar phenotypic changes. Augmenting the immunogenicity of cancer cells through the CD40L/CD40 pathway has been shown to induce an endogenous antitumor response in previously published vaccine studies using the infusion of autologous CLL tumor cells transduced with an adenovirus vector encoding CD40L (Ad-CD40L-modified CLL cells).26 In a similar manner, infusions of CAR T cells further modified to constitutively express CD40L could induce an endogenous antitumor response limiting the ability of the tumors to escape elimination through the downregulation/loss of target antigen(s). Additionally, upregulation of IL-21R by CD40L-modified T cells has potentially important implications as the IL-21/IL-21R pathway has been shown to prevent CD8+ T-cell exhaustion.22
DC function (migration, maturation, and antigen presentation) is impeded within the tumor microenvironment.6 In fact, DC's within the suppressive tumor microenvironment have a paradoxical function of inducing Tregs and tolerizing tumor-specific T cells.37 To overcome this the CD40L/CD40 pathway can bolster DC antigen presentation, IL-12 secretion, and promote CD8+ T-cell cytotoxic function.18,19 In support of this agonistic CD40 antibodies have previously been shown to activate DCs and augment CD8+ T-cell response.25 Furthermore, CD40L-modified tumor-specific CD8+ T cells have been shown to stimulate the maturation of DCs and augment the antitumor responses of adoptively transferred CD8+ T cells in tumor-bearing mice.38 We now demonstrate CD40L-modified T cells stimulate DC maturation with concomitant secretion of IL-12p70 from autologous moDCs. Importantly, IL-12 has several immune-stimulatory functions including the ability to enhance T-cell proliferation, cytotoxic capacity, and mediate resistance to Treg suppression as we and others have previously shown.39,40 The ability of CAR/40L T cells to stimulate maturation as well as IL-12 production from moDCs may translate into an improved antitumor effect of adoptively transferred CAR T cells as well as the recruitment of endogenous tumor-specific T cells and natural killer cells. By promoting IL-12 production in close proximity to the tumor, we anticipate minimal systemic IL-12-related toxicity in contrast to prior studies showing severe toxicity following systemic IL-12 administration. In addition to stimulating IL-12 production, CD40L-modified T cells promote DC maturation which in the context of CAR T-cell cytotoxicity should further enhance DC tumor antigen uptake and presentation resulting in recruitment/activation of an endogenous antitumor response by effector T cells and natural killer cells (Figure 4). Taken together enhanced DC function could translate into enhanced antitumor efficacy of CAR-modified tumor-specific T cells.
We next developed a retroviral vector containing both the anti-CD19 CAR (1928z) and the CD40L genes and demonstrated constitutive expression of both CAR and CD40L by T cells is readily achievable. Significantly, when testing the cytotoxic potential of 1928z/40L T cells against a panel of CD19+ targets (DOHH2, Raji) we noted increased in vitro cytotoxicity compared to T cells modified with the 1928z CAR alone (Figure 5c, Supplementary Figure S6). Recently, Laurin et al.41 reported enhanced cytotoxicity by CAR T cells against tumor cell lines following CD40/IL-4-dependent upregulation of surface adhesion molecules providing one explanation for our in vitro results. Proliferation of our 1928z/40L T cells compared to 1928z T cells following stimulation on AAPC was not found to be different in vitro which in part may be due to strength of the CD28 costimulatory signal in the presence of antigen (Supplementary Figure S7). To test the in vivo potential of CAR/CD40L T cells, a xenotransplant model using an aggressive transformed follicular lymphoma cell line (DOHH2) was studied. This model has been historically resistant to eradication by 1928z T cells (unpublished data; Figure 6). However, 1928z/CD40L T cells extended the survival of tumor-bearing mice when compared to mice treated with 1928z T cells alone and resulted in long-term survival in the 1928z/CD40L T-cell treated group (Figure 6). While modest (the majority of mice demonstrated progression of systemic lymphoma) this immunocompromised model has several inherent limitations including the inability of human CD40L-expressing T cells to activate the murine endogenous immune system given that hCD40L is unable to bind mouse CD40.42 Therefore, this model is unable to demonstrate measurement of DC activation/IL-12 secretion by adoptively infused 1928z/CD40L T cells or if phenotypic alteration of the tumor cells in vivo can result in recruitment of endogenous antitumor effectors. The observed enhanced antitumor efficacy in this model could be related to the enhanced cytotoxicity of CAR T cells (Figure 5c, Supplementary Figure S6) but the true in vivo mechanism is not fully defined. To overcome the limitations of this model and fully appreciate the impact constitutive CD40L expression by CAR T cells has on the tumor microenvironment, we have developed a fully immune competent mouse model of systemic lymphoma using C57/B6 transgenic mice that express both the human CD19 antigen and mouse CD19 on their B cells. This model will allow us to investigate if constitutive expression of CD40L by CAR T cells will result in auto-/trans-costimulation of CAR T cells, enhance IL-21/IL-21R signaling, alter the phenotype of systemic malignancy, activate an endogenous immune response including effective recruitment of DC and endogenous immune effectors such as tumor-infiltrating lymphocytes and/or natural killer cells. Investigating this more clinically relevant model of systemic lymphoma is the current focus of our research with a goal of assessing the full antitumor potential of adoptively transferred 1928z/CD40L T cells.
