Abstract
Preeclampsia is characterized by maternal endothelial dysfunction (e.g., increased maternal vascular permeability caused by the disassembly of endothelial junction proteins). However, it is unclear if preeclampsia is associated with impaired vascular growth and expression of endothelial junction proteins in human placentas. Herein, we examined vascular growth in placentas from women with normal term (NT) and preeclamptic (PE) pregnancies using two endothelial junction proteins as endothelial markers: CD31 and vascular endothelial-cadherin (VE-Cad). We also compared protein and mRNA expression of CD31 and VE-Cad between NT and PE placentas, and determined the alternatively spliced expression of CD31 using PCR. We found that CD31 and VE-Cad were immunolocalized predominantly in villous endothelial cells. However, capillary number density (total capillary number per unit villous area) and capillary area density (total capillary lumen area per unit villous area) as well as CD31 and VE-Cad protein and mRNA levels were similar between NT and PE placentas. PCR in combination with sequence analysis revealed a single, full-length CD31, suggesting that there are no alternatively spliced isoform of CD31 expressed in placentas. These data indicate that preeclampsia does not significantly affect vascular growth or the expression of endothelial junction proteins in human placentas.
Keywords: preeclampsia, placenta, vasculature, CD31, VE-cadherin
Introduction
Preeclampsia is a major cause of maternal and neonatal morbidity and mortality (Eiland et al. 2012). The etiology of preeclampsia is unclear; however, preeclampsia is characterized by increased endothelial permeability in maternal vasculature (Mayhew et al. 2004) and possibly also in placental vasculature (Wang et al. 2002). Such endothelial dysfunction is caused primarily by disorganized and diminished expression of endothelial cell junction proteins (Wang et al. 2002; Zhang et al. 2003) in association with aberrant expression and distribution of angiogenesis-related factors, including vascular endothelial growth factor (VEGF), soluble Flt1 (a VEGF inhibitor), and soluble endoglin (another anti-angiogenic factor) in the maternal circulation (Ahmad and Ahmed 2004; Chung et al. 2004; Kumazaki et al. 2002; Levine et al. 2006; Levine et al. 2004).
The reports on placental vascular growth in women with preeclamptic (PE) pregnancies are controversial. Increased villous capillary branching has been reported in placentas from PE versus normal term (NT) pregnancies (Boyd and Scott 1985). In contrast, other investigators have reported decreased microvessel counts in PE placentas (Uras et al. 2012) or unchanged vascular densities between PE and NT placentas (Burton et al. 1996; Mayhew et al. 2003; Teasdale 1985). The evidence, however, is lacking as to whether PE pregnancies affect the expression of endothelial junction proteins, thereby impairing endothelial integrity within human placentas; although, Wang et al. (2002) previously demonstrated that human umbilical cord vein endothelial cells (HUVECs) isolated from PE pregnancies exhibited increased permeability in association with an disorganized and decreased expression of vascular endothelial-cadherin (VE-Cad) and occludin, two cell junctional proteins.
CD31, a junctional protein, has been widely used as an endothelial marker (Newman 1997). CD31 contains 16 exons encoding a 5’-untranslated region and the signal peptide, an extracellular domain, a transmembrane domain, and a cytoplasmic domain, the latter of which is the major functional domain of CD31 (Newman and Newman 2003). The cytoplasmic domain of CD31 (exons 9-16) can undergo alternative splicing, resulting in different isoforms (Bergom et al. 2008; Dejana and Giampietro 2012; DeLisser et al. 1994; Newman and Newman 2003; Wang et al. 2003). Among these isoforms, the full-length CD31 is the predominant form expressed in humans, whereas CD31 without exons 14 and 15 is the major form expressed in mice (Privratsky and Newman 2014). The alternatively spliced forms of CD31 may have different cellular and tissue distributions and altered cellular functions (Bergom et al. 2008; Newman 1997, Newman and Newman 2003). VE-Cad is an endothelial-specific adhesion protein that is located at junctions between endothelial cells and also serves as an endothelial marker (Lampugnani et al. 1992; Vestweber 2008).
CD31 is an important endothelial adhesion protein mediating endothelial integrity and other functions, including vascular permeability, regulation of bioavailability of nitric oxide (NO), and angiogenesis (Bagi et al. 2005; Carrithers et al. 2005; Liu et al. 2006; Maas et al. 2005; Wong et al. 2005). For instance, the CD31 knockout mice exhibit enhanced vascular permeability (Carrithers et al. 2005; Maas et al. 2005; Wong et al. 2005). VE-Cad also mediates vascular stability and angiogenesis (Carmeliet et al. 1999; Dejana and Giampietro 2012; Matheny et al. 2000; Vestweber 2008). The importance of VE-Cad in vasculature is further supported by the observation that either targeted deficiency or cytosolic truncation of VE-Cad in mice impairs VEGF-induced endothelial survival and angiogenesis (Burkhardt et al. 2010; Carmeliet et al. 1999). Moreover, the overexpression of VE-Cad is also associated with increased permeability of human brain vasculature (Burkhardt et al. 2010; Carmeliet et al. 1999).
