Abstract
Tumefactive multiple sclerosis (MS) is a rare variant of MS characterized by the presence of large demyelinating plaques of more than 2 cm, identified with magnetic resonance imaging (MRI). Distinguishing tumefactive lesions from other etiologies of intracranial space occupying lesions is necessary to avoid the inadvertent intervention. We had a 14-year-old girl who presented to us with two episodes of subacute hemiparesis over a span of 6 months. Her MRI brain showed large lesions, which were hyperintense on T2-weighted/flair images with incomplete ring enhancement open towards the gray matter in postgadolinium images with minimal surrounding edema and mass effect. We treated her as a case of tumefactive demyelination (TD) with steroids after which patient recovered with minimal deficits. TD occurs more commonly in women and young adults and is reported rarely. TD in a young girl with recurrence in such short span causing bilateral hemiparesis has never been reported.
Keywords: Demyelination, multiple sclerosis, tumefactive
Introduction
Tumefactive demyelination (TD) is defined as large solitary demyelinating lesion mimicking neoplasm clinically and radiographically.[1] These features include size more than 2 cm, mass effect, edema, and/or ring enhancement. Distinguishing tumefactive lesions from other etiologies of intracranial space occupying lesions is necessary to avoid inadvertent chemotherapeutic or surgical intervention. TD occurs more commonly in women and young adults and is reported rarely. Here we report a case of TD in a young girl who developed recurrent episodes in a span of 6 months causing bilateral hemiparesis. TD in the young girl with recurrence in such short span has never been reported.
Case Report
An apparently normal 14-year-old girl presented to us with complaints of subacute onset hemiparesis of 3 weeks duration. For the initial three to 4 days along with weakness, she also had severe throbbing headache associated with vomiting and intermittent blackouts. History was negative for fever, trauma, seizures, vision loss or weight loss.
On examination, she was conscious, cooperative and her vitals were stable. Fundus examination showed bilateral papilledema. Systemic examination revealed hypertonia on the right side with strength of 4/5 in upper limb and 3/5 in lower limb (Medical Research Council grading) and extensor plantar. Rest of the examination was normal. With differentials of mass lesion, abscess and stroke we went ahead with investigations.
Laboratory reports were normal for hemogram, random blood sugar, renal and liver function tests, serum electrolytes, serology for HIV, HBsAg, hepatitis B virus and venereal disease research laboratory. Magnetic resonance imaging (MRI) of brain [Figures 1 and 2] showed large poorly marginated lesion in the left parieto-occipital lobe measuring 6.3 × 5.6 × 5.4 cm, hypointense on T1-weighted, hyperintense on T2-weighted/flair images and postgadolinium images showing incomplete ring enhancement which is open towards the gray matter. Surrounding edema and mass effect was little compared to the size of the lesion. Magnetic resonance spectroscopy (MRS) [Figure 3] revealed abnormally elevated choline and mildly decreased creatine, with increased choline creatine ratio. N-acetyl aspartate peak was mildly reduced with abnormal lactate doublet on long TE spectrum Philips Achieva 1.5 Tesla MRI. With the possibility of tumefactive multiple sclerosis (MS) we evaluated her further for serum antinuclear antibody, antinuclear cytoplasmic antibodies, rheumatoid factor, C3, angiotensin converting enzyme levels, aquaporin-4 antibodies, which were all negative. Cerebrospinal fluid analysis showed normal glucose (46 mg), raised protein (76 mg) and positive for oligoclonal bands. To confirm the diagnosis biopsy was planned but was refused by her parents.
Figure 1.