The constitutive expression of CD40L on bone marrow or thymic cells has been shown to result in T-lymphoproliferative disorders following infusion into CD40L-deficient mice.43 The authors of this study suggest the clonal populations arose within the thymus following unremitting CD40L stimulation of thymocytes leading to malignant transformation (rather than the insertional oncogenesis of CD40L-modified cells). While we have noted minimal toxicity and the absence of malignant transformation following infusion of CAR/CD40L T cells in our SCID/Beige model, our focus on utilizing a syngeneic model will allow us to define toxicity such as uncontrolled proliferation or cytokine release. Furthermore, given the concerns regarding malignant T-cell transformation we recognize that future application of this therapy, particularly to the clinical setting, will require an effective suicide gene, such as inducible Caspase 9, to be expressed by the CAR/CD40L T cell.44
In conclusion, we present a novel approach to adoptive T-cell immunotherapy wherein CAR T cells constitutively express CD40L. This approach delivers a potent immune-modulating agent directly into the tumor microenvironment with the ability to enhance T-cell proliferation/cytotoxicity, enhance tumor cell immunogenicity, bolster antigen-presenting DC function with the potential to recruit an endogenous antitumor immune response to augment the antitumor responses mediated by CAR/CD40L T cells. The true significance of this novel approach is not in the modest increase in CD19-targeted cytotoxicity but in the concept that constitutive expression of CD40L by a tumor-specific T cells could have profound effects on the tumor microenvironment. Preclinical testing using a fully immune competent mouse model is currently underway to validate this hypothesis with a goal of translating this promising immunotherapy to the clinic.
Materials and Methods
Cell culture. DoHH2, Raji, and NALM-6 (American Type Culture Collection, Manassas, VA) tumor cell lines were maintained in RPMI 1640 medium (GIBCO, Life Technologies, Gaithersburg, MD) supplemented with 10% heat-inactivated fetal bovine serum, nonessential amino acids, sodium pyruvate, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer, and 2-Mercaptoethanol (Invitrogen, Carlsbad, CA). The 293GP-GLV9 retroviral producer cell lines have been described previously and were cultured in Dulbecco's modified Eagle's medium (GIBCO) supplemented with 10% fetal bovine serum.45 NIH-3T3 AAPCs were cultured in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated donor calf serum as described previously.27 Human T cells were isolated from peripheral blood of healthy donors under Memorial Sloan Kettering institutional review board-approved protocol 95-054 using BD Vacutainer CPT tubes (BD Medical, Franklin Lakes, NJ) as per the manufacturer's instructions. Patient T cell and CLL cells were obtained from patients undergoing treatment under Memorial Sloan Kettering institutional review board-approved protocol 06-138 (NCT00466531) and isolated using Dynabeads ClinExVivo CD3/CD28 beads (Invitrogen). T cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum and 20 IU/ml IL-2 (R&D Systems, Minneapolis, MN). moDCs were obtained from tissue culture plastic-adherent peripheral blood mononuclear cells of healthy donors and cultured in RPMI 1640 supplemented with 1% pooled human A/B serum, HEPES buffer, 2-Mercaptoethanol (Invitrogen), IL-4 (500 IU/ml—R&D Systems), and granulocyte–monocyte colony-stimulating factor (1,000 IU/ml—R&D Systems) as previously described.46 All media were supplemented with 2 mmol/l l-glutamine (Invitrogen), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen).
