Abstract
Background: Serum troponin elevation, characteristic of ischemic myocardial injury, has been observed in some acute ischemic stroke (AIS) patients. Its cause and significance are still controversial. The purpose of this study is to find determinants of troponin elevation and its relationship with stroke severity and location.
Methods: Between January 2013 and August 2013, 114 consecutive AIS patients confirmed by diffusion-weighted magnetic resonance imaging were recruited in this study. Serum troponin T level was measured as part of routine laboratory testing on admission. Ten lead standard electrocardiogram (ECG) was performed and stoke severity was assessed based on National Institutes of Health Stroke Scale (NIHSS).
Results: Troponin T was elevated in 20 (17.6%) of 114 patients. Patients with elevated troponin were more likely to have higher age, higher serum creatinine and ischemic ECG changes. Troponin levels were higher in patients with more severe stroke measured by NIHSS [7.96 (6.49-9.78) vs. 13.59 (10.28-18.00)]. There was no association between troponin and locations of stroke and atrial fibrillation. There were 6 (5%) patients with elevated troponin in the presence of normal creatinine and ECG.
Conclusion: Stroke severity, not its location, was associated with higher troponin levels. Abnormal troponin levels are more likely, but not exclusively, to be due to cardiac and renal causes than cerebral ones.
Key Words: Troponin, Stroke, Location, National Institutes of Health Stroke Scale, Electrocardiography, Creatinine
Introduction
Troponin is a sensitive marker of myocardial injury.1 Rise in serum troponin is characteristic for myocardial ischemic injury; however it can rise in several other conditions (e.g. renal failure, heart failure, pulmonary edema, and sepsis).2,3 In the last decade, much interest has been drawn to the importance of serum troponin level in acute stroke. Previous studies have shown that troponin is elevated in 10-30% of acute stroke patients.4-6 This rise can be due to concomitant coronary artery disease and myocardial infarction (MI), congestive heart failure, renal insufficiency or direct neurogenic myocardial injury.7,8 Some researchers have found association between troponin level and location and size of infarction, severity of stroke [measured by National Institutes of Health Stroke Scale (NIHSS)], ischemic electrocardiogram (ECG) changes and increased mortality.9-12 The purpose of this study is to investigate the relationship between cardiac troponin T and severity and location of a stroke.
Materials and Methods
Subjects with a diagnosis of acute ischemic stroke (AIS) presenting to Shariati Hospital Tehran, Iran from January 2013 to August 2013 were enrolled. Stroke patients were diagnosed according to World Health Organization definition (sudden neurological deficit that has a presumable vascular etiology) and were diagnosed as AIS if their brain computed tomography (CT) scan was normal or showed acute ischemic changes. Ischemic stroke was confirmed by showing diffusion restriction on diffusion-weighted imaging magnetic resonance imaging (MRI) using Siemens Magnetom Avanto 1.5 Tesla (Siemens Medical Solutions, Erlangen, Germany). When MRI could not be performed (e.g. cardiac pacemaker), acute stroke was confirmed by showing new hypodensity on repeat brain CT scan 4 days later. Stoke severity was assessed based on NIHSS.
Serum troponin T was measured as part of routine laboratory testing on admission. Levels of Troponin T were measured by Elecsys and cobas e analyzer (Roche diagnostics) and was considered abnormal if it was ≥ 24 ng/l. Ten lead standard ECG was performed and repeated every hour if there was any sign of ischemic changes (i.e., ST-T changes and Left Bundle Branch Block).
Descriptive statistics was shown as mean. P-P plot (which plots a variable cumulative proportion against cumulative proportions of a normal distribution, if the selected variable follows a normal distribution, points cluster around a straight line) and Kolmogorov–Smirnov test were used to assess normal distribution violation of variables. T-test and Kruskal–Wallis test, when non-parametric test had to be used, were used to compare groups in their troponin levels. Spearman correlation coefficient was used to assess the correlation between NIHSS and troponin level. Univariate general linear model was used to control for confounding variables in assessment of the association between stroke severity and troponin level. P < 0.500 was considered as significant. All analyses were done using SPSS software (version 21, SPSS Inc., Chicago, IL, USA).