The expression of CD31 and VE-Cad in human placentas has been evaluated largely through immunohistochemistry. It has been reported that CD31 immunoreactivity is decreased in PE placentas as compared with control pregnancies (Goksu Erol et al. 2012; Uras et al. 2012). However, no difference in CD31 immunoreactivities has been observed in spiral arteries of placental bed biopsies from PE versus control pregnancies (Tziotis et al. 2002). For VE-Cad, Groten et al. (2010) have previously demonstrated that VE-Cad immunoreactivity in syncytiotrophoblasts from late onset preeclampsia is significantly elevated as compared with the controls. However, HUVECs isolated from PE placentas have decreased VE-Cad expression and disturbed cellular distribution in association with significantly increased cellular permeability (Wang et al. 2002).
It is still unclear if PE pregnancies alter vascular growth and the expression of CD31 and VE-Cad in human placentas. Thus, the aims of the current study are 1) to determine vascular growth using capillary number density (CND, total capillary number per tissue unit) and capillary area density (CAD, total capillary lumen area per unit tissue area) as indexes; 2) to quantify CD31 and VE-Cad protein and mRNA expression using western blotting and qPCR; and 3) to examine alternative splicing of CD31 using PCR in combination with sequence analysis in human NT and PE placentas.
Materials & Methods
Collection of Placental Tissues
Two sets of placental samples were collected from two different sites. The first set of samples were collected from NT (n=10; nine were from vaginal delivery and one from cesarean section delivery) and PE (n=10; all were from cesarean section delivery) pregnancies in the Meriter Hospital, Madison, Wisconsin, as previously described (Jiang et al. 2010; Zhao et al. 2014). The tissue collection was approved by the IRB of Meriter Hospital, and the Health Sciences IRB of the University of Wisconsin-Madison. Preeclampsia was defined according to standard criteria (National High Blood Pressure Education Program 2000). This set of placental samples was used for immunohistochemistry and western blotting analyses.
The second set of samples was obtained from NT (n=5) and PE (n=5) pregnancies from patients at the Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai, China. Collection of the placentas was approved by the Ethics Committee of Shanghai First Maternity and Infant Hospital. Written informed consent to participate in the study was obtained from each patient. This set of placental samples was used for reverse transcription (RT)-qPCR and PCR analyses.
All PE samples were collected during late onset pre-eclampsia. Preeclampsia was considered severe if one or more of the following criteria were present: maternal blood pressure higher than or equal to 160/110 mmHg on two separate readings or proteinuria more than 2+ by dipstick or more than 2 g/24 hr. None of the study subjects had signs of infection. Smokers were excluded. Patient ages were similar (23 ± 0.9 years) between NT and PE pregnancies. Gestational ages for NT pregnancies (39 ± 0.2 weeks) were significantly higher (p<0.05) than those for PE pregnancies (34 ± 0.7 weeks), as were fetal weights for NT pregnancies (3404 ± 90.9 g) over PE pregnancies (2198 ± 236.4 g; p <0.05). Based on the most common definition of intrauterine growth restriction (IUGR) of fetal weight below the 10th percentile for gestational age (GA), the fetuses in the PE group were not growth restricted, as determined using ultrasound (Williams et al. 1982). APGAR scores at 1 and 5 min in PE pregnancies were 6.1 ± 0.59 and 7.9 ± 0.23, respectively, each of which was lower (p < 0.05) than that in NT pregnancies (8.8 ± 0.13 and 8.9 ± 0.23, respectively). Each of these parameters was analyzed using the Student’s t-test.
Placental villi were quickly dissected (~10 g each), snap-frozen, and stored in liquid nitrogen for western blot analysis. Additional placental tissues were fixed overnight at 4°C in 4% paraformaldehyde in 10 mM PBS, and embedded in paraffin for immunohistochemistry. HUVECs were also isolated from NT pregnancies (Jiang et al. 2014; Jiang et al. 2013). These HUVECs express CD31 and VE-Cad (Jiang et al. 2014), and were therefore used as standards in Western blotting.