Construction retroviral constructs. Human CD40L complementary DNA was PCR amplified from isolated healthy donor peripheral blood mononuclear cells using the following primers (i) 5′-CACGTGCATGATCGAAACATACAACCAAACTTCTCCCCGATCTGC-3′ and (ii) 5′-CTCGAGGGATCCTCAGAGTTTGAGTAAGCCAAAGGA-3′. A gamma-retroviral vector encoding human CD40L was constructed using the SFG vector backbone (Figure 1a).47 Construction of 1928z and Pz1 (antiprostate-specific membrane antigen CAR; anti-PSMA) SFG-vector has been previously described.48,49 Construction of 1928z-IRES-40L and Pz1-IRES-40L SFG-gamma-retroviral vector was generated using overlapping PCR (Figure 5a).50
Retroviral transduction of human T lymphocytes. Generation of stable 293GP-GLV9 retroviral producer cell lines and genetic modification of human T cells has been previously described.45,51 For T-cell transduction, isolated healthy donor peripheral blood mononuclear cells were activated with phytohemagglutinin at 2 μg/ml (Sigma, St. Louis, MO), whereas patient-derived T cells were isolated, activated, and expanded using Dynabeads ClinExVivo CD3/CD28 beads following the manufacturer's recommendations. Activated T cells were retrovirally transduced on retronectin-coated (Takara Bio, Shiga, Japan) nontissue culture-treated plates as previously described.51 Gene transfer was assessed on day 7 by flow cytometry. Control mock-transduced T cells were generated in the same manner except supernatant was derived from empty 293GP-GLV9 cell cultures. Proliferation of CD40L-modified T cells for three independent experiments was assessed by the guava EasyCyte cell analyzer with guava ViaCount reagent (EMD Millipore, Billerica, MA) and using CellTrace CFSE Cell Proliferation Kit as per manufacturer's instructions. Supernate was also obtained for cytokine analysis over the first 3 days of the proliferation assay for three independent experiments. Expansion of modified T cells for in vivo experiments was performed using AAPCs derived from NIH-3T3 murine fibroblast genetically engineered to express the target antigen (CD19 or PSMA) along with a costimulatory ligand (CD80) as previously described.27
Coculture assays. Tumor cells (DOHH2, Raji, Ph+ ALL 3.1, NALM-6) were cocultured at a ratio of 5:1 with CD40L-modified T cells and mock-transduced T cells. Flow cytometry was performed after 3 days to determine phenotype of tumor cells. moDCs (2.5 × 105) were cocultured with autologous CD40L-modified T cells or mock-transduced T cells at a 1:5 ratio and tissue culture supernatant was analyzed after 24 hours for IL-12p70 on a Luminex IS100 system. moDCs were also cocultured at a ratio of 5:1 with CD40L-modified T cells and mock-transduced T cells after which the phenotype of moDCs was analyzed by flow cytometry at 24 hours.
Cytotoxicity assay. The cytolytic capacity of transduced T cells from three independent experiments using three separate donors was determined using standard 51Cr release assay as previously described.49 T cells utilized in the cytotoxicity assay did not undergo prestimulation on AAPCs.
Cytokine detection assays. Cytokine detection in tissue culture supernatant was assessed using the MILLIPLEX Human Cytokine Detection System (EMD Millipore) in conjunction with the Luminex IS100 system and IS 2.3 software (EMD Millipore) as per manufacturer's instructions.