Results
A total of 120 AIS patients were enrolled. After review of patients’ records, 6 of them were excluded because their troponin levels were not measured. Compared with included patients, they were non-significantly younger (59.67 ± 17.33 vs. 66.34 ± 14.98), more masculine (83.3 vs. 55.3%), and had non-significant less severe strokes (6.17 ± 5.04 vs. 8.18 ± 6.59).
Table 1 shows patients’ basic characteristics. The precise location of a stroke could not be determined in 9 (7.9%) patients because MRI could not be done and repeated CT was inconclusive. Mean time of onset to admission was 23.76 ± 31.01 h. If 6 patients who were admitted more than three days after onset of symptoms were excluded, mean time of onset to admission would be 17.75 ± 17.85.
Table 1.
Basic characteristics of enrolled acute ischemic stroke (AIS) patients
| Basic characteristic | Value |
|---|---|
| Age (mean ± SD) | 66.34 ± 14.98 |
| Female sex [n (%)] | 51 (44.7) |
| Time from onset (Hour) [n (%)] | |
| < 5 | 17 (14.9) |
| 5-12 | 39 (34.2) |
| 13-24 | 24 (21.1) |
| 25-48 | 11 (9.6) |
| 49-72 | 5 (4.4) |
| ≥ 73 | 6 (5.3) |
| Unknown | 12 (10.5) |
| NIHSS [n (%)] | |
| 0-9 | 74 (64.9) |
| 10-19 | 31 (27.2) |
| 20-42 | 9 (7.9) |
| Use of rTPA [n (%)] | 5 (4.4) |
| Abnormal serum creatinine [n (%)] | 9 (7.9) |
| Serum troponin (ng/l) | |
| Minimum | 1 |
| Maximum | 356 |
| Mean ± SD | 22.61 ± 43.63 |
| Median | 11.75 |
| Abnormal [n (%)] | 20 (17.5) |
NIHSS: National Institutes of Health Stroke Scale, rTPA: Recombinant tissue plasminogen activator
Table 2 shows ECG changes seen among patients. ECG ischemic changes were seen in 20% of patients.
Table 2.
Electrocardiogram (ECG) changes of enrolled acute ischemic stroke (AIS) patients
| ECG changes | n (%) |
|---|---|
| ST elevation | 3 (2.5) |
| ST depression | 6(5.0) |
| T inversion | 16(14.2) |
| ST-T changes | 20(16.7) |
| Atrial fibrillation | 15(12.5) |
| Dynamic ECG changes | 3(2.5) |
| LBBB | 5(4.2) |
| RBBB | 3(2.5) |
| Ischemic changes (ST-T, LBBB) | 25(21.9) |
ECG: Electrocardiogram; LBBB: Left bundle branch block; RBBB: Right bundle branch block
Table 3 shows brain areas that were affected by infarcts. About two-third of strokes occurred in posterior circulation.
Table 3.
Infarct locations of enrolled acute ischemic stroke (AIS) patients
| Location of stroke | n (%) |
|---|---|
| Right hemisphere | 59 (56.2) |
| Left hemisphere | 52 (49.5) |
| Brainstem | 21 (20.0) |
| Cerebellum | 13 (12.4) |
| Frontal | 52 (49.5) |
| Parietal | 32 (30.5) |
| Temporal | 8 (7.6) |
| Occipital | 7 (6.7) |
| Insula | 24 (22.9) |
| Basal ganglia | 21 (20.0) |
| Internal capsule | 13 (12.4) |
| Thalamus | 5 (4.8) |
| Lacunar | 22 (21.0) |
| Cortical | 51 (48.6) |
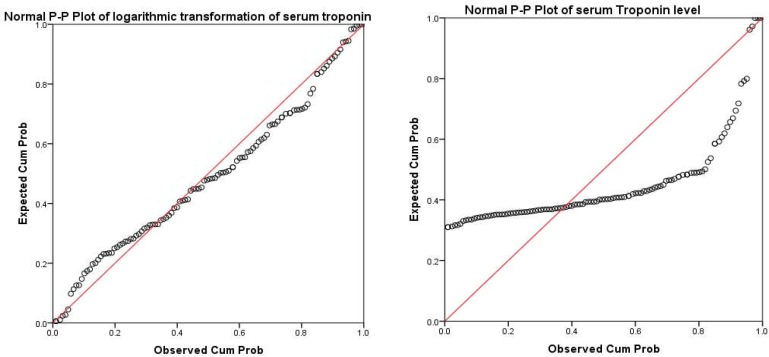
Mean of serum troponin was 22.61 ± 43.63. It was abnormal in 20 (17.5%) patients. Figure 1 shows that serum troponin level did not have a normal distribution (P < 0.001). After logarithmic transformation it had a normal distribution (P = 0.260), and was used in the transformed shape in further analyses.
