Abstract
A 60-year-old man who abused corticosteroids developed thoracic-distribution zoster. Varicella zoster virus (VZV) DNA was found in non-healing skin 3 months later. He died suddenly 2 months later. Skin was ulcerated and necrotic. VZV was widespread in organs and arteries, particularly coronary arteries and aorta, with VZV vasculopathy in the posterior cerebral artery.
Keywords: Corticosteroids, VZV dissemination, Sudden death
1. Why this case is important
Primary infection with VZV usually results in varicella, after which virus becomes latent in cranial nerve ganglia, dorsal root ganglia and autonomic ganglia along the entire neuraxis. VZV reactivation in elderly and immunocompromised individuals causes herpes zoster and other neurological diseases, including stroke (VZV vasculopathy). Zoster is also associated with an increased risk of myocardial infarction [1].
Herein is a case of sudden death in a zoster patient who abused steroids. Autopsy revealed VZV dissemination and asymptomatic VZV vasculopathy. Extensive VZV infection in cardiovascular structures may have produced a cardiac arrhythmia that led to sudden death.
2. Case description
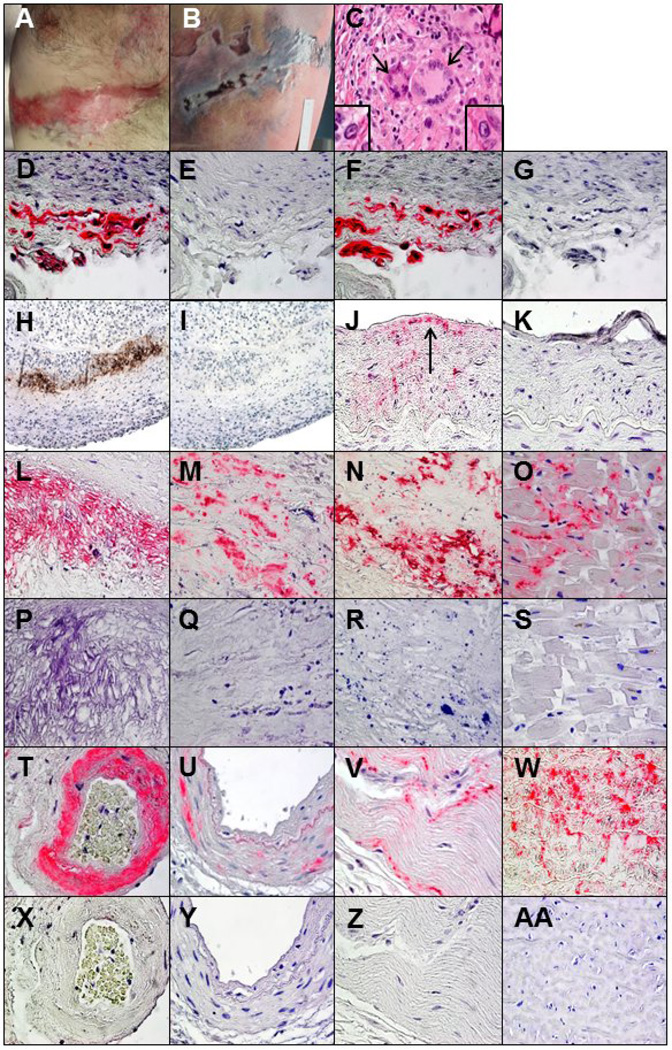
In August 2013, a 60-year-old man developed right-sided T6–7 distribution zoster. He was treated with valacyclovir and oral prednisone, taken near-continuously for pain relief. Zoster lesions below the right nipple (Fig. 1A) and in the same posterior dermatome (Fig. 1B) became ulcerated and did not heal, and VZV DNA was detected by PCR 3 months later. For 7 years before zoster, he self-administered corticosteroids repeatedly for ill-defined musculoskeletal aches; from 2011–13, he consumed at least 13 prednisone packs (20 50-mg tablets/pack). He died suddenly 5 months after zoster without known preceding chest or arm pain, dizziness, syncope, nausea, vomiting or other neurological symptoms.
Fig. 1.
Postmortem examination showed linear T6–7 distribution scarred lesions at the site of earlier zoster just below the nipple (A) along with ulcerative necrotic lesions in the same dermatome on the back (B) that were confirmed by PCR to contain VZV. In the right posterior cerebral artery, hematoxylin and eosin staining revealed inflammation and 2 giant cells (C, arrows) in the media adjacent to the disrupted internal elastic lamina, as well as Cowdry A inclusion bodies (C, insets). To confirm the specificity of binding to VZV antigen, immunoperoxidase and immunohistochemical staining were performed with 3 different anti-VZV antibodies. Immunohistochemical staining of a positive control VZV-infected cadaveric cerebral artery with mouse anti-VZV gE IgG1 antibody revealed VZV antigen (D, pink color) that was not seen with mouse isotype IgG1 antibody (E). Immunostaining of the same artery with rabbit anti-VZV IE 63 antibody also revealed VZV antigen (F, pink color) that was not seen with normal rabbit serum (G). In the right posterior cerebral artery, immunoperoxidase staining with a mouse anti-VZV antibody directed against multiple VZV antigens revealed virus in the arterial media (H, brown color) that was not seen when anti-HSV antibody was substituted for anti-VZV antibody (I). Immunohistochemical staining with mouse anti- VZV gE IgG1 antibody also revealed VZV antigen in the thickened arterial intima (J, arrow) that was not seen when adjacent sections were immunostained with mouse isotype IgG1 antibody (K). All other arteries and tissues were immunostained with mouse anti-VZV gE IgG1 antibody, and the presence of VZV antigen in some tissues was confirmed with rabbit anti-VZV IE 63 antibody. Immunostaining with mouse anti-VZV gE IgG1 antibody revealed viral antigen in the media of the aorta (L), the thickened intima of the left anterior descending coronary artery (M), the proximal left circumflex coronary artery (N), the bundle of His (O), the intima and media of an artery adjacent to the adrenal gland (T), the media of the renal artery (U) and the ileum (V), that was not seen when adjacent sections were immunostained with mouse isotype IgG1 antibody (P-S and X-Z). Immunostaining with rabbit anti-VZV IE 63 antibody revealed VZV antigen in sections of the aorta adjacent to that shown in panel L (W), which was not seen when adjacent sections were immunostained with normal rabbit serum (AA). 600× magnification.
2.1. Postmortem findings
At autopsy, the heart was hypertrophic with focal fibrosis of the posterior left ventricular wall. There was patchy, mild, non-stenotic atherosclerosis of the coronary arteries without arteritis. The brain was slightly edematous without inflammation, infarction or hemorrhage. All arteries of the Circle of Willis were mildly atherosclerotic. Grossly, the right posterior cerebral artery was moderately narrowed; histopathology showed fibrinoid necrosis of the media, disruption of the elastic lamina, intimal proliferation and transmural mononuclear cell inflammatory infiltrates with multinucleated giant cells and Cowdry A inclusion bodies (Fig. 1C). Cerebral parenchyma in the corresponding vascular distribution showed no pathology.
2.2. Virological findings
The posterior cerebral arteries were initially examined by immunoperoxidase staining for VZV antigen using a Bond-III immunostainer (Leica, Buffalo Grove, IL). Slides were pretreated with Bond Enzyme Pretreatment Kit (Leica) for 10 minutes at room temperature, incubated with 1:100 dilution of mouse anti-VZV antibody directed against multiple VZV antigens (Cell Marque, Rocklin, CA) for 30 minutes at room temperature, processed with biotinylated secondary antibody followed by horseradish peroxidase and diaminobenzidine (DAB) using the Bond Polymer Refine Detection Kit (Leica). Control was provided by substitution of anti-VZV primary antibody with 1:100 dilution of anti-HSV antibody (Cell Marque).
Further immunohistochemical analysis for VZV antigen was performed on 19 formalin-fixed, paraffin-embedded arteries and 17 tissue specimens listed in Table 1. One hundred 5-µm sections of all specimens were cut and baked for 1 hour at 60°C (1520 total sections). From each block, alternating sections (50 sections/specimen) were deparaffinized, immunostained with 1:500 dilution of mouse monoclonal anti-VZV gE IgG1 antibody (Santa Cruz Biotechnology, Dallas, TX) for 2 hours, rinsed in 1X PBS 3 times for 3 minutes each time, incubated with 1:1000 dilution of secondary biotinylated goat anti-mouse antibody (Dako, Carpinteria, CA) for 1 hour, rinsed as above, and incubated with prediluted streptavidin-alkaline phosphatase (BD Biosciences, San Diego, CA) for 1 hour then rinsed as above. All incubations were at room temperature. Color reaction was developed under a light microscope using fresh fuchsin substrate system (Dako) with levamisole (Dako; 24 µg/mL). Slides were counterstained with 1X hematoxylin for 2 minutes. When a section contained VZV antigen, at least 2 adjacent sections were stained as above except that mouse anti-VZV gE IgG1 antibody was replaced with 1:500 dilution of control mouse IgG1 antibody (Dako). To confirm the presence of VZV antigen, some sections from multiple tissues were immunostained as described above with 1:10,000 dilution of rabbit polyclonal anti-VZV IE 63 antibody followed by 1:1000 dilution of secondary biotinylated polyclonal goat anti-rabbit antibody (Dako); when a section contained VZV antigen, at least 2 adjacent sections were stained as above except that rabbit anti-VZV IE 63 antibody was replaced with 1:10,000 dilution of normal rabbit serum (Jackson ImmunoResearch, West Grove, PA). Positive controls consisted of VZV-infected cadaveric cerebral arteries maintained for 14 days in vitro and stained with either mouse anti-VZV gE IgG1 (Fig. 1D) or rabbit anti-VZV IE63 (Fig. 1F) antibody as above. For each section that contained VZV antigen, an adjacent section (within 5 µm) was stained with hematoxylin and eosin (H&E).
Table 1.
Distribution of varicella zoster virus antigen in arteries and other tissues.
| Artery | VZV antigen |
Skip areasa |
|---|---|---|
| Basilar artery | + | 0 |
| Left ACA | - | |
| Right ACA | - | |
| Left MCA | + | 0 |
| Right MCA | - | |
| Left PCA | - | |
| Right PCA | + | 0 |
| Left ICA | + | 9 |
| Right ICA | + | 0 |
| Aorta | + | 7 |
| Coronary, LAD | + | 23 |
| Coronary, proximal left circumflex | + | 48 |
| Coronary, central left circumflex | + | 0 |
| Coronary, distal left circumflex | + | 21 |
| Coronary, right | + | 37 |
| Pulmonary | + | 5 |
| Mesenteric | - | |
| Renal | + | 17 |
| Adrenal gland | + | 14 |
| Tissue | VZV antigen |
|---|---|
| Medulla | + |
| Parietal lobe | - |
| Salivary gland | - |
| Paratracheal lymph node | - |
| Mesenteric lymph note | - |
| Esophagus | + |
| Left lung | - |
| Right lung | ++ |
| AV node | ++ |
| Bundle of HIS | ++ |
| Myocardium, left and right posterior wall | + |
| Small intestine | ++ |
| Liver | - |
| Gallbladder | + |
| Spleen | + |
| Kidney | + |
| Adrenal gland | - |
Abbreviations: ACA, anterior cerebral artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; ICA, internal carotid artery; LAD, left anterior descending; AV, atrioventricular
Skip areas are defined as at least 2 VZV antigen-positive regions flanked by VZV antigen-negative regions.
In the right posterior cerebral artery, immunoperoxidase staining revealed VZV antigen in arterial media (Fig. 1H) and immunohistochemical staining showed VZV antigen in thickened intima (Fig. 1J, arrow). Immmunohistochemical staining also revealed VZV antigen in 5 (56%) of 9 intracerebral arteries examined (Table 1; representative arteries and tissues in Fig. 1), including the basilar artery, left middle cerebral artery, right posterior cerebral artery, left internal carotid artery (ICA), and right ICA; 9 skip areas (defined as at least 2 VZV antigen-positive regions flanked by VZV antigen-negative regions) were found in the left ICA. The aorta, left anterior descending coronary artery, proximal, central and distal left circumflex coronary artery, and right coronary artery contained VZV antigen in 7, 23, 48, 0, 21 and 37 skip areas, respectively. VZV antigen was particularly abundant in the aorta (Fig. 1L and 1W), left anterior descending coronary artery (Fig. 1M) and proximal left circumflex coronary artery (Fig. 1N). VZV antigen was also present in arteries of the lung, adrenal gland (Fig. 1T) and kidney (Fig. 1U) in 5, 14 and 17 skip areas, respectively.
Immuohistochemical staining also revealed VZV antigen in 10 (59%) of 17 tissues examined, including the brainstem medulla, esophagus, left and right posterior wall of the myocardium, gallbladder, spleen and kidney, right lung, atrioventricular node, bundle of His (Fig. 1O) and ileum (Fig. 1V). Except for the posterior cerebral artery, no histopathological abnormalities were seen in sections adjacent to those containing VZV antigen.
3. Other similar and contrasting cases in the literature
There have been no previously reported cases of VZV antigen in systemic arteries and cardiovascular structures of subjects with corticosteroid abuse, chronic VZV infection and sudden death.
4. Discussion
We describe an extraordinary case of sudden death in a 60-year-old man 5 months after thoracic-distribution zoster. Several features are remarkable. First was a 7-year history of frequent corticosteroid use, before and particularly during the interval between zoster and death, which likely potentiated VZV infection [2], as evidenced by amplifiable VZV DNA from skin 3 months after zoster, when rash has usually resolved, as well as by ulceration and necrosis of skin over the site of earlier zoster, indicative of impaired wound healing, both most likely attributable to long-term corticosteroid use.
VZV antigen was found in multiple organs, particularly in arteries supplying those organs, most abundant in the aorta and coronary arteries, underscoring the predilection of VZV for vascular tissue. To our knowledge, our analysis of 1520 slide sections from 17 tissues and 19 arteries represents the most extensive virological study of a single patient undertaken to date. Most noteworthy was the presence of VZV vasculopathy in the posterior cerebral artery, as evidenced by necrosis and inflammation, Cowdry A inclusions, multinucleated giant cells and viral antigen. This is the first demonstration of intracerebral VZV vasculopathy in the absence of neurological symptoms and signs.
Because there were no clinical or pathological features of cerebral hemorrhage, pulmonary embolism, inflammatory heart disease or coronary artery thrombosis, the exact cause of the patient‘s sudden death is unknown. However, widespread VZV infection in the aorta, coronary arteries and myocardium, including the bundle of His, raises the possibility that VZV induced a cardiac arrhythmia. Two reports describe cardiac arrhythmia associated with zoster. The first was a 64-year old man who developed right T8-distribution zoster followed 2 months later by complete heart block [3]. The second was a 34-year-old man who simulaneously developed ventricular fibrillation and disseminated zoster whose skin, throat and nose swabs contained VZV DNA [4]. Because both patients survived, tissues were not analyzed virologically. Overall, our case not only confirms previous clinical associations of truncal-distribution zoster with putative cardiac arrhythmia, but also provides virological evidence of extensive VZV infection in cardiovascular structures.
Thoracic sympathetic ganglia (TSG) supply postganglionic fibers to blood vessels, skin, heart, lung, pancreas, gastrointestinal tract, liver, spleen, adrenal glands, kidneys, ureters and bladder and connect to dorsal root ganglia via gray communicating rami [5, 6]. VZV DNA was found in TSG in all of 15 subjects studied [7]. Thus, one explanation for the considerable abundance of VZV antigen in cardiovascular structures is infection via transaxonal spread after reactivation of virus from dorsal root ganglia and autonomic ganglia. Such a pathway for virus-induced visceral disease is evidenced by detection of VZV antigen and Cowdry type A inclusion bodies in TSG of a patient with pancreatitis [8], as well as additional reports of pancreatitis [9], hepatitis and gastritis [10] temporally associated with zoster.
Overall, this case not only underscores the two-edged sword nature of corticosteroid treatment for zoster, but also shows that VZV vasculopathy may develop without neurological symptoms and signs. Finally, the case shows that VZV infection of cardiovascular structures can potentially lead to sudden death.
Highlights.
Long-term corticosteroid use can potentiate VZV infection and dissemination.
VZV has a predilection for arteries.
Early VZV vasculopathy can occur without clinical features.
Acknowledgements
The authors thank Marina Hoffman for editorial assistance and Cathy Allen for manusript preparation.
Funding
This work was supported by the National Institutes of Health (AG032958 to D.G. and M.A.N., AG006127 to D.G., and NS067070 to M.A.N.).
Abbreviations
- IE
immediate-early
- TSG
thoracic sympathetic ganglia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors contribution
M.A.N., D.L. and D.G. reviewed the literature, wrote the manuscript, contributed to the conception and design of the study, and acquired, analyzed and interpreted the data. T.W., N.K. and A.H. acquired, analyzed and interpreted data. P.J.B. analyzed and interpreted the data. All authors have approved the final manuscript.
Competing interests
All authors declare no competing interests.
Ethical approval
Not required.
Contributor Information
Maria A. Nagel, Email: maria.nagel@ucdenver.edu.
Daniela Lenggenhager, Email: Daniela.lenggenhager@usz.ch.
Teresa White, Email: Teresa.m.white@ucdenver.edu.
Nelly Khmeleva, Email: nelly.khmeleva@ucdenver.edu.
Anna Heintzman, Email: anna.heintzman@gmail.com.
Philip J. Boyer, Email: Philip.boyer@ucdenver.edu.
Don Gilden, Email: don.gilden@ucdenver.edu.
References
- 1.Breuer J, Pacou M, Gauthier A, Brown MM. Herpes zoster as a risk factor for stroke and TIA: a retrospective cohort study in the UK. Neurology. 2014;82:206–212. doi: 10.1212/WNL.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smitten AL, Choi HK, Hochberg MC, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum. 2007;57:1431–1438. doi: 10.1002/art.23112. [DOI] [PubMed] [Google Scholar]
- 3.Ma TS, Collins TC, Habib G, Bredikis A, Carabello BA. Herpes zoster and its cardiovascular complications in the elderly – another look at a dormant virus. Cardiology. 2007;107:63–67. doi: 10.1159/000093777. [DOI] [PubMed] [Google Scholar]
- 4.Dennison P, Zaremba E. Varicella zoster induced cardiac dysfunction: a case report. Emerg Med J. 2007;24:682–683. doi: 10.1136/emj.2006.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Standing S. Gray’s Anatomy. 40th ed. London: Churchill Livingston, Elsevier; 2008. [Google Scholar]
- 6.Marieb EN, Wilhelm PB, Mallatt J. The Autonomic Nervous System and Visceral Sensory Neurons. 6th ed. San Francisco: 2011. [Google Scholar]
- 7.Nagel MA, Rempel A, Huntington J, Kim F, Choe A, Gilden D. Frequency and abundance of α-herpesvirus DNA in human thoracic sympathetic ganglia. J Virol. 2014;88:8189–8192. doi: 10.1128/JVI.01070-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dueland AN, Devlin M, Martin JR, et al. Fatal varicella zoster virus meningoadiculitis without skin involvement. Ann Neurol. 1991;29:569–572. doi: 10.1002/ana.410290520. [DOI] [PubMed] [Google Scholar]
- 9.Pulik M, Teillet F, Teillet-Thiebaud F, Lionnet F, Genet P, Petitdidier C. Varicella zoster virus pancreatitis in hematologic diseases. Ann Med Interne (Paris) 1995;146:292–294. [PubMed] [Google Scholar]
- 10.Remmerswaal RG, de Vries AC, Ramsoekh D, van Buuren HR. Varicella zosterassociated gastric ulcers, hepatitis and pancreatitis in an immunocompromised patient. Endoscopy. 2012;44(Suppl 2) doi: 10.1055/s-0030-1256934. UCTN:E140. [DOI] [PubMed] [Google Scholar]