Abstract
The objective of this study is to establish the utilization trends of CT, MRI, and FDG-PET/CT for evaluation of oropharyngeal squamous cell carcinoma (OPSCC) patients. A total of 173 patients with newly diagnosed stage III or IV OPSCC between 2003 and 2009 were included. Frequency of imaging modality use, divided into four time periods (2003-04, 2005-06, 2007-08 and 2009), was evaluated. For initial staging, percentage of PET/CT use was 64.6%, 87.5%, 94.1% and 96.3%, with an increasing trend (p < 0.001). The CT (p = 0.762) and MRI (p = 0.224) use demonstrated no change in trend. For post-treatment imaging, percentage of PET/CT use was 59.5%, 68.6%, 89.7% and 100%, with an increasing trend (p < 0.001). The CT use demonstrated a decreasing trend (p = 0.004) and MRI showed no trend change (p = 0.231). PET/CT is used with an increasing trend for initial staging and has become a central imaging modality for follow up evaluation after treatment, for advanced OPSCC.
Keywords: Oropharyngeal squamous cell carcinoma (OPSCC), PET/CT, FDG, cancer, magnetic resonance imaging (MRI)
Introduction
Head and neck cancers are relatively rare cancers with estimated 36,000 new patients in the United States in 2013 by American Cancer Society [1]. Oropharyngeal cancers related to human papillomavirus infection (HPV) has been reported to be increasing [2,3]. The five-year survival rate is 40-55% for advanced stage diseases [1]. The precise evaluation of disease invasion in primary lesion and metastases especially to neck lymph nodes is critical for the disease management. At present, CT with intravenous contrast and MRI are considered to be conventional imaging modalities, and PET/CT has been proven as a useful option [2,4]. The usefulness of PET/CT has been recognized especially after it was introduced into clinical setting in the last decade [5]. Moreover, as Hillner et al reported, after Medicare reimbursement for PET use in head and neck cancer management was approved in 2001, PET has been more frequently utilized [6].
There are several guidelines by professional groups such as NCCN [2] and Clinical Oncology [7], in which PET/CT is considered as an additional option for advanced head and neck cancers besides CT and MRI, based on current evidences. It is unclear how these imaging modalities are utilized in clinical settings. Hence, the objective of this study is to establish the temporal utilization trends of CT, MRI and PET/CT at baseline and follow up of patients with advanced oropharyngeal cancer from one of the leading, tertiary care referral centers for head and neck and otolaryngology, in the United States- the Johns Hopkins Hospital.
Materials and methods
Study design overview
This is a retrospective data analysis from medical records and imaging database at Johns Hopkins Hospital. These data was used for evaluation of the changes in use of advanced imaging modalities, CT, MRI and PET/CT for advanced oropharyngeal cancer patients (stage III and IV) between 2003 and 2009. Institutional Review Board approved the study and informed consent was waived.
Patients
From the institutional imaging database, all the cancer patients who received CT neck with intravenous contrast, MRI, PET/CT for the evaluation of head and neck region between January 2003 and December 2009 were extracted. Using information both from radiological reports and medical records, a total of 195 oropharyngeal cancer patients who were newly diagnosed during the study period were identified. To decrease the heterogeneity in stages, 173 patients with AJCC (7th edition) stage III or IV and biopsy-proven oropharyngeal squamous cell carcinoma were included in this analysis.
Data collection
The following information was collected as baseline characteristics: age, sex, race, smoking status, subsites, AJCC staging at the time of biopsy, treatment and recurrence. As to imaging information, the timing and utilization of CT, MRI and PET/CT were collected for the initial evaluation before treatment (baseline) and follow up during first two years after completion of treatment (post-treatment). Imaging performed outside our institution for evaluation of the disease was included if the images were evaluated and stored in the institutional imaging database and reports in the electronic medical records. Since 2005, FDG PET/CT of the head and neck has been performed with intravenous contrast and diagnostic CT neck at the same time as PET/CT, if clinicians request and the test is coded as ‘PET/CT with contrast’ in this study. For post-treatment analysis, 148 patients with at least six months follow up were included.