Immunohistochemistry
Immunolocalization of CD31 and VE-Cad were detected as described (Jiang et al. 2010; Zhao et al. 2014). For each sample, two adjacent tissue sections (5-μm thick) were mounted on one glass slide (Fisher Scientific; Pittsburgh, PA). The sections were deparaffinized, dehydrated, and boiled in a 10 mM citrate buffer solution (pH 6.0) in a microwave for 10 min for antigen retrieval. Endogenous peroxidase activity was quenched by immersing the tissue array in 3% H2O2 in methanol for 10 min. After blocking nonspecific binding sites, one tissue section per slide was probed with a goat polyclonal antibody against the C-terminus of human CD31 (2 μg/ml, sc-1505, Santa Cruz Biotechnology; Dallas, TX) or a mouse monoclonal antibody against the C-terminus of human VE-Cad (2 μg/ml, sc-9989, Santa Cruz Biotechnology) for 1 hr. The second tissue section on the same slide was probed with the corresponding pre-immune goat or mouse IgG (Vector Laboratories; Burlingame, CA) at the same concentration as the primary antibody as a negative control. After washing, the tissue sections were incubated with a biotinylated horse anti-goat antibody for CD31 or anti-mouse antibody for VE-Cad (Vector Laboratories) for 30 min. The specific immunoreactivity was visualized by 3-amino-9-ethylcarbazole (Vector Laboratories).
Morphological Analysis
Using CD31 as an endothelial marker, the CND and the CAD in the placentas were analyzed as described (Grazul-Bilska et al. 2010). Five images per tissue section were taken using a Nikon inverted microscope connected to a CCD camera with a 20× objective. For each image, the capillary number was counted and the lumen area was measured using the Image-J imaging analysis software (NIH; Bethesda, MD). The units of villous area were also determined using the Image-J.
Western Blotting
Western blotting was conducted as described (Jiang et al. 2010; Zhao et al. 2014). Placental tissues were pulverized in liquid nitrogen using a mortar and pestle, followed by homogenization in buffer [50 mM HEPES, 0.1 M NaCl, 10 mM EDTA, 4 mM sodium pyrophosphate, 10 mM sodium fluoride, 2 mM sodium orthovanadate (pH 7.5), 1 mM phenyl methyl sulfonyl fluoride, 1% Triton X-100, 5 μg/ml leupeptin, 5 μg/ml aprotinin] using a rotor-stator homogenizer (PawerGen700, Fisher Scientific; Hampton, NH), and further lysed by sonication. After centrifugation, protein concentrations of the supernatant were determined with BSA (fraction V; Sigma-Aldrich; St. Louis, MO) as standards. The protein samples (150 μg) were subjected to electrophoresis and electrotransfer. The membranes were probed with an antibody against the C-terminus of human CD31 (1:1000; Santa Cruz Biotechnology, sc-1505), against the N-terminus of human CD31 (1:100; Santa Cruz Biotechnology, sc-133091) or against human VE-Cad (1:200, sc-9989, Santa Cruz Biotechnology) followed by re-probing with a β-actin antibody (1:5000, cat # 4967s, Cell Signaling Technology; Danvers, MA). Protein samples from HUVECs (20 μg each) were used as positive controls. Proteins were visualized using enhanced chemiluminescence reagents (Amersham Biosciences; Piscataway, NJ), followed by exposure to chemiluminescence films. The immunoreactive signals were analyzed by densitometry using Image-J.
RT-qPCR, PCR and Sequence Analysis
To quantify CD31 and VE-Cad mRNA levels in placentas, RT-qPCR was carried out. Total RNA were extracted from the placentas from women with NT (n=5) and PE (n=5) pregnancies using Trizol and an RNA Simple Total RNA Kit (Tiangen Biotech; Beijing, China), as described (Wang et al. 2013). Total RNA was quantified using a spectrophotometer. Samples of total RNA (1 μg for each) were reverse transcribed to cDNA in a 20-μl reaction volume with PrimeScript RT Reagent Kit (TaKaRa; Dalian, China), as previously described (Wang et al. 2013). Each PCR reaction was performed with 50 ng cDNA and 2×Taq PCR MasterMix Kit (Tiangen), according to the manufacturer’s instructions. The volume of the reaction mixture for qPCR was 20 μl and contained 15 ng cDNA, SYBR Premix Ex Taq (TaKaRa) and 10 μM of each primer. Primers were synthesized by Sangon Inc. (Shanghai, China) as follows: CD31 for qPCR (Sense: 5’-AACAGTGTTGACATGAAGAGCC-3’; antisense: 5’-TGTAAAACAGCACGTCATCCTT-3’); CD31 for PCR (Sense: 5’-AGGAAAGCCAAGGCCAGG-3’; Antisense: 5’-CCTTGCTGTCTAAGTTCC-3’), which encompassed the entire C-domain, thus having the potential to amplify all CD31 isoforms as reported (Wang et al. 2003); VE-Cad for qPCR (Sense 5’-TGTGGGCTCTCTGTTTGTTG-3’; Antisense: 5’-AATGACCTGGGCTCTGTTTC-3’) and GAPDH for qPCR (Sense: 5’-GGAGCGAGATCCCTCCAAAAT-3’; Antisense: 5’-GGCTGTTGTCATACTTCTCATGG-3’). The PCR cycling conditions for CD31 were 5 min at 94°C followed by 30 sec at 94°C, 40 sec at 56°C, and 59 sec at 72°C for 35 cycles. This was followed by a final extension of 7 min at 72°C.
