Abstract
Objective
Pupillometry has been used to assess both pain intensity and response to analgesic medications in adults. The aim of this observational study was to explore proof-of-concept for the use of this technique in paediatric patients. Changes in pupil parameters before and after opioid exposure also were evaluated.
Design and Setting
This was a single-center, prospective study conducted at an academic paediatric medical center.
Patients
Children 9-17 years of age undergoing elective surgical correction of pectusexcavatum were enrolled into a protocol approved by the human ethical committee (IRB).
Interventions
Pupil size and reactivity were measured using a hand-held pupillometer. Pain was assessed using age-appropriate, validated pain self-report scales.
Results
Thirty patients were enrolled. Each point change on a 10 cm visual analog pain intensity scale was associated with a statistically significant mean change of 0.11 mm/s in maximum pupil constriction velocity, and of approximately 0.4% in pupil diameter. As expected, there was an association between total opioid dose (expressed as morphine equivalents) and pupil diameter. Age, sex, and baseline anxiety scores did not correlate significantly with pupillary response.
Conclusion
The association of both maximum pupillary constriction velocity and diameter with pain scores illustrates the potential for using pupillometry as a non-invasive method to objectively quantitate pain response/intensity in children. The technique holds promise as a pharmacodynamic “tool” to assess opioid response in paediatric patients.
Keywords: pain, pharmacology, analgesia, technology
Background
Research over the past several decades has led to significant improvement in the recognition and treatment of pain in children.1,2 However, despite significant advances in the understanding of the mechanisms of pain,3 considerable gaps remain in our knowledge of how pain is interpreted and expressed by children. As a consequence of the challenges in quantifying pain in children, management often is suboptimal.4-6
Self-reported pain ratings are the primary metric for quantifying pain because of their ease of use and compatibility with the commonly held belief that pain is best defined by the individual's own perception.7 However, self- reports of pain by children reflect an array of developmental, psychological, and social influences that may obscure the information required to make a reliable assessment of pain and analgesic response.8-10 Furthermore, self-reporting tools are not useful in non-verbal infants or children.
In adults, pupillometry has been studied as a method to quantitate pain11 and to assess opioid pharmacodynamics.12,13 The extent of pupil dilatation can provide an index of nociceptive input via autonomic innervation of the iris muscles,14,15 while the extent of attenuation in this pupillary response during exposure to opioid analgesics can provide an index of pharmacological effect by reflecting the extent of occupancy of mu and kappa opioid receptors in the central nervous system.16 Currently available handheld pupillometers are safe, well-tolerated by children, easy to use, and modestly priced.17 They have potential for providing an objective measurement of pain intensity and treatment response in children. To determine their utility in paediatric patients, we assessed pupillary response in a cohort of patients following a painful surgical procedure by comparing pupillometry measurements with simultaneous self-reported pain intensity.
Methods
This was a single-center, prospective, within-subjects design study approved by the local institutional review board and conducted at an academic paediatric medical center. Written permission/assent was obtained from all participants. Participants were enrolled from a population of children undergoing pectusexcavatum repair with bar placement. All patients were given the opportunity to participate in the study. Postoperatively, all patients received either patient controlled analgesia (PCA) or analgesia via epidural catheter.18 Participants were excluded if they had inadequate baseline cognitive functioning or understanding of the English language to adequately understand the pain self-report questionnaires, or if they had any known allergies to the pain medications used in the post-operative pain management protocol. The final sample contained 30 patients between the ages of 9 and17 years (63% male; 97% Caucasian; 3% African-American). Of the study participants, 13 received epidural analgesia while 16 were on intravenous PCA pumps. During the study, five participants that initially had an epidural switched to PCA.
Pre-operative medication and anesthesia was standardized in all patients. At the conclusion of surgery, participants receiving PCA were started on IV hydromorphone by PCA (approximately 5-6 mcg/kg continuous infusion, 5-6 mcg/kg demand dose, with a 6 minute lockout) with a loading dose if necessary. An additional 8 mcg/kg dose of hydromorphone was available every 2 hours for self-reported pain scores greater than 4/10. For participants who received epidural analgesia, a thoracic epidural catheter was placed at the level of T6 to T8. An initial bolus of approximately 0.3 mL/kg of 0.1% ropivacaine, fentanyl (1-1.2 mcg/kg), and clonidine (1.8-2 mcg/kg) was administered, followed by an infusion of 0.1% ropivacaine, fentanyl (2 mcg/mL) or hydromorphone (4 mcg/mL), and clonidine (1 or 1.5 mcg/mL) solution at approximately 0.3 mL/kg/hour. Post-operatively, bolus doses of 0.05 mL/kg of the ropivacaine/fentanyl/clonidine solution were available every 20 minutes for pain scores greater than 4/10 (maximum of 2 mL/dose or a cumulative dose of 14 mL/hour). Patients still in pain received ropivacaine 0.2%, lidocaine 1%, or a bolus of clonidine (1 mcg/kg; maximum every 8 hours). If pain relief while on the epidural protocol was judged to be inadequate, participants were then switched to IV analgesia via morphine or hydromorphone PCA. No pupillometry assessments were collected on post-operative day 0 in order to avoid confounding pupillometry readings by medications administered perioperatively. Starting on post-operative day 1, the cumulative dose of opioid analgesics administered three hours prior to each pain assessment was recorded and converted to intravenous parenteral morphine-equivalent doses expressed in milligrams using published conversion factors (0.15 for hydromorphone, 0.01 for fentanyl, and a factor of 3 for epidural to systemic conversion).19 The total equianalgesic dose was divided by patient weight to derive a standardized dose equivalent expressed as mg/kg of morphine equivalent.
Self-Report Pain Measures
Pain intensity was assessed at rest at baseline (pre-operatively) and every 3 hours during post-operative days 1-3. At each pain assessment, self-reported pain scores were recorded using a 10 cm horizontal visual analog scale (VAS) with anchors of no pain (0) and worst possible pain (10).20 Pain scores related to movement (i.e. coughing) were not assessed in the study participants. Information on emotional status was assessed at each pupillometry reading using an abbreviated form of the Positive and Negative Affect Schedule for Children (PANAS-C), a previously validated technique.21
Pupillometry Technique
Pupillometry measurements were obtained by a research coordinatorat baseline (pre-operatively) and then simultaneously with self-reported pain intensity every 3 hours beginning at 0800 and ending at 2000 during post-operative days 1-3 (5 readings per day). Each pupillometry measurement was performed in triplicate, and the average of the three measurements served as the final data point for each time period. Potential bias associated with operator error was minimized by having only three individuals, all fully trained in the use of the pupillometer, record all observations.
ANeurOptics PLR-100® infrared pupillometer (NeurOptics Inc., Irvine, CA) was used to measure dark-adapted pupil diameter and pupillary response dynamics under controlled dim light conditions using the standardized procedure recommended by the manufacturer. The procedure involves positioning the soft rubber cup of the instrument over the eye to be used for measurement. The resting (maximum) pupil diameter (mm) is then recorded, followed by measurement of the pupil constriction velocity (mm/s), minimum diameter (mm), time to minimum diameter (s), constriction amplitude (resting minus minimum diameter), relaxation velocity (mm/s), and final diameter (mm) in response to a standard light stimulus. The procedure is brief, non-invasive, has no associated risk, and has been validated as a measure of analgesic drug effect in adults.16,22
Data Analysis
The primary hypothesis tested was that pupillometry measurements would correlate with post-operative self-report pain scores. A secondary hypothesis was that pupillometry measurements would correlate with opioid exposure expressed as morphine equivalents. Hierarchical linear modeling (HLM) was used to test the primary and secondary study hypotheses. HLM is a recommended analytic approach used to establish associations for variables measured serially over time within each individual.23 In HLM models, the dependent variable (a given pupil parameter) is regressed onto predictor variables (pain and opioid exposure) for each individual at every time point while controlling for unknown sources of measurement error. Individual models are then averaged together to determine the overall unique relationship between each predictor and the outcome variable (i.e., the expected change in the outcome variable for each unit change in the given predictor variable) while controlling for error from unspecified individual differences. To test the primary study hypothesis, model b-coefficients representing the average relationship between pain intensity and pupil parameters (while controlling for opioid exposure) were evaluated for statistical significance against a t-distribution. If significant, this was interpreted as support for the primary study hypothesis. Similarly, model b-coefficients representing the average relationship between opioid exposure and pupil parameters (while controlling for pain) were evaluated for statistical significance against a t-distribution. If significant, this was interpreted as support for the secondary study hypothesis. Child age, sex, and baseline anxiety were evaluated in all models as covariates and removed if they did not significantly moderate findings. Estimates of central tendency (mean, median) and variability (standard deviation, quartiles) were computed on the primary study variables for descriptive purposes and for evaluating statistical assumptions. HLM was conducted using HLM6 for Windows® (Scientific Software International, Inc., Skokie, IL, 2004). All other statistical analyses were conducted using subroutines available in SPSS v.18 (SPSS for Windows®, SPSS Inc., Chicago, IL, 2004). The significance limit set for all statistical analyses was α = 0.05.
Results
With one exception of a subject who refused to provide assent, all of the remaining patients who were initially approached agreed to participate, thus yielding a 97% accrual. No discomfort, injury or other apparent study-related adverse effects occurred with any study participant and no pupillometry measurements were refused at any point during the study.
Age, sex, and anxiety scores were found to not significantly correlate with pupillary response and were excluded from subsequent analyses. Maximum pupil size, percent change in pupil size, and maximum constriction velocity were directly correlated with the VAS (while controlling for opioid dose and baseline pupillary measurements)(Table 1). The two most robust of these were maximum constriction velocity and percent change in pupil size. As illustrated in Figure 1, each point change on the 10 point VAS was associated with a mean change of 0.11 mm/s in maximum constriction velocity (p=0.004). Likewise, as illustrated in Figure 2, each point change on the 10 point VAS was associated with a mean change of 0.39% in pupil size (p=0.018). Relationships between maximum constriction velocity and pain at the level of an individual are displayed in Figures 3 and 4 for the patients with the highest and lowest correlation between these variables, respectively. As would be expected on a physiologic basis regarding pupil function, the regression of maximum constriction velocity onto resting pupil diameter demonstrated a statistically significant moderate association (p < 0.01), with 56% of the variability in pupil diameter being unique from maximal constriction velocity (r2 = 0.441, standard error of the estimate = 0.597; illustration not shown).
Table 1. Results of Hierarchical Linear Modeling evaluating the association of pain intensity with pupillometry metrics.
| Pupillometry measurement | Change in pupil parameter per each point change in pain VAS | Standard error | t-ratio | p-value |
|---|---|---|---|---|
| Maximum Pupil Size (mm) | 0.04 | 0.02 | 2.00 | 0.046 |
| Minimum Pupil Size (mm) | 0.01 | 0.01 | 1.07 | 0.284 |
| Average Constriction Velocity (mm/s) | 0.03 | 0.02 | 1.65 | 0.101 |
| Dilation Velocity (mm/s) | 0.02 | 0.01 | 1.75 | 0.081 |
| Percent Change in Pupil Size | 0.39 | 0.16 | 2.37 | 0.018 |
| Maximum Constriction Velocity (mm/s) | 0.11 | 0.04 | 2.94 | 0.004 |
Figure 1.
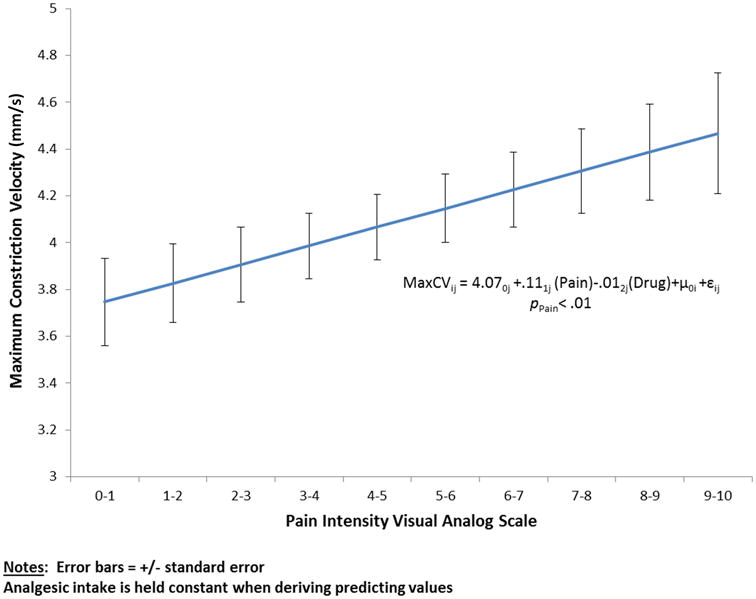
The association of maximum pupil constriction velocity with pain intensity determined by patient self-report using a validated visual analog scale (VAS). The association was statistically significant (p < 0.01). The error bars reflect the ± standard error of the mean for each point.
Figure 2.
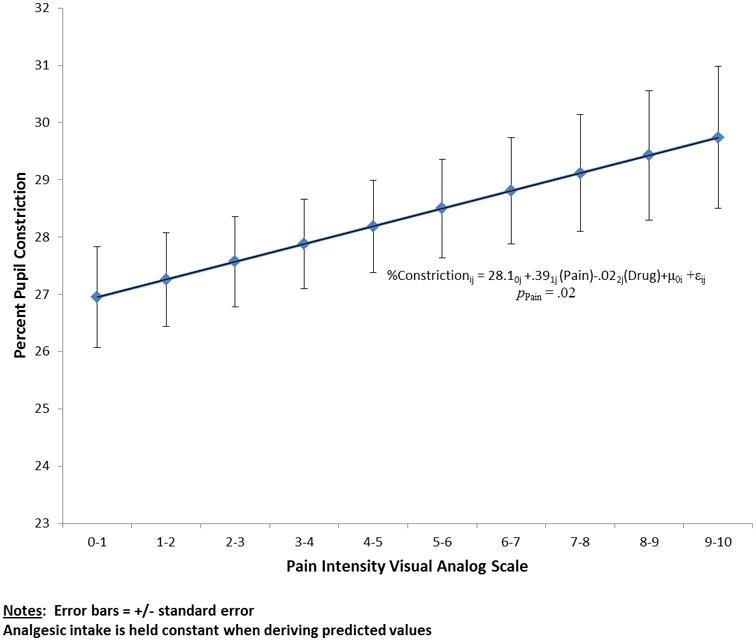
The association of the percent of pupil constriction with pain intensity determined by patient self-report using a validated visual analog scale (VAS). The association was statistically significant (p < 0.01). The error bars reflect the ± standard error of the mean for each point.
Figure 3.
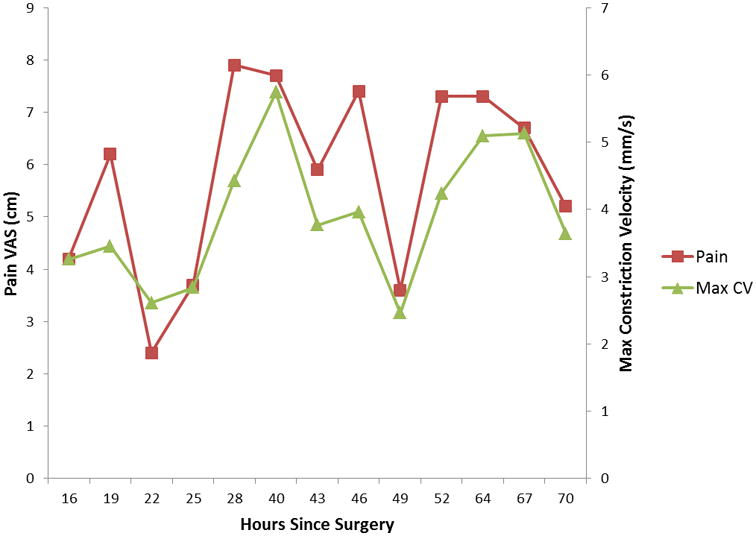
The relationship between pain intensity and maximum constriction velocity over time for the individual with the highest and lowest relationship between these variables.
Figure 4.
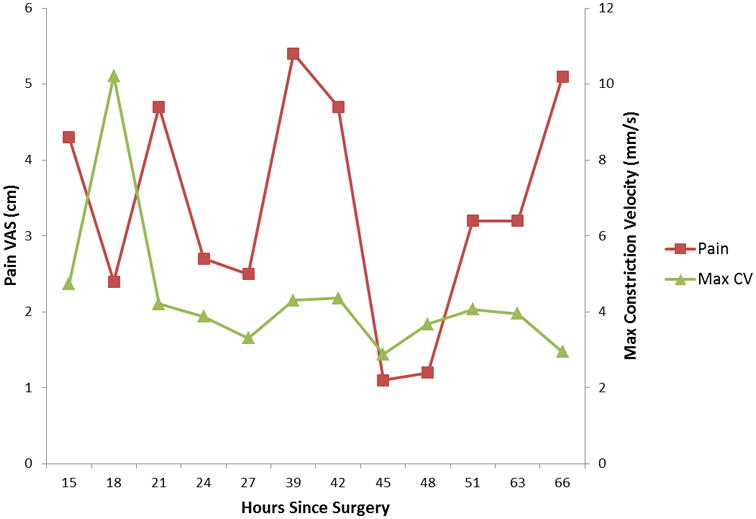
The relationship between pain intensity and maximum constriction velocity over time for the individual with the lowest relationship between these variables.
The exploratory (i.e., secondary) hypothesis tested was to explore the potential associations between pupillary response and opioid exposure. As expected given the effects of opioids on pupillary function, all measurements of pupillary response were inversely associated with opioid dose (Table 2). As illustrated in Figure 5, every 1 mcg/kg increase in opioid dose was associated with an average 2.02 mm reduction in pupil size. For every mcg/kg increase in opioid dose, minimum pupil size decreased 0.82 mm, overall change in pupil size (before and after light stimulus) decreased 17.88%, maximum constriction velocity decreased 2.64 mm/s, average constriction velocity decreased 1.26 mm/s, and dilation velocity decreased 0.89 mm/s as compared to baseline (Table 2).
Table 2. Pupillary parameter values before and during analgesic medication administration.
| Pupillometry Measurement | Without Analgesic Medication | Without Analgesic Medication | ||||
|---|---|---|---|---|---|---|
| Mean (95% CI) | Minimum | Maximum | Mean (95% CI) | Minimum | Maximum | |
| Maximum Pupil Size (mm) | 6.39 (4.55 - 8.23) | 4.13 | 7.93 | 3.63 (2.61 - 4.65) | 2.75 | 5.04 |
| Minimum Pupil Size (mm) | 3.89 (2.62 - 5.16) | 2.73 | 5.27 | 2.56 (1.93 - 3.19) | 2.02 | 3.24 |
| Percent Change in Pupil Size | 38.8 (29.59 - 48.01) | 28.30 | 46.00 | 28.09 (19.05 - 37.13) | 18.06 | 37.04 |
| Average Constriction Velocity (mm/s) | 2.65 (1.73 - 3.57) | 1.47 | 3.28 | 1.48 (0.77 - 2.19) | 0.90 | 2.35 |
| Maximum Constriction Velocity (mm/s) | 6.34 (3.05 - 9.63) | 1.41 | 10.89 | 4.07 (2.54 - 5.60) | 2.53 | 5.95 |
| Dilation Velocity (mm/s) | 1.46 (0.83 - 2.09) | 0.81 | 2.34 | 0.81 (0.38 - 1.24) | 0.38 | 1.26 |
| Response Latency (s) | 0.62 (0.27 - 0.97) | 0.38 | 1.33 | 0.22 (0.18 - 0.26) | 0.18 | 0.27 |
P < .01 for all variables
Figure 5.
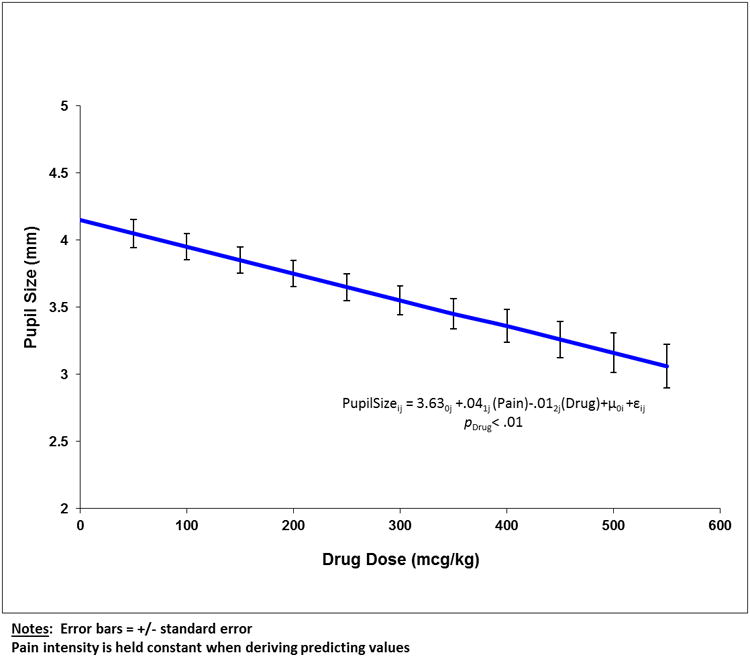
The association between baseline pupil size and daily drug dose (expressed as mcg/kg morphine equivalents). The association was statistically significant (p < 0.01). The error bars reflect the ± standard error of the mean for each point.
Discussion
We found significant associations between self-reported pain intensity and several measures of pupillary response in children who were evaluated after a uniform, painful surgical procedure. Of the associations tested, the relationship between maximum pupillary constriction velocity and VAS pain intensity (Figure 1) provided the best surrogate for use of quantitating pain intensity in children.
Expressing pupillary function as a percentage (increase) in pupil constriction functionally provides a measure of maximum effect (Emax) for a given observation (Figure 2). This “ceiling” could potentially be impacted by differences in the concentration versus effect profile for agents used to manage pain (e.g., opioids, clonidine, ropivacaine, lidocaine) in our cohort. This is especially pertinent to the use of clonidine, a centrally acting α2 adrenergic agonist which has been shown to have pupillary effects.24 Maximum constriction velocity on the other hand is a dynamic parameter, which reflects how efficiently the pupil responds to a given stimulus. Given that an increase of 2 or more on the 0-10 VAS scale is associated with a clinically significant change in pain intensity,25 an observed change in maximal pupil constriction velocity of ≥ 0.22 mm/sec could be used to guide intervention to optimize pain management. It could also provide an objective surrogate to aid in the differentiation of pain perception vs. actual pain intensity. Finally, it is important to recognize that either maximal constriction velocity or the percent of pupil constriction is dependent upon the baseline status of the pupil, as illustrated by our finding of a significant correlation between maximum constriction velocity and resting pupil diameter. This caveat does not limit the utility of pupillometry, especially when maximum constriction velocity is used as the surrogate endpoint.
The association between daily drug dose (expressed as morphine equivalents) and pupil size (Figure 3) was expected. Nonetheless, controlling for opioid effect in HLM analyses, we were able to demonstrate the independent effect of pain on pupillary response. However, further research is needed to support the use of pupil size as a predictor of analgesic dose requirement given potential confounders in our uncontrolled study (e.g., different drug combinations including non-opioid compounds).
Our findings are consistent with previous studies that have examined pupillometry with traditional physiologic surrogates of pain assessment in both adults and children. Barvais et al. compared pupil response with arterial pressure, heart rate, and bispectral index (BIS) variations following a painful stimulus in healthy adult patients receiving propofol and remifentanil. They found that in response to a noxious stimulus, pupil dilatation was more sensitive and better correlated with opioid concentrations than heart rate, blood pressure, and BIS monitoring.16 In a similar study performed in anesthetized children, Constant et al.14 compared changes in pupil diameter with heart rate, blood pressure, and BIS monitor variation in response to a skin incision on the lower limb. Similar to the adult data,16 these investigators were able to show that the change in pupil diameter had a greater effect size than BIS variation or hemodynamic markers.
While precise pupil measurements and subjective pain assessment have not been previously compared in children, Aissou et al. performed a study in 100 adults comparing changes in the pupil dilatation reflex and self-reported pain scores via a 5 point verbal rating scale.26 Shortly after extubation, patients receiving intravenous morphine in a postoperative setting had their pupil size monitored and recorded for 10 seconds during a standardized stimulus of constant pressure (200 kPa) within 2-3 cm from their skin incision. A significant relationship was observed between the VAS and maximum pupil diameter, as well as a threshold of percent change in pupil diameter in patients who required additional doses of morphine.
As with all physiologic surrogates, pupillometry does have limitations. These include the lack of specificity of the pupil dilatation reflexin response to pain and clinical situations in which a patient is in pain but has constricted pupils. Also, as our study enrolled verbal, cognitively appropriate participants capable of self-reporting pain, additional research is needed to generalize findings to non-verbal patients or those with significant cognitive impairment.
Summary and Conclusions
Our data preliminarily illustrate the utility of pupillometry as a physiologic surrogate capable of assessing pain intensity in paediatric post-surgical patients ranging in age from 9 to 17 years. The device was well-accepted, easy to use and produced reliable measurements of several parameters reflective of pupillary function. Of all the parameters evaluated, maximum constriction velocity appeared to have the greatest association with VAS pain intensity ratings and provided the most dynamic measurement. Our findings suggest that this technique may not only have value for assessing pain intensity and/or response to treatment in nonverbal patients but also in patient populations experiencing different types of pain (e.g., nociceptive pain vs. complex pain; acute vs. chronic pain; surgical vs. traumaticpain). Pupillometry may also prove to demonstrate its application as a pharmacodynamic surrogate capable of exploring the impact of age on the exposure-response relationship for opioids in paediatric patients.
What is already known
Current pain assessment in children can be challenging and current strategies are not always accurate.
A single study in adults found that the pupil dilation reflex in response to a painful stimulus correlated with subjective pain scores.
What this study adds
In post-operative children certain quantitative pupillometry readings significantly correlated with self-reported pain scores.
Pupillometry may be a viable tool for objective pain assessment in children.
Acknowledgments
Disclosure: This study was supported in part by a grant from the Katherine B. Richardson Endowment fund. The guidance and technical assistance provided by Dr. Ralph Kauffman in the preparation of this manuscript is gratefully acknowledged.
Footnotes
MAC was significantly involved in study design, data collection, and data analysis/interpretation, as well as drafting and revising the manuscript. JTB assisted with interpretation of the data and drafting/revising the manuscript. GLK assisted with interpretation of the data and drafting/revising the manuscript. RAA had significant involvement in data collection. SDSP had significant involvement in the study conception/design. KAN was significantly involved in study design, data collection, and data analysis/interpretation, in addition to revising the manuscript, approving the final version for publication, and the accuracy and integrity of all aspects of the research.
References
- 1.IASP. Global Year Against Pain in Children. [Accessed January 30th, 2007];2005 at http://iasp-pain.org/GlobalDay-2005.htm.
- 2.McGrath P. Children - not simply “little adults”. In: Merskey H, Loeser J, Dubner R, editors. The Paths of Pain 1975-2005. Seattle: IASP Press; 2005. pp. 443–6. [Google Scholar]
- 3.Schecter N, Berde C, Yaster M. Pain in infants, children, and adolescents. 2nd. Philadelphia: Lippincott, Williams, and Wilkins; 2003. [Google Scholar]
- 4.Howard RF. Current status of pain management in children. JAMA. 2003;290:2464–9. doi: 10.1001/jama.290.18.2464. [DOI] [PubMed] [Google Scholar]
- 5.Stevens BJ, Harrison D, Rashotte J, Yamada J, Abbott LK, Coburn G, et al. Pain assessment and intensity in hospitalized children in Canada. J Pain. 2012;13:857–965. doi: 10.1016/j.jpain.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Brand K, Canchi N. Pain assessment in children. Anaesth Intens Care. 2013;14:228–31. [Google Scholar]
- 7.Schiavenato M, Craig KD. Pain assessment as a social transaction beyond the “gold standard”. Clin J Pain. 2010;26:667–76. doi: 10.1097/AJP.0b013e3181e72507. [DOI] [PubMed] [Google Scholar]
- 8.Connelly M. The verbal numeric rating scale in the pediatric emergency department: what do the numbers really mean? Pain. 2010;149:167–8. doi: 10.1016/j.pain.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Hodgins MJ. Interpreting the meaning of pain severity scores. Pain Res Manag. 2002;7:192–8. doi: 10.1155/2002/971935. [DOI] [PubMed] [Google Scholar]
- 10.de C Williams AC, Davies HT, Chadury Y. Simple pain rating scales hide complex idiosyncratic meanings. Pain. 2000;85:457–63. doi: 10.1016/S0304-3959(99)00299-7. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand AL, Santos Garcia JB, Viera EB, Santos AM, Bertrand RH. Pupillometry: the influence of gender and anxiety on the pain response. Pain Physician. 2013;16:E257–E266. [PubMed] [Google Scholar]
- 12.MacLeod DB, Habib AS, Ikeda K, Spyker DA, Cassella JV, Ho KY, Gan TJ. Inhaled fentanyl aerosol in healthy volunteers: pharmacokinetics and pharmacodynamics. Anesth Analg. 2012;115:1071–1077. doi: 10.1213/ANE.0b013e3182691898. [DOI] [PubMed] [Google Scholar]
- 13.Setnik B, Sommerville K, Goli V, Han L, Webster L. Assessment of pharmacodynamic effects following oral administration of crushed morphine sulfate and naltrexone hydrochloride extended-release capsules compared with crushed morphine sulfate controlled-release tablets and placebo in nondependent recreational opioid users. Pain Med. 2013;14:1173–1186. doi: 10.1111/pme.12148. [DOI] [PubMed] [Google Scholar]
- 14.Constant I, Nghe MC, Boudet L, Berniere J, Schrayer S, Seeman R, et al. Reflex pupillary dilatation in response to skin incision and alfentanil in children anaesthetized with sevoflurane: a more sensitive measure of noxious stimulation than the commonly used variables. Br J Anaesth. 2006;96:614–9. doi: 10.1093/bja/ael073. [DOI] [PubMed] [Google Scholar]
- 15.Höfle M, Kenntner-Mabiala R, Pauli P, Alpers GW. You can see pain in the eye: pupillometry as an index of pain intensity under different luminance conditions. Int J Psychophysiol. 2008;70:171–5. doi: 10.1016/j.ijpsycho.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Barvais L, Engelman E, Eba JM, Coussaert E, Cantraine F, Kenny GN. Effect site concentrations of remifentanil and pupil response to noxious stimulation. Br J Anaesth. 2003;91:347–52. doi: 10.1093/bja/aeg178. [DOI] [PubMed] [Google Scholar]
- 17.Boev AN, Fountas KN, Karampelas I, Boev C, Machinis TG, Feltes C, et al. Quantitative pupillometry: normative data in healthy pediatric volunteers. J Neurosurg. 2005;103(6 Suppl):496–500. doi: 10.3171/ped.2005.103.6.0496. [DOI] [PubMed] [Google Scholar]
- 18.St Peter SD, Weesner KA, Weissend EE, Sharp SW, Valusek PA, Sharp RJ, et al. Epidural vs patient-controlled analgesia for postoperative pain after pectusexcavatum repair: a prospective, randomized trial. J PediatrSurg. 2012;47:148–53. doi: 10.1016/j.jpedsurg.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Patanwala AE, Duby J, Waters D, Erstad BL. Opioid conversions in acute care. Ann Pharmacother. 2007;41:255–66. doi: 10.1345/aph.1H421. [DOI] [PubMed] [Google Scholar]
- 20.McGrath PA, Seifert CE, Speechley KN, Booth JC, Stitt L, Gibson MC. A new analogue scale for assessing children's pain: an initial validation study. Pain. 1996;64:435–43. doi: 10.1016/0304-3959(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 21.Ebesutani C, Regan J, Smith A, Reise S, Higa-McMillan C, Chorpita BF. The 10-Item Positive and Negative Affect Schedule for Children, Child and Parent Shortened Versions: Application of Item Response Theory for More Efficient Assessment. J Psychopathol Behav Assess. 2012;34:191–203. [Google Scholar]
- 22.Tegeder I, Meier S, Burian M, Schmidt H, Geisslinger G, Lötsch J. Peripheral opioid analgesia in experimental human pain models. Brain. 2003;126:1092–102. doi: 10.1093/brain/awg115. [DOI] [PubMed] [Google Scholar]
- 23.Raudenbush S, Bryk A, Cheong Y, Congdon R, du Toit M. HLM6: Linear and non-linear modeling. Chicago: Scientific Software International; 2004. [Google Scholar]
- 24.Phillips MA, Szabadi E, Bradshaw CM. Comparison of the effects of clonidine and yohimbine on pupillary diameter at different illumination levels. Br J Clin Pharmacol. 2000;50:65–68. doi: 10.1046/j.1365-2125.2000.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powell CV, Kelly AM, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Ann Emerg Med. 2001;37:28–31. doi: 10.1067/mem.2001.111517. [DOI] [PubMed] [Google Scholar]
- 26.Aissou M, Snauwaert A, Dupuis C, Atchabahian A, Aubrun F, Beassier M. Objective assessment of the immediate postoperative analgesia using pupillary reflex measurement: a prospective and observational study. Anesthesiology. 2012;116:1006–12. doi: 10.1097/ALN.0b013e318251d1fb. [DOI] [PubMed] [Google Scholar]