Statistical analysis
We present central tendencies as mean ± standard deviation (SD) or as median (interquartile range) when data was skewed, or frequency and percentage for categorical variables. The data was divided into four cohorts: 2003-2004 (n = 48), 2005-2006 (n = 64), 2007-2008 (n = 34), 2009 (n = 27) for analysis purposes, and frequency of each imaging modality use was evaluated at initial staging and post-treatment, respectively, using trend test. P-value less than 0.05 was considered as significant. All statistical analyses were performed using Stata12 (Stata Corp., College Station, Texas, USA).
Results
Baseline characteristics
There were 134 males and 39 females and the mean age of patients was 55.6 years (SD 9.71). More than three-fourth of the patients was male (77.5%). Tonsil was the most common subsite in 77 patients (44.5%) and the second common site was base of tongue in 68 patients (39.3%). Treatment methods was chemoradiotherapy (CRT) or radiotherapy alone (RT) for 96 patients (55.5%). Seventy patients (40.5%) received surgery with or without CRT or RT as either neoadjuvant or adjuvant therapy. A total of 17 patients (9.8%) had recurrence during the first two years after completion of treatment. Baseline characteristics of whole patients and each cohort were shown in Table 1.
Table 1.
Baseline characteristics of patients included in the study by year of diagnosis
| Year | 2003-4 | 2005-6 | 2007-8 | 2009 | Total |
|---|---|---|---|---|---|
| Number | 48 | 64 | 34 | 27 | 173 |
| Age (mean, SD) | 31-79 (55.2, 9.84) | 28-88 (54.7, 10.52) | 45-75 (57.3, 7.11) | 29-70 (56.0, 10.51) | 28-88 (55.6, 9.71) |
| Sex | |||||
| Male (%) | 38 (79.2) | 46 (71.9) | 28 (82.4) | 22 (81.5) | 134 (77.5) |
| Female (%) | 10 (20.8) | 18 (28.1) | 6 (17.6) | 5 (18.5) | 39 (22.5) |
| Race | |||||
| White (%) | 38 (79.2) | 49 (76.6) | 25 (77.8) | 21 (77.8) | 133 (76.9) |
| Black (%) | 9 (18.8) | 14 (21.9) | 8 (23.5) | 3 (11.1) | 34 (19.6) |
| Other (%) | 1 (2.1) | 1 (1.6) | 1 (2.9) | 3 (11.1) | 6 (3.5) |
| Smoker | |||||
| Ever (%) | 25 (52.1) | 25 (39.1) | 8 (23.5) | 8 (29.6) | 64 (37.0) |
| Never (%) | 23 (47.9) | 35 (54.7) | 14 (41.2) | 12 (44.4) | 86 (49.7) |
| NA (%) | 0 (0.0) | 4 (6.2) | 12 (35.3) | 7 (25.9) | 23 (13.3) |
| Double cancer (%) | 8 (16.7) | 8 (12.5) | 5 (14.7) | 4 (14.8) | 25 (14.4) |
| HPV | |||||
| Positive (%) | 5 (10.4) | 10 (15.6) | 20 (58.8) | 22 (81.5) | 57 (33.0) |
| Negative (%) | 2 (4.2) | 8 (12.5) | 1 (2.9) | 0 (0.0) | 11 (6.4) |
| NA (%) | 41 (85.4) | 46 (71.9) | 13 (38.2) | 5 (18.5) | 105 (60.7) |
| Subsites | |||||
| Tonsil (%) | 19 (39.6) | 32 (50.0) | 15 (44.1) | 11 (40.7) | 77 (44.5) |
| Base of tongue (%) | 18 (37.5) | 20 (31.2) | 16 (47.0) | 14 (51.8) | 68 (39.3) |
| Other (%)*1 | 11 (22.9) | 12 (18.8) | 3 (8.8) | 2 (7.4) | 28 (16.2) |
| Stage 3 (%) | 10 (20.8) | 6 (9.4) | 3 (8.8) | 5 (18.5) | 24 (13.9) |
| 4 (%) | 38 (79.2) | 58 (90.6) | 31 (91.2) | 22 (81.5) | 149 (86.1) |
| Treatment*2 | |||||
| RT (%) | 23 (47.9) | 33 (51.6) | 22 (64.7) | 18 (66.7) | 96 (55.5) |
| Surgery (%) | 23 (47.9) | 27 (42.2) | 11 (32.4) | 9 (33.3) | 70 (40.5) |
| Other (%) | 2 (4.2) | 4 (6.2) | 1 (2.9) | 0 (0.0) | 7 (4.0) |
| Recurrence (%) | 7 (14.6) | 6 (9.4) | 3 (8.8) | 1 (3.7) | 17 (9.8) |
Other: include subsite unspecified.