qPCR was carried out with DNA Engine Opticon2 Fluorescence Detector (MJ Research, Walthman, MA). To confirm the amplification specificity, the PCR products were subjected to a melting curve analysis. Levels of mRNA expression were analyzed using the 2−Δ ΔCT method and gene levels were normalized to GAPDH (Wang et al. 2013).To examine if there were alternatively spliced forms of CD31, the CD31 amplicons (20 ng) from NT (n=5) and PE (n=5) pregnancies were run on an agarose gel and visualized. To confirm the PCR amplicon, the CD31 PCR bands from four samples randomly chosen from NT (n=2) and PE (n=2) groups were extracted, purified using Wizard SV Gel and PCR Clean-up System (Promega; Madison, WI), and were sequenced using an ABI 3730XL Genetic Analyzer (Applied Biosystems; Foster City, CA) by Saiin Biotechnology Inc. (Shanghai, China). The CD31 amplicon sequences obtained were further analyzed using Seqman software (DNAstar Inc.; Madison, WI).
Statistics Procedures
Data were analyzed using the Student’s t-test (SigmaStat; Jandel Co.; San Rafael, CA). p<0.05 was considered significant.
Results
Morphological Analysis of Vasculature
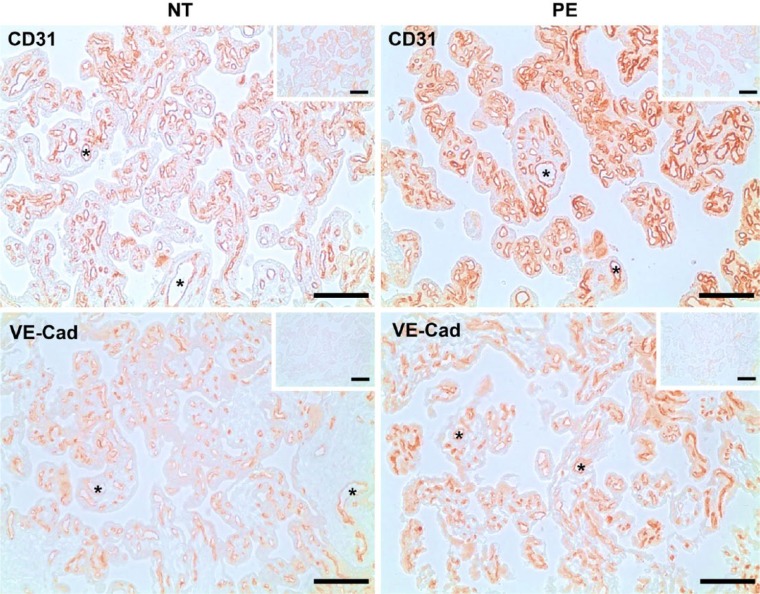
To compare the vascular density in NT (n=10) and PE (n=10) placentas, immunohistochemical analysis was performed using CD31 and VE-Cad as endothelial markers. We observed positive staining of CD31 and VE-Cad predominantly in endothelial cells of placental villi (Fig. 1). No staining was observed in the IgG control (see the small inset in each panel in Fig. 1). CND and CAD in placentas were analyzed in the tissue sections stained for CD31. No difference was detected in CND between NT (2.77 ± 0.22 capillary/1000 µm2 villous area) and PE (2.83 ± 0.13 capillary/1000 µm2 villous area) placentas as well as in CAD between NT (184.00 ± 8.86 µm2/1000 µm2 villous area) and PE (201.91 ± 9.93 µm2/1000 µm2 villous area) placentas.
Figure 1.
Immunolocalization of CD31 and VE-Cad in human placentas from women with normal term (NT) and preeclamptic (PE) pregnancies. The adjacent tissue sections were incubated with antibodies against CD31 or VE-Cad, or pre-immune goat or mouse IgG (insets). Reddish color indicates positive staining for the CD31 and VE-Cad. Representative images are shown. *lumen of blood vessels. n=10 for each group. Scale, 100 μm.
Western Blotting
To determine the protein expression of CD31 and VE-Cad in NT (n=10) and PE (n=10) placentas, western blot analysis was carried out. The C-terminal-binding CD31 antibody detected one band at ~130 kD (CD31c), which corresponds to the molecular mass of CD31, as reported (Newman 1997). In contrast, the N-terminal-binding CD31 antibody detected two bands at ~130 and 110 kD (CD31n) in NT and PE placentas (Fig. 2A). VE-Cad was also detected at ~130 kD, as reported (Gulino et al. 1998) (Fig. 2A). No difference in CD31c, CD31n (Fig. 2B) or VE-Cad (Fig. 2C) protein levels was observed between NT and PE placentas.
Figure 2.
