Abstract
The polycyclic aromatic hydrocarbon (PAH), dibenzo[def,p]chrysene (DBC; also known as dibenzo[a,l]pyrene), is a potent carcinogen in animal models and a class 2A human carcinogen. Recent investigations into DBC-mediated toxicity identified DBC as a potent immunosuppressive agent similar to the well-studied immunotoxicant 7,12-dimethylbenz[a]anthracene (DMBA). DBC, like DMBA, is bioactivated by cytochrome P450 (CYP) 1B1 and forms the reactive metabolite DBC-11,12-diol-13,14-epoxide (DBCDE). DBCDE is largely responsible for the genotoxicity associated with DBC exposure. The immunosuppressive properties of several PAHs are also linked to genotoxic mechanisms. Therefore, this study was designed to identify DBCDE-DNA adduct formation in the spleen and thymus of wild-type and cytochrome P450 1b1 (Cyp1b1) knockout (KO) mice using a highly sensitive stable-isotope dilution UHPLC-MS/MS method. Stable-isotope dilution UHPLC-MS/MS identified the major DBC adducts (±)-anti-cis-DBCDE-dA and (±)-anti-trans-DBCDE-dA in the lung, liver, and spleen of both WT and Cyp1b1 KO mice. However, adduct formation in the thymus was below the level of quantitation for our method. Additionally, adduct formation in Cyp1b1 KO mice was significantly reduced compared to wild-type (WT) mice receiving DBC via oral gavage. In conclusion, the current study identifies for the first time DBCDE-dA adducts in the spleen of mice supporting the link between genotoxicity and immunosuppression, in addition to supporting previous studies identifying Cyp1b1 as the primary CYP involved in DBC bioactivation to DBCDE. The high levels of DBC-DNA adducts identified in the spleen, along with the known high levels of Cyp1b1 expression in this organ, supports further investigation into DBC-mediated immunotoxicity.
Keywords: dibenzo[def,p]chrysene; immunosuppression; DNA adduct; Cyp1b1; mass spectrometry; spleen
1. Introduction
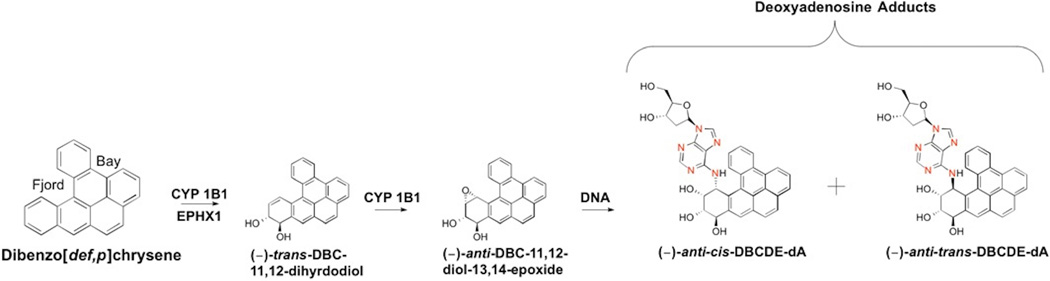
Dibenzo[def,p]chrysene (DBC; also know as dibenzo[a,l]pyrene) is a commonly identified component of polycyclic aromatic hydrocarbon (PAH) mixtures associated with cigarette smoke, coal smoke, and outdoor air pollution [1–3]. The International Agency for Research on Cancer (IARC) classifies DBC as a class 2A probable human carcinogen [4]. In laboratory animals DBC is 30 times more carcinogenic than the well studied benzo[a]pyrene (BaP) [4]. Previous reports have shown wild-type (WT) mice orally gavaged with DBC develop cancerous lesions in several tissues including ovaries, lymphoid tissue, and skin [5, 6]. The schematic in Figure 1 represents DBC bioactivation by cytochrome P450 (CYP1) 1B1 and epoxide hydrolase (EPHX1) to form the ultimate carcinogen DBCDE [7, 8]. CYP1B1 is primarily responsible for DBC bioactivation evidenced by several in vitro studies [9, 10]. Using a Cyp1b1 knockout (KO) mouse model Buters et al. [5] reported oral exposure to DBC in mice lacking Cyp1b1 resulted in lower tumor formation and restriction of carcinogenic lesions primarily to the lung. Additionally, DBC preferentially forms stable (−)-anti-cis-DBCDE-dA and (−)-anti-trans-DBCDE-dA adducts at the N6 position of deoxyadenosine (N6dA) rather than the N2 position on deoxyguanine (N2dG) as with BaP due to the presence of a sterically hindered fjord region [11]. The preferential formation of N6dA adducts contributes to DBC carcinogenicity as these DNA lesions are more resistant to nucleotide excision repair than N2dG adducts [12].
Figure 1. Schematic of DBC bioactivation to DBC-11,12-diol-13,14-epoxide (DBCDE) by cytochrome P450 1B1 and EPHX1.
In the presence of DNA DBCDE binds covalently to DNA preferentially at the N6-position of deoxyadenosine forming the major adducts (−)-anti-cis-DBCDE-dA and (−)-anti-trans-DBCDE-dA. Location of stable 15N isotopes incorporated into the internal standards are shown in red.
PAH genotoxicity is not only associated with carcinogenesis, but also correlated with the immunosuppressive properties of PAHs [13, 14]. Recently, DBC was reported to be strongly immunosuppressive as measured by the T-cell dependent antibody response (TDAR) ex vivo model at levels comparable to the proven immunotoxicant, 7,12,-dimethylbenz[a]anthracene (DMBA) [15, 16]. Considering the established immunosuppressive properties of DBC this study was designed to examine adduct formation in the spleen and thymus of WT and Cyp1b1 KO mice using a highly sensitive stable-isotope dilution UHPLC-MS/MS method.
2. Materials and Methods
2.1 Animals
All animal work was performed with approval from the Institutional Animal and Care Use Committee at Oregon State University. Eight to ten week old male WT (n = 4) (Jackson Laboratories, Bar Harbor, ME) and Cyp1b1 KO (n = 5) (Dr. Frank Gonzalez, NCI) mice on the same C57BL/6J genetic background were maintained on a 12-hour light/dark cycle with rodent chow and water ad libitum. Mice were dosed with 10 mg/kg DBC via oral gavage for 5 consecutive days. The mice were euthanized via CO2 asphyxiation 3 days after the final exposure. Target tissues (spleen, thymus, lung, liver) were collected, snap-frozen in liquid nitrogen, and stored at −80°C until analysis.
2.2 Reagents and Chemicals
Dibenzo[def,p]chrysene was purchased from Midwest Research Institute (Kansas City, MO). Unlabeled (±)-anti-cis-DBCDE-dA and (±)-anti-trans-DBCDE-dA standards, and 15N-labeled (±)-anti-cis-DBCDE-dA and (±)-anti-trans-DBCDE-dA standards were synthesized by the laboratory of Dr. Shantu Amin at Penn State University.
2.3 Preparation of DNA
DNA was isolated from frozen tissue using Qiagen Genomic Tip 500/G (Qiagen, Germantown, MD) or Roche DNA Isolation Kit for Cells and Tissues (Roche, Indianapolis, IN) as described by the manufacturer. One hundred µg of DNA in a final volume of 450 µl water with 10 mM MgCl2 was submitted to a series of enzymatic steps to prepare individual deoxynucleotides: 1) 375 units Dnase I (Life Technologies, Carlsbad, CA) at 37°C for 1.5 hours; 2) 7 units nuclease P1 (Sigma-Alrich, St. Louis, MO) at 37°C for 1.5 hours; 3) 30 units shrimp alkaline phosphatase (Thermo Scientific, Waltham, MA) at 37°C for 1 hours. After DNA digestion, a 50 µl aliquot was set aside for HPLC base analysis. Forty pg of 15N-labeled (±)-anti-cis-DBCDE-dA and (±)-anti-trans-DBCDE-dA was spiked into each sample to serve as an internal standard. One ml ethanol was added to each sample and the sample was incubated at −20°C overnight. The following day the samples were centrifuged at 14,000g for 10 minutes at 4°C. The supernatant was then transferred to a new 1.7 ml microcentrifuge tube and evaporated under a nitrogen stream to concentrate the sample to 50 µl. Fifty µl of methanol was added to bring the samples to 100 µl (1 µg DNA/µl). The samples were filtered through a costar-X spin tube before being transferred to an autosampler vial.
2.4 HPLC Base Analysis
The aliquot of digested DNA reserved from sample preparation for adduct analysis was subjected to HPLC analysis using an Alliance 2695 HPLC (Waters, Milford, MA) with a 2996 photodiode array detector (PDA), and a Luna 3 µm C18, 150 × 3 mm column (Phenomenex, Torrance, CA). Solvent A was 20 mM ammonium formate (pH 4.5), and solvent B was acetonitrile. The flow rate was 0.4 ml per minute. The gradient used was 99% A and 1% B for 3 minutes, followed by a 12 minute ramp from 99% A to 60% A and 1% B to 40% B. The column and samples were kept at 30°C during the run. A wavelength of 254 ηm was chosen for sample detection. Deoxyguanosine (dG) and deoxyadenosine (dA) standards eluted at 9.3 and 10.2 minutes respectively. A calibration curve for each standard was constructed and sample base levels were calculated by interpolation of the curve using Empower 2 software (Waters, Milford, MA). The number of dA calculated in each sample was used to present the data as DBCDE-dA/1×108 dA.
2.5 Stable isotope dilution UHPLC-MS/MS
Adduct analysis was performed using a 4000 QTRAP hybrid triple quadrupole/linear ion trap LC-MS/MS system (AB Sciex, Redwood City, CA) interfaced with a Flexar UHPLC system (Perkin-Elmer, Waltham, MA) using an Acuity UPLC 1.7 µm BEH C18, 100 × 2.1 mm column (Waters, Milford, MA). The UHPLC solvent method utilized a gradient of solvent A (5 mM NH4OAc + 0.1% formic acid in HPLC grade water) and solvent B (acetonitrile) at a flow rate of 500 µl/min. The 7.5 minute gradient was as follows: 1) 0.5 minute 80% A and 20% B; 2) 6 minutes to 10% A and 90% B; 3) 0.1 min 10% A and 90% B; 4) 0.9 minute wash out 10% A and 90% B. The MS parameters were set as follows: electrospray ionization – positive mode; electrospray source temperature – 600°C; declustering potential – 60 eV; collision energy – 30 eV; entrance potential – 10 eV; cell exit potential – 10 eV; collision activated dissociation gas – high; curtain gas – 20 psi. Adducts were analyzed in multiple reaction monitoring (MRM) mode with the following transitions 604.2/335.0, 604.2/317.0, 604.2/289.1 (targeted adducts) and 609.2/335.0, 609.2/317.0, 609.2/289.1 (internal standards). Peak integration was performed using Analyst 1.5.2 Software (AB Sciex, Redwood City, CA).
2.5.1 Method Validation
The UHPLC-MS/MS method was validated by spiking 100 µg of control sample matrix with fixed amounts of (±)-anti-cis-DBCDE-dA internal standard and increasing amounts of unlabeled (±)-anti-cis-DBCDE-dA (0.05, 0.12, 0.17, 0.50, 1.66 fmol/µl) and plotting the observed concentrations against the spiked concentrations.
2.5.2 Calibration Curve
A calibration curve for each adduct isomer was set up using unlabeled (±)-anti-cis-DBCDE-dA and (±)-anti-trans-DBCDE-dA adducts (0.1, 0.3, 3.0 pg/µl) mixed with 0.4 pg/µl 15N-labeled adducts in a 100 µg DNA matrix.
2.6 Statistical Analysis
Statistical analysis was carried out using Prism 6.0 (Graphpad, La Jolla, CA). One-way Analysis of Variance (Anova) with Tukey’s multiple comparisons test was carried out to determine significant difference in adduct formation between tissues in treatments groups and isomers within the same treatment. A p-value of ≤ 0.05 was considered significant. Percentage of adducts was calculated using the following equation: (Total Adducts WT) – (Total Adducts KO)/(Total Adducts WT).
3. Results
3.1 UHPLC-MS/MS Method Validation
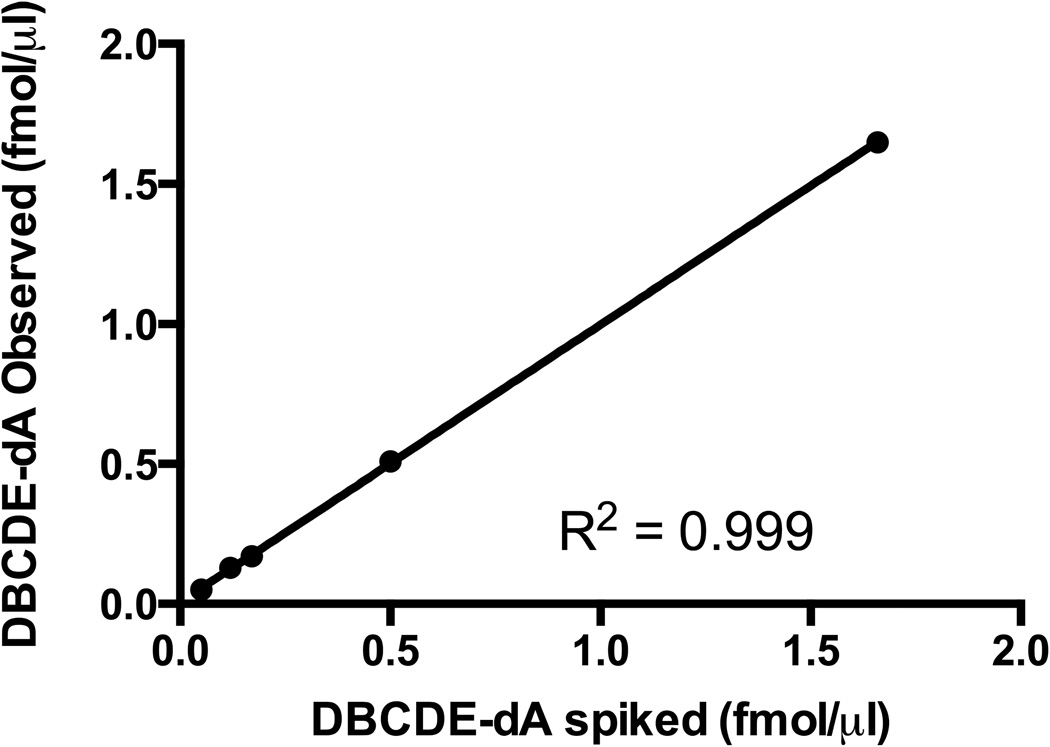
The UHPLC-MS/MS method was validated using a spiked control matrix (0.05, 0.12, 0.17, 0.50, 1.66 fmol/µl) and plotting the measured analyte concentrations against the spiked concentrations. Linear regression revealed an R2 of 0.999 with a slope of 0.997 indicating accurate quantitation with the developed protocol (Figure 2). The limit of quantitation (LOQ) was determined to be 0.12 fmol/µg DNA (10 times signal-to-noise ratio) using standards prepared as described in Materials and Methods 2.5.1.
Figure 2. UHPLC-MS/MS method validation.
The stable-isotope dilution UHPLC-MS/MS method was validated using 100 µg spiked control matrix (0.05, 0.12, 0.17, 0.50, 1.66 fmol/µl (±)-anti-cis-DBCDE-dA) and plotting the measured analyte concentrations against the spiked concentrations. Linear regression revealed an R2 of 0.999 with a slope of 0.997 indicating accurate quantitation with the developed protocol. The limit of quantitation (LOQ) was determined to be 0.12 fmol/µg DNA (10 times signal-to-noise ratio).
Although the (−)-anti-cis and (−)-anti-trans isomers are the major stereoisomers formed after CYP 1B1 metabolism in mammalian systems, our 7.5 minute UHPLC-MS/MS method did not separate the 15N-labeled optically distinct (−)-anti-cis from the (+)-anti-cis, nor the (−)-anti-trans from the (+)-anti-trans internal standards. As a result we are not able to definitively distinguish the (−) and (+) stereoisomers, but are confident the overwhelming majority of the adducts are (−)-anti isomers based on previous studies characterizing DBC bioactivation and adduction [6, 17, 18].
3.2 Identification and Quantitation of DBCDE-dA adducts after oral exposure
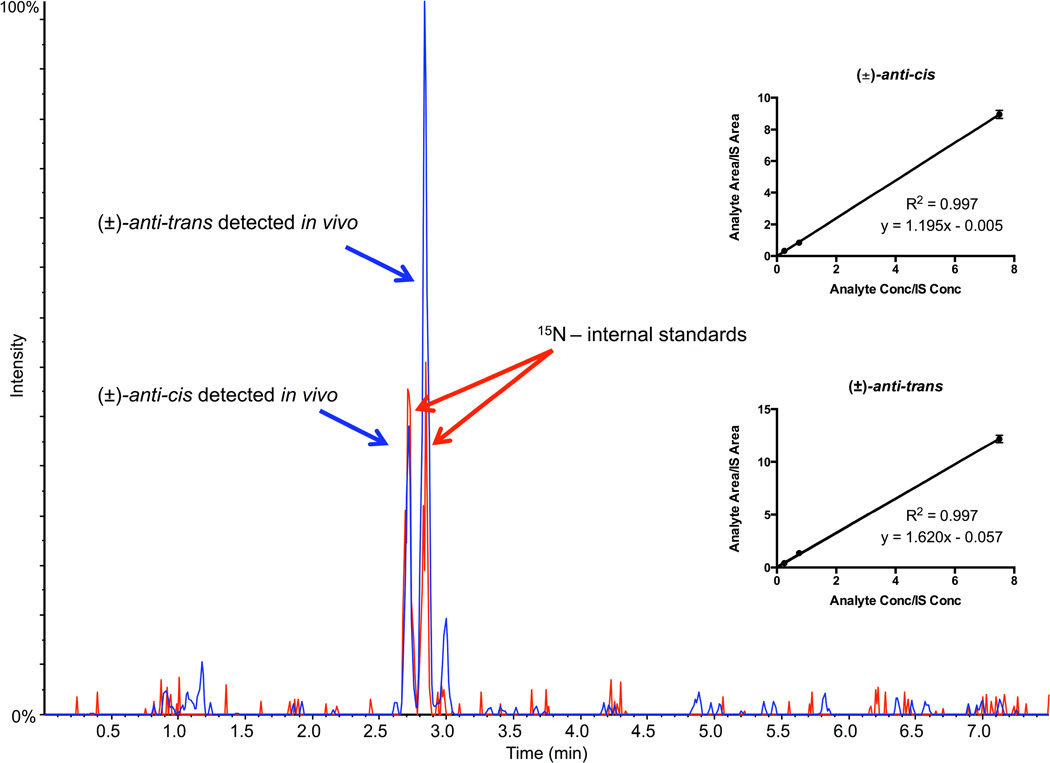
Successful identification of the target (±)-anti-cis-DBCDE-dA and (±)-anti-trans-DBCDE-dA adduct isomers with co-elution of spiked internal standards is indicated by the two peaks in the representative MRM chromatogram using liver DNA from a WT gavaged mouse (Figure 3). Calibration curves for both isomers appear to be linear (Figure 3 inset).
Figure 3. Representative MRM chromatogram of (±)-anti-DBCDE-dA adducts obtained from mice orally exposed to 10 mg/kg DBC for 5 days and euthanized 3 days after final exposure.
The chromatogram shows separation of major adducts (±)-anti-cis-DBCDE-dA and (±)-anti-trans-DBCDE-dA using the 7.5-minute stable-isotope dilution UPLC-MS/MS method. The blue peaks represent the target adducts (m/z 604.2 → m/z 335.0) detected in vivo, while the red peaks represent the spiked 15N-labeled (±)-anti-cis-DBCDE-dA and (±)-anti-trans-DBCDE-dA internal standards (m/z 609.2 → m/z 335.0). Figure insets represent linear calibration curves for each (±)-anti-cis-DBCDE-dA and (±)-anti-trans-DBCDE-dA adduct.
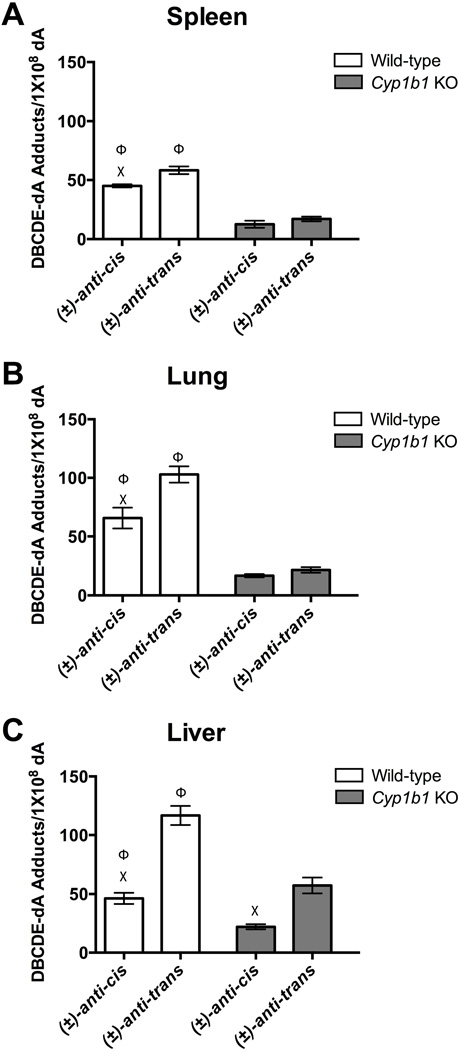
Figure 4A represents adduct formation in the spleen of WT and Cyp1b1 KO mice. Statistical analysis indicates the formation of (±)-anti-cis-DBCDE-dA and (±)-anti-trans-DBCDE-dA adducts are significantly decreased in the spleen of Cyp1b1 KO mice compared to gavaged WT mice (Figure 4A). Additionally, the (±)-anti-trans adduct is formed preferentially over the (±)-anti-cis adduct in the gavaged WT group. Adduct levels in the thymus of WT and Cyp1b1 KO mice were below the LOQ, making meaningful interpretation unreliable.
Figure 4. DBCDE-dA Adduct Quantitation.
Quantitation of (±)-anti-cis-DBCDE-dA and (±)-anti-trans-DBCDE-dA adducts in spleen (A), lung (B), and liver (C) of wild-type (n = 4) or Cyp1b1 KO (n = 5) mice exposed orally to 10 mg/kg DBC daily for 5 days and euthanized 3 days after the final exposure. All tissue from WT mice gavaged (white bars) had significantly higher levels of (±)-anti-trans-DBCDE-dA adduct formation compared to (±)-anti-cis-DBCDE-dA adducts consistent with previous studies. Additionally, the loss of Cyp1b1 (light grey bars) significantly reduced both adducts in all three tissues. One-way Analysis of Variance (Anova) with Tukey’s multiple comparisons test was carried out to determine significant difference in adduct formation between tissues within different treatment groups and isomers within the same treatment. A p-value of ≤ 0.05 was considered significant. (Χ = significant difference between (±)-anti-cis-DBCDE-dA and (±)-anti-trans-DBCDE-dA adducts of the same tissue and treatment group; Φ = significant difference between indicated isomer for treatment and Cyp1b1 KO group
Furthermore, we measured adduct formation in the liver and lung for both treatment groups (Figure 4B and 4C). In gavaged WT mice the (±)-anti-trans adduct is formed at significantly higher levels than the (±)-anti-cis adduct in both organs consistent with adduct formation in the spleen. Cyp1b1 KO adducts in the liver and lung are significantly reduced in each tissue as expected based on predominant DBCDE formation by Cyp1b1 [9, 20]. The (±)-anti-trans isomer is preferentially formed in Cyp1b1 KO liver, whereas the (±)-anti-cis and (±)-anti-trans isomers in Cyp1b1 KO lung are formed at comparable levels.
4. Discussion
The primary mechanism for DBC induced carcinogenesis occurs through the formation of covalently bound DBCDE-DNA adducts resulting in genotoxicity [21]. Additionally, PAH genotoxicity is correlated with immunotoxicity [14]. This study provides the first evidence of DBC-adduct formation in the spleen of mice treated orally with DBC (Figure 4). Adduct identification provides mechanistic support for the immunosuppressive properties of DBC established previously using T-cell dependent and independent antibody response assays [15, 16]. Using a UHPLC-MS/MS method we are able to quantitate (±)-anti-cis-DBCDE-dA (45 DBCDE-dA/1×108 dA) and (±)-anti-trans-DBCDE-dA (58 DBCDE-dA/1×108 dA) adducts in the spleen of WT mice euthanized 3 days after 5 consecutive days of oral gavage with 10 mg/kg DBC. Identification of the (±)-anti-cis and (±)-anti-trans adducts is consistent with the stereoselectivity reported in other DBC target tissues (Figure 3) [6, 7, 11, 18]. Direct application of DBC to the oral cavity by Zhang et al. [18] resulted in high levels of adduct formation surpassing the current study’s maximum measured level (116 (±)-anti-trans-DBCDE-dA/1×108 dA). However, our route of administration (oral gavage) differs significantly compared to direct application of the target tissue making direct comparison of the two studies difficult.
In the current study DBCDE-dA adducts detected in the thymus were below the LOQ for the method. Lauer et al. [15], reported DBC exposure results in a greater T-cell-dependent antibody response than a T-cell-independent antibody response suggesting low level adduct formation in the thymus may contribute to the T-cell dependent-immunosuppression identified in the spleen. However, we do not know the level of adduct formation required for DBC-mediated immunosuppression emphasizing the need for future studies investigating DBC genotoxicity and immunosuppression. Furthermore, we provide the first evidence using a highly sensitive UHPLC-MS/MS method that the loss of Cyp1b1 expression significantly reduces adduct formation in the spleen, lung, and liver (Figure 4). Reduced adduct formation in Cyp1b1 KO mice support earlier studies identifying Cyp1b1 as the major Cyp responsible for DBCDE-dA adduct formation [5, 9, 11]. Cyp1b1 is also critical for DMBA-induced spleen cell immunotoxicity in mice [22]. The spleen is known for high levels of constitutive Cyp1b1 expression, and as a result is susceptible to genotoxicity through bioactivation [14, 23]. The reduced adduct formation identified in the spleen would suggest Cyp1b1 KO mice should be less susceptible to DBC-mediated splenic immunotoxicity.
DBC adduct formation in the liver of Cyp1b1 KO mice retained the cis:trans (2:5) ratio identified in the liver of the gavaged WT group, whereas lung and spleen isomer formation occurred at equal levels. Adduct formation in the lung of Cyp1b1 KO animals is lower than expected based on a previous study using Cyp1b1 KO mice reporting oral treatment with DBC reduces tumor incidence and restricts carcinogenic lesions to the lung [5]. Although adduct formation was not examined, the high number of lung lesions found by Buters et al. [5], were attributed to Cyp1a1 bioactivation and DBCDE-adduct genotoxicity. Supporting studies performed using V79 Chinese hamster cells stably expressing either human, rat, or mouse CYP1A1 or CYP1B1 indicate only mouse Cyp1a1 is able to form the nonpolar DBCDE-adducts associated with Cyp1b1 bioactivation [5, 20]. Using Cyp1b1 KO mice, the current study reports additional mouse Cyp enzymes (most likely 1a1) are capable of forming DBCDE-adducts, but at low levels suggesting other factors may be contributing substantially to DBC carcinogenicity in the lung. Further investigation is necessary to fully understand CYP1B1 and 1A1 mechanism(s) of carcinogenicity and immunotoxicity.
5. Conclusion
Using a highly sensitive UHPLC-MS/MS method we identified for the first time DBCDE-dA adduct formation in the spleen of mice providing linkage between DBC exposure and immunotoxicity. Furthermore, utilizing Cyp1b1 KO mice we show Cyp1b1 is responsible for 71% of DBCDE-dA adducts formed in the spleen. Taking Cyp1b1 bioactivation into account the current study suggests Cyp1b1 KO mice should be less susceptible to DBC-induced immunotoxicity. The high levels of DBC-DNA adducts identified in the spleen, along with the known high levels of Cyp1b1 expression in this organ, supports further investigation into DBC-mediated immunotoxicity.
Highlights.
Stable-isotope dilution UHPLC-MS/MS was used to detect DBCDE-dA adducts
DBCDE-dA adducts were identified at high levels in the spleen of wild-type mice
Cyp1b1 KO mice have significantly reduced adducts in liver, lung, and spleen
Cyp1b1 accounts for 71% of DBCDE-dA adducts formed in the spleen
Acknowledgements
The authors would like to thank Dr. Frank Gonzalez of the National Cancer Institute for providing the Cyp1b1 KO mice and Dr. Shantu Amin for providing the DBCDE-dA standards. Additionally, the authors would like to thank Beth Siddens for her help with the DNA digestion protocol, and Erin Madeen for her help with the HPLC base analysis protocol. This study was supported by the NIH grants P42 ES016465, P30 ES000210, R01 ES019968 and P01CA90890. TAH was supported by T32 ES007060.
Abbreviations
- DBC
dibenzo[def,p]chrysene
- DBCDE
dibenozo[def,p]chrysene-(±)-11,12-dihydrodiol-13,14-epoxide
- PAH
polycyclic aromatic hydrocarbon
- BaP
benzo[a]pyrene
- DMBA
7,12,-dimethylbenz[a]anthracene
- EPHX1
epoxide hydrolase
- TDAR
T cell-dependent antibody response
- UHPLC
ultra-high performance liquid chromatography
- MS/MS
tandem mass spectrometry
- MRM
multiple reaction monitoring
- LOQ
limit of quantitation
- CYP
cytochrome P450
- dA
deoxyadenosine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CYP1B1 denotes human protein; Cyp1b1 denotes mouse protein; CYP1B1 and Cyp1b1 denotes human and mouse gene, respectively
Contributor Information
Tod A. Harper, Jr., Email: tod.harper@oregonstate.edu.
Jeff Morré, Email: jeff.morre@oregonstate.edu.
Fredine T. Lauer, Email: flauer@salud.unm.edu.
Tammie J. McQuistan, Email: tammie.mcquistan@oregonstate.edu.
Jessica M. Hummel, Email: hummelj@onid.oregonstate.edu.
Scott W. Burchiel, Email: sburchiel@salud.unm.edu.
David E. Williams, Email: david.williams@oregonstate.edu.
References
- 1.Jia Y, Stone D, Wang W, Schrlau J, Tao S, Simonich SL. Estimated reduction in cancer risk due to PAH exposures if source control measures during the 2008 Beijing Olympics were sustained. Environmental Health Perspectives. 2011;119:815–820. doi: 10.1289/ehp.1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mumford JL, Li X, Hu F, Lu XB, Chuang JC. Human exposure and dosimetry of polycyclic aromatic hydrocarbons in urine from Xuan Wei, China with high lung cancer mortality associated with exposure to unvented coal smoke. Carcinogenesis. 1995;16:3031–3036. doi: 10.1093/carcin/16.12.3031. [DOI] [PubMed] [Google Scholar]
- 3.Orris L, Van Duuren BL, Kosak AI, Nelson N, Schmitt FL. The carcinogenicity for mouse skin and the aromatic hydrocarbon content of cigarette-smoke condensates. Journal of the National Cancer Institute. 1958;21:557–561. [PubMed] [Google Scholar]
- 4.IARC. Some non-heterocyclic polycyclic aromatic hydrocarbons and some realted exposures. IARC monographs on the evaluation of carcinogenic risks to humans / World Health Organization, International Agency for Research on Cancer. 2010;92:1–853. [PMC free article] [PubMed] [Google Scholar]
- 5.Buters JT, Mahadevan B, Quintanilla-Martinez L, Gonzalez FJ, Greim H, Baird WM, Luch A. Cytochrome P450 1B1 determines susceptibility to dibenzo[a,l]pyrene-induced tumor formation. Chemical Research in Toxicology. 2002;15:1127–1135. doi: 10.1021/tx020017q. [DOI] [PubMed] [Google Scholar]
- 6.Chen KM, Zhang SM, Aliaga C, Sun YW, Cooper T, Gowdahalli K, Zhu J, Amin S, El-Bayoumy K. Induction of ovarian cancer and DNA adducts by Dibenzo[a,l]pyrene in the mouse. Chemical Research in Toxicology. 2012;25:374–380. doi: 10.1021/tx2004322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ralston SL, Lau HH, Seidel A, Luch A, Platt KL, Baird WM. The potent carcinogen dibenzo[a,l]pyrene is metabolically activated to fjord-region 11,12-diol 13,14-epoxides in human mammary carcinoma MCF-7 cell cultures. Cancer Research. 1994;54:887–890. [PubMed] [Google Scholar]
- 8.Arif JM, Gupta RC. Microsome-mediated bioactivation of dibenzo[a,l]pyrene and identification of DNA adducts by 32P-postlabeling. Carcinogenesis. 1997;18:1999–2007. doi: 10.1093/carcin/18.10.1999. [DOI] [PubMed] [Google Scholar]
- 9.Luch A, Coffing SL, Tang YM, Schneider A, Soballa V, Greim H, Jefcoate CR, Seidel A, Greenlee WF, Baird WM, Doehmer J. Stable expression of human cytochrome P450 1B1 in V79 Chinese hamster cells and metabolically catalyzed DNA adduct formation of dibenzo[a,l]pyrene. Chemical Research in Toxicology. 1998;11:686–695. doi: 10.1021/tx970236p. [DOI] [PubMed] [Google Scholar]
- 10.Luch A, Kishiyama S, Seidel A, Doehmer J, Greim H, Baird WM. The K-region trans-8,9-diol does not significantly contribute as an intermediate in the metabolic activation of dibenzo[a,l]pyrene to DNA-binding metabolites by human cytochrome P450 1A1 or 1B1. Cancer Research. 1999;59:4603–4609. [PubMed] [Google Scholar]
- 11.Ralston SL, Seidel A, Luch A, Platt KL, Baird WM. Stereoselective activation of dibenzo[a,l]pyrene to (−)-anti (11R,12S,13S,14R)- and (+)-syn(11S,12R,13S,14R)-11,12-diol-13,14-epoxides which bind extensively to deoxyadenosine residues of DNA in the human mammary carcinoma cell line MCF-7. Carcinogenesis. 1995;16:2899–2907. doi: 10.1093/carcin/16.12.2899. [DOI] [PubMed] [Google Scholar]
- 12.Kropachev K, Kolbanovskiy M, Liu Z, Cai Y, Zhang L, Schwaid AG, Kolbanovskiy A, Ding S, Amin S, Broyde S, Geacintov NE. Adenine-DNA adducts derived from the highly tumorigenic Dibenzo[a,l]pyrene are resistant to nucleotide excision repair while guanine adducts are not. Chemical Research in Toxicology. 2013;26:783–793. doi: 10.1021/tx400080k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page TJ, O'Brien S, Holston K, MacWilliams PS, Jefcoate CR, Czuprynski CJ. 7,12-Dimethylbenz[a]anthracene-induced bone marrow toxicity is p53-dependent. Toxicological Sciences. 2003;74:85–92. doi: 10.1093/toxsci/kfg115. [DOI] [PubMed] [Google Scholar]
- 14.Uno S, Dalton TP, Dragin N, Curran CP, Derkenne S, Miller ML, Shertzer HG, Gonzalez FJ, Nebert DW. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Molecular Pharmacology. 2006;69:1103–1114. doi: 10.1124/mol.105.021501. [DOI] [PubMed] [Google Scholar]
- 15.Lauer FT, Walker MK, Burchiel SW. Dibenzo[def,p]chrysene (DBC) suppresses antibody formation in spleen cells following oral exposures of mice. Journal of Toxicology and Environmental Health. Part A. 2013;76:16–24. doi: 10.1080/15287394.2012.722521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, Lauer FT, Liu KJ, Hudson LG, Burchiel SW. Low-dose synergistic immunosuppression of T-dependent antibody responses by polycyclic aromatic hydrocarbons and arsenic in C57BL/6J murine spleen cells. Toxicology and Applied Pharmacology. 2010;245:344–351. doi: 10.1016/j.taap.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luch A. On the impact of the molecule structure in chemical carcinogenesis. Exs. 2009;99:151–179. doi: 10.1007/978-3-7643-8336-7_6. [DOI] [PubMed] [Google Scholar]
- 18.Zhang SM, Chen KM, Aliaga C, Sun YW, Lin JM, Sharma AK, Amin S, El-Bayoumy K. Identification and quantification of DNA adducts in the oral tissues of mice treated with the environmental carcinogen dibenzo[a,l]pyrene by HPLC-MS/MS. Chemical Research in Toxicology. 2011;24:1297–1303. doi: 10.1021/tx200188j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahadevan B, Luch A, Bravo CF, Atkin J, Steppan LB, Pereira C, Kerkvliet NI, Baird WM. Dibenzo[a,l]pyrene induced DNA adduct formation in lung tissue in vivo. Cancer Letters. 2005;227:25–32. doi: 10.1016/j.canlet.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 20.Luch A, Schober W, Soballa VJ, Raab G, Greim H, Jacob J, Doehmer J, Seidel A. Metabolic activation of dibenzo[a,l]pyrene by human cytochrome P450 1A1 and P450 1B1 expressed in V79 Chinese hamster cells. Chemical Research in Toxicology. 1999;12:353–364. doi: 10.1021/tx980240g. [DOI] [PubMed] [Google Scholar]
- 21.Melendez-Colon VJ, Luch A, Seidel A, Baird WM. Cancer initiation by polycyclic aromatic hydrocarbons results from formation of stable DNA adducts rather than apurinic sites. Carcinogenesis. 1999;20:1885–1891. doi: 10.1093/carcin/20.10.1885. [DOI] [PubMed] [Google Scholar]
- 22.Gao J, Lauer FT, Dunaway S, Burchiel SW. Cytochrome P450 1B1 is required for 7,12-dimethylbenz(a)-anthracene (DMBA) induced spleen cell immunotoxicity. Toxicological Sciences. 2005;86:68–74. doi: 10.1093/toxsci/kfi176. [DOI] [PubMed] [Google Scholar]
- 23.Bieche I, Narjoz C, Asselah T, Vacher S, Marcellin P, Lidereau R, Beaune P, de Waziers I. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenetics and Genomics. 2007;17:731–742. doi: 10.1097/FPC.0b013e32810f2e58. [DOI] [PubMed] [Google Scholar]