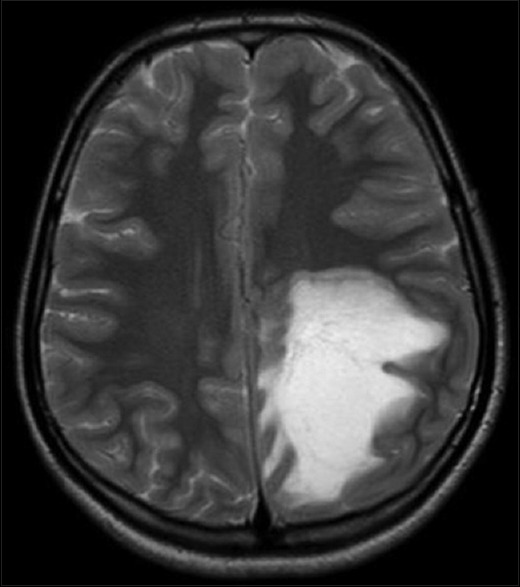
Magnetic resonance imaging of brain showing large poorly marginated lesion in the left parieto-occipital lobe measuring 6.3 × 5.6 × 5.4 cm, hyperintense on flair image
Figure 2.
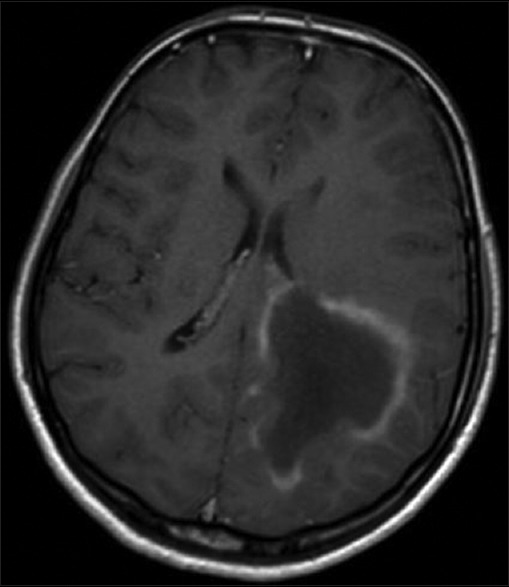
Magnetic resonance imaging of brain with gadolinium showing incomplete ring enhancement which is open towards the gray matter
Figure 3.
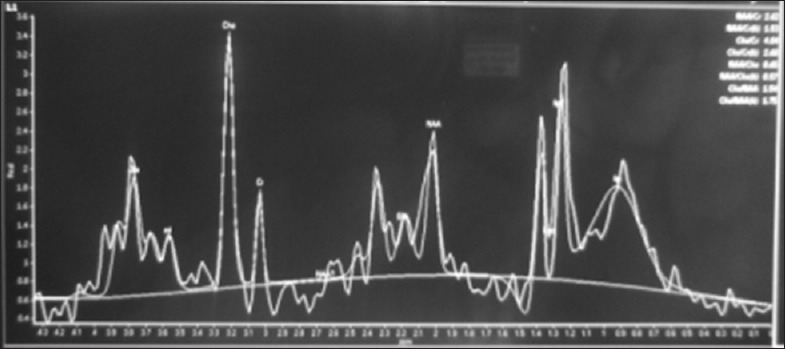
Magnetic resonance spectroscopy showing abnormally elevated choline and mildly decreased creatine, with increased choline creatine ratio. N-acetyl aspartate peak was mildly reduced with abnormal lactate doublet on long TE spectrum
Patient was treated with intravenous methyl prednisolone 1 g daily for 5 days and was put on oral Prednisone 40 mg for 15 days, and 10 mg was tapered every 15 days. Patient improved remarkably with strength of 4/5 in the right upper and lower limb, and was on regular follow-up every month for 3 months after discharge.
Six months after the first event patient came to us again with complaints of subacute onset left hemiparesis of 2 weeks duration. In view of her history of TD we went ahead directly with brain imaging [Figures 4 and 5], which revealed circumscribed lesion in paramedian high right frontal lobe measuring about 2 × 2 cm with hyperintense T2-weighted/flair images and incomplete ring on diffusion weighted images, along with encephalomalacia and gliosis in left high parietal region. She had a relapse of TD in 6 months. We treated her again with steroids after which she improved over 2 weeks.
