Abstract
Background:
Dashamoola, in the form of arishta and kwath, is a commonly used classical Ayurvedic multi-ingredient formulation for management of pain, arthritis and inflammatory disorders.
Objective:
To study analgesic, anti-inflammatory and anti-platelet activity of Dashamoola and its combination with aspirin.
Materials and Methods:
Wistar albino rats (180-200 g) and Swiss albino mice (20-25 g) of either sex were divided randomly into five groups: Distilled water, aspirin (500mg/kg in rats; 722.2 mg/kg in mice), Dashamoolarishta (1.8 mL/kg in rats; 2.5 mL/kg in mice) and Dashamoolarishta with aspirin. Anti-inflammatory activity was measured by change in paw volume in carrageenan-induced inflammation, protein content in model of peritonitis and granuloma weight in cotton pellet granuloma. Analgesic effect was evaluated by counting number of writhes in writhing model. Maximum platelet aggregation and percentage inhibition of ADP and collagen-induced platelet aggregation were estimated in vitro. Statistical analysis was done using one way ANOVA (post hoc Tukey's test) and P < 0.05 was considered significant.
Results:
Dashamoolarishta and its combination with aspirin showed significantly (P < 0.01) less number of writhes. It showed significant (P < 0.001) anti-inflammatory activity by paw edema reduction in rats, decrease in proteins in peritoneal fluid (P < 0.001) and decrease in granuloma weight (P < 0.05) as compared to respective vehicle control groups. Dashamoola kwath alone and in combination with aspirin inhibited maximum platelet aggregation and percent inhibition of platelets as compared to vehicle (P < 0.001).
Conclusion:
Dashamoola formulation alone and its combination with aspirin showed comparable anti-inflammatory, analgesic and anti-platelet effects to aspirin.
Keywords: Aspirin, carrageenan, cotton pellet granuloma, peritonitis, platelet aggregation, writhing
INTRODUCTION
The search for ideal analgesic and anti-inflammatory agents is still on despite introduction of numerous new drugs over the last century. None of the available agents, however, have appreciable safety profiles.[1] Often patients turn to the traditional systems of medicine for treatment of chronic inflammatory disorders like osteoarthritis or rheumatoid arthritis. Scientific evidence for the claimed medicinal properties of treatments or drugs from the traditional systems of medicine is sparse.
Dashamoola as the name suggests contains roots of ten different plants. Of these, five are known as brihad panchamoola and the remaining as laghoo panchamoola.[2] It is used in the form of kwath or arishta according to Ayurveda.[3,4] These formulations are used by the Ayurvedic practitioners in various conditions for headache, relief of pain and swelling related to arthritis, pyrexia, abdominal distension and costo-chondral pains. It is described as an analgesic, anti-arthritic and anti-rheumatic combination.[5] It is believed that the 10 ingredients in Dashamoola may be serving different roles like adjuvant, carrier agent and stabilizer etc.[6] Some of these ingredients have been evaluated in experimental models of inflammation and pain and have shown to possess anti-inflammatory and analgesic activities.[5,7,8]
After reviewing the modern literature, it was found that there were very few studies carried out to validate claims of Dashamoola as a whole combination for anti-inflammatory and analgesic activities. Hence the present study was planned to assess anti-inflammatory and analgesic activity of commercially available standard preparation of Dashamoola, which was prepared as per the recommended method in Ayurveda. Keeping in view the possibility of simultaneous consumption of Dashamoola with modern anti-inflammatory-analgesic drugs by patients, their combined activities were evaluated in experimental models of inflammation and pain. The results of earlier studies in which Dashamoola has consistently shown efficacy in models of acute inflammationhinted at the possibility of prostaglandin synthesis inhibition as the probable mechanism of action. By virtue of the same mechanism, it may have anti-platelet effect. If found to be effective, Dashamoola may be a useful therapeutic adjuvant or alternative for prevention of myocardial infarction and stroke. The possibility of anti-platelet activity of Dashamoola is hitherto unreported and untested. Hence an in-vitro study was conducted to test the anti-platelet activity of Dashamoola.
MATERIALS AND METHODS
The study was carried out in 2 Stages. Approval of Institutional Animal Ethics Committee (AEC/307) for stage 1 (parts I to IV; animal experiments) and Institutional Ethics Committee (EC/266/07) for stage 2 (parts V and VI; in vitro) were obtained prior to commencement of the study.
Stage 1
In the first stage, experimental studies were done and the second stage included in vitro study. The first stage was divided into the following parts: Part I: Carrageenan-induced rat paw edema; Part II: Acetic-acid–induced peritonitis in mice, Part III: Cotton pellet granuloma in rats and Part IV: Acetic acid induced writhing in mice.
Animals
Wistar albino rats of either sex weighing 180-200 g and Swiss albino mice of either sex weighing 20-25 g were used. The animals used in the study were bred from the Central Animal House of Seth G.S. Medical College and K.E.M. Hospital, Mumbai (registration number 60/1999) registered with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. The guidelines recommended by the CPCSEA were carefully followed during the entire study. The animals were housed in clean polypropylene cages and provided with standard laboratory conditions such as room temperature at 25 ± 2°C, humidity at 30-70% and with 12 h light and dark cycle. They were fed with normal rat chow and Aquaguard pure water in polyethylene bottles given ad libitum.
Study drugs
Dashamoolarishta (DA) was received as a gift sample from Sandu Pharmaceuticals and was stored at room temperature throughout the experiment. Certificate of analysis was provided by the manufacturer (Batch No: GA-09; Date of Mfg: 12/07; A.R. No: F/N/236/07). It was administered orally once in the dose of 1.8 mL/kg to rats and 2.5 mL/kg to mice.[4] Commercially available aspirin was the control used in the study and was administered orally as 1mL, once a day. The doses of aspirin used were 500 mg/kg/day in rats and 722.2 mg/kg/day in mice.[9] Distilled water served as the vehicle control.
Experimental groups
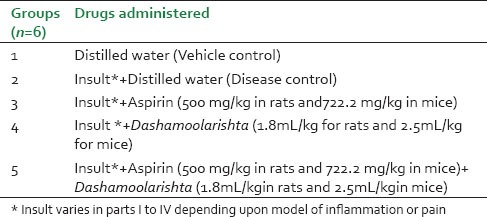
The animals were divided into 5 groups as shown in Table 1.
Table 1.
Experimental Groups
Study procedure
Model of acute inflammation-Carrageenan-induced paw edema in rats
A set of 30 rats, kept for 12 h fasting prior to experiment were randomly divided into 5 groups as shown in Table 1. The study drugs were administered to the respective groups one hour prior to insult. In this model, 0.1 mL of subcutaneous injection of commercially available carrageenan, freshly prepared (30 minutes prior to injection) as suspension in 1% CMC was injected in the center of plantar region of right hind paw of the rats.[10] The paw volume of each rat was measured before and 3 hours after the carrageenan injection using mercury plethysmograph.[11,12] The percent edema was calculated using the following formula:[13]
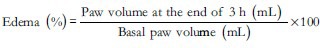
Model of acute inflammation - Acetic acid induced peritonitis in mice
A set of 30 mice after an overnight fasting (12 h) were randomly allocated to 5 experimental groups [Table 1] to receive the study drugs one hour prior to insult. Freshly prepared 1.2% acetic acid was injected intra-peritoneally in a dose of 0.25 mL/mouse to induce peritonitis.[14] After 3 hours, the animals were sacrificed to perform laparotomy. Washings of peritoneal lavage, given with 5 mL of saline containing 0.1 mM EDTA, were collected and processed for protein content estimation using microprotein kits on a semi-auto analyzer.[15]
Protein content was calculated as:
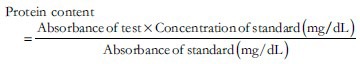
Model of chronic inflammation-Cotton pellet granuloma in rats
A set of 24 rats after an overnight fasting (12 h) were randomly allocated to 4 experimental groups (similar to groups 2, 3, 4 and 5 shown in Table 1). Cotton pellets weighing 50 ± 1 mg were autoclaved and soaked in 0.2 mL of distilled water containing penicillin (0.1 mg) and streptomycin (0.13 mg) and implanted subcutaneously bilaterally in axilla on ventral aspect of each rat. The procedure was carried under stringent aseptic precautions. From the next day, the groups then received respective study drugs for 7 days. On day 8th, the granulation tissue identified as firm vascular tissue surrounding and adherent to the inserted pellets were dissected out. The pellets were then dried in hot air oven at 60°C for 24 h and weighed to record the dry weight. Granuloma weight was obtained by subtracting the weight of cotton pellet on day 0 (before the experiment) from the weight of cotton pellet on eighth day (at the end of the experiment).[16]
Model of pain - Acetic acid induced writhing in mice
After an overnight fasting (for 12 h), a set of 30 mice received study drugs as given in Table 1. One hour later they were subjected to insult by injecting 0.25 mL freshly prepared 3% acetic acid solution intra-peritoneally. Writhing movements were counted 5 min after the injection of acetic acid up to 15 min by an observer who was blinded to the treatment group. Writhing movement was defined as a sustained stretching of the abdomen along with extension of at least one of the hind limbs.[17,18]
After analyzing the results of the above-mentioned parts, anti-platelet effects of Dashamoola were evaluated in an in vitro study (Phase 2), as follows:
Stage 2
In the second stage the following parts were carried out.
Part V: Platelet aggregation inhibition study with Dashamoola.
Part VI: Platelet aggregation inhibition study with best effective concentration of Dashamoola in combination with aspirin.
Subject population
Six normal male volunteers, between 18 and 55 years of age were included after taking written informed consent. The volunteers with history of any bleeding disorder or of taking NSAID or any other drug that would interfere with platelet function in the past 7 days, smoking and alcohol intake etc., were excluded from this study.
Study drugs
Aqueous extract of Dashamoola (kwath-DK) was purchased commercially. Two concentrations of DK (Undiluted and 1:5 dilution) were selected and their non-toxicity for platelets was confirmed by carrying out the platelet viability study based on the lactate dehydrogenase release assay.[19] The concentration of aspirin used was 0.025 mg/mL.[20] ADP and collagen served as the platelet aggregating agents. The concentrations of ADP and collagen were 2.5 μM and 1 μg/mL, respectively, which were identified on the basis of work done earlier in the department.[21]
Platelet aggregation inhibition study
The recruited volunteers were asked to report to the laboratory after overnight fasting. Twenty milliliter of venous blood was collected taking aseptic precautions, in a citrated buffer tube (3.8% tri-sodium citrate) and centrifuged at 800 rpm for 8 minutes to obtain platelet-rich plasma (PRP). Platelets from the PRP were counted using automated cell counter and adjusted to 2 × 105 platelets per μL using autologous platelet poor plasma. This working platelet-rich plasma (WPRP) was used for studying platelet aggregation by the turbidimetric method[22] on a platelet aggregocorder using ADP (2.5 μM) and Collagen (1 μg/mL) as aggregating agents.[23,24]
Ten microliter of distilled water (vehicle) or aspirin (0.025 mg/mL) or undiluted DK or diluted DK (1:5) were added to the WPRP cuvettes and incubated at 37°C in the incubation wells for 3 minutes. The time for starting the aggregation after adding the aggregating agent and the time for stopping the procedure were set. The aggregation pattern was recorded as percent aggregation versus time for a period of 8 minutes for both ADP and collagen. The maximum platelet aggregation (MPA) was recorded as an optical curve on the aggregocorder. After analyzing the results, the single best effective concentration of DK was tested again in combination with aspirin (0.025 mg/mL) using the same methodology. The percentage inhibition (PI) shown by the vehicle control and the test drug groups were calculated from the values of MPA using the formula:
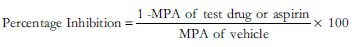
Statistical analysis
The readings noted in different groups in all the parts were expressed as mean ± SD. The data were compared by one-way ANOVA followed by post-hoc Tukey's test (GraphPad InStat Version 3.06). A ‘p’ value of < 0.05 was considered statistically significant.
RESULTS
Model of acute inflammation - Carrageenan-induced paw edema in rats
At 0 hour, paw volume was comparable between all the groups (P > 0.05). The rats injected with carrageenan (disease control group) developed significant edema of paw (P < 0.001) after 3 hours as compared to distilled water control group. Aspirin pre-treated rats developed significantly less edema (P < 0.01) compared to the disease control group. The rats receiving DA and those which received aspirin with DA also showed comparable results (P < 0.001) [Table 2 and Figure 1].
Table 2.
Percent edema of paw in rats

Figure 1.
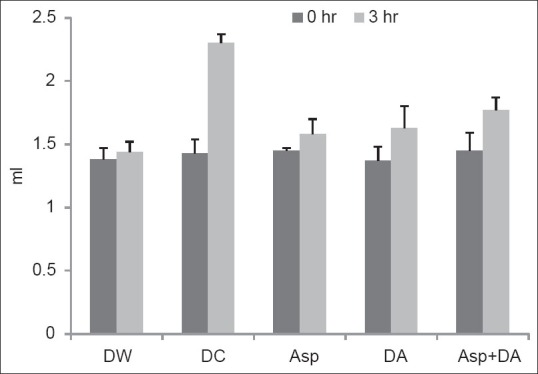
Paw volume in rats. Bars represent mean and error bars represents SD values. ANOVA followed by post hoc Tukey's test. dP< 0.001 as compared to distilled water. *P<0.01 as compared todisease control. DW: Distilled water, DC: Disease control, Asp: Aspirin, DA: Dashamoolaristha
Model of acute inflammation - acetic acid induced peritonitis in mice
Protein content of the peritoneal lavage fluid procured from the mice injected with acetic acid (disease control) was significantly high (P < 0.001) after 3 h as compared to distilled water treated group [Table 3]. Pre-treatment with aspirin significantly prevented this rise (P < 0.001). Mice receiving DA and those pre-treated with the combination of aspirin with DA also had significantly less protein content (P < 0.001) compared to the disease control group. The effects of aspirin and the combination groups were found to be comparable to one another and exceeded the effect of DA alone.
Table 3.
Protein content in mice
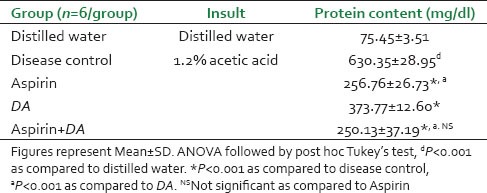
Model of chronic inflammation - Cotton pellet granuloma in rats
As depicted in Table 4, the groups of rats administered either aspirin or the DA following cotton pellet implantation demonstrated comparable weight of granuloma after 8 days. These granuloma weights were significantly less (P < 0.05) as compared to distilled water group. The combination group, however, did not show statistically significant difference compared to the vehicle group, though a trend towards decrease in weight was observed in this group.
Table 4.
Granuloma weight in rats

Model of pain - acetic-acid - induced writhing in mice
Distilled water injected intraperitoneally in mice did not produce any writhing response. The mice injected with acetic acid showed 30.00 ± 9.23 writhing responses when observed for 10 min. The aspirin (P < 0.001) as well as DA (P < 0.01) pre-treatment significantly prevented the writhing responses compared to distilled water pre-treated group [Figure 2]. Prior treatment with aspirin and DA given concomitantly also showed a significant (P < 0.001) decrease in number of writhes. This effect was better than DA alone (P < 0.05) as compared to DA, but was comparable to that of aspirin.
Figure 2.
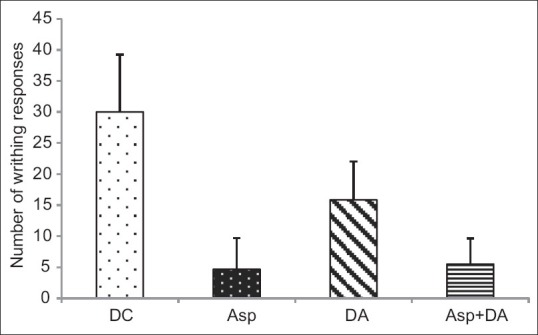
Number of writhes in mice. Figures represent mean ± SD, ANOVA followed by post hoc Tukey's test. **P<0.001, *P<0.01 as compared to distilled water pre-treated group. aP<0.05 as compared to DA. NSNon significant as compared to Aspirin. DW: Distilled water, DC: Disease control, Asp-aspirin, DA: Dashamoolaristha
Effect of Dashamoola kwath on platelet aggregation in human volunteers
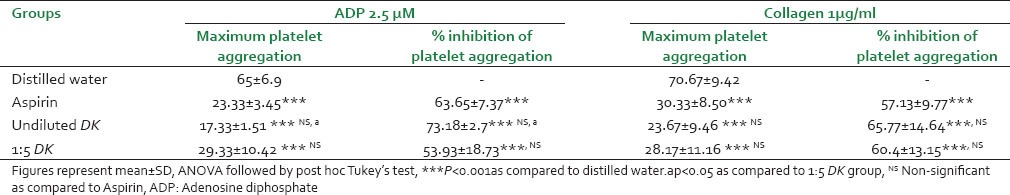
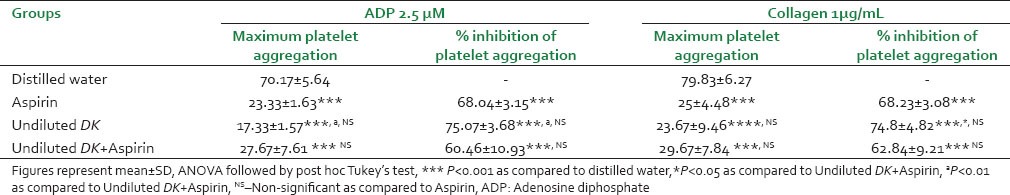
As shown in Table 5, aspirin significantly (P < 0.001) inhibited the maximum platelet aggregation (MPA) induced by ADP and collagen as compared to vehicle control. Both undiluted DK and 1:5 DK showed comparable effects to aspirin on MPA and percent inhibition of platelet aggregation by ADP and collagen. However, between the two DK groups, undiluted DK showed significantly more (P < 0.05) percent inhibition of ADP-induced platelet aggregation as compared to 1:5 DK group.
Table 5.
Effect of DK on platelet aggregation in human volunteers
Effect of Dashamoola kwath in combination with aspirin on platelet aggregation in human volunteers
As shown in Table 6, the combination of aspirin and undiluted DK showed significant percent inhibition of platelet aggregation as compared to distilled water. Undiluted DK as well as the combination group showed comparable effects to aspirin alone on percent inhibition of platelet aggregation and MPA by both ADP and collagen. Undiluted DK showed significantly better inhibition of ADP (P < 0.01) and collagen (P < 0.05) induced platelet aggregation than the combination group.
Table 6.
Effect of DK with aspirin on platelet aggregation in human volunteers
DISCUSSION
A number of animal models which simulate biological and biochemical characteristics of pain and inflammation are available for screening of analgesic and anti-inflammatory agents. We selected time tested models of acute exudative phase of inflammation like paw edema induced by the injection of phlogistic agent like carrageenan[10,11,12,13] and acetic-acid–induced peritonitis.[14] These models are sensitive to steroidal and non-steroidal anti-inflammatory agents. Granulation tissue formation is the key feature in chronic inflammation and has been employed for screening anti-arthritic drugs. Foreign body implantation like cotton pellet implantation[16] induces initial transudative and exudative responses ultimately culminating into proliferative cellular response within 3-6 days of implantation. Injection of irritant like acetic acid in the peritoneal cavity develops writhing, which is a very sensitive model for screening analgesic drugs that mainly act peripherally.[17,18]
In the carrageenan-induced paw edema, acetic-acid–induced peritonitis and writhing models, a single dose pretreatment was given. Pretreatment used in these models evaluated the prophylactic role of Dashamoolarishta, in prevention of induced inflammation and pain. While, in the model of cotton pellet induced granuloma, therapy with either of the agents was given for the period of 7 days after insertion of pellets, which was as per the standard protocol used in the experimental studies. Our results showed that Dashamoolarishta demonstrated comparable anti-inflammatory and analgesic effects as shown by aspirin. In carrageenan-induced paw edema, acetic-acid–induced peritonitis and writhing models, prostaglandins are involved in mediating inflammation.[25,26,27] The granulomatous chronic inflammation is also driven by prostaglandins, which are liberated from the inflammatory neutrophils and macrophages migrating to the site of foreign bodies like cotton pellets.[28] It is likely that Dashamoolarishta probably has the same mechanism of action as aspirin i.e., prostaglandin inhibition.
When Dashamoolarishta was co-administered with aspirin, there was decreased inflammation and pain in all the four models when compared to control group. The effects of combination group were comparable to the aspirin group in all the three models viz. carrageenan-induced paw edema, peritonitis and writhing. On repeated dosing as in the chronic model of cotton pellet induced granuloma, the combination showed lesser effects than aspirin alone. These findings also indicate the possibility that because of similar mechanism of action, there was no synergism when Dashamoolarishta and aspirin were used together.
Comparison of Dashamoolarishta and its combination showed significantly better findings than Dashamoolarishta alone in only acetic-acid-induced peritonitis and writhing models in mice unlike in the other two models i.e., carrageenan-induced rat paw edema and cotton pellet granuloma. Considering the fact that the combination did not supersede effects seen in aspirin group, these seemingly better results with combination group versus Dashamoolarishta alone could be due to variable responses of animal species.
Our study findings corroborated the evidence generated by some of the recent studies done in last 2 years by other researchers. In one study, Dashamoola decoction (2.88 mL/200g rat) showed significant reduction in carrageenan-induced rat paw edema and significant reduction in cotton pellet granuloma formation when compared to control group in rats.[29] In another study, Dashamoola kwath (750 mg/kg in rats) showed 11.4% while phenylbutazone showed 17.1% reduction in carrageenan-induced rat paw edema but it did not show any effect in granuloma pouch model of chronic inflammation.[30] Three Dashamoola marketed formulations (2.34 mL/kg in rats) were shown to cause significant reduction in paw edema in the study by Pawar et al.[31] Singh et al. demonstrated that Dashamoola kwath formulation (0.41 mL/20g mice) led to reduction in the phenylquinone-induced number of writhes in mice and also (500 mg/kg in rats) showed significant reduction in yeast-induced pyrexia in rats to a comparable extent toaspirin.[32] In a study by Dawane and others, Dashamoola formulation (2.90 mL/200 g rat) showed antipyretic activitycomparable to diclofenac as early as 1 h and lasting for 4 h in Brewer's yeast-induced pyrexia in rats.[33]
In all the above-mentioned studies, different formulations and varied doses of Dashamoola were used. This makes comparison of the available data with ours difficult except for one study wherein marketed formulation of Dashamoolarishta was used.[31] The dose used in this study was higher (2.35mL/kg for rat) than the dose used by us (1.8 mL/kg for rat). We preferred to adhere to formulation and doses described in the Ayurvedic pharmacology. Lower ranges of doses are used for arishta formulation.[34,35,36] Arishta formulation is preferred clinically over kwath as it is more palatable and effective in long-term use.[37] An additional spectrum of Dashamoolarishta was evaluated in the present study which has not been studied earlier. It was felt that if Dashamoolarishta has similar mechanism of action as aspirin, then it would exhibit anti-platelet activity. However, while evaluating this activity using in vitro technique, it was not possible to use Dashamoolarishta. Dashamoola as arishta, i.e. an alcoholic preparation would have affected the viability of platelets and vitiated the results. Hence, an aqueous extract (kwath) of Dashamoola was selected. The concentrations of Dashamoola kwath for this in vitro study were selected arbitrarily as there was no prior reported study in the literature. The selected concentrations, the undiluted and diluted, as 1:5 were found to be non-toxic to platelets as confirmed by the results of platelet viability study. Both the concentrations of the Dashamoola kwath inhibited platelet aggregation to a significant extent. This was a novel finding and is being reported for the first time. The percentage inhibition of aggregation induced by ADP and collagen was found to be comparable to aspirin. Between the two, the undiluted dose was found to be significantly better than the diluted one. Hence it was chosen for the combination dose with aspirin. As in the earlier in vivo experiments, the co-administration of undiluted Dashamoola kwath with aspirin although prevented aggregation of platelets which was comparable to aspirin alone (for both, ADP and collagen) or Dashamoola kwath alone (for collagen). The combination does not show any better effect than the individual drugs as the target site of action may be common for these drugs. If the mechanisms were different then potentiation of each others’ effects on co-administration would have been evident as they are observed on co-administration of anti-platelet agents such as aspirin (cyclooxygenase inhibition) and clopidogrel (P2Y12 inhibitor)[38] or aspirin and dipyridamole (phosphodiesterase inhibitor).[39]
Ayurvedic texts mention beneficial effects of Dashamoola especially the kwath preparation in various cardiovascular diseases.[40,41] The study findings indicate that Dashamoola can be beneficial in medical management of conditions like cardiovascular and cerebrovascular diseases viz. myocardial infarction and stroke. As anti-platelet drugs are most commonly used drugs for the above-mentioned diseases for prevention of thrombosis and aspirin remains the first choice, Dashamoola can be a safer alternative treatment. It may be possible to use it as an add-on therapy to aspirin, which will help clinicians to achieve better efficacy and reduce doses of aspirin. Future experimental and clinical studies are needed to explore this possibility.
Further exploratory studies with Dashamoola to evaluate its effects on cyclooxygenase, inflammatory mediators, other anti-aggregatory mechanisms and on gastrointestinal mucosal membrane would strengthen its position in therapeutics.
CONCLUSION
The present study demonstrated that Dashamoola had anti-inflammatory, analgesic and anti-platelet effects comparable to that of aspirin. Combination of Dashamoola with aspirin did not offer any advantage over aspirin alone. Further studies needed to prove Dashamoola to be a useful alternative to currently available non-steroidal anti-inflammatory drugs.
ACKNOWLEDGEMENT
Authors acknowledge gift sample of Dashamoola from Sandu Pharmaceuticals Ltd, Mumbai for this study.
Footnotes
Source of Support: Post graduate student fund by D.J.S.T. Seth G.S. Medical College and K.E.M. Hospital
Conflict of Interest: None declared.
REFERENCES
- 1.Furst DE, Ulrich RW, Prakash S. Non steroidal Anti-inflammatory Drugs, Disease Modifying Anti-rheumatoid Drugs, Non-opiod Analgesics, and Drugs Used in Gout. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic and Clinical Pharmacology. 12th ed. New Delhi: McGraw-Hill Companies; 2012. pp. 635–57. [Google Scholar]
- 2.Sastri A. 8th ed. Varanasi: Chaukhamba Amarabharati Prakashan; 1988. Rasaratna Samuchchaya of Sri Vaghbhatacharya; pp. 95–8. [Google Scholar]
- 3.Sharma PV. In: Dravyaguna – Vigyan. Vol. 2. Varanasi: Chaukhamba Prakashan; 2001. Tritiya Adhyay Hridyadi Varga; pp. 221–2. [Google Scholar]
- 4.Gogte VM. Ayurvedic Pharmacology and therapeutic uses of medicinal plants. In: Ramkrishnan S, editor. Dravyagunavidnyan. Mumbai: Bharatiya Vidya Bhavan; 2000. pp. 287–8. [Google Scholar]
- 5.Jabbar S, Khan MT, Choudhuri MS, Sil BK. Bioactivity studies of the individual ingredients of the Dashamularishta. Pak J Pharm Sci. 2004;17:9–17. [PubMed] [Google Scholar]
- 6.Puranik GV, Dhamankar PV. Chapter 11 – Ayurvediya Aushadhee Pathasanyojana. In: Puranik GV, Dhamanskar PV, editors. Ayurvediya Aushadheekaran (Agam ani Pratyaksha) Part 2. 2nd ed. Mumbai: Dhootpapeshwar Publication; 1964. pp. 49–512. [Google Scholar]
- 7.Niranjan A, Tiwari SK. Phytochemical composition and antioxidant potential of Desmodium gangeticum (Linn.) DC. Nat ProdRediance. 2008;7:35–9. [Google Scholar]
- 8.Bose LV, Varghese GK, Habtemariam S. Identification of acteoside as the active antioxidant principle of Premna serratifolia root wood tissues. Phytopharmacology. 2013;4:228–36. [Google Scholar]
- 9.Dang GK, Parekar RR, Kamat SK, Scindia AM, Rege NN. Antiinflammatory activity of Phyllanthus emblica, Plumbago zeylanica and Cyperus rotundus in acute models of inflammation. Phytother Res. 2011;25:904–8. doi: 10.1002/ptr.3345. [DOI] [PubMed] [Google Scholar]
- 10.Winter CA, Risley EA, Nuss GW. Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 11.Vogel HG. 2nd ed. Berlin: Springer-Verlag; 2002. Drug Discovery and Evaluation: Pharmacological Assays; p. 761. [Google Scholar]
- 12.Parmar NS, Prakash S. 5th ed. New Delhi: Narosa Publishing House; 2011. Screening Methods in Pharmacology; p. 214. [Google Scholar]
- 13.Mandal SC, Maity TK, Das J, Saba BP, Pal M. Anti-inflammatory evaluation of Ficus racemosa Linn. Leaf extract. J Ethnopharmacol. 2000;72:87–92. doi: 10.1016/s0378-8741(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 14.Tomisawa S, Sato NL. Preventive and curative effects of non-steroidal anti-inflammatory drugs on experimental peritoneal inflammation in mice. Jpn J Pharmacol. 1973;23:453–8. doi: 10.1254/jjp.23.453. [DOI] [PubMed] [Google Scholar]
- 15.Fujita Y, Mori I, Kitano S. Color reaction between pyrogallol red-molybdenum (VI) complex and protein. Bunseki Kagaku. 1983;32:E379–86. [Google Scholar]
- 16.Meir R, Schuler W, Desaulles P. [On the mechanism of cortisone inhibition of connective tissue proliferation] Experientia. 1950;6:469–71. doi: 10.1007/BF02154110. [DOI] [PubMed] [Google Scholar]
- 17.Whittle BA. The use of changes in capillary permeability in mice to distinguish between narcotic and non-narcotic analgesics. Br J Pharmacol. 1964;22:246–53. doi: 10.1111/j.1476-5381.1964.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koster R, Anderson M, De-Beer EJ. Acetic acid for analgesic screening. Fed Proc. 1959;18:412–8. [Google Scholar]
- 19.Snyder EL, Hezzey A, Katz AJ, Bock J. Occurrence of the release reaction during preparation and storage of platelet concentrates. Vox Sang. 1981;41:172–7. doi: 10.1111/j.1423-0410.1981.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 20.Stuart RK. Platelet function studies in human being receiving 300 mg of aspirin per day. J Lab Clin Med. 1970;75:463–71. [PubMed] [Google Scholar]
- 21.Menon S. MD thesis submitted to the University of Mumbai on “Evaluation of Anti. Platelet Activity of Phyllanthus emblica, Plumbago zeylanicus, Cyperus rotundus and Embelia ribes.”. 2006:46–9. [Google Scholar]
- 22.Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;462:927–9. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- 23.Bhat R. MD thesis submitted to the University of Mumbai on “Effect of prostacyclin mimetics and Indian medicinal plants on platelet function.”. 2004:19–22. [Google Scholar]
- 24.Sawant DS. PhD thesis submitted to the University of Mumbai on “Effect of selected Indian medicinal plants and neutraceuticals on lipid metabolism.”. 2002:53–59. [Google Scholar]
- 25.Buodonpri W, Wichitnithad W, Rojsitthisak P, Towiwat P. Synthetic curcumin inhibits carrageenan-induced paw edema in rats. J Health Res. 2009;23:11–6. [Google Scholar]
- 26.Aiyelero OM, Ibrahim ZG, Yaro AH. Analgesic and anti-inflammatory properties of the methanol leaf extract of Ficus ingens (Moraceae) in rodents. Niger J Pharm Sci. 2009;8:79–86. [Google Scholar]
- 27.Paschapur M, Patil S, Patil S, Kumar R, Patil MB. Evaluation of the analgesic and antipyretic activities of ethanolic extract of male flowers (inflorescence) of Borassus flabellifer L. (Arecaceae) Int J Pharm Pharm Sci. 2009;1:98–106. [Google Scholar]
- 28.Srinivasan K, Muruganandan S, Lal J, Chandra S, Tandan SK, Prakash VR. Evaluation of anti-inflammatory activity of Pongamia pinnata leaves in rats. J Ethnopharmacol. 2001;78:151–7. doi: 10.1016/s0378-8741(01)00333-6. [DOI] [PubMed] [Google Scholar]
- 29.Singh RS, Ahmad M, Wafai ZA, Seth V, Moghe VV, Upadhyaya P. Anti-inflammatory effects of Dashmula, an Ayurvedic preparation, versus Diclofenac in animal models. J Chem Pharm Res. 2011;3:882–8. [Google Scholar]
- 30.Gupta RA. Pharmacological Studies on Dasamula Kvatha-Part 2. Indian J Anc Med Yoga. 2012;5:103–8. [Google Scholar]
- 31.Pawar N, Kogje A, Bhondave P, Nagarkar B, Kulkarni O, Harsulkar A, et al. Comparative free radical scavenging and anti-inflammatory potential of branded market samples of an Ayurvedic formulation: Dashamoolarishta. Int J Pharm Biol Sci. 2013;4:789–99. [Google Scholar]
- 32.Singh RS, Ahmad M, Wafai ZA, Khan ZY, Sharma M, Seth V. Analgesic effects of Dashamula, an Ayurvedic preparation, versus Diclofenac sodium in animal models. J Clin Diagn Res. 2012;6:547–50. [Google Scholar]
- 33.Dawane JS, Pandit V, Borole K. Experimental evaluation of antipyretic activity of aqueous extract of Dashamula. Spatula DD. 2012;2:17–21. [Google Scholar]
- 34.Tripathi B, editor. Sharangdhara-Samhita. Varanasi: Chaukhambha Surbharti Prakashan; 2001. Sharangdhacharya. Sandhan-Kalpana; pp. 247–62. [Google Scholar]
- 35.Sharma PV. In: Dravyaguna-Vidnyan. Varanasi: Chaukhambha Surbharti Prakashan; 1998. Impurities of drugs and their purification; pp. 353–9. [Google Scholar]
- 36.Khandal S. In: Rasa. Bhaishajyakalpana Vigyana. 3rd ed. Jaipur: Publication Scheme; 2000. Sandhankalpana Vidnyan; pp. 469–83. [Google Scholar]
- 37.Nandre BN, Bakliwal SR, Rane BR, Pawar SP. Traditional fermented formulations-Asava and Aristha. Int J Pharm Biol Arch. 2012;3:1313–9. [Google Scholar]
- 38.Mannacio VA, Di Tommaso L, Antignan A, De Amicis V, Vosa C. Aspirin plus clopidogrel for optimal platelet inhibition following off-pump coronary artery bypass surgery: Results from the CRYSSA (prevention of Coronary arteRYbypaSS occlusion After off-pump procedures) randomised study. Heart. 2012;98:1710–5. doi: 10.1136/heartjnl-2012-302449. [DOI] [PubMed] [Google Scholar]
- 39.Eisert WG. Dipyridamole in anti-thrombotic treatment. Adv Cardiol. 2012;47:78–86. doi: 10.1159/000338053. [DOI] [PubMed] [Google Scholar]
- 40.Shastri B, editor. Yogratnakar- Part 2. Varanasi: Chaukhambha Surbharti Prakashan; 2011. Hrudrogchikitsa; p. 47. [Google Scholar]
- 41.Tripathi B, editor. Charaka Samhita. Varanasi: Chaukhambha Surbharti Prakashan; 2011. Trimarmiyachikitsa, Chiitsastahn 26; p. 882. [Google Scholar]