(A) Western blot analysis of CD31 and VE-Cad proteins in human placentas from women with normal term (NT) and preeclamptic (PE) pregnancies. Proteins (150 μg for each placental sample, and 20 μg for HUVECs) were subjected to Western blotting. (B–D) Data normalized to β-actin are expressed as the mean ± SEM as a fold-difference with respect to that of the NT pregnancy samples. CD31c and CD31n: indicate antibodies against the C- or N-terminus, respectively, of human CD31. n=10 for each group.
RT-qPCR, PCR and Sequence Analyses
We next performed RT-qPCR to quantify mRNA expression of CD31 and VE-Cad in NT (n=5) and PE (n=5) placentas. Similar to the western blotting results (Fig. 2), the RT-qPCR analysis showed no difference in CD31 and VE-Cad mRNA levels between NT and PE placentas (data not shown).
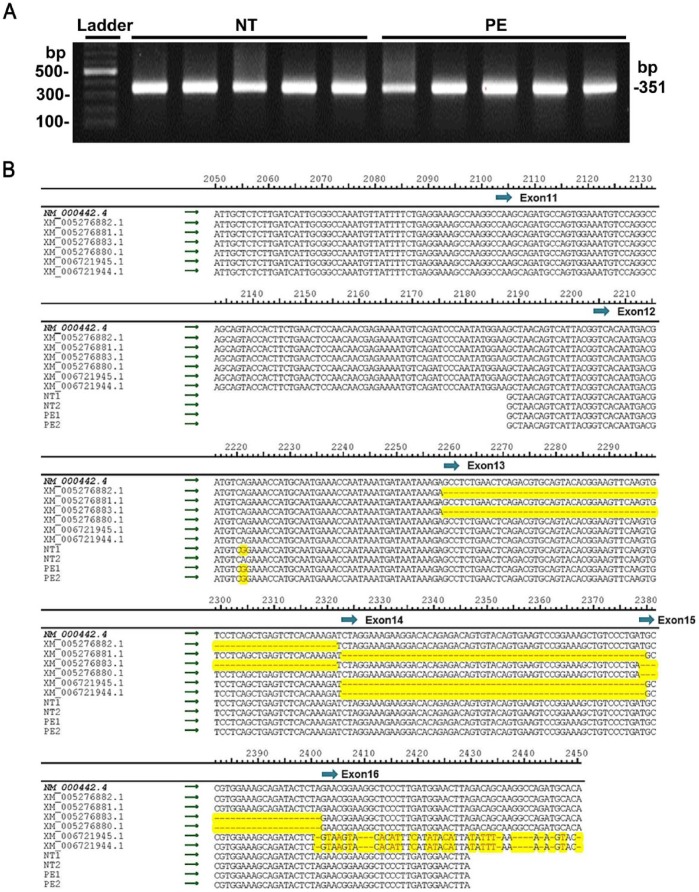
To examine if there was any difference in the expression of alternative splice variants of CD31 in NT and PE placentas, PCR was conducted targeting the 3’ end between exon 11 to exon 16 of the human CD31 transcript. PCR products from all NT and PE placentas showed a single band at ~350 bp in the agarose gel analysis (Fig. 3A), which is in consistent with the estimated size of the targeted human CD31 sequence (GenBank accession # NM_000442.4); this suggests that only the full-length CD31 mRNA is expressed in human placentas.
Figure 3.
Sequence analyses of CD31 PCR products from the placentas of women with normal term (NT) and preeclamptic (PE) pregnancies. (A) The PCR products along with a DNA ladder were run on a 1.5% agarose gel. n=5 for each group. (B) Multiple sequence alignment among CD31 PCR products from NT and PE placentas (n=2 for each group), the full-length human CD31 mRNA (NM_000442.4: CD31), and 6 of the human CD31 transcript variants mRNA sequences (XM_005276880.1: CD31, transcript variant X2; XM_005276881.1: CD31, transcript variant X3; XM_005276882.1: CD31, transcript variant X4; XM_005276883.1: CD31, transcript variant X5; XM_006721944.1: CD31, transcript variant X6; XM_006721945.1: CD31, transcript variant X7). Sequences that differ from the full-length human CD31 mRNA are shown in red and highlighted in yellow. The numbers above the sequence alignment indicate the corresponding sequence positions in the full-length human CD31 mRNA sequence. The position of exons 11–16 are shown in blue arrows.
To further confirm this PCR result, the CD31 PCR product bands from NT (n=2) and PE (n=2) placentas were extracted and directly sequenced. These CD31 sequences from placentas were compared with the full-length human CD31 mRNA sequence (NM_000442.4) along with the other 6 human CD31 transcription variants mRNA sequences (XM_005276880.1, XM_005276881.1, XM_005276882.1, XM_005276883.1, XM_006721944.1, XM_006721945.1) (Fig. 3B). The results showed that the CD31 sequences from placentas were identical to the full-length CD31 mRNA sequence, whereas they differed from all of the other 6 CD31 transcript variants. All of these results suggest that full-length CD31 is the only form of CD31 transcript expressed in human placentas from both NT and PE pregnancies.