RT: chemoradiotherapy (CRT) or radiotherapy alone (RT).
Surgery: surgery alone or surgery with neoadjuvant or adjuvant CRT or RT.
HPV status
HPV status was available for 68 patients (39.3%), and 57 patients (83.8%) were HPV positive. The proportion of the patients who had HPV status performed was 14.6% in 2003-4 and increased over the study period up to 81.5% in 2009 with statistically significant increasing trend (p < 0.001).
Imaging modalities utilization trends at baseline
Initial staging
For initial staging, the percentage of PET/CT use was 64.6% in 2003-4, 87.5% in 2005-6, 94.1% in 2007-8 and 96.3% in 2009, with an increasing trend (p < 0.001). The percentage of CT use was 68.8% in 2003-4, 34.4% in 2005-6, 58.8% in 2007-8 and 59.3% in 2009, while that of MRI use was 16.7% in 2003-4, 35.9% in 2005-6, 32.4% in 2007-8 and 29.6% in 2009. The percentage of PET/CT with contrast was 0% in 2003-4, 12.5% in 2005-6, 50.0% in 2007-8 and 48.2% in 2009 with an increasing trend (p < 0.001) (Figure 1). There was no obvious change over the study period in CT (p = 0.762) or MRI (p = 0.224) use for baseline evaluation.
Figure 1.
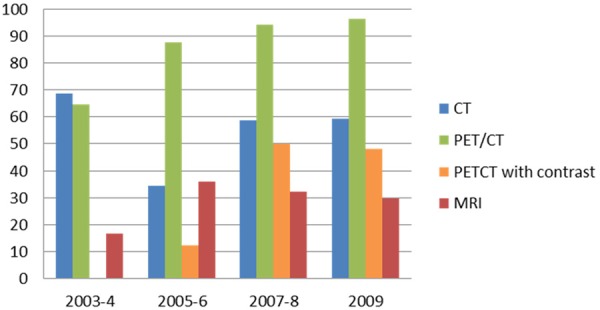
Percentage of imaging modalities used at initial staging (n = 173).
In subgroup analysis by treatment methods (RT or Surgery), the percentage of PET/CT use in RT group was 60.9% in 2003-4, 87.9% in 2005-6, 95.4% in 2007-8 and 100.0% in 2009, with an increasing trend (p < 0.001). The percentage of CT and MRI use in RT group was 82.6% and 21.7% in 2003-4, 39.4% and 45.4% in 2005-6, 59.1% and 31.8% in 2007-8 and 66.7% and 22.2% in 2009, respectively. The percentage of PET/CT with contrast was 0% in 2003-4, 18.2% in 2005-6, 40.9% in 2007-8 and 33.3% in 2009 with an increasing trend (p = 0.002) (Figure 2A). Similar to the results of whole patients analysis, neither CT nor MRI use showed any changing trend (p = 0.581 for CT use and p = 0.786 for MRI use). In Surgery group, the percentage of PET/CT use was 65.2% in 2003-4, 85.2% in 2005-6, 90.9% in 2007-8 and 88.9% in 2009. The trend test failed to reach statistical significance with p = 0.065. The percentage of CT use was 52.2% in 2003-4, 29.6% in 2005-6, 54.6% in 2007-8 and 44.4% in 2009, with no obvious trend. The use of MRI showed an increase in this subgroup with 13.0% in 2003-4, 29.6% in 2005-6, 36.4% in 2007-8 and 44.4% in 2009 (p = 0.048). The percentage of PET/CT with contrast was 0% in 2003-4, 7.4% in 2005-6, 63.6% in 2007-8 and 77.8% in 2009 with an increasing trend (p < 0.001) (Figure 2B).