Flow cytometry. Flow cytometry was performed using a BD FACS Calibur cytometer and data analyzed using FlowJo version 9.2 software (Tree Star, Ashland, OR). CAR expression was detected using CAR-specific Armenian hamster monoclonal antibody 19E3 (1928z) and 12D11 (1928z and Pz1, Memorial Sloan Kettering monoclonal antibody facility). CD40L expression was detected using mouse antihuman CD154 (BD Biosciences, San Jose, CA). Human T cells were stained with mouse antihuman CD3, IL-21R, PD-1 (BD Biosciences), CD4, and CD8 (Invitrogen). moDCs were stained using mouse antihuman CD11b, HLA-DR, CD83, and CD86 (Invitrogen). DOHH2, Raji, and NALM6 tumor cell phenotype was detected using mouse antihuman CD19, CD40, CD54, CD80 CD86, HLA Class I, and HLA-DR (Invitrogen), CD58, CD70, and CD95 (BD Biosciences).
CAR T-cell in vivo studies. We inoculated 8–12 weeks old SCID/Beige (CB17.Cg-PrkdcscidLystbg-J/Crl) mice (Charles River Laboratories, Wilmington, MA) with DOHH2 tumor cells (5 × 105 cells) by intravenous injection. Two days later mice were infused intravenously with transduced T cells (1 × 107 CAR+ T cells). Tumor progression was monitored clinically and mice were euthanized when disease became clinically evident (development of hind limb paralysis and/or decreased response to stimuli). All murine studies were done in accordance with a Memorial Sloan Kettering Institutional Animal Care and Use Committee approved protocol (00-05-065).
Statistical analysis. All analyses were calculated using Graphpad Prism 5.0 software; survival data were assessed using a log-rank analysis and all other analyses were achieved with a Mann–Whitney test (one-tailed).
SUPPLEMENTARY MATERIAL Figure S1. CD3+ T-cell expression in CD40L-modfied T-cell cultures. Figure S2. CD40L-modified T cells have enhanced proliferation. Figure S3. CD40L-modified T cells have downregulation of PD1 on cell surface. Figure S4. Augmented immunogenicity of CD40+ tumor cells by sCD40L. Figure S5. Increased IL-21R expression by CD40L-modified T cells. Figure S6. 1928z/CD40L T-cell cytotoxicity. Figure S7. Proliferation of first and second generation CAR T with constitutive expression of CD40L. Figure S8. Secretion of IL-12 from monocyte-derived dendritic cells (moDCs) following coculture with 1928z/40L T cells. Table S1. Range of CD40L expression by donor CD40L-modified T cells.
Acknowledgments
This study was supported by the National Institutes of Health (grants CA095152, CA138738, CA059350, CA008748); St. Baldrick's Foundation - AVM Traders Scholar's Award (K.J.C.); William Lawrence and Blanche Hughes Foundation (R.J.B. and K.J.C.), The Emerald Foundation (R.J.B.); VSB Scholarship (B.A.S.), Dutch Cancer Society Scholarship (B.A.S.), Damon Runyon Clinical Investigator Award (R.J.B.); The Annual Terry Fox Run for Cancer Research (New York, NY) organized by the Canada Club of New York; Kate's Team; Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Cancer Foundation for Research, the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center; and the Geoffrey Beene Cancer Foundation. The authors would like to thank Joe Olechnowicz, Mythili Koneru, Sarwish Rafiq, and Eric Smith for their thoughtful advice and critical review of this manuscript. R.J.B. and K.J.C. designed the concept and interpreted the data. K.J.C., B.A.S., Y.N., D.G.L., T.P., and R.Y. performed experiments. H.J.P. and Y.U. provided critical reagents; K.J.C. and R.J.B. wrote the paper. R.J.B. is a cofounder, stock holder, and consultant for Juno Therapeutics. The other authors have no conflict of interest to disclose.