Figure 1.
P-P plot of serum troponin level and its logarithmic transformation
Table 4 shows association of different variables with serum troponin levels in bivariate analyses. Serum troponin was significantly higher among older, male, uremic stroke patients, or patients who had ischemic changes in their ECGs.
Table 4.
Association of different variables with serum troponin levels among acute ischemic stroke (AIS) patients
| Variable | Troponin (ng/l) | Logarithm of troponin | P (on logarithm of troponin) |
|---|---|---|---|
| Sex | 0.008 | ||
| Male | 29.44 ± 56.10 | 2.73 ± 0.99 | |
| Female | 14.18 ± 16.44 | 2.24 ± 0.92 | |
| Age | 0.002 | ||
| < 70 | 22.56 ± 56.70 | 2.23 ± 1.08 | |
| ≥ 70 | 22.67 ± 25.03 | 2.79 ± 0.80 | |
| Serum creatinine (mg/dl) | 0.001 | ||
| ≤ 1.5 | 20.25 ± 42.78 | 2.43 ± 0.94 | |
| > 1.5 | 52.51 ± 49.39 | 3.56 ± 1.00 | |
| Ischemic changes | < 0.001 | ||
| No | 14.63 ± 15.00 | 2.32 ± 0.86 | |
| Yes | 53.43 ± 87.44 | 3.16 ± 1.20 | |
| NIHSS | 0.140 | ||
| 0-9 | 24.08 ± 52.52 | 2.41 ± 1.08 | |
| 10-42 | 19.90 ± 18.46 | 2.70 ± 0.76 | |
| Right hemisphere | 0.760 | ||
| No | 17.00 ± 17.04 | 2.49 ± 0.86 | |
| Yes | 25.96 ± 57.75 | 2.44 ± 1.09 | |
| Left hemisphere | 0.250 | ||
| No | 23.84 ± 57.75 | 2.35 ± 1.06 | |
| Yes | 20.20 ± 26.12 | 2.57 ± 0.91 | |
| Brainstem | 0.750 | ||
| No | 22.43 ± 47.17 | 2.48 ± 0.99 | |
| Yes | 20.47 ± 34.53 | 2.40 ± 1.02 | |
| Cerebellum | 0.045 | ||
| No | 14.41 ± 14.78 | 2.34 ± 0.82 | |
| Yes | 76.00 ± 110.10 | 3.33 ± 1.58 | |
| Insula | 0.240 | ||
| No | 21.44 ± 47.41 | 2.40 ± 1.00 | |
| Yes | 24.05 ± 35.26 | 2.67 ± 0.93 | |
| Frontal | 0.690 | ||
| No | 25.57 ± 57.65 | 2.50 ± 1.00 | |
| Yes | 18.44 ± 25.98 | 2.42 ± 0.99 | |
| Parietal | 0.740 | ||
| No | 23.50 ± 50.52 | 2.44 ± 1.06 | |
| Yes | 18.71 ± 28.00 | 2.51 ± 0.80 | |
| Occipital | 0.410 | ||
| No | 22.77 ± 46.19 | 2.48 ± 1.00 | |
| Yes | 11.85 ± 12.03 | 2.16 ± 0.80 | |