Figure 4.
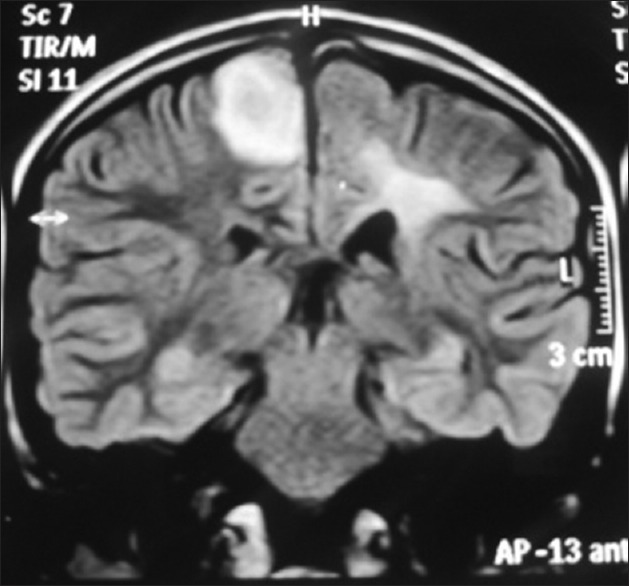
Magnetic resonance imaging brain showing circumscribed lesion in paramedian high right frontal lobe measuring about 2 × 2 cm with hyperintense flair images along with encephalomalacia and gliosis in left high parietal region
Figure 5.
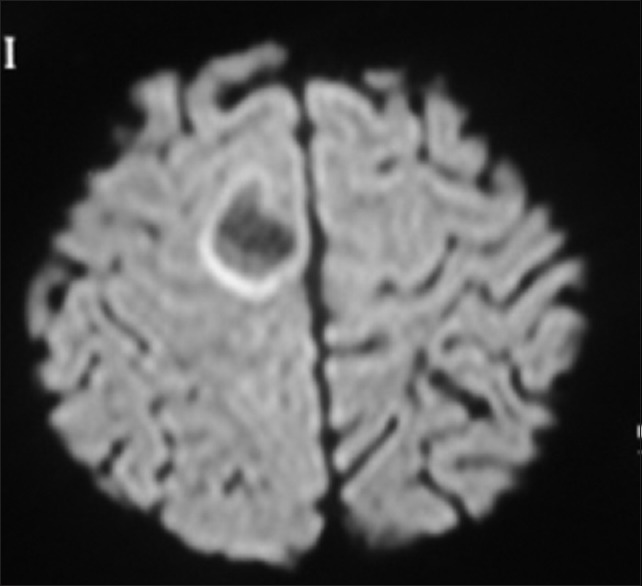
Magnetic resonance imaging brain showing incomplete ring on diffusion weighted image
Discussion
Tumefactive lesions are an uncommon manifestation of demyelinating disease and are frequently misdiagnosed in patients without a preexisting diagnosis of MS. The prevalence of TD is estimated to be approximately 1–2 per 1000 cases of MS.[2] There is no clear gender predilection and the patient group comprises of adults in their 20 s and 30 s although pediatric and older patients have been described.
Our patient was a young girl of 14 years which was atypical for TD. As she presented with headaches, vomiting and subacute hemiparesis our differentials at the time of presentation were abscess, neoplasm and stroke. It has been mentioned in the literature that patients may present clinically with symptomatology that is atypical of MS and tends to resemble that of space occupying lesions. Depending on the anatomical site, features that may suggest a TD lesion include altered sensorium, seizures, cognitive dysfunction, visual field deficits, hemiparesis and hemisensory disturbance.[3] Headache and vomiting are found to be common in children.
Magnetic resonance imaging brain with spectroscopy has a major role in making the diagnosis of TD. Certain imaging characteristics may help in diagnosing TD over a neoplasm or abscess. Commonly TD lesions are well circumscribed supratentorial lesions with a predilection for the frontal and parietal lobes. Mass effect and perilesional edema in TD lesions are usually less substantial than that seen with malignancy.[4] The majority of lesions range in size from 2 cm to 6 cm and enhance with gadolinium. Open ring enhancement with the incomplete portion of the ring on the grey matter side of the lesion is an important diagnostic clue to a tumefactive lesion. Other features include a T2-weighted hypointense rim, peripheral restriction on diffusion weighted imaging. The above mentioned features were found in the imaging of our patient.
The role of MRS, in distinguishing tumefactive lesions, is yet to be clearly defined. TD lesions may cause an increased choline to N-acetyl-aspartate ratio, but this is also a common finding in neoplastic lesions. Increased glutamate/glutamine peaks appear to favor TD but this has not been extensively studied.[5]
Natural clinical course according to the available evidence suggests that around 65% patients go on to develop relapsing remitting course typical of conventional MS after a first TD lesion. Remaining patients will have no further attacks of demyelination. But our patient developed another attack of TD in 6 months which was unusual. Though tumefactive relapses may occur in the context of other more typical MS demyelinating lesions, some patients may only experience relapse with TD lesions.[6] It may be that recurrent TD is simply a phenotypic variant of conventional MS but the possibility exists that patients with recurrent TD lesions may have a distinct subtype of demyelinating disease. This is the first report, to our knowledge, describing recurrent tumefactive lesions in a young patient without MS. Whether early intervention with immunomodulation is beneficial in patients of TD is a matter of debate. Further research is warranted.
Conclusion
Tumefactive demyelination is a rare variant of MS which can have atypical course. Although tumefactive MS has been described as a monophasic disease, relapses do occur. Misdiagnosis is common in TD, so clinicians should be aware of this entity to prevent inadvertent interventions.
Acknowledgments
Dr. Gaurav Kasundra, DM neuroresident, Department of Neurology, Dr. S. N. Medical College, Jodhpur, Dr. Bharat Bhushan, DM neuroresident, Department of Neurology, Dr. S. N. Medical College, Jodhpur.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Kalanie H, Harandi AA, Bakhshandehpour R, Heidari D. Multiple large tumefactive MS plaques in a young man: A diagnostic enigma and therapeutic challenge. Case Rep Radiol 2012. 2012 doi: 10.1155/2012/363705. 363705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poser S, Lüer W, Bruhn H, Frahm J, Brück Y, Felgenhauer K. Acute demyelinating disease. Classification and non-invasive diagnosis. Acta Neurol Scand. 1992;86:579–85. doi: 10.1111/j.1600-0404.1992.tb05490.x. [DOI] [PubMed] [Google Scholar]
- 3.Hardy TA, Chataway J. Tumefactive demyelination: An approach to diagnosis and management. J Neurol Neurosurg Psychiatry. 2013;84:1047–53. doi: 10.1136/jnnp-2012-304498. [DOI] [PubMed] [Google Scholar]
- 4.Lucchinetti CF, Gavrilova RH, Metz I, Parisi JE, Scheithauer BW, Weigand S, et al. Clinical and radiographic spectrum of pathologically confirmed tumefactive multiple sclerosis. Brain. 2008;131:1759–75. doi: 10.1093/brain/awn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cianfoni A, Niku S, Imbesi SG. Metabolite findings in tumefactive demyelinating lesions utilizing short echo time proton magnetic resonance spectroscopy. AJNR Am J Neuroradiol. 2007;28:272–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Häne A, Bargetzi M, Hewer E, Bruehlmeier M, Khamis A, Roelcke U. Recurrent tumefactive demyelination without evidence of multiple sclerosis or brain tumour. J Neurol. 2011;258:318–20. doi: 10.1007/s00415-010-5722-1. [DOI] [PubMed] [Google Scholar]