Figure 2.
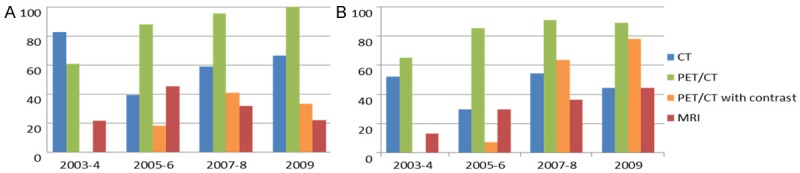
A: Percentage of imaging modalities used at initial staging in patients who received radiation therapy (n = 96). B: Percentage of imaging modalities used at initial staging in patients treated with Surgery (n = 70).
Post treatment imaging
As for post-treatment evaluation, the percentage of PET/CT use within two years was 59.5% in 2003-4, 68.6% in 2005-6, 89.7% in 2007-8 and 100% in 2009, with an increasing trend (p < 0.001). The percentage of PET/CT use within first six months after completion of treatment has also significantly increased: 50% in 2003-4, 47.1% in 2005-6, 82.8% in 2007-8 and 100% in 2009 (p < 0.001). On the other hand, the percentage of CT use within first two years was 76.2% in 2003-4, 88.2% in 2005-6, 62.1% in 2007-8 and 50.0% in 2009, which showed a decreasing trend (p = 0.004). The use of MRI was much less than other two modalities, with 19.0% in 2003-4, 11.8% in 2005-6, 13.8% in 2007-8 and 7.7% in 2009, and showed no significant increase or decrease (p = 0.231). The percentage of ‘PET/CT with contrast’ within first two years was 4.6% in 2003-4, 33.3% in 2005-6, 72.4% in 2007-8 and 88.5% in 2009 with an increasing trend (p < 0.001) (Figure 3).
Figure 3.
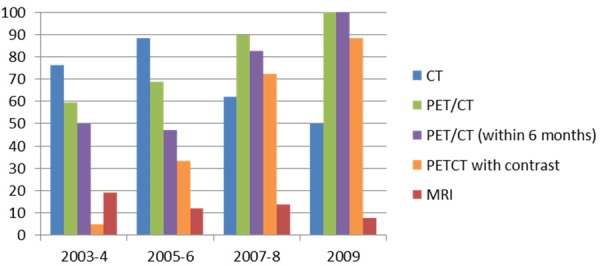
Percentage of imaging modalities used post-treatment (n = 148).
In treatment subgroup analyses, overall trends were similar to the results of whole patients. In RT group, the percentage of PET/CT use within first two years was 66.7% in 2003-4, 72.0% in 2005-6, 95.0% in 2007-8 and 100% in 2009, and that of PET/CT use within six months was 52.4% in 2003-4, 60.0% in 2005-6, 95.0% in 2007-8 and 100% in 2009, both with a significant increasing trend (p < 0.001). The percentage of PET/CT with contrast was 4.8% in 2003-4, 40.0% in 2005-6, 75.0% in 2007-8 and 100.0% in 2009 with an increasing trend (p < 0.001). On the other hand, the percentage of CT use was 81.0% in 2003-4, 84.0% in 2005-6, 55.0% in 2007-8 and 50.0% in 2009, with a decreasing trend (p = 0.008). The percentage of MRI use was 19.0% in 2003-4, 4.0% in 2005-6, 15.0% in 2007-8 and 11.1% in 2009, with no obvious trend (p = 0.718) (Figure 4A).
Figure 4.