| Temporal | 0.660 | ||
| No | 22.52 ± 46.49 | 2.45 ± 1.01 | |
| Yes | 16.18 ± 10.47 | 2.61 ± 0.63 | |
| Thalamus | 0.080 | ||
| No | 22.78 ± 45.76 | 2.50 ± 0.98 | |
| Yes | 7.20 ± 4.31 | 1.70 ± 1.00 | |
| Basal ganglia | 0.150 | ||
| No | 20.62 ± 46.38 | 2.39 ± 0.97 | |
| Yes | 27.75 ± 38.18 | 2.74 ± 1.04 | |
| Internal capsule | 0.390 | ||
| No | 23.49 ± 47.55 | 2.49 ± 1.03 | |
| Yes | 11.77 ± 10.17 | 2.24 ± 0.65 | |
| Cortical | 0.980 | ||
| No | 25.57 ± 57.16 | 2.46 ± 1.10 | |
| Yes | 18.30 ± 26.09 | 2.46 ± 0.87 |
NIHSS: National Institute of Health Stroke Scale
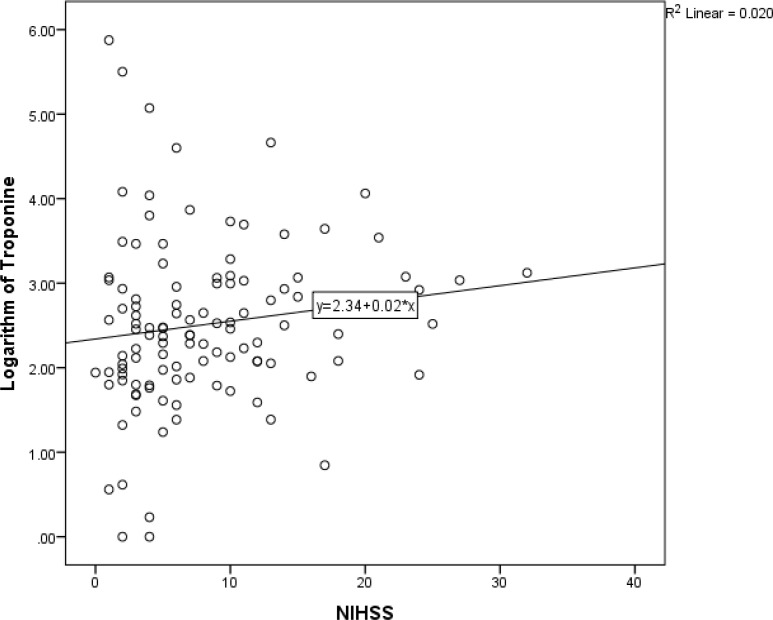
Although dichotomizing NIHSS did not yield significant association between this variable and serum troponin, figure 2 shows that there was a weak linear association between NIHSS and serum troponin (spearman correlation coefficient = 0.20; P = 0.030). Among other stroke variables, only cerebellum strokes were significantly associated with serum troponin.
Figure 2.
Association between National Institute of Health Stroke Scale (NIHSS) and serum troponin level among acute ischemic stroke patients
Four stroke variables which had P < 0.200 in univariate analyses were entered in a multivariate analysis after exclusion of subjects who had either abnormal creatinine levels or ischemic ECG changes. Only NIHSS remained a significant predictor of troponin levels. After adjustment by age and sex, NIHSS was still a significant predictor (P = 0.002). However, the effect of NIHSS on troponin level was too small in a way that mean of troponin among stroke patients with NIHSS < 10 was 7.96 [95% confidence interval (CI) = 6.49-9.78] compared with 13.59 (95% CI = 10.28-18.00) in patients with NIHSS ≥ 10. And, among subjects with ECG ischemic signs, NIHSS was not a significant predictor of troponin (71.55 ± 112.05 vs. 29.88 ± 29.35 in subjects with NIHSS 0-9 vs. 10-42, respectively).
There were 78 AIS subjects without any evidence of renal impairment or ECG ischemic signs. 8 of them (10%) had abnormal troponin levels; 6 of them were older than 70 and 4 had NIHSS more than 9. All 8 subjects had at least one of these predictors.
Discussion
Stroke is the second-fourth most common cause of death, after ischemic heart disease (IHD);13 meanwhile, IHD is the second most common cause of death after stroke.14,15 While stroke and IHD share the same risk factors (i.e., hypertension, hyperlipidemia, diabetes mellitus and smoking), it is not unexpected to see both diseases in one patient.16 Many studies have shown elevated serum troponin in significant proportion of acute stroke patients (11-36.4%).4,17-19
17.5% of acute stroke patients in our study had elevated serum troponin level which was congruent with previous studies. Associations, causes and value of troponin rise have been the core of many studies. It has been demonstrated that troponin elevation could be associated with higher age, more severe stroke, larger stroke, specific stroke locations, renal dysfunction, previous IHD, ECG changes and poor outcome.20-23 In our study, this association was found just between troponin T and age, renal impairment, ECG changes and stroke severity (i.e. NIHSS).
We did not measure infarction size, and outcome determination was not in design of our study. Renal impairment and ischemic ECG changes were greater determinant than NIHSS; in a way that in patients with high creatinine or ischemic ECG changes, effect of NIHSS on troponin was not significant. However in patients without ischemic ECG or renal problem, higher troponin was seen in patients with more severe stroke. This effect was independent and could not be explained by other factors.
It has been hypothesized that damage to centers regulating autonomic function can cause autonomic dysregulation resulting in sympathetic overflow and neurogenic myocardial injury.24 Therefore, insula and brainstem were center of attention of many researchers.25 Some studies have shown that troponin level is higher in the right insular, brainstem or middle cerebral artery territory infarction.5,10,26,27 Nevertheless, our findings along with the study of Barber and Morton. did not corroborate those results, and we found no correlation between stroke location and troponin level.28
Apart from associations of it, the possible causes of troponin elevation in AIS have been investigated previously. Jensen et al.7 and Scheitz et al.29 proposed that the most likely causes of increased troponin in AIS patients are silent acute MI before stroke, heart failure and renal insufficiency. Our data also show that most troponin elevations in AIS patients occurred in the context of renal or cardiac dysfunction and 3 (2.5%) of the patients had concomitant acute MI. However, there were 8 patients with elevated troponin (mean 43.75) at the presence of normal ECG and renal function. In these patients, troponin elevation could not be attributed to renal or cardiac problem, and neurogenic cardiac injury could be suspected. Nonetheless, we did not go through those cases and coronary status was not evaluated to be sure of the absence of coronary artery disease as predisposing factor for troponin elevation.
According to our findings, although there are some AIS patients with possible neurogenic myocardial injury, it is prudent to be vigilant in those with high troponin and perform appropriate cardiac and renal evaluation.
This was the first Troponin-Stroke relationship study in Iranian population. The number of patients (sample volume) was limited to 114, which made specific location stroke groups small, and might have made probable associations statistically insignificant. As mentioned, we did not evaluate cardiac structural (echocardiography) and coronary status; therefore the number of real neurogenic myocardial injury patients might have been over- or underestimated.
Conclusion
Troponin T elevation in AIS patients was associated with higher age, creatinine, ECG changes and severity of stroke, but location of stroke was not a determinant factor. Cardiac and renal impairment were the cause of troponin elevation in the majority of patients; however, there are some patients with possible neurogenic myocardial injury.
Conflict of Interests
The authors declare no conflict of interest in this study.
Acknowledgments
We acknowledge all residents of the Neurology Ward of Shariati Hospital who participated in this study.
Notes
How to cite this article: Abdi S, Oveis Gharan Sh, Sinaei F, Ghorbani A. Elevated troponin T after acute ischemic stroke: Association with severity and location of infarction. Iran J Neurol 2015; 14(1): 35-40.
References
- 1.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858–67. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 2.Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med. 2005;142(9):786–91. doi: 10.7326/0003-4819-142-9-200505030-00015. [DOI] [PubMed] [Google Scholar]
- 3.Kelley WE, Januzzi JL, Christenson RH. Increases of cardiac troponin in conditions other than acute coronary syndrome and heart failure. Clin Chem. 2009;55(12):2098–112. doi: 10.1373/clinchem.2009.130799. [DOI] [PubMed] [Google Scholar]
- 4.Jensen JK, Kristensen SR, Bak S, Atar D, Hoilund-Carlsen PF, Mickley H. Frequency and significance of troponin T elevation in acute ischemic stroke. Am J Cardiol. 2007;99(1):108–12. doi: 10.1016/j.amjcard.2006.07.071. [DOI] [PubMed] [Google Scholar]
- 5.Ghali J, Allison D, Kleinig T, Ooi SY, Bastiampillai S, Ashby D, et al. Elevated serum concentrations of troponin T in acute stroke: what do they mean? J Clin Neurosci . 2010;17(1):69–73. doi: 10.1016/j.jocn.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Apak I, Iltumur K, Tamam Y, Kaya N. Serum cardiac troponin T levels as an indicator of myocardial injury in ischemic and hemorrhagic stroke patients. Tohoku J Exp Med. 2005;205(2):93–101. doi: 10.1620/tjem.205.93. [DOI] [PubMed] [Google Scholar]
- 7.Jensen JK, Atar D, Mickley H. Mechanism of troponin elevations in patients with acute ischemic stroke. Am J Cardiol. 2007;99(6):867–70. doi: 10.1016/j.amjcard.2006.10.052. [DOI] [PubMed] [Google Scholar]
- 8.Freda BJ, Tang WH, Van LF, Peacock WF, Francis GS. Cardiac troponins in renal insufficiency: review and clinical implications. J Am Coll Cardiol. 2002;40(12):2065–71. doi: 10.1016/s0735-1097(02)02608-6. [DOI] [PubMed] [Google Scholar]
- 9.Wira CR, II, Rivers E, Martinez-Capolino C, Silver B, Iyer G, Sherwin R, et al. Cardiac complications in acute ischemic stroke. West J Emerg Med. 2011;12(4):414–20. doi: 10.5811/westjem.2011.2.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song HS, Back JH, Jin DK, Chung PW, Moon HS, Suh BC, et al. Cardiac troponin T elevation after stroke: relationships between elevated serum troponin T, stroke location, and prognosis. J Clin Neurol. 2008;4(2):75–83. doi: 10.3988/jcn.2008.4.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darki A, Schneck MJ, Agrawal A, Rupani A, Barron JT. Correlation of elevated troponin and echocardiography in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22(7):959–61. doi: 10.1016/j.jstrokecerebrovasdis.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Balaney B, Batal O, Kolia N, Hickey G, Dardari Z, Reddy V, et al. Troponin I elevation in acute ischemic stroke. J Am Coll Cardiol. 2013;61(10_S) doi: 10.1016/j.jcrc.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Minino AM, Murphy SL. Death in the United States, 2010, NCHS Data Brief No. 99 [Online] [cited 2012]. Available from: URL: http://www.cdc.gov/nchs/data/databriefs/db99.pdf. [PubMed]
- 14.Kaarisalo MM, Immonen-Raiha P, Marttila RJ, Salomaa V, Kaarsalo E, Salmi K, et al. Atrial fibrillation and stroke. Mortality and causes of death after the first acute ischemic stroke. Stroke. 1997;28(2):311–5. doi: 10.1161/01.str.28.2.311. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann A, Rundek T, Mast H, Paik MC, Boden-Albala B, Mohr JP, et al. Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study. Neurology. 2001;57(11):2000–5. doi: 10.1212/wnl.57.11.2000. [DOI] [PubMed] [Google Scholar]
- 16.Amarenco P, Lavallee PC, Labreuche J, Ducrocq G, Juliard JM, Feldman L, et al. Prevalence of coronary atherosclerosis in patients with cerebral infarction. Stroke. 2011;42(1):22–9. doi: 10.1161/STROKEAHA.110.584086. [DOI] [PubMed] [Google Scholar]
- 17.Nijjer SS, Banerjee G, Barker J, Banerjee S, Connolly S, Fox KF. 4 A rational approach to raised troponins on a hyperacute stroke unit: coping with the impact on cardiology services. Heart. 2011;97:A7. [Google Scholar]
- 18.Beaulieu-Boire I, Leblanc N, Berger L, Boulanger JM. Troponin elevation predicts atrial fibrillation in patients with stroke or transient ischemic attack. J Stroke Cerebrovasc Dis. 2013;22(7):978–83. doi: 10.1016/j.jstrokecerebrovasdis.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Kral M, Sanak D, Veverka T, Hutyra M, Vindis D, Kuncarova A, et al. Troponin T in acute ischemic stroke. Am J Cardiol. 2013;112(1):117–21. doi: 10.1016/j.amjcard.2013.02.067. [DOI] [PubMed] [Google Scholar]
- 20.Furtner M, Hammerer-Lercher A, Kiechl S, Mair J. High-sensitivity troponin T is predictive of adverse outcome after acute ischemic stroke. Eur Heart J. 2013;34(suppl 1) [Google Scholar]
- 21.Maliszewska M, Fiszer U, Palasik W, Morton M, Tadeusiak W. Elevated troponin I level-a predictor of poor prognosis after ischemic stroke**. Postepy Nauk Medycznych. 2013;10:667–72. [Google Scholar]
- 22.Anders B, Alonso A, Artemis D, Schafer A, Ebert A, Kablau M, et al. What does elevated high-sensitive troponin I in stroke patients mean: concomitant acute myocardial infarction or a marker for high-risk patients? Cerebrovasc Dis. 2013;36(3):211–7. doi: 10.1159/000353875. [DOI] [PubMed] [Google Scholar]
- 23.Hasirci B, Okay M, Agircan D, Kocer A. Elevated troponin level with negative outcome was found in ischemic stroke. Cardiovasc Psychiatry Neurol. 2013;2013:953672. doi: 10.1155/2013/953672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeppellini R, Salsa F, Gheno G, Cucchini F. Cardiac injury in acute cerebral vasculopathy. Ann Ital Med Int. 2001;16(2):73–81. [PubMed] [Google Scholar]
- 25.Colivicchi F, Bassi A, Santini M, Caltagirone C. Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvement. Stroke. 2004;35(9):2094–8. doi: 10.1161/01.STR.0000138452.81003.4c. [DOI] [PubMed] [Google Scholar]
- 26.Ay H, Koroshetz WJ, Benner T, Vangel MG, Melinosky C, Arsava EM, et al. Neuroanatomic correlates of stroke-related myocardial injury. Neurology. 2006;66(9):1325–9. doi: 10.1212/01.wnl.0000206077.13705.6d. [DOI] [PubMed] [Google Scholar]
- 27.Fayard C, Fayard M, Osseby GV, Cochet A, Giroud M. 326 Elevated troponin in acute ischaemic stroke: prevalence, predictive factors, mortality and applicability of cardiac magnetic resonance imaging. Archives of Cardiovascular Diseases Supplements. 2011;3(1):109–10. [Google Scholar]
- 28.Barber M, Morton JJ, Macfarlane PW, Barlow N, Roditi G, Stott DJ. Elevated troponin levels are associated with sympathoadrenal activation in acute ischaemic stroke. Cerebrovasc Dis. 2007;23(4):260–6. doi: 10.1159/000098325. [DOI] [PubMed] [Google Scholar]
- 29.Scheitz JF, Endres M, Mochmann HC, Audebert HJ, Nolte CH. Frequency, determinants and outcome of elevated troponin in acute ischemic stroke patients. Int J Cardiol. 2012;157(2):239–42. doi: 10.1016/j.ijcard.2012.01.055. [DOI] [PubMed] [Google Scholar]