Supplementary Material
References
- Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med. 2012;14:405–415. doi: 10.1002/jgm.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage RJ, Fanslow WC, Strockbine L, Sato TA, Clifford KN, Macduff BM, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–82. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- Schönbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001;58:4–43. doi: 10.1007/PL00000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun FM, Gajl-Peczalska K, Myers DE, Jaszcz W, Haissig S, Ledbetter JA. Temporal association of CD40 antigen expression with discrete stages of human B-cell ontogeny and the efficacy of anti-CD40 immunotoxins against clonogenic B-lineage acute lymphoblastic leukemia as well as B-lineage non-Hodgkin's lymphoma cells. Blood. 1990;76:2449–2456. [PubMed] [Google Scholar]
- Gruss HJ, Ulrich D, Braddy S, Armitage RJ, Dower SK. Recombinant CD30 ligand and CD40 ligand share common biological activities on Hodgkin and Reed-Sternberg cells. Eur J Immunol. 1995;25:2083–2089. doi: 10.1002/eji.1830250742. [DOI] [PubMed] [Google Scholar]
- Zong YS, Lin H, Choy DT, Sham JS, Wei W, Chan KH, et al. Nasopharyngeal carcinoma and lymphoinfiltration. Oncology. 1991;48:290–296. doi: 10.1159/000226945. [DOI] [PubMed] [Google Scholar]
- Lollini PL, Landuzzi L, Frabetti F, Rossi I, Nicoletti G, Scotlandi K, et al. Expression of functional CD40 on human osteosarcoma and Ewing's sarcoma cells. Clin Cancer Res. 1998;4:1843–1849. [PubMed] [Google Scholar]
- van den Oord JJ, Maes A, Stas M, Nuyts J, Battocchio S, Kasran A, et al. CD40 is a prognostic marker in primary cutaneous malignant melanoma. Am J Pathol. 1996;149:1953–1961. [PMC free article] [PubMed] [Google Scholar]
- Wingett DG, Vestal RE, Forcier K, Hadjokas N, Nielson CP. CD40 is functionally expressed on human breast carcinomas: variable inducibility by cytokines and enhancement of Fas-mediated apoptosis. Breast Cancer Res Treat. 1998;50:27–36. doi: 10.1023/a:1006012607452. [DOI] [PubMed] [Google Scholar]
- Ciaravino G, Bhat M, Manbeian CA, Teng NN. Differential expression of CD40 and CD95 in ovarian carcinoma. Eur J Gynaecol Oncol. 2004;25:27–32. [PubMed] [Google Scholar]
- Altenburg A, Baldus SE, Smola H, Pfister H, Hess S. CD40 ligand-CD40 interaction induces chemokines in cervical carcinoma cells in synergism with IFN-gamma. J Immunol. 1999;162:4140–4147. [PubMed] [Google Scholar]
- Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SR. The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity. J Leukoc Biol. 2000;67:607–614. doi: 10.1002/jlb.67.5.607. [DOI] [PubMed] [Google Scholar]
- Cayabyab M, Phillips JH, Lanier LL. CD40 preferentially costimulates activation of CD4+ T lymphocytes. J Immunol. 1994;152:1523–1531. [PubMed] [Google Scholar]
- Peng X, Kasran A, Warmerdam PA, de Boer M, Ceuppens JL. Accessory signaling by CD40 for T cell activation: induction of Th1 and Th2 cytokines and synergy with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26:1621–1627. doi: 10.1002/eji.1830260732. [DOI] [PubMed] [Google Scholar]
- Bhadra R, Gigley JP, Khan IA. Cutting edge: CD40-CD40 ligand pathway plays a critical CD8-intrinsic and -extrinsic role during rescue of exhausted CD8 T cells. J Immunol. 2011;187:4421–4425. doi: 10.4049/jimmunol.1102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- Khong A, Nelson DJ, Nowak AK, Lake RA, Robinson BW. The use of agonistic anti-CD40 therapy in treatments for cancer. Int Rev Immunol. 2012;31:246–266. doi: 10.3109/08830185.2012.698338. [DOI] [PubMed] [Google Scholar]
- Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierda WG, Cantwell MJ, Woods SJ, Rassenti LZ, Prussak CE, Kipps TJ. CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood. 2000;96:2917–2924. [PubMed] [Google Scholar]
- Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- Frentsch M, Stark R, Matzmohr N, Meier S, Durlanik S, Schulz AR, et al. CD40L expression permits CD8+ T cells to execute immunologic helper functions. Blood. 2013;122:405–412. doi: 10.1182/blood-2013-02-483586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. 2014Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor J Clin Oncol pii:JCO.2014.56.2025. [DOI] [PMC free article] [PubMed]