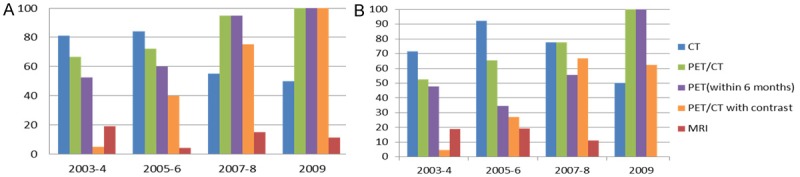
A: Percentage of imaging modalities used post-treatment in patients received radiation therapy (n = 96). B: Percentage of imaging modalities used post-treatment in patients treated with surgery (n = 70).
In Surgery group, the percentage of PET/CT use within two years was 52.4% in 2003-4, 65.4% in 2005-6, 77.8% in 2007-8 and 100% in 2009, and that of PET/CT within six months was 47.6% in 2003-4, 34.6% in 2005-6, 55.6% in 2007-8 and 100% in 2009, both with an increasing trend (p = 0.012 and 0.017, respectively). The percentage of PET/CT with contrast was 4.6% in 2003-4, 26.9% in 2005-6, 66.7% in 2007-8 and 62.5% in 2009 with an increasing trend (p < 0.001). The percentage of CT use was 71.4% in 2003-4, 92.3% in 2005-6, 77.8% in 2007-8 and 50.0% in 2009, with no statistical significant trend in this subgroup (p = 0.34). The percentage of MRI use was 19.0% in 2003-4, 19.2% in 2005-6, 11.1% in 2007-8 and 0% in 2009, showing no significant trend (p = 0.207) (Figure 4B).
Discussion
FDG PET/CT is useful in management of patients with many human solid tumors including diagnosis, treatment and follow up evaluation [8-13]. The analyses of data from Medicare beneficiaries between 2004 and 2008 by Hillner et al showed increase in use of PET for six types of cancers including head and neck cancers without change in use of CT or MRI, suggesting the additional role of PET to other imaging modalities in clinical setting [6]. Our data indicated similar trends that PET/CT use increased over the study period without obvious changes in CT or MRI use overall for initial staging evaluation. However, MRI utilization demonstrated increasing trends at the initial staging for the subgroup of patients who had primary surgical management. For post-treatment evaluation, CT use declined with increase in PET/CT use. Our result suggests that PET/CT played an additional role to CT and MRI for initial staging evaluation, and it was used as a replacement for CT for post-treatment follow up evaluation in most patients with oropharyngeal SCC.
In the head and neck, for initial staging of squamous cell cancers, PET/CT has been considered to be additional [2,7,14,15] or complementary test. Recent meta-analysis comparing imaging modalities in N0 neck showed similar diagnostic accuracy among CT, MRI, PET and US as a single modality to diagnose neck nodal metastasis [16]. However, a meta analysis of studies comparing studies in which both FDG PET and conventional diagnostic tests were performed, sensitivity and specificity of FDG PET were 80% and 86%, respectively, and of conventional diagnostic tests were 75% and 79%, respectively [17]. In addition, a prospective and multicentric trial on PET for the initial staging of head and neck squamous cell carcinoma indicated that additional PET improved initial staging and altered management for 13.7% of the study patients [15]. Thus, the most important contribution of PET/CT in head and neck cancer patients at baseline is for neck nodal staging and for identifying distant metastases.
However, the increasing trend of contrast enhanced head and neck PET/CT being performed at initial staging (Figure 5), it contributes to the primary site staging and surgical planning as appropriately, in addition to the neck nodal and distant staging. This obviate the need for contrast enhanced CT and PET/CT performed separately [18]. The increasing trend of MRI utilization at initial staging evaluation for the subgroup of patients who undergo primary surgical management could be explained by use of surgical robotic management and the requirement for excellent soft tissue delineation, including the need to exclude tongue muscle invasion before surgery.
Figure 5.