Discussion
In this study, we have found both CD31 and VE-Cad are present predominantly in villous endothelial cells, confirming that CD31 and VE-Cad are reliable endothelial markers in human placentas. However, using CD31 as an endothelial marker, we did not observe a significant difference in either CND or CAD between NT and PE placentas. We also, for the first time, quantified protein and mRNA expression levels of CD31 and VE-Cad in NT and PE placentas, although no substantial difference was detected. We further demonstrated that human placentas predominantly expressed a single, full-length CD31. These data indicate that preeclampsia does not significantly alter the vascular growth and expression of CD31 and VE-Cad in placentas as compared with NT pregnancies. Thus, given that the expression of either CD31 or VE-Cad is critical for the maintenance of endothelial integrity and the regulation of endothelial functions, such as angiogenesis and vasodilation (Bagi et al. 2005; Carrithers et al. 2005; Liu et al. 2006; Maas et al. 2005; Wong et al. 2005), the current data suggest that preeclampsia does not strongly impair endothelial integrity or function in human placentas.
The reports on vascular growth in PE placentas are controversial, possibly in part due to the complicated nature of preeclampsia (Mayhew et al. 2004). Several investigators have shown that PE pregnancies are not associated with impaired placental vascular growth using the classic stereological techniques without labeling endothelial cells with any endothelial marker (Burton et al. 1996; Mayhew et al. 2003; Teasdale 1985). In particular, Burton et al. (1996) demonstrated that the placental capillary volume fraction, capillary surface density, and capillary length density were all similar between PE pregnancies and GA-matched (38–40 weeks) controls, even though 20% of the PE placentas were collected from mild PE pregnancies and the fetal weights were similar between PE pregnancies and the normotensive control. Mayhew et al. (2003) reported that the capillary volume, surface area, and diameter of placentas from severe PE pregnancies, which had a shorter GA (36 vs. 39 weeks) and reduced fetal weight (2800 vs. 3460 g), were similar to those in the control; however, all of these vascular parameters were decreased in IUGR and PE pregnancies that also presented with IUGR. Teasdale (1985) also found that severe PE placentas were morphologically very similar to the GA-matched (40 weeks) controls in terms of capillary surface density, weight, and parenchymal and cellular contents. In the current study, we directly counted the capillary number and measured the capillary lumen diameter in placentas after labeling endothelial cells with CD31, which, in combination with the use of computerized imaging software, might more acutely determine placental vascular changes. Our findings of unchanged CND and CAD between NT and severe PE placentas are in agreement with those previous reports (Burton et al. 1996; Mayhew et al. 2003; Teasdale 1985), indicating no association of severe PE pregnancies with impaired placental vascular growth.
Other reports have claimed that PE pregnancies are associated with either increased (Boyd and Scott 1985) or decreased (Uras et al. 2012) placental vascular densities. In this regard, Boyd and Scott (1985) showed that the capillary volume density was increased in severe PE versus the normal control placentas from women with similar GA at ~35 weeks; however, such a short GA in the normal control apparently should be considered as a preterm birth, another complication pregnancy. Uras et al. (2012) evaluated placentas from severe PE women (GA at ~37 weeks) using Factor VIII and CD31 as endothelial markers, and found decreased placental microvessel counts based on the Factor VII and CD31 staining intensities in PE placentas as compared with the control placentas from normal pregnancies (GA at ~38 weeks). These observations are in contrast to previous studies (Burton et al. 1996; Mayhew et al. 2003; Teasdale 1985) and our current data. It is unclear what factors cause these discrepancies. However, the relatively small size, not-so closely matched samples from the PE subjects and controls, and the use of different analysis methods may partially contribute to these discrepancies.
No previous study has quantitatively evaluated CD31 and VE-Cad protein and mRNA expression between NT and PE placentas using western blotting and qPCR. In this study, we quantified the expression of CD31 and VE-Cad protein using two different CD31 antibodies and one VE-Cad antibody. The C-terminal CD31 antibody detects only one band, whereas the N-terminal CD31 antibody detects two bands of the CD31 protein. PCR, along with sequencing analysis, revealed a single form of CD31 expressed in NT and PE placentas; the two bands of CD31 detected in the current study are highly likely to represent two forms of CD31 with different maturities, as it is known that CD31 is synthesized as a 110-kDa precursor before fully maturing into its 130-kDa form by glycosylation (DeLisser et al. 1994; Wang et al. 2003).
Our observations of unaltered CD31 and VE-Cad expression between NT and PE placentas are contrary to the previous report showing decreased VE-Cad expression in HUVECs, which are isolated from PE pregnancies and cultured in vitro until reaching the first passage (Wang et al. 2002). This discrepancy is not surprising as endothelial cells of different origins (e.g., microvasculature, arteries, and veins) could have highly variable phenotypes, including distinct gene expression profiles (Chi et al. 2003) as well as distinct morphologies and responses to certain growth factors (Lang et al. 2003). In addition, this unaltered CD31 and VE-Cad expression between NT and PE placentas is in parallel with unaltered CND and CAD. These data suggest that the expression of either CD31 or VE-Cad is correlated with changes in villous capillary density and size. Nonetheless, it is noteworthy that unchanged expression of total CD31 and VE-Cad protein and mRNA may not always reflect an absence of a change in CD31 and VE-Cad functions, as posttranslational modifications (such as phosphorylation) can also robustly alter functions of CD31 (Newman and Newman 2003; Privratsky and Newman 2014) and VE-Cad (Dejana and Giampietro 2012).
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is partially supported by the National Institutes of Health Grants HD38843 (RRM/IMB/JZ), and the R & D grant from Department of Ob/Gyn, University of Wisconsin-Madison (JZ).
References
- Ahmad S, Ahmed A. (2004). Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res 95:884-891. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. (2005). PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol 25:1590-1595. [DOI] [PubMed] [Google Scholar]
- Bergom C, Paddock C, Gao C, Holyst T, Newman DK, Newman PJ. (2008). An alternatively spliced isoform of PECAM-1 is expressed at high levels in human and murine tissues, and suggests a novel role for the C-terminus of PECAM-1 in cytoprotective signaling. J Cell Sci 121:1235-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd PA, Scott A. (1985). Quantitative structural studies on human placentas associated with pre-eclampsia, essential hypertension and intrauterine growth retardation. Br J Obstet Gynaecol 92:714-721. [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Schmidt D, Schoenauer R, Brokopp C, Agarkova I, Bozinov O, Bertalanffy H, Hoerstrup SP. (2010). Upregulation of transmembrane endothelial junction proteins in human cerebral cavernous malformations. Neurosurg Focus 29:E3. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Reshetnikova OS, Milovanov AP, Teleshova OV. (1996). Stereological evaluation of vascular adaptations in human placental villi to differing forms of hypoxic stress. Placenta 17:49-55. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. (1999). Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98:147-157. [DOI] [PubMed] [Google Scholar]
- Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. (2005). Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol 166:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi J-T, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. (2003). Endothelial cell diversity revealed by global expression profiling. Proceedings of the National Academy of Sciences 100:10623-10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Song Y, Wang Y, Magness RR, Zheng J. (2004). Differential expression of vascular endothelial growth factor (VEGF), endocrine gland derived-VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. J Clin Endocrinol Metab 89:2484-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E, Giampietro C. (2012). Vascular endothelial-cadherin and vascular stability. Curr Opin Hematol 19:218-223. [DOI] [PubMed] [Google Scholar]
- DeLisser HM, Chilkotowsky J, Yan HC, Daise ML, Buck CA, Albelda SM. (1994). Deletions in the cytoplasmic domain of platelet-endothelial cell adhesion molecule-1 (PECAM-1, CD31) result in changes in ligand binding properties. J Cell Biol 124:195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland E, Nzerue C, Faulkner M. (2012). Preeclampsia 2012. J Pregnancy 2012:586578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goksu Erol AY, Nazli M, Elis Yildiz S. (2012). Significance of platelet endothelial cell adhesion molecule-1 (PECAM-1) and intercellular adhesion molecule-1 (ICAM-1) expressions in preeclamptic placentae. Endocrine 42:125-131. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Borowicz PP, Johnson ML, Minten MA, Bilski JJ, Wroblewski R, Redmer DA, Reynolds LP. (2010). Placental development during early pregnancy in sheep: vascular growth and expression of angiogenic factors in maternal placenta. Reproduction 140:165-174. [DOI] [PubMed] [Google Scholar]
- Groten T, Gebhard N, Kreienberg R, Schleussner E, Reister F, Huppertz B. (2010). Differential expression of VE-cadherin and VEGFR2 in placental syncytiotrophoblast during preeclampsia - New perspectives to explain the pathophysiology. Placenta 31:339-343. [DOI] [PubMed] [Google Scholar]
- Gulino D, Delachanal E, Concord E, Genoux Y, Morand B, Valiron MO, Sulpice E, Scaife R, Alemany M, Vernet T. (1998). Alteration of endothelial cell monolayer integrity triggers resynthesis of vascular endothelium cadherin. J Biol Chem 273:29786-29793. [DOI] [PubMed] [Google Scholar]
- Jiang YZ, Li Y, Wang K, Dai CF, Huang SA, Chen DB, Zheng J. (2014). Distinct roles of HIF1A in endothelial adaptations to physiological and ambient oxygen. Mol Cell Endocrinol 391:60-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YZ, Wang K, Fang R, Zheng J. (2010). Expression of aryl hydrocarbon receptor in human placentas and fetal tissues. J Histochem Cytochem 58:679-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YZ, Wang K, Li Y, Dai CF, Wang P, Kendziorski C, Chen DB, Zheng J. (2013). Transcriptional and functional adaptations of human endothelial cells to physiological chronic low oxygen. Biol Reprod 88:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazaki K, Nakayama M, Suehara N, Wada Y. (2002). Expression of vascular endothelial growth factor, placental growth factor, and their receptors Flt-1 and KDR in human placenta under pathologic conditions. Hum Pathol 33:1069-1077. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco LP, Dejana E. (1992). A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J Cell Biol 118:1511-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang I, Pabst MA, Hiden U, Blaschitz A, Dohr G, Hahn T, Desoye G. (2003). Heterogeneity of microvascular endothelial cells isolated from human term placenta and macrovascular umbilical vein endothelial cells. Eur J Cell Biol 82:163-173. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA. (2006). Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 355:992-1005. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. (2004). Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350:672-683. [DOI] [PubMed] [Google Scholar]
- Liu Y, Bubolz AH, Shi Y, Newman PJ, Newman DK, Gutterman DD. (2006). Peroxynitrite reduces the endothelium-derived hyperpolarizing factor component of coronary flow-mediated dilation in PECAM-1-knockout mice. Am J Physiol Regul Integr Comp Physiol 290:R57-R65. [DOI] [PubMed] [Google Scholar]
- Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ. (2005). Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol 288:H159-H164. [DOI] [PubMed] [Google Scholar]
- Matheny HE, Deem TL, Cook-Mills JM. (2000). Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J Immunol 164:6550-6559. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Charnock-Jones DS, Kaufmann P. (2004). Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta 25:127-139. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Ohadike C, Baker PN, Crocker IP, Mitchell C, Ong SS. (2003). Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta 24:219-226. [DOI] [PubMed] [Google Scholar]
- Newman PJ. (1997). The biology of PECAM-1. J Clin Invest 100:S25-S29. [PubMed] [Google Scholar]
- Newman PJ, Newman DK. (2003). Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol 23:953-964. [DOI] [PubMed] [Google Scholar]
- Privratsky JR, Newman PJ. (2014). PECAM-1: Regulator of endothelial junctional integrity. Cell Tissue Res 355:607-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale F. (1985). Histomorphometry of the human placenta in maternal preeclampsia. Am J Obstet Gynecol 152:25-31. [DOI] [PubMed] [Google Scholar]
- Tziotis J, Malamitsi-Puchner A, Vlachos G, Creatsas G, Michalas S. (2002). Adhesion molecules expression in the placental bed of pregnancies with pre-eclampsia. BJOG 109:197-201. [DOI] [PubMed] [Google Scholar]
- Uras N, Oguz SS, Zergeroglu S, Akdag A, Polat B, Dizdar EA, Dilmen U. (2012). CD31 and Factor VIII in angiogenesis of normal and pre-eclamptic human placentas. J Obstet Gynaecol 32:533-536. [DOI] [PubMed] [Google Scholar]
- Vestweber D. (2008). VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol 28:223-232. [DOI] [PubMed] [Google Scholar]
- Wang K, Li Y, Jiang YZ, Dai CF, Patankar MS, Song JS, Zheng J. (2013). An endogenous aryl hydrocarbon receptor ligand inhibits proliferation and migration of human ovarian cancer cells. Cancer Lett 340:63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gu Y, Granger DN, Roberts JM, Alexander JS. (2002). Endothelial junctional protein redistribution and increased monolayer permeability in human umbilical vein endothelial cells isolated during preeclampsia. Am J Obstet Gynecol 186:214-220. [DOI] [PubMed] [Google Scholar]
- Wang Y, Su X, Sorenson CM, Sheibani N. (2003). Tissue-specific distributions of alternatively spliced human PECAM-1 isoforms. Am J Physiol Heart Circ Physiol 284:H1008-H1017. [DOI] [PubMed] [Google Scholar]
- Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. (1982). Fetal growth and perinatal viability in California. Obstet Gynecol 59:624-632. [PubMed] [Google Scholar]
- Wong MX, Hayball JD, Hogarth PM, Jackson DE. (2005). The inhibitory co-receptor, PECAM-1 provides a protective effect in suppression of collagen-induced arthritis. J Clin Immunol 25:19-28. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gu Y, Li H, Lucas MJ, Wang Y. (2003). Increased endothelial monolayer permeability is induced by serum from women with preeclampsia but not by serum from women with normal pregnancy or that are not pregnant. Hypertens Pregnancy 22:99-108. [DOI] [PubMed] [Google Scholar]
- Zhao YJ, Zou QY, Li Y, Li HH, Wu YM, Li XF, Wang K, Zheng J. (2014). Expression of G-protein subunit alpha-14 is increased in human placentas from preeclamptic pregnancies. J Histochem Cytochem 62:347-354. [DOI] [PMC free article] [PubMed] [Google Scholar]