Abstract
Background
This study was aimed to investigate whether ATP-sensitive potassium channel (KATP) is involved in curcumin’s anti-proliferative effects against gastric cancer.
Methods
In an in vitro study, gastric cancer cell line SGC-7901 was treated with curcumin at serial concentrations and co-administrated with the KATP opener, diazoxide. The effect of curcumin and diazoxide on proliferation were assessed by MTT assay. Mitochondrial membrane potential (MMP) was studied by flow cytometry detection of rhodamine 123 staining. Apoptosis was evaluated by flow cytometry detection of Annexin V propidium iodide double staining. In an in vivo study, SGC-7901 cells were planted into nude mice as xenografts. Animals were treated with curcumin co-administered with diazoxide. Tumor volume and tumor weight were observed.
Results
Curcumin incubation significantly induced loss of MMP in SGC-7901 cells in a dose- dependent manner (P < 0.05); the cell apoptotic rate also dramatically increased after curcumin incubation in a dose-dependent manner (P < 0.05). After co-administration with diazoxide, however, we found that both the MMP-loss-inducing and the apoptosis-inducing effects of curcumin in SGC-7901 cells were significantly impaired (all P < 0.05). As a result, the proliferation of SGC-7901 cells was maintained by diazoxide treatment.
Conclusions
Impaired mitoKATP opening causes MMP loss, and is involved in curcumin-induced apoptosis in gastric cancer.
Keywords: apoptosis, curcumin, gastric cancer, KATP
Background
As the third leading cause of death in men and fourth in women with malignant tumors, gastric cancer is now threatening people’s lives worldwide [1]. Gastric cancer is one of the highly malignant carcinomas arising from epithelial cells, and is characterized by therapeutic inefficiency and poor prognosis [2]. Like most malignant tumors, the malignancy of gastric cancer is caused by uncontrolled cell proliferation, invasion, and metastasis. The therapeutic options for gastric cancer are often limited because most patients with gastric cancer are diagnosed at an advanced stage [3]. Currently, chemotherapy and radiotherapy are employed to treat gastric cancer at an advanced stage. However, the side effects of these therapies restrict their application and lower the quality of life of patients [4]. Traditional Chinese medicine has been effectively applied in treating malignant diseases for a long time in Eastern Asia [5]. Several monomers, including curcumin, discovered from traditional Chinese medicine formulas have been shown to have anti-cancer activities in previous studies [6]. However, the molecular mechanisms for these activities are still unclear.
Natural extracts or products have attracted attention and become the focus of anti-cancer drug research because over two-thirds of novel anti-cancer drugs discovered in recent decades are of natural original [7]. Curcumin, also known as diferuloylmethane, is extracted from the rhizome of Curcuma longa. The spectrum of biological activities of curcumin is thought to be wide, including anti-angiogenic, anti-oxidant, anti-inflammatory and anti-diabetic effects [8]. Previous studies have shown that the expression of oncogenes [9], transcription factors [10], cytokines, and growth factors [11] was modulated in the anti-cancer effects of curcumin. Although several mechanisms regulating proliferation and apoptosis of curcumin have been investigated, many more studies are still needed.
ATP-sensitive potassium channels, also referred to as KATP, are distributed throughout the organs and tissues, including skeletal muscle, cardiac muscle, brain, and kidney [12]. KATP is characterized by a hetero-octameric molecular structure; it is composed of four Kir6 and four sulphonylurea receptor subunits [13]. It is now believed that the fundamental function of KATP is to adjust membrane excitability to cellular metabolic status [14]. KATP located on the mitochondrial membrane is called mitoKATP, the opening of which reduces the mitochondrial transition opening permeability by increasing the activity of inwardly rectifying potassium channels under stressful conditions [15, 16]. Thus, opening of the mitoKATP could attenuate cell apoptosis by maintaining mitochondrial membrane potential (MMP) [17].
A recent study preliminarily established the relationship between mitoKATP and the proliferation of malignant cancer cells, such as glioma cells [18]. This association aroused our interests in exploring whether the possibility that curcumin’s anti-cancer effect on gastric cancer is related to mitoKATP. In this study, we investigated the effect of curcumin and the selective mitoKATP opener diazoxide on proliferation, mitochondrial transition opening permeability, and apoptosis in human gastric cancer cells SGC-7901 in vitro. A corresponding in vivo study was also implemented. Our results will contribute to a deepened understanding of the molecular mechanisms of curcumin’s anti-cancer activity.
Methods
Cell culture and treatment
Human gastric cancer cell line SGC-7901 was purchased from the American Type Culture Collection and cultured in DMEM (Gibco) supplemented with 10% FBS (Gibco). The cells were maintained in a humidified cell incubator (Thermo Scientific, Pittsburgh, PA, USA) containing 5% CO2 at 37°C. Equal numbers of cells were divided into seven independent groups: a control group (C), a low-dose curcumin group (LCur), a medium-dose curcumin group (MCur), a high-dose curcumin group (HCur), a low-dose curcumin group treated with diazoxide (LCur + DZ), a medium-dose curcumin group treated with diazoxide (MCur + DZ) and a high-dose curcumin group treated with diazoxide (HCur + DZ).
In the control group, cells were maintained in culture medium, as described; in LCur, cells were treated with curcumin (Sigma-Aldrich, St. Louis, MO, USA) solution at concentration of 15 μmol/l; in MCur, cells were treated with curcumin solution at a concentration of 30 μmol/l; in HCur, cells were treated with curcumin at a concentration of 60 μmol/l; in LCur + DZ, cells were treated with diazoxide (Sigma-Aldrich) at a concentration of 100 μmol/l together with curcumin at a concentration of 15 μmol/l; in MCur + DZ, cells were treated with diazoxide at a concentration of 100 μmol/l together with curcumin at a concentration of 30 μmol/l; in HCur + DZ, cells were treated with diazoxide at concentration of 100 μmol/l together with curcumin at a concentration of 60 μmol/l.
Cell proliferation assessment
A 3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium-bromide (MTT) assay was employed to assess the proliferation of SGC-7901 cells. Briefly, 1 × 104 cells per well were planted in a 96-well culturing plate (Corning Costar, Corning, NY, USA) for 24 hours and then treated with diazoxide and curcumin, as described. Then 20 μl MTT (Sigma-Aldrich, 5 mg/ml, dissolved in PBS) was added to each well and 150 μl dimethylsulfoxide (Sigma-Aldrich) was added to replace medium from each well. Absorbance at 450 nm (A450) was measured using a plate reader (Bio-Rad, Hercules, CA, USA). The growth inhibition rate was calculated using the formula:
Cell apoptosis assay
The apoptosis assessment of SGC-7901 cells in each group was carried out by flow cytometry using an Annexin V-FITC Apoptosis Detection Kit (BD, San Jose, CA, USA) as described previously [19]. Briefly, equal numbers of cells from each group were washed by PBS and titrated by binding buffer to concentrations of 1 × 106 cell/ml. Then 100 μl of this cell suspension, 5 μl Annexin V-FITC solution and 5 μl propidium iodide were added to a culture tube for 15 min incubation in a dark chamber. After 400 μl binding buffer was added to each tube, apoptosis was then analyzed by a fluorescence-activated cell sorting flow cytometer (BD, San Jose, CA, USA).
Mitochondrial membrane potential (MMP) detection
The MMP in SGC-7901 cells was determined by detection of rhodamine 123 staining by flow cytometry following the protocols described in a previous study [19]. Cells from each group were washed by PBS twice then titrated to 1 × 106 cells/ml. Rhodamine 123 solution (Beyotime, Shanghai, China) was then added to the cell suspension at a final concentration of 1 μmol/l. After incubation at 37°C in a dark chamber for 30 min, the fluorescent signal of rhodamine 123 released from cells was analyzed by a fluorescence-activated cell sorting flow cytometer (BD, San Jose, CA, USA) at 529 nm.
In vivoxenograft tumor study
Cultured SGC-7901 cells were suspended and titrated in PBS at a concentration of 1 × 107/ml. 100 μl of the cell suspension was subcutaneously injected into the right armpits of male BALB/C nude mice (Animal Experimental Center of the Fourth Military Medical University) with a mean body weight of (17.64 ± 5.19) g. After the volumes of the xenograft tumors exceeded 100 mm3, 18 mice were divided into three independent groups randomly and evenly (six mice per group); namely, a control group (C), a curcumin treatment group (Cur) and curcumin and diazoxide co-treatment group (Cur + DZ). In Cur + DZ, mice received peritumoral injections of curcumin (50 mg/kg) applied together with peritumoral injections of diazoxide (10 mg/kg) twice per day for 2 weeks. In Cur, mice received peritumoral injections of curcumin (50 mg/kg) twice per day for 2 weeks. In C, mice received peritumoral injections of physiological saline twice per day for 2 weeks. The animal treatment protocols were in accordance with our pre-experimental results (not shown) and several previous studies [20, 21]. The volume of tumor was measured twice per week and calculated by the formula:
The tumor was also weighted after the mice were sacrificed. All animal experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Statistical considerations
All data collected in this study are expressed as mean ± standard deviation. The statistical analysis was processed by software SPSS (version 16.0) by analysis of variance (ANOVA), least-significant difference and Fisher exact tests. P < 0.05 was considered statistically significant when comparing differences.
Results
Curcumin inhibited proliferation of SGC-7901 cells but this was alleviated by diazoxide co-administration
As shown in Figure 1, according to the results of the MTT assay, curcumin elevated inhibition rate in a concentration-dependent manner in LCur (9.5% ± 2.10%), MCur (23.2% ± 7.21%), and HCur (39.4% ± 9.43%) compared with C (1.20% ± 0.25) (all P < 0.05). However, after co-administration of diazoxide, the inhibition rate was decreased in LCur + DZ (6.72% ± 1.80%), MCur + DZ (16.55% ± 6.11%), and HCur + DZ (31.43 ± 8.15%) compared with corresponding LCur, MCur, and HCur (all P < 0.05).
Figure 1.
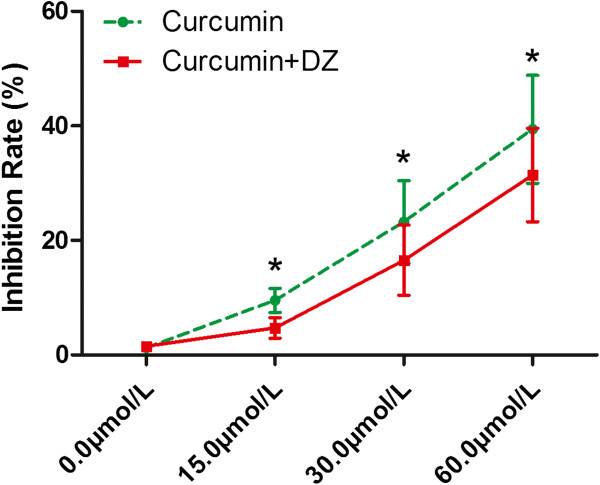
Effects of curcumin and its co-administration with diazoxide on proliferation of SGC-7901 cells. Cell proliferation was assessed by MTT assay. Graphs shows inhibition rate of SGC-7901 cells treated by curcumin (red) and co-administration of curcumin and diazoxide (green). Values are represented as mean ± standard deviation. *, values are significantly different when compared with control; #, values are significantly different when compared with curcumin treatment.
Co-administration of diazoxide impaired curcumin’s effects of decreasing mitochondrial membrane potential (MMP) in SGC-7901 cells
In a concentration-dependent manner (Figure 2), curcumin lowered MMP in SGC-7901 cells in LCur (77.51% ± 8.13%), MCur (62.07% ± 4.52%), and HCur (59.18% ± 9.30%) compared with C (95.40% ± 7.62%) (all P < 0.05). However, MMP was significantly higher with the co-administration of diazoxide; LCur + DZ (87.33% ± 4.68%), MCur + DZ (59.18% ± 5.92%), and HCur + DZ (65.29% ± 6.10%) (all P < 0.05).
Figure 2.
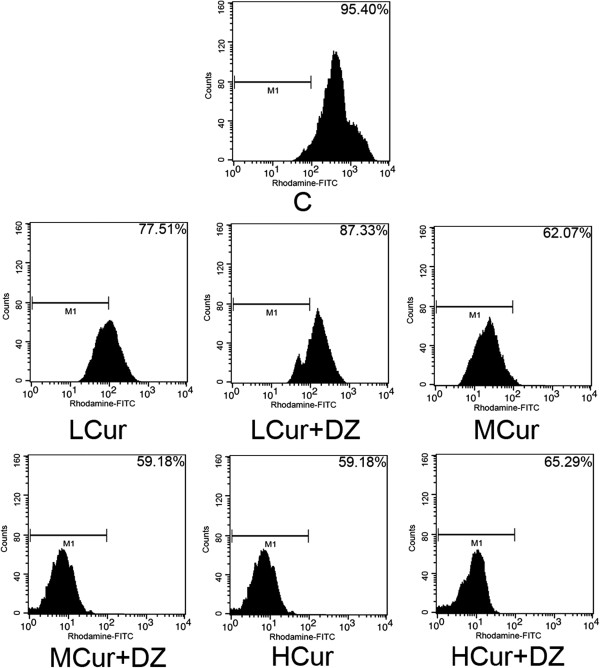
Effects of curcumin and its co-administration with diazoxide on mitochondrial membrane potential in cultured SGC-7901 cells. Cells were stained with rhodamine 123 and then detected by a flow cytometer. Cells with decreased MMP show less detected fluorescence from rhodamine 123. Charts of flow cytometry from top to bottom and from left to right show the detected rhodamine 123 fluorescence of C, LCur, LCur + DZ, MCur, MCur + DZ, HCur, and HCur + DZ. C, control; DZ, diazoxide; FITC, fluorescein isothiocyanate; HCur, high-dose curcumin; LCur, low-dose curcumin; MCur, medium-does curcumin; MMP, mitochondrial membrane potential.
Co-administration of diazoxide mitigated apoptosis in curcumin-incubated SGC-7901 cells
Detected by flow cytometry, compared with C (2.44% ± 0.79%) the apoptosis rate of SGC-7901 cells was increased by curcumin incubation in a concentration-dependent manner in LCur (18.94% ± 2.32%), MCur (35.21% ± 2.77%), and HCur (63.09% ± 3.05%) (all P < 0.05). When applied together, diazoxide alleviated the curcumin-induced apoptosis in LCur + DZ (13.51% ± 1.85%), MCur + DZ (27.81% ± 2.24%), and HCur + DZ (54.22% ± 2.72%). The results are shown in Figure 3.
Figure 3.
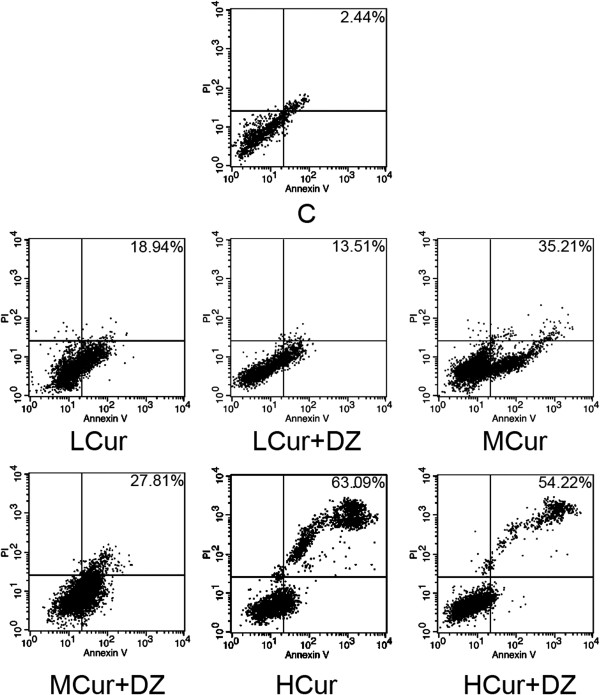
Effects of curcumin and its co-administration with diazoxide on apoptosis of SGC-7901 cells. Cells were double-stained with Annexin V and propidium iodide, and apoptosis rates were analyzed by flow cytometry. Percentage on the right top corner of each chart stands for apoptosis rate. Charts of flow cytometry from top to bottom, from left to right show the detected rhodamine 123 fluorescence of C, LCur, LCur + DZ, MCur, MCur + DZ, HCur and HCur + DZ, respectively. C, control; DZ, diazoxide; HCur, high-dose curcumin; LCur, low-dose curcumin; MCur, medium-does curcumin, PI; propidium iodide.
Growth of xenograft tumor was reduced by curcumin but reversed by co-administration of diazoxide in vivo
At the end of 3 weeks after initiation of curcumin and diazoxide treatment, all mice were alive. After sacrifice, tumors were isolated. Compared with C, tumor volume was significantly reduced in Cur starting from day 7 (P < 0.05). However, tumor volume in Cur + DZ was significantly higher than that in Cur (P < 0.05). In Cur, curcumin administration showed a significant inhibitory effect on the weight of xenografts compared with C (P < 0.05). However, this effect was mitigated by co-administration of diazoxide (P < 0.05). The results are shown in Figure 4.
Figure 4.
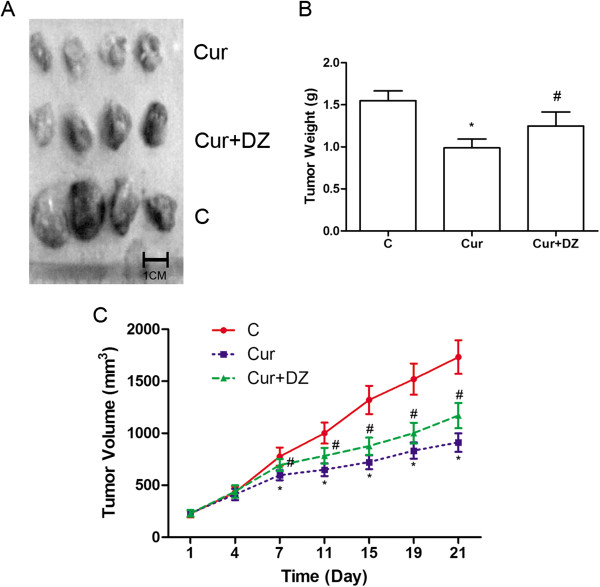
Effects of curcumin and its co-administration with diazoxide on growth of SGC-7901 tumor xenografts. (A) Isolated tumor xenografts from nude mice of Cur, Cur + DZ, and C at day 21. (B) Weight of isolated xenografts from nude mice of Cur, Cur + DZ, and C at day 21. (C) Tumor volume of SGC-7901 tumor xenografts in Cur (blue), Cur + DZ (green) and C (red) from day 1 to day 21. Values are represented as mean ± standard deviation. *, values are significantly different compared with C; #, values are significantly different compared with Cur. C, control; Cur, curcumin; DZ, diazoxide.
Discussion
We explored the involvement of KATP in the anti-proliferation effects of curcumin in gastric cancer. It was found that curcumin could inhibit the proliferation of gastric cancer cells by inducing apoptosis. Our in vitro results showed that curcumin-induced apoptosis of SGC-7901 cells by facilitating the collapse of MMP, which was believed to initiate the mitochondria-dependent apoptotic pathway. However, the co-administration of diazoxide, which is a mitoKATP selective opener, alleviated the collapse of MMP in curcumin-incubated SGC-7901 cells. In our in vivo study, the reduction in both volume and weight of xenograft tumor by curcumin was also reversed by co-administration of diazoxide. These results indicated that curcumin could induce apoptosis of gastric cancer cells via deactivating mitoKATP, which would expedite the collapse of MMP.
With the improvement of modern medical technology and cancer prevention, the incidence of gastric cancer has decreased remarkably in the past few years [22]. However, globally, gastric cancer is now the second leading cause of mortality in malignant diseases [1]. The prognosis of patients with gastric cancer is poor, especially in patients with metastatic lymph nodes and low serum albumin levels, who are considered not suitable for surgical treatment [23]. Owing to the unapparent and sneaky clinical manifestations of early stage gastric cancer, patients are often only diagnosed when the cancer is at an advanced stage [24]. Thus, the current most curative therapy, surgery [25], is excluded from treatment strategies. Alternative therapies, including chemotherapy, radiotherapy, and radiochemotherapy, though effective, are none of them curative. In recent decades, several natural products originating from medicinal herbs have broadened our understanding because of their extensive biological activities [26]. Such drugs as emodin [27], curcumin [28], and matrine [29] have been demonstrated to have anti-cancer effects by inhibiting proliferation, invasion, and metastasis of multiple malignant cancers. Although many studies revealed their pharmacological mechanisms, much research is still needed.
Used as a coloring agent, spice, and flavoring, curcumin has also been widely applied since ancient times in medical systems in Eastern and Southeastern Asia as an important ingredient of medicinal formulas [30]. Modern medical studies found that this bioactive agent, extracted from the rhizome of a herb named Curcuma Longa Linn, exerted potent anti-cancer activity by inhibiting cell proliferation in vitro and in vivo[31]. The mechanisms involved in curcumin’s proliferation inhibition were thought to be complicated: multiple pathways and molecular mediators were proved relevant. It was believed that three canonical apoptotic pathways, namely the death receptor [32], mitochondrial [33], and endoplasmic reticulum stress [34] pathways, were activated to induce apoptosis of cancer cells. Some transcriptional factor-related mechanisms, such as downregulation of TNF-induced nuclear factor κB mediated gene expressions [35] by curcumin were also indicated [36]. A recent report of the involvement of mitoKATP in regulating proliferation, invasion, and metastasis in glioma cells [37] provided new clues to the mechanisms of curcumin’s anti-proliferation effects.
It was suggested that KATP channels were distributed throughout the body, located on cell membranes and mitochondrial membranes, including malignant cells [38]. The major function of mitoKATP was supposed to be to adjust mitochondrial membrane functions to external stressors by opening the ion channel. Most studies concerning mitoKATP have been on cardiac ischemia-reperfusion injuries [39]. Results from these studies suggest that opening of mitoKATP channels could protect against apoptosis [40]. At the early stage of apoptosis, the opening of mitoKATP could inhibit depolarization of mitochondrial membrane to maintain MMP [41]. Thus, the mitochondrial membrane was stabilized to prevent further apoptotic chain reaction, such as transition pore formation, cytochrome c release or caspase cascade activation. Diazoxide is a widely used antihypertensive agent that acts as a selective opener of mitoKATP to modulate the loss of MMP [42, 43]. As a result, diazoxide could further protect cells from apoptosis.
In this study, to test the participation of mitoKATP in curcumin-induced apoptosis of SGC-7901 cells, diazoxide was co-administrated with curcumin to incubate SGC-7901 cells. The results of an in vitro study indicated that diazoxide co-administration alleviated the apoptosis of SGC-7901 by stabilizing MMP. The co-administration of diazoxide impaired curcumin’s inhibitory effects against xenograft tumor growth. The results of an in vivo study consolidated the findings of the in vitro study that impaired mitoKATP is one of the possible mechanisms of curcumin’s anti-proliferative effects against gastric cancer.
Conclusions
We can conclude that:
Curcumin inhibits proliferation of gastric cancer by inducing apoptosis.
Impaired mitoKATP opening causes MMP loss, and is involved in curcumin-induced apoptosis in gastric cancer.
Acknowledgements
None.
Abbreviations
- C
control
- Cur
curcumin
- DMEM
Dulbecco’s modified Eagle’s medium
- DZ
diazoxide
- FBS
fetal bovine serum
- HCur
high-dose curcumin
- KATP
ATP-sensitive potassium channel
- LCur
low-dose curcumin
- MCur
medium-does curcumin
- MMP
mitochondrial membrane potential
- MTT
3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium-bromide
- PBS
phosphate-buffered saline
- TNF
tumor necrosis factor.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XL and KS conducted all the experiments presented in this work. HC conducted the revision of the manuscript. XH and XYZ made central contributions to the conception and design of the studies, and to the analysis and interpretation of data. AS and XZ were involved in drafting the manuscript. All authors gave final approval of the version to be published. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content.
Contributor Information
Xiaohong Liu, Email: onlyhawk@163.com.
Kai Sun, Email: 810927700@qq.com.
Ailin Song, Email: renhongdoctor@gmail.com.
Xiaoyun Zhang, Email: zhangxiaoyun1981@foxmail.com.
Xu Zhang, Email: Zhangxu64@163.com.
Xiaodong He, Email: hxd@lzu.edu.cn.
References
- 1.Piazuelo MB, Correa P. Gastric cancer: overview. Colomb Med (Cali) 2013;44:192–201. [PMC free article] [PubMed] [Google Scholar]
- 2.Choi YY, An JY, Kim HI, Cheong JH, Hyung WJ, Noh SH. Current practice of gastric cancer treatment. Chin Med J (Engl) 2014;127:547–553. [PubMed] [Google Scholar]
- 3.Choi Y, Choi HS, Jeon WK, Kim BI, Park DI, Cho YK, Kim HJ, Park JH, Sohn CI. Optimal number of endoscopic biopsies in diagnosis of advanced gastric and colorectal cancer. J Korean Med Sci. 2012;27:36–39. doi: 10.3346/jkms.2012.27.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takashima A, Shirao K, Hirashima Y, Takahari D, Okita NT, Nakajima TE, Kato K, Hamaguchi T, Yamada Y, Shimada Y. Sequential chemotherapy with methotrexate and 5-fluorouracil for chemotherapy-naive advanced gastric cancer with disseminated intravascular coagulation at initial diagnosis. J Cancer Res Clin Oncol. 2010;136:243–248. doi: 10.1007/s00432-009-0655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy - from TCM theory to mechanistic insights. Planta Med. 2010;76:1118–1131. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 6.Yin SY, Wei WC, Jian FY, Yang NS. Therapeutic applications of herbal medicines for cancer patients. Evid Based Complement Alternat Med. 2013;2013:302426. doi: 10.1155/2013/302426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Chen LX, Ouyang L, Cheng Y, Liu B. Plant natural compounds: targeting pathways of autophagy as anti-cancer therapeutic agents. Cell Prolif. 2012;45:466–476. doi: 10.1111/j.1365-2184.2012.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Priyadarsini KI. Chemical and structural features influencing the biological activity of curcumin. Curr Pharm Des. 2013;19:2093–2100. doi: 10.2174/138161213805289228. [DOI] [PubMed] [Google Scholar]
- 9.Lin JK. Suppression of protein kinase C and nuclear oncogene expression as possible action mechanisms of cancer chemoprevention by curcumin. Arch Pharm Res. 2004;27:683–692. doi: 10.1007/BF02980135. [DOI] [PubMed] [Google Scholar]
- 10.Yu LL, Wu JG, Dai N, Yu HG, Si JM. Curcumin reverses chemoresistance of human gastric cancer cells by downregulating the NF-κB transcription factor. Oncol Rep. 2011;26:1197–1203. doi: 10.3892/or.2011.1410. [DOI] [PubMed] [Google Scholar]
- 11.Lin SS, Lai KC, Hsu SC, Yang JS, Kuo CL, Lin JP, Ma YS, Wu CC, Chung JG. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and vascular endothelial growth factor (VEGF) Cancer Lett. 2009;285:127–133. doi: 10.1016/j.canlet.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 12.Hu HL, Zhang ZX, Zhao JP, Wang T, Xu YJ. Effect of opening of mitochondrial ATP-sensitive K+ channel on the distribution of cytochrome C and on proliferation of human pulmonary arterial smooth muscle cells in hypoxia. Sheng Li Xue Bao. 2006;58:262–268. [PubMed] [Google Scholar]
- 13.Grover GJ, Garlid KD. ATP-sensitive potassium channels: a review of their cardioprotective pharmacology. J Mol Cell Cardiol. 2000;32:677–695. doi: 10.1006/jmcc.2000.1111. [DOI] [PubMed] [Google Scholar]
- 14.Kurachi Y, Yamada M. Relationship between function and structure of ATP-sensitive K+ (KATP) channels. Nihon Yakurigaku Zasshi. 2005;126:311–316. doi: 10.1254/fpj.126.311. [DOI] [PubMed] [Google Scholar]
- 15.Shen F, Wu LP, Liu WG, Lu Y, Liang HW, Xia Q. Effect of activation of mitochondrial ATP sensitive potassium channel and calcium activated potassium channel on the permeability transition of mitochondria from both normal and ischemic rat brain. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2007;23:14–18. [PubMed] [Google Scholar]
- 16.Wu L, Shen F, Lin L, Zhang X, Bruce IC, Xia Q. The neuroprotection conferred by activating the mitochondrial ATP-sensitive K+ channel is mediated by inhibiting the mitochondrial permeability transition pore. Neurosci Lett. 2006;402:184–189. doi: 10.1016/j.neulet.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Zhou F, Ding JH, Zhou XQ, Sun XL, Hu G. ATP-sensitive potassium channel opener iptakalim protects against MPP-induced astrocytic apoptosis via mitochondria and mitogen-activated protein kinase signal pathways. J Neurochem. 2007;103:569–579. doi: 10.1111/j.1471-4159.2007.04775.x. [DOI] [PubMed] [Google Scholar]
- 18.Ru Q, Tian X, Wu YX, Wu RH, Pi MS, Li CY. Voltage-gated and ATP-sensitive K+ channels are associated with cell proliferation and tumorigenesis of human glioma. Oncol Rep. 2014;31:842–848. doi: 10.3892/or.2013.2875. [DOI] [PubMed] [Google Scholar]
- 19.Chen HY, Zhang X, Chen SF, Zhang YX, Liu YH, Ma LL, Wang LX. The protective effect of 17β-estradiol against hydrogen peroxide-induced apoptosis on mesenchymal stem cell. Biomed Pharmacother. 2012;66:57–63. doi: 10.1016/j.biopha.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Wakiyama H, Cowan DB, Toyoda Y, Federman M, Levitsky S, McCully JD. Selective opening of mitochondrial ATP-sensitive potassium channels during surgically induced myocardial ischemia decreases necrosis and apoptosis. Eur J Cardiothorac Surg. 2002;21:424–433. doi: 10.1016/S1010-7940(01)01156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu WD, Qin Y, Yang C, Li L, Fu ZX. Effect of curcumin on human colon cancer multidrug resistance in vitro and in vivo. Clinics (Sao Paulo) 2013;68:694–701. doi: 10.6061/clinics/2013(05)18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dassen AE, Dikken JL, Bosscha K, Wouters MW, Cats A, van de Velde CJ, Coebergh JW, Lemmens VE. Gastric cancer: decreasing incidence but stable survival in the Netherlands. Acta Oncol. 2014;53:138–142. doi: 10.3109/0284186X.2013.789139. [DOI] [PubMed] [Google Scholar]
- 23.Isik A, Okan I, Firat D, Yilmaz B, Akcakaya A, Sahin M. A new prognostic strategy for gastric carcinoma: albumin level and metastatic lymph node ratio. Minerva Chir. 2014;69:147–153. [PubMed] [Google Scholar]
- 24.Thrumurthy SG, Chaudry MA, Hochhauser D, Mughal M. The diagnosis and management of gastric cancer. BMJ. 2013;347:f6367. doi: 10.1136/bmj.f6367. [DOI] [PubMed] [Google Scholar]
- 25.Mahar AL, Coburn NG, Singh S, Law C, Helyer LK. A systematic review of surgery for non-curative gastric cancer. Gastric Cancer. 2012;15(Suppl 1):S125–S137. doi: 10.1007/s10120-011-0088-3. [DOI] [PubMed] [Google Scholar]
- 26.Arslan D, Tural D, Akar E. Herbal administration and interaction of cancer treatment. J Palliat Med. 2013;16:1466–1476. doi: 10.1089/jpm.2013.0126. [DOI] [PubMed] [Google Scholar]
- 27.Yu JQ, Bao W, Lei JC. Emodin regulates apoptotic pathway in human liver cancer cells. Phytother Res. 2013;27:251–257. doi: 10.1002/ptr.4703. [DOI] [PubMed] [Google Scholar]
- 28.Bimonte S, Barbieri A, Palma G, Luciano A, Rea D, Arra C. Curcumin inhibits tumor growth and angiogenesis in an orthotopic mouse model of human pancreatic cancer. Biomed Res Int. 2013;2013:810423. doi: 10.1155/2013/810423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang P, Wang Z, Chong T, Ji Z. Matrine inhibits proliferation and induces apoptosis of the androgen-independent prostate cancer cell line PC-3. Mol Med Rep. 2012;5:783–787. doi: 10.3892/mmr.2011.701. [DOI] [PubMed] [Google Scholar]
- 30.Kurien BT, Singh A, Matsumoto H, Scofield RH. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev Technol. 2007;5:567–576. doi: 10.1089/adt.2007.064. [DOI] [PubMed] [Google Scholar]
- 31.Johnson SM, Gulhati P, Arrieta I, Wang X, Uchida T, Gao T, Evers BM. Curcumin inhibits proliferation of colorectal carcinoma by modulating Akt/mTOR signaling. Anticancer Res. 2009;29:3185–3190. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu S, Li T, Tan J, Yan X, Zhang D, Zheng C, Chen Y, Xiang Z, Cui H. Bax is essential for death receptor-mediated apoptosis in human colon cancer cells. Cancer Biother Radiopharm. 2012;27:577–581. doi: 10.1089/cbr.2012.1256. [DOI] [PubMed] [Google Scholar]
- 33.Kim KY, Yu SN, Lee SY, Chun SS, Choi YL, Park YM, Song CS, Chatterjee B, Ahn SC. Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. Biochem Biophys Res Commun. 2011;413:80–86. doi: 10.1016/j.bbrc.2011.08.054. [DOI] [PubMed] [Google Scholar]
- 34.Chiu SC, Chen SP, Huang SY, Wang MJ, Lin SZ, Harn HJ, Pang CY. Induction of apoptosis coupled to endoplasmic reticulum stress in human prostate cancer cells by n-butylidenephthalide. PLoS One. 2012;7:e33742. doi: 10.1371/journal.pone.0033742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bours V, Bentires-Alj M, Hellin AC, Viatour P, Robe P, Delhalle S, Benoit V, Merville MP. Nuclear factor-kappa B, cancer, and apoptosis. Biochem Pharmacol. 2000;60:1085–1089. doi: 10.1016/S0006-2952(00)00391-9. [DOI] [PubMed] [Google Scholar]
- 36.Zong H, Wang F, Fan QX, Wang LX. Curcumin inhibits metastatic progression of breast cancer cell through suppression of urokinase-type plasminogen activator by NF-κB signaling pathways. Mol Biol Rep. 2012;39:4803–4808. doi: 10.1007/s11033-011-1273-5. [DOI] [PubMed] [Google Scholar]
- 37.Paino T, Gangoso E, Medina JM, Tabernero A. Inhibition of ATP-sensitive potassium channels increases HSV-tk/GCV bystander effect in U373 human glioma cells by enhancing gap junctional intercellular communication. Neuropharmacology. 2010;59:480–491. doi: 10.1016/j.neuropharm.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Bodenstine TM, Vaidya KS, Ismail A, Beck BH, Diers AR, Edmonds MD, Kirsammer GT, Landar A, Welch DR. Subsets of ATP-sensitive potassium channel (KATP) inhibitors increase gap junctional intercellular communication in metastatic cancer cell lines independent of SUR expression. FEBS Lett. 2012;586:27–31. doi: 10.1016/j.febslet.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khimenko PL, Moore TM, Taylor AE. ATP-sensitive K+ channels are not involved in ischemia-reperfusion lung endothelial injury. J Appl Physiol (1985) 1995;79:554–559. doi: 10.1152/jappl.1995.79.2.554. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Zhu QL, Wang GZ, Deng TZ, Chen R, Liu MH, Wang SW. The protective roles of mitochondrial ATP-sensitive potassium channels during hypoxia-ischemia-reperfusion in brain. Neurosci Lett. 2011;491:63–67. doi: 10.1016/j.neulet.2010.12.065. [DOI] [PubMed] [Google Scholar]
- 41.Garg V, Hu K. Protein kinase C isoform-dependent modulation of ATP-sensitive K+ channels in mitochondrial inner membrane. Am J Physiol Heart Circ Physiol. 2007;293:H322–H332. doi: 10.1152/ajpheart.01035.2006. [DOI] [PubMed] [Google Scholar]
- 42.Jia D. The protective effect of mitochondrial ATP-sensitive K+ channel opener, nicorandil, combined with Na+/Ca2+ exchange blocker KB-R7943 on myocardial ischemia-reperfusion injury in rat. Cell Biochem Biophys. 2011;60:219–224. doi: 10.1007/s12013-010-9142-8. [DOI] [PubMed] [Google Scholar]
- 43.Pringle MJ, Sanadi DR. Effects of Cd2+ on ATP-driven membrane potential in beef heart mitochondrial H+-ATPase: a study using the voltage-sensitive probe oxonol VI. Membr Biochem. 1984;5:225–241. doi: 10.3109/09687688409150280. [DOI] [PubMed] [Google Scholar]