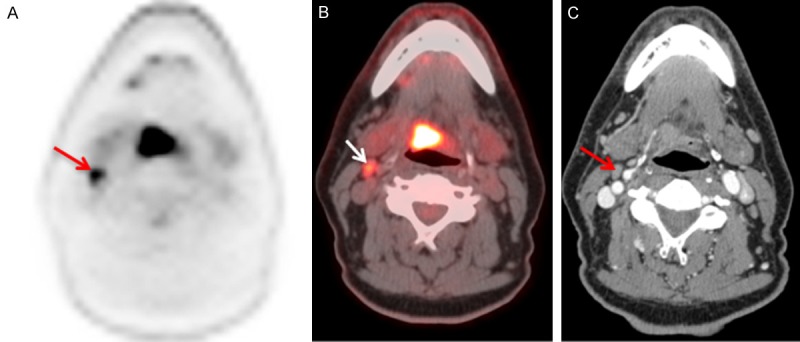
Value of contrast enhanced FDG PET/CT- Axial PET (A), fused PET/CT (B), contrast enhanced CT (C) images of a 60 year old gentleman with HPV associated squamous cell carcinoma of the tongue base who underwent a staging FDG PET/CT study. PET/CT shows hypermetabolic base of tongue mass (SUVmax – 4.6), hypermetabolic right level II cervical node. The contrast enhanced CT of the neck showed no invasion of the tongue muscles from the primary tumor and subcentimeter right level II cervical node. Contrast enhanced PET/CT provided the clinical information about the primary tumor as well as the nodal metastasis required for management decisions in a single imaging study.
PET/CT is increasingly used for radiotherapy planning, therapy assessment and identifying recurrence [19-21] in head and neck cancer, leading to his primary role after treatment among imaging modalities. In order to reduce side effect of radiotherapy, use of advanced radiotherapy technologies such as intensity-modulated radiotherapy (IMRT) is preferred [2]. It is reported that addition of PET/CT significantly altered treatment planning [22]. According to the most recent systematic review and meta analysis by Gupta et al, the pooled sensitivity, specificity, PPV and NPV of PET/CT for detecting residual or recurrence of head and neck squamous cell carcinoma after treatment were 79.9%, 87.5%, 58.6% and 95.1% [20]. Scans done > 12 weeks after completion of definitive therapy had moderately higher diagnostic accuracy in this meta-analysis.
Recently, PET/CT has been performed simultaneously with CT using intravenous contrast, which could have been the reason that CT as a stand alone modality has decreased for post-treatment evaluation. This has been observed in other institutions as well [18]. This might pose a question to be considered about PET/CT use. If PET/CT is an additional option besides CT as described in guidelines and recommendations, it should be performed sequentially after conventional evaluation by CT. On the other hand, PET/CT accuracy is superior in detecting the nodal and distant metastasis at initial staging, therapy response and follow up than conventional imaging by CT or MRI. In addition, performing the contrast enhanced PET/CT combines the benefits of PET/CT and contrast enhanced CT in one examination and further improves the anatomic characterization of primary tumor and adjacent structures. Hence contrast enhanced PET/CT needs to be considered as the modality of choice in staging and assessing therapy response. This has distinct advantages of improved work flow, convenience to patients, integration of functional and structural information to clinicians and potential radiation reduction [18].
Besides imaging modalities, a trend about HPV test was also clear in this study. The proportion of the patients who had assessment of HPV status increased dramatically during the study period. Since HPV testing became “recommended” in the latest NCCN Guideline (2), it is suggested that the clinical trend and the guideline have becoming consistent. It has been reported that HPV positive patients tend to have better prognosis than HPV negative patients [23-25]. Considering the high proportion of HPV positive patients among newly diagnosed oropharyngeal patients, the precise evaluation of disease status and role of PET/CT or contrast enhanced PET/CT need to be established in this context.
There are several major limitations in our study. Since this is a study at a single institution, the sample size is limited. In addition, head and neck cancers are highly heterogeneous diseases including a number of primary sites whose probability of metastases and prognosis varies considerably and hence the imaging utilization. As our intention was to evaluate a homogenous patient population as possible, we included only advanced oropharyngeal cancer, which limited the sample size. Another limitation is the retrospective nature of the study and we did not obtain individual information of health care insurance status, which may affect the utilization of expensive imaging modalities. Regardless of relatively small sample size, limited data source, and single institutional experience, the results of our study showed clear trends of increasing PET/CT use for advanced oropharyngeal cancer management in clinical setting. It is expected that this trend would continue, and more evaluation of imaging modalities utilization, especially economical evaluation, is required.