- Stephan MT, Ponomarev V, Brentjens RJ, Chang AH, Dobrenkov KV, Heller G, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121:734–744. doi: 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek TB, van Kooyk Y, van Vliet SJ, Renes MH, Raymakers RA, Figdor CG. High frequency of adhesion defects in B-lineage acute lymphoblastic leukemia. Blood. 1999;94:754–764. [PubMed] [Google Scholar]
- Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- Schultze JL, Cardoso AA, Freeman GJ, Seamon MJ, Daley J, Pinkus GS, et al. Follicular lymphomas can be induced to present alloantigen efficiently: a conceptual model to improve their tumor immunogenicity. Proc Natl Acad Sci USA. 1995;92:8200–8204. doi: 10.1073/pnas.92.18.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattner EJ, Mascarenhas J, Bishop J, Yoo DH, Chadburn A, Crow MK, et al. CD4+ T-cell induction of Fas-mediated apoptosis in Burkitt's lymphoma B cells. Blood. 1996;88:1375–1382. [PubMed] [Google Scholar]
- O'Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–2246. doi: 10.1182/blood-2003-12-4392. [DOI] [PubMed] [Google Scholar]
- Higham EM, Wittrup KD, Chen J. Activation of tolerogenic dendritic cells in the tumor draining lymph nodes by CD8+ T cells engineered to express CD40 ligand. J Immunol. 2010;184:3394–3400. doi: 10.4049/jimmunol.0903111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Laurin D, Marin V, Biagi E, Pizzitola I, Agostoni V, Gallot G, et al. Upregulation of adhesion molecules on leukemia targets improves the efficacy of cytotoxic T cells transduced with chimeric anti-CD19 receptor. J Immunother. 2013;36:181–189. doi: 10.1097/CJI.0b013e318288f8c1. [DOI] [PubMed] [Google Scholar]
- Bossen C, Ingold K, Tardivel A, Bodmer JL, Gaide O, Hertig S, et al. Interactions of tumor necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem. 2006;281:13964–13971. doi: 10.1074/jbc.M601553200. [DOI] [PubMed] [Google Scholar]
- Brown MP, Topham DJ, Sangster MY, Zhao J, Flynn KJ, Surman SL, et al. Thymic lymphoproliferative disease after successful correction of CD40 ligand deficiency by gene transfer in mice. Nat Med. 1998;4:1253–1260. doi: 10.1038/3233. [DOI] [PubMed] [Google Scholar]
- Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghani K, Wang X, de Campos-Lima PO, Olszewska M, Kamen A, Rivière I, et al. Efficient human hematopoietic cell transduction using RD114- and GALV-pseudotyped retroviral vectors produced in suspension and serum-free media. Hum Gene Ther. 2009;20:966–974. doi: 10.1089/hum.2009.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzinger G, Reagan JL, Heller G, Busam KJ, Young JW. Differential CD52 expression by distinct myeloid dendritic cell subsets: implications for alemtuzumab activity at the level of antigen presentation in allogeneic graft-host interactions in transplantation. Blood. 2003;101:1422–1429. doi: 10.1182/blood-2002-04-1093. [DOI] [PubMed] [Google Scholar]
- Rivière I, Brose K, Mulligan RC. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci USA. 1995;92:6733–6737. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13 18 Pt 1:5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- Gong MC, Latouche JB, Krause A, Heston WD, Bander NH, Sadelain M. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999;1:123–127. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos EB, Yeh R, Lee J, Nikhamin Y, Punzalan B, Punzalan B, et al. Sensitive in vivo imaging of T cells using a membrane-bound Gaussia princeps luciferase. Nat Med. 2009;15:338–344. doi: 10.1038/nm.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintás-Cardama A, Yeh RK, Hollyman D, Stefanski J, Taylor C, Nikhamin Y, et al. Multifactorial optimization of gammaretroviral gene transfer into human T lymphocytes for clinical application. Hum Gene Ther. 2007;18:1253–1260. doi: 10.1089/hum.2007.088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.