Abstract
Though hemangiomas of the bone are quite common, calvarial (skull) cavernous hemangiomas are relatively rare pathologies. Calvarial hemangiomas are usually small and asymptomatic. However, they may occasionally grow in size to achieve large sizes and can present as a palpable swelling. We present a child with massive temporo-parieto-occipital calvarial cavernous hemangioma, who was managed with a multimodal approach with excellent cosmetic and neurologic outcome.
Keywords: Calvarial hemangioma, giant, outcome, surgery
Introduction
Vascular malformations are developmental anomalies that occur when embryonic vascular networks fail to differentiate. Cavernous malformations (CM), which are also termed cavernous hemangiomas, cavernous angiomas, and cavernomas, are one of the many known vascular malformations. They can occur within the parenchyma of the central nervous system, in intracranial extra-axial locations, in bone (especially the vertebrae and skull) and in soft tissues of the body. Extensive involvement of adjacent tissues is unusual. We present a patient with a giant temporo-parietal CM involving the extracranial soft tissues, skull, and extradural space.
Case Report
A 9-year-old girl presented to us with a large swelling over the left side of her skull that was progressively increasing in size for 2 years. Medical advice was sought when the swelling became quite large posing cosmetic problems.
On inspection, there was a swelling on the left temporal region that was 15 × 15 cm in size with signs of inflammation over the skin. There were two linear scar marks of previous biopsies that she was subjected to at other hospitals (their report was inconclusive). The swelling was disfiguring and pushing the left ear downwards and outwards [Figure 1]. On palpation, the overlying scalp was freely mobile over the swelling. The swelling was firm, and nontender, and no bruit could be heard on auscultation. Neurological examination was unremarkable.
Figure 1.
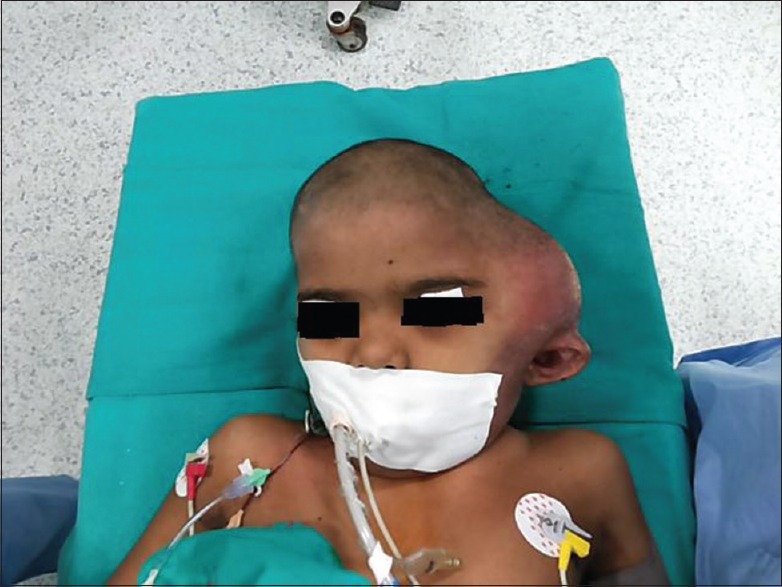
Preoperative photograph
Noncontrast computerized tomography scan [Figure 2] showed an extra-axial heterogenous lesion extending up to middle cranial fossa, posterior fossa up to cerebellopontine angle and the extracranial temporal and infratemporal fossa with intense enhancement following contrast administration. Magnetic resonance imaging (MRI) of brain revealed a large extra-axial lesion with epicenter in left temporal bone (size 114 × 65 × 80 mm), predominantly solid with few cystic areas with heterogenous signal intensity. Intracranially the lesion extended into the left cerebellopontine angle posteriorly and middle cranial fossa anteriorly. The lesion displaced left temporal lobe, left cerebellar hemisphere and brainstem to the contralateral side. Perilesional soft tissue mass was also seen in the left temporo-parieto-occipital scalp region. The lesion was predominantly hypointense on T1-weighted images (T1-WI) and hyperintense on T2-WI [Figure 2]. The lesion was extending both inside and outside the calvarium with destruction of left temporal bone. The lesion showed heterogenous enhancement on gadolinium injection. Areas of T1 hyperintensity, suggestive of subacute hemorrhage, were also seen. The cystic areas showed peripheral hypointense hemosiderin rim, suggestive of chronic hemorrhage. The whole lesion bloomed on saturation weighted images. On magnetic resonance venography, left transverse sinus, left sigmoid sinus and left jugular vein were not visualized probably due to thrombosis.
Figure 2.
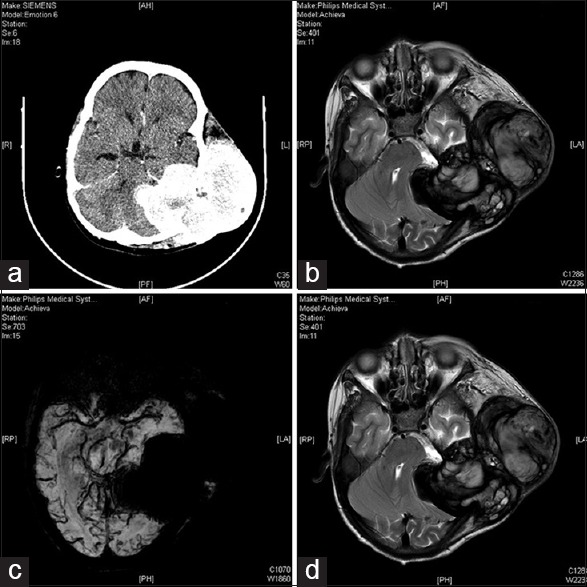
(a) Preoperative contrast-enhanced computed tomography brain. (b) Preoperative magnetic resonance imaging T2 axial. (c) Preoperative magnetic resonance imaging saturation weighted images axial. (d) Preoperative magnetic resonance imaging T1 postgadolinium axial
She was found to be severely anemic with blood hemoglobin 4.0 g/dL. She was transfused packed red blood cells before taking her for surgery. Rest of the hematological and biochemical parameters were within normal limits.
A tru-cut biopsy was planned, but it was nonconclusive and showed clotted and degenerated blood products. An incisional biopsy was then planned, and it showed some ill-defined blood-filled cavernous structures with no conclusive report. On both the occasions, no malignant/round cells could be found, which we were expecting.
During surgery, she was positioned in the lateral decubitus position and head was fixed in 3-pin head holder. An inverted J-shaped incision was given straddling the left ear pinna, and the scalp flap was raised subgaleally. Soft, reddish-brown, highly vascular lesion was seen in the left temporo-parietal region with large areas of coagulated blood within it. The lesion had stripped out the temporalis muscle from its attachment to the partially lysed squamous temporal bone. The whole extracranial portion was removed, and the patient lost 2.5 L of blood [Figure 3]. In view of massive blood loss, it was planned to remove the intracranial portion of the tumor at a later stage. In the second stage, the intracranial part was explored. As previously mentioned there was a defect in the temporo-parietal bone, this was extended using a pneumatic drill. The lesion was found to be completely extradural extending up to the left petrous apex, shifting the posterior fossa structures toward the right side. Gross total excision of the tumor was achieved. Postoperative computed tomography (CT) brain was done after 4 h and it showed no operative site hematoma [Figure 4]. She was discharged on postoperative day 7 without any significant postoperative events.
Figure 3.
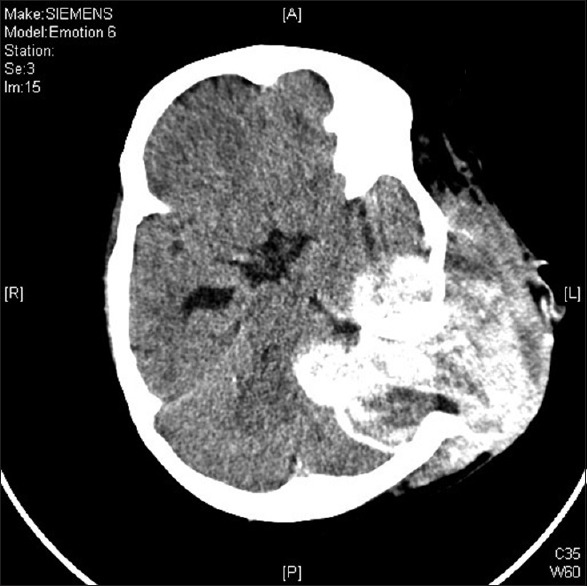
Post first surgery computed tomography brain
Figure 4.
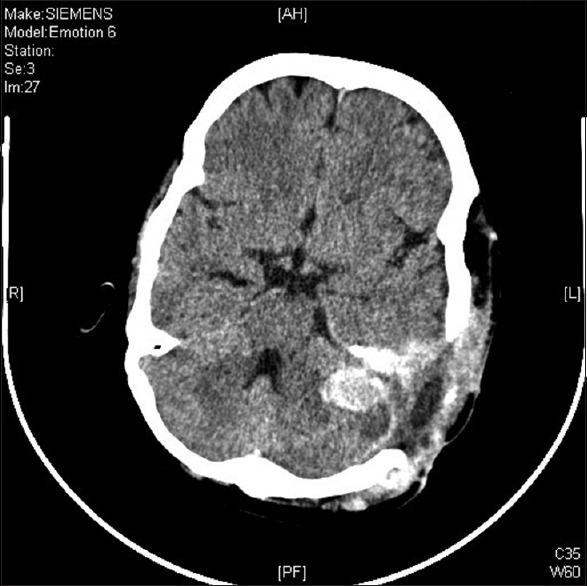
Post second surgery computed tomography brain
Postoperative contrast enhanced magnetic resonance imaging brain was done after 2 months that suggested a small residual lesion at the left cerebellopontine angle [Figure 5], for which stereotactic radiosurgery using Leksell Gamma Knife (Elekta AB, Box 7593, SE-103 93 Stockholm, Sweden) is planned.
Figure 5.
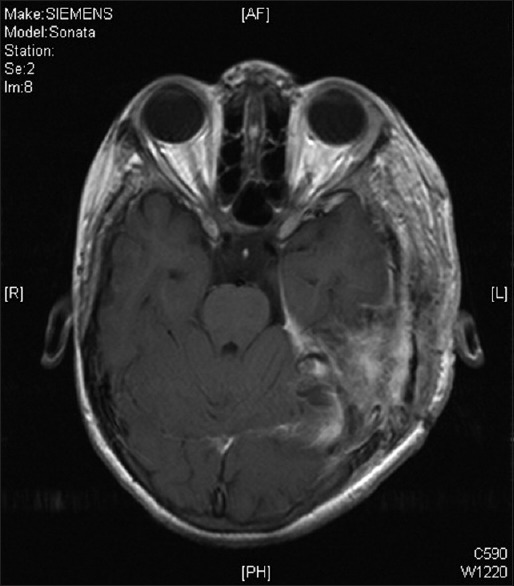
Postoperative magnetic resonance imaging T1 postgadolinium
Discussion
Intraosseous hemangiomas are rare neoplasms and comprise about 0.7% of all the bone tumors. The most commonly involved site is the vertebral column, followed by the skull. Hemangiomas of the calvarial bones account for 0.2% of all bone neoplasms.[1,2,3] These cranial vascular lesions are more prevalent in females than males (3:1 ratio)[1,4] and have a predilection for the frontal and parietal bones in the skull.[2,4,5] Although they may be seen in any age group, the peak incidence is during the second through fourth decades.
Calvarial cavernous hemangiomas arise from vessels in the diploic space and are supplied by the branches of the external carotid artery (ECA), arising in the skull vault. The middle meningeal and superficial temporal arteries are the main sources of blood supply.[4,6]
Hemangiomas vary in size from microscopic to massive and may be classified histologically as either capillary or cavernous. In 15% of cases, the lesions are multiple. Skull hemangiomas are usually small and asymptomatic but may evolve into a visible and palpable area of tenderness and swelling. The scalp overlying the lesion is typically freely mobile.
Histologically, cavernous hemangiomas are unencapsulated dilated sinusoidal channels that are lined by a single layer of flattened endothelium and are interspersed among bony trabeculae. Capillary hemangiomas are similar but contain more capillary-sized vessel loops; frequently, a mixture of both histologic forms is present. The bony trabeculae are thought to be caused by osteoclastic remodeling in response to stress from the enlarging vascular malformation.[6] Hemangiomas are always benign and may remain static for a long period. When they do grow, one of two growth patterns is typically exhibited: A “sessile” pattern, the commoner variety, involves extension along the diploe or a “globular” pattern with expansion of the skull and eventual extension into the surrounding soft tissues.[3] Association of skull hemangiomas with hemangiomas elsewhere in the body (including other bones), such as in the liver, kidney, spleen, and adrenal gland, has been reported.
Calvarial hemangiomas tend to involve the outer table of the skull and the diploë, with relative sparing of the inner table of the skull.[7] More extensive involvement of the inner table and extradural space is very unusual. Most are solitary, and one-third have perilesional sclerosis and there may be a halo of decalcification.[8] The classic appearance of a hemangioma on radiographs are a “honeycomb” pattern, denoting rounded area of rarefaction.[6] This pattern can perhaps best be appreciated on bone windows of a CT scan. A “sunray” pattern of radiating trabeculae is sometimes present on tangential views. Enhancement with contrast material is intense on both CT and MRI. Angiography may demonstrate feeding of the hemangioma by branches of the ECA and often by the superficial temporal artery or the middle meningeal artery, and it may suggest a pathway for preoperative embolization. Radionuclide bone scanning is of little use in the assessment of these lesions.[3] En bloc surgical excision with establishment of normal bony margins, when indicated, typically results in cure. Simple curettage may increase the chance of leaving residual disease and tumor recurrence. Some have advocated radiation treatment for residual tumor or for skull base lesions that are difficult to access surgically.[9]
Intracranial, extra-axial CMs are uncommon, making up 0.4–2% of all vascular malformations. Most reported cases are found in the middle fossa, arising within the cavernous sinus.[7,10,11,12] They occur predominantly in females (male: female ratio −11:1). Other reported sites of origin include the subarachnoid space, the tentorium, the convexity dura,[8,9] the cerebellopontine angle, Meckel's cave, and other cranial nerves. Dural CMs found outside the middle fossa have no gender predilection. Erosion of adjacent bone is often found with all these lesions, and they are histologically similar to intraparenchymal malformations.[12]
In the present case, the lesion had its epicenter in the left temporal bone, suggesting the possibility that the temporal bone was the site of origin. Another possibility is that the malformation might have originated in the soft tissue and subsequently invaded structures underneath. Sinonasal and cavernous sinus CMs have been shown to erode adjacent parts of the skull.[4,7] Okada, et al., reported a frontal skull cavernous hemangioma with components extending extracranially as well as extradurally. Dandy reported a 1-month-old infant with a large intradural posterior fossa cavernous hemangioma that extended through the dura and skull into the subgaleal space in 1928.
Conclusion
As the present case demonstrates, extracerebral CMs may undergo significant growth and cross tissue planes. They may cause cosmetic deformity and produce mass effect on the brain. Surgical excision is the treatment of choice and is challenging in large lesions due to high vascularity, but the outcome is good.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Okada J, Hara M, Takeuchi K. Dural haemangioma with extracranial component. Acta Neurochir. 1977;36:111–5. doi: 10.1007/BF01405992. [DOI] [PubMed] [Google Scholar]
- 2.Peterson DL, Murk SE, Story JL. Multifocal cavernous hemangioma of the skull: Report of a case and review of the literature. Neurosurgery. 1992;30:778–81. [PubMed] [Google Scholar]
- 3.Yoshida D, Sugisaki Y, Shimura T, Teramoto A. Cavernous hemangioma of the skull in a neonate. Childs Nerv Syst. 1999;15:351–3. doi: 10.1007/s003810050410. [DOI] [PubMed] [Google Scholar]
- 4.Kawai K, Fukui M, Tanaka A, Kuramoto S, Kitamura K. Extracerebral cavernous hemangioma of the middle fossa. Surg Neurol. 1978;9:19–25. [PubMed] [Google Scholar]
- 5.Ohtomo K, Itai Y, Yoshikawa K, Kokubo T, Yashiro N, Iio M. Hepatic haemangioma: Dynamic MRI using gadolinium-DTPA. Eur J Radiol. 1987;7:257–9. [PubMed] [Google Scholar]
- 6.Hook SR, Font RL, McCrary JA, Harper RL. Intraosseous capillary hemangioma of the frontal bone. Am J Ophthalmol. 1987;103:824–7. doi: 10.1016/s0002-9394(14)74401-0. [DOI] [PubMed] [Google Scholar]
- 7.Khanam H, Lipper MH, Wolff CL, Lopes MB. Calvarial hemangiomas: Report of two cases and review of the literature. Surg Neurol. 2001;55:63–7. doi: 10.1016/s0090-3019(00)00268-8. [DOI] [PubMed] [Google Scholar]
- 8.Pang D, Tse HH, Zwienenberg-Lee M, Smith M, Zovickian J. The combined use of hydroxyapatite and bioresorbable plates to repair cranial defects in children. J Neurosurg. 2005;102:36–43. doi: 10.3171/ped.2005.102.1.0036. [DOI] [PubMed] [Google Scholar]
- 9.Isla A, Roda JM, Alvarez F, Muñoz J, García E, Blázquez MG. Intracranial cavernous angioma in the dura. Neurosurgery. 1989;25:657–9. doi: 10.1097/00006123-198910000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Harper DG, Buck DR, Early CB. Visual loss from cavernous hemangiomas of the middle cranial fossa. Arch Neurol. 1982;39:252–4. doi: 10.1001/archneur.1982.00510160058014. [DOI] [PubMed] [Google Scholar]
- 11.Linskey ME, Sekhar LN. Cavernous sinus hemangiomas: A series, a review, and an hypothesis. Neurosurgery. 1992;30:101–8. doi: 10.1227/00006123-199201000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Lombardi D, Giovanelli M, de Tribolet N. Sellar and parasellar extra-axial cavernous hemangiomas. Acta Neurochir (Wien) 1994;130:47–54. doi: 10.1007/BF01405502. [DOI] [PubMed] [Google Scholar]