Conclusion
FDG-PET/CT has become the main modality with an increasing trend both for initial evaluation before treatment and for follow up evaluation for advanced oropharyngeal cancer at a leading institution for head and neck cancer and otolaryngology, over the period between 2003 and 2009. No significant change in the utilization rate of CT and MRI at initial staging, except for the subgroup of patients underwent primary surgical management. The utilization rate of CT as a stand alone imaging modality, for post treatment follow up, has declined significantly, partly due to increasing trend of contrast enhanced head and neck PET/CT is being performed for evaluation of head and neck cancer patients which improves the specificity of PET/CT in post treatment settings.
References
- 1.American Cancer Society. What are the key statistics about oral cavity and oropharyngeal cancers. Accessed on 23th March, 2013. [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Head and Neck Cancers version1. 2012. [Google Scholar]
- 3.Hammarstedt L, Lindquist D, Dahlstrand H, Romanitan M, Dahlgren LO, Joneberg J, Creson N, Lindholm J, Ye W, Dalianis T, Munck-Wikland E. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 4.Kubicek GJ, Champ C, Fogh S, Wang F, Reddy E, Intenzo C, Dusing RW, Machtay M. FDG-PET staging and importance of lymph node SUV in head and neck cancer. Head Neck Oncol. 2010;2:19. doi: 10.1186/1758-3284-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poeppel TD, Krause BJ, Heusner TA, Boy C, Bockisch A, Antoch G. PET/CT for the staging and follow-up of patients with malignancies. Eur J Radiol. 2009;70:382–392. doi: 10.1016/j.ejrad.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 6.Hillner BE, Tosteson AN, Song Y, Tosteson TD, Onega T, Goodman DC, Siegel BA. Growth in the use of PET for six cancer types after coverage by medicare: additive or replacement? J Am Coll Radiol. 2012;9:33–41. doi: 10.1016/j.jacr.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo J, Henderson S, Walker-Dilks C. Evidence-based guideline recommendations on the use of positron emission tomography imaging in head and neck cancer. Clin Oncol (R Coll Radiol) 2013;25:e33–66. doi: 10.1016/j.clon.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of liver metastases, part 1. AJR Am J Roentgenol. 2011;197:W256–259. doi: 10.2214/AJR.10.6331. [DOI] [PubMed] [Google Scholar]
- 9.Dibble EH, Karantanis D, Mercier G, Peller PJ, Kachnic LA, Subramaniam RM. PET/CT of Cancer Patients: Part 1, Pancreatic Neoplasms. AJR Am J Roentgenol. 2012;199:952–967. doi: 10.2214/AJR.11.8182. [DOI] [PubMed] [Google Scholar]
- 10.Paidpally V, Chirindel A, Lam S, Agrawal N, Quon H, Subramaniam RM. FDG-PET/CT imaging biomarkers in head and neck squamous cell carcinoma. Imaging Med. 2012;4:633–647. doi: 10.2217/iim.12.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davison JM, Subramaniam RM, Surasi DS, Cooley T, Mercier G, Peller PJ. FDG PET/CT in patients with HIV. AJR Am J Roentgenol. 2011;197:284–294. doi: 10.2214/AJR.10.6332. [DOI] [PubMed] [Google Scholar]
- 12.Mirpour S, Mhlanga JC, Logeswaran P, Russo G, Mercier G, Subramaniam RM. The Role of PET/CT in the Management of Cervical Cancer. AJR Am J Roentgenol. 2013;201:W192–205. doi: 10.2214/AJR.12.9830. [DOI] [PubMed] [Google Scholar]
- 13.Kruse M, Sherry SJ, Paidpally V, Mercier G, Subramaniam RM. FDG PET/CT in the Management of Primary Pleural Tumors and Pleural Metastases. AJR Am J Roentgenol. 2013;201:W215–226. doi: 10.2214/AJR.13.10572. [DOI] [PubMed] [Google Scholar]
- 14.de Bree R, Castelijns JA, Hoekstra OS, Leemans CR. Advances in imaging in the work-up of head and neck cancer patients. Oral Oncol. 2009;45:930–935. doi: 10.1016/j.oraloncology.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Lonneux M, Hamoir M, Reychler H, Maingon P, Duvillard C, Calais G, Bridji B, Digue L, Toubeau M, Gregoire V. Positron emission tomography with [18F] fluorodeoxyglucose improves staging and patient management in patients with head and neck squamous cell carcinoma: a multicenter prospective study. J. Clin. Oncol. 2010;28:1190–1195. doi: 10.1200/JCO.2009.24.6298. [DOI] [PubMed] [Google Scholar]
- 16.Liao LJ, Lo WC, Hsu WL, Wang CT, Lai MS. Detection of cervical lymph node metastasis in head and neck cancer patients with clinically N0 neck-a meta-analysis comparing different imaging modalities. BMC Cancer. 2012;12:236. doi: 10.1186/1471-2407-12-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyzas PA, Evangelou E, Denaxa-Kyza D, Ioannidis JP. 18F-fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma: a meta-analysis. J Natl Cancer Inst. 2008;100:712–720. doi: 10.1093/jnci/djn125. [DOI] [PubMed] [Google Scholar]
- 18.Subramaniam RM, Agarwal A, Colucci A, Ferraro R, Paidpally V, Mercier G. Impact of Concurrent Diagnostic Level CT With PET/CT on the Utilization of Stand-Alone CT and MRI in the Management of Head and Neck Cancer Patients. Clin Nucl Med. 2013;38:790–4. doi: 10.1097/RLU.0b013e31829f8ca5. [DOI] [PubMed] [Google Scholar]
- 19.Isles MG, McConkey C, Mehanna HM. A systematic review and meta-analysis of the role of positron emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol. 2008;33:210–222. doi: 10.1111/j.1749-4486.2008.01688.x. [DOI] [PubMed] [Google Scholar]
- 20.Gupta T, Master Z, Kannan S, Agarwal JP, Ghsoh-Laskar S, Rangarajan V, Murthy V, Budrukkar A. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38:2083–2095. doi: 10.1007/s00259-011-1893-y. [DOI] [PubMed] [Google Scholar]
- 21.Schoder H, Fury M, Lee N, Kraus D. PET monitoring of therapy response in head and neck squamous cell carcinoma. J Nucl Med. 2009;50(Suppl 1):74S–88S. doi: 10.2967/jnumed.108.057208. [DOI] [PubMed] [Google Scholar]
- 22.Garg MK, Glanzman J, Kalnicki S. The evolving role of positron emission tomography-computed tomography in organ-preserving treatment of head and neck cancer. Semin Nucl Med. 2012;42:320–327. doi: 10.1053/j.semnuclmed.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Tahari AK, Alluri KC, Quon H, Koch W, Wahl RL, Subramaniam RM. FDG PET/CT Imaging of Oropharyngeal Squamous Cell Carcinoma: Characteristics of Human Papillomavirus-Positive and -Negative Tumors. Clin Nucl Med. 2013;39:225–31. doi: 10.1097/RLU.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paidpally V, Tahari AK, Lam S, Alluri K, Marur S, Koch W, Wahl RL, Subramaniam RM. Addition of 18F-FDG PET/CT to Clinical Assessment Predicts Overall Survival in HNSCC: A Retrospective Analysis with Follow-up for 12 Years. J Nucl Med. 2013;54:2039–2045. doi: 10.2967/jnumed.113.121285. [DOI] [PubMed] [Google Scholar]
- 25.Subramaniam RM, Alluri KC, Tahari AK, Aygun N, Quon H. PET/CT Imaging and Human Papilloma Virus-Positive Oropharyngeal Squamous Cell Cancer: Evolving Clinical Imaging Paradigm. J Nucl Med. 2014;55:431–8. doi: 10.2967/jnumed.113.125542. [DOI] [PubMed] [Google Scholar